 A new iridium catalyst containing an imine–diphosphine ligand was developed for the hydrogenation of CO2 to formate.
A new iridium catalyst containing an imine–diphosphine ligand was developed for the hydrogenation of CO2 to formate.
Abstract
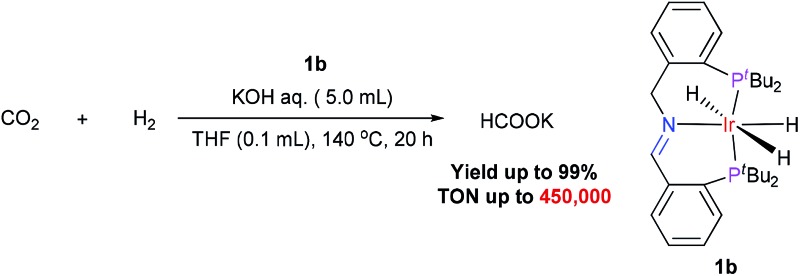
A new iridium catalyst containing an imine–diphosphine ligand has been developed, which showed high efficiency for the hydrogenation of CO2 to formate (yield up to 99%, TON up to 450 000). A possible catalytic mechanism is proposed, in which the imine group of the catalyst plays a key role in the cleavage of H2 and the activation of CO2.
Carbon dioxide (CO2), an economical, safe, environmentally friendly, and renewable carbon source, is an ideal one-carbon building block for organic chemicals, including carbohydrates and fuels.1 However, its thermodynamic and kinetic stability presents a fundamental obstacle to the use of CO2 in both academia and industry.2 High-energy reagents, harsh reaction conditions, and special activation mechanisms are typically required for transforming CO2 into other chemicals. Hydrogen is a green high-energy material that can be used to convert CO2 to valuable secondary energy carriers, such as methane, methanol, and formic acid. Formic acid is widely used in agriculture and in the leather and dye industries, as well as in fuel cells and synthetic chemistry.3
Homogeneous catalytic hydrogenation of CO2 into formic acid catalyzed by transition-metal complexes based on rhodium,4 ruthenium,5 iridium,6 iron,7 and cobalt8 has been extensively investigated since it was first reported in 1976 by Inoue et al.,4a who used Wilkinson's catalyst, RhCl(PPh3)3, to accomplish the reaction. In the early 1990s, Graf and Leitner4b achieved turnover numbers (TONs) as high as 3400 for CO2 hydrogenation reactions catalyzed by rhodium phosphine complexes. Noyori et al. 5a,b found that with RuH2(PMe3)4 as a catalyst, the reaction was more efficient in supercritical CO2 than in traditional organic media. Half-sandwich iridium(iii) complexes containing a strong electron donor ligand such as 4,4′-dihydroxy-2,2′-bipyridine or 4,7-dihydroxy-1,10-phenanthroline were used for the hydrogenation of CO2 by Himeda et al.,6b who reported a TON of 2.2 × 105. Subsequently, a significant breakthrough was made by Nozaki et al.,6c who discovered that an iridium(iii) trihydride complex with a pyridine-based PNP–pincer ligand has extraordinary catalytic activity (TON 3.5 × 106). However, from the practical point of view, the reported catalysts exhibited high activity only at harsh conditions such as high temperature and high pressure. Hence the research focus in the field of hydrogenation of CO2 is still the search for efficient catalysts, especially those with new activation models.
Late-transition-metal–amine complexes, which have a nitrogen atom with strong nucleophilicity, basicity, and donor ability, have been used as metal–ligand cooperative catalysts.9 A noteworthy example is the iridium hydride catalyst containing an amine–diphosphine ligand developed by Hazari et al.,6e which shows high activity for the hydrogenation of CO2. As part of our exploration of stable and efficient metal–ligand cooperative catalysts,10 we designed a new type of iridium(iii) catalysts containing imine–diphosphine ligands, which exhibit high activity for the hydrogenation of CO2. Herein, we report the preparation and characterization of iridium(iii)/imine–diphosphine catalysts 1 and their application for the hydrogenation of CO2 to formate.
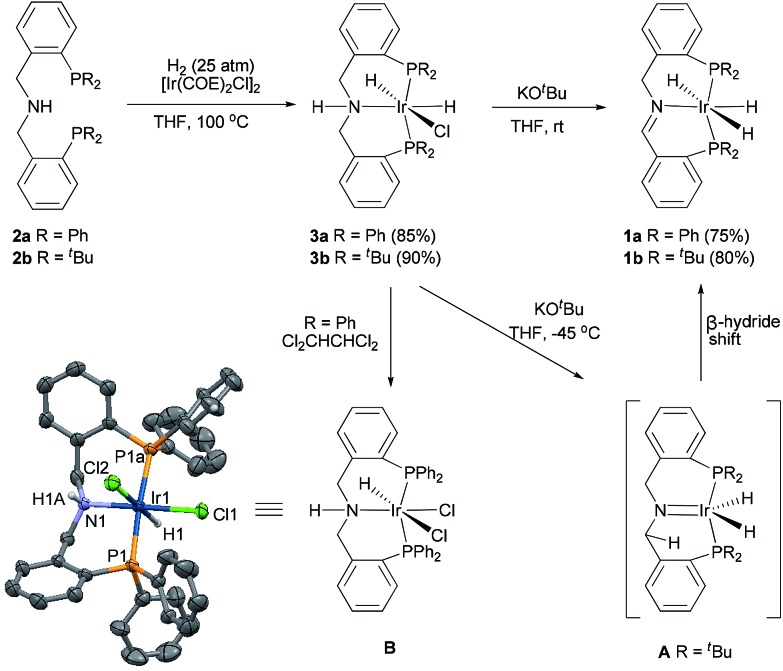
Catalysts 1 were synthesized in high yields by the complexation of tridentate amine–diphosphine ligands 2 (ref. 11) with [Ir(COE)2Cl]2 under hydrogen and subsequent treatment with base (Scheme 1). Complexation in THF at 100 °C produced complexes 3 (85–90% yield), which were stable solids that could be kept for several months under a nitrogen atmosphere. Catalysts 1 were obtained in 75–80% yield from the reaction of 3 with KO t Bu in THF. Interestingly, when we monitored the reaction of 3b by NMR at –45 °C, we observed a new species characterized by a singlet at δ = 54.84 ppm in the 31P NMR spectrum and a double-doublet at δ = –16.87 ppm (J = 21.8, 13.1 Hz) in the 1H NMR spectrum, neither of which belonged to 3b [31P NMR: δ = 40.74 ppm (t, J = 10.4 Hz); 1H NMR: δ = –21.87 ppm (td, J = 15.8, 7.4 Hz, 1H), –26.04 ppm (td, J = 16.9, 7.8 Hz, 1H)] or to 1b [31P NMR: δ = 52.98 ppm (dd, J = 13.0, 346.8 Hz), 59.83 ppm (dd, J = 7.3, 346.8 Hz); 1H NMR: δ = –10.77 ppm (t, J = 17.4 Hz, 2H), –19.39 ppm (t, J = 15.2 Hz, 1H)] (ESI). We speculated that this species was a 16-electron iridium dihydride complex, IrH2[( t Bu2PC6H4CH2)2N] (A),12 generated by the elimination of HCl from 3b upon treatment with KO t Bu. Complex A was unstable and rapidly converted to an 18-electron iridium trihydride complex of imine–diphosphine complex 1b by means of a shift of a hydride from CH2 to iridium.13
Scheme 1. The preparation of iridium catalysts 1 containing imine–diphosphine ligands and the X-ray structure of complex B. The H atoms, except for the hydride ligands, and disorder were omitted for clarity. Thermal ellipsoids shown at the 30% probability level. Selected bond lengths [Å] and angles [°] for complex B: Ir1–P1a 2.319 (2), Ir1–N1 2.115(7), Ir1–Cl1 2.391(3), Ir1–Cl2 2.579(3); P1a–Ir1–N1 91.59(4), P1a–Ir1–P1 174.90(9), N1–Ir1–Cl2 82.5(2), N1–Ir1–Cl1 177.3(2).
Attempts to grow crystals of 3 and 1 were unsuccessful. Fortunately, 3a slowly underwent an exchange of hydride to chloride in 1,1,2,2-tetrachloroethane, affording dichloride complex B as a crystal (Scheme 1).14 X-ray crystallographic analysis of B confirmed the structure of 3a as having one nitrogen atom and two phosphorus atoms coordinated to iridium, as well as two six-membered heterometallic rings. In the crystal of B, two phosphines were coordinated to iridium in a trans arrangement (P1a–Ir–P1 174.90(9)°), and two chlorides were in a cis arrangement (Cl1–Ir–Cl2 100.24(8)°). One chloride (Cl2) was cis to the nitrogen (Cl2–Ir–N 82.5(2)°), and the other chloride (Cl1) was trans to the nitrogen (Cl1–Ir–N 177.3(2)°). The bite angles of the ligands in the complexes 3 and 1 are larger than those in the conventional PNP ligands. 6c,e The flexibility of the ligands in the complexes 3 and 1 is important because a proton is released from the ligand during the catalytic process, which changes the conformation of the ligand.
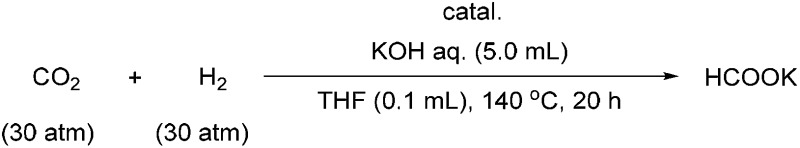
The activity of the iridium catalysts 1 for the hydrogenation of CO2 to formate was evaluated. The initial experiments were carried out under 60 atm of 1 : 1 CO2/H2 in aqueous KOH in the presence of 0.1 mol% iridium catalyst at 140 °C for 20 h. Catalysts 1a and 1b gave 93% and 99% yields of formate, respectively (Table 1, entries 1 and 2). When the catalyst loading was reduced to 0.001 mol%, 1b, with four tert-butyl groups on the phosphorus atoms, it still afforded an 81% yield (entry 4); however, 1a, which has four phenyl groups on the phosphorus atoms, gave only a 23% yield (entry 3). Increasing the reaction temperature and extending the reaction time have a negligible improvement on the yield of the reaction. The use of K3PO4 and other weaker bases resulted in a lower yield. We were delighted to find that 1b could be generated in situ from 3b under the reaction conditions, and the yield (78%) with the in situ-generated catalyst was similar to that obtained from the isolated catalyst (entries 4 and 6). The use of the stable catalyst precursor 3b, instead of the active catalyst 1b itself, offered an additional advantage for the reaction performance.
Table 1. The hydrogenation of CO2 to formate a .
| |||
| Entry | Catalyst (mol%) | Yield b (%) | TON c (×103) |
| 1 | 1a (0.1) | 93 | 0.9 |
| 2 | 1b (0.1) | 99 | 1 |
| 3 | 1a (0.001) | 23 | 23 |
| 4 | 1b (0.001) | 81 | 81 |
| 5 | 3a (0.001) | 22 | 22 |
| 6 | 3b (0.001) | 78 | 78 |
| 7 | 4 (0.001) | 5 | 5 |
| 8 | 5a (0.001) | 10 | 10 |
| 9 | 5b (0.001) | 14 | 14 |
| 10 | 6a (0.001) | 2 | 2 |
| 11 | 6b (0.001) | 8 | 8 |
| 12 | 7a (0.001) | 6 | 6 |
| 13 | 7b (0.001) | 1 | 1 |
| 14 | 7c (0.001) | 2 | 2 |
aGeneral reaction conditions: 5 mL aqueous 1 M KOH solution, 0.1 mol% or 0.001 mol% iridium catalyst relative to KOH in 100 μL THF, 60 atm of H2 : CO2 (1 : 1, initial), 140 °C, 20 h.
bYield, which represents conversion of the added KOH, based on 1H NMR analysis with sodium 3-(trimethylsilyl)-1-propanesulfonate as an internal standard.
cTON = turnover number; number of moles of product formed per mole of catalyst.
Using the most active catalyst (1b), we optimized the conditions for the hydrogenation of CO2 to formate (Table 2). First, we investigated the influence of the initial pressures of H2 and CO2 and found that when the total initial pressure of a 1 : 1 mixture of H2 and CO2 was lowered from 60 to 40 atm, the yield of formate decreased from 81% to 35% (entries 1 and 2). Raising the H2/CO2 ratio from 1 : 1 to 2 : 1 or to 5 : 1, at the same total initial pressure, markedly increased the yield (to 99%, entries 3 and 4). Increasing the initial concentration of KOH from 1 M to 2 M or 5 M at the catalyst loading of 0.0001 mol% (relative to KOH) dramatically increased the yield and TON of the reaction (entries 6 and 7 vs. 5).
Table 2. Optimization of reaction conditions a .
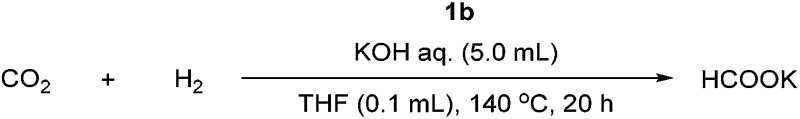
| ||||
| Entry | P(H2)/P(CO2) (atm) | KOH (M) | Yield b (%) | TON c (×103) |
| 1 | 30/30 | 1 | 81 | 81 |
| 2 | 20/20 | 1 | 35 | 35 |
| 3 | 40/20 | 1 | 99 | 99 |
| 4 | 50/10 | 1 | 99 | 99 |
| 5 d | 40/20 | 1 | 20 | 200 |
| 6 d | 40/20 | 2 | 34 | 340 |
| 7 d | 40/20 | 5 | 45 | 450 |
aGeneral reaction conditions: 5 mL aqueous KOH solution, 0.001 mol% iridium catalyst 1b relative to KOH in 100 μL THF, under the desired CO2 : H2 pressure, 140 °C, 20 h.
bYield, which represents conversion of the added KOH, based on 1H NMR analysis with sodium 3-(trimethylsilyl)-1-propanesulfonate as an internal standard.
cTON = turnover number; number of moles of product formed per mole of catalyst.
dCatalyst loading was 0.0001 mol% relative to KOH.
Under the optimal reaction conditions (P(H2)/P(CO2) 40/20 atm, 5 M KOH, 140 °C, 0.0001 mol% 1b), formate was obtained in 45% yield (TON = 450 000, turnover frequency = 22 500 h–1, entry 7). This result showed that 1b was a very efficient catalyst for the conversion of CO2 to formate under relatively mild reaction conditions.
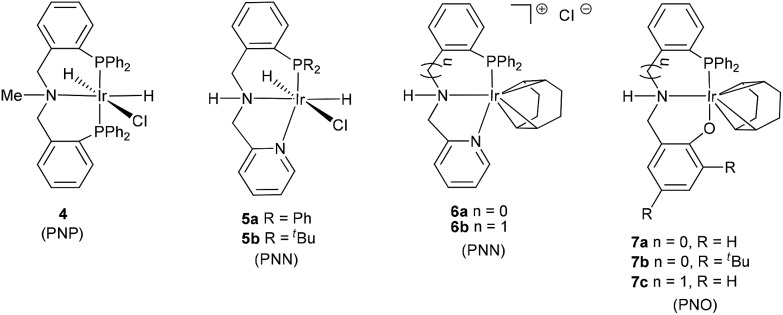
For a comparison, the iridium complexes with tridentate PNP, PNN,15 and PNO16 ligands (4–7, Fig. 1) were also synthesized (for details, ESI†), and their catalytic activities for the hydrogenation of CO2 to formate were studied. The very low activity of complex 4 containing N-methyl (entry 7, Table 1) indicates that the NH moiety in catalyst 3 was essential for the formation of iridium(iii)/imine–diphosphine catalysts 1. The iridium complexes with PNN ligands (5 and 6) and PNO ligands (7), which can also form iridium(iii)/imine–diphosphine catalysts, showed extremely low activity for the hydrogenation of CO2 (Table 1, entries 8–14).
Fig. 1. Other iridium complexes containing tridentate PNP, PNN, and PNO ligands.
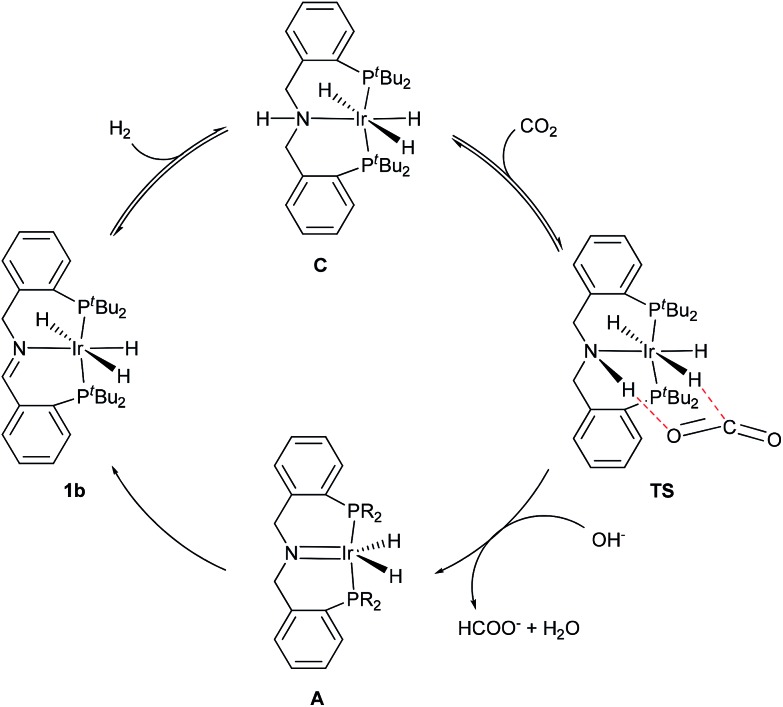
On the basis of the above-described experimental results for the hydrogenation of CO2 catalyzed by iridium(iii)/imine–diphosphine, we proposed a mechanism involving metal–imine cooperative catalysis (Scheme 2).17 First, a molecule of H2 adds to the C N double bond of 1b to generate iridium–trihydride complex C, which was isolated in 75% yield and was identified (ESI†). Base-mediated addition of the N–H proton and the Ir–H hydride of C to the carbonyl group of CO2 via a six-membered-ring transition state (TS) generates formic acid and complex A, which was detected by NMR (vide ante). A rapid shift of hydride from α-CH2 to iridium regenerated the catalyst 1b and finished a catalytic cycle. An experiment with C and CO2 showed the formation of intermediate A, which was quickly converted to complex 1b in 69% yield. This metal–imine bi-functional catalysis provides a highly efficient hydrogenation of CO2.
Scheme 2. Proposed mechanism for the hydrogenation of CO2 catalyzed by 1b.
In summary, we developed a new type of iridium catalyst containing an imine–diphosphine ligand, which showed high activity for the hydrogenation of CO2 to formate (yields up to 99%, TONs up to 450 000). The Ir–imine–diphosphine-catalyzed hydrogenation of CO2 proceeded through a metal–imine bi-functional mechanism. This activation model should be applicable for other transformations.
Acknowledgments
We thank the National Natural Science Foundation of China, the National Basic Research Program of China (2012CB821600), and the “111” project (B06005) of the Ministry of Education of China for financial support.
Footnotes
References
- (a) Gibson D. H. Chem. Rev. 1996;96:2063. doi: 10.1021/cr940212c. [DOI] [PubMed] [Google Scholar]; (b) Aresta M., in Carbon Dioxide as Chemical Feedstock, Wiley-VCH, Weinheim, 2010. [Google Scholar]; (c) Aresta M., Dibenedetto A., Angelini A. Chem. Rev. 2014;114:1709. doi: 10.1021/cr4002758. [DOI] [PubMed] [Google Scholar]
- (a) Leitner W. Coord. Chem. Rev. 1996;153:257. [Google Scholar]; (b) Sakakura T., Choi J. C., Yasuda H. Chem. Rev. 2007;107:2365. doi: 10.1021/cr068357u. [DOI] [PubMed] [Google Scholar]
- (a) Federsel C., Jackstell R., Beller M. Angew. Chem., Int. Ed. 2010;49:6254. doi: 10.1002/anie.201000533. [DOI] [PubMed] [Google Scholar]; (b) Peters M., Köhler B., Kuckshinrichs W., Leitner W., Markewitz P., Müller T. E. ChemSusChem. 2011;4:1216. doi: 10.1002/cssc.201000447. [DOI] [PubMed] [Google Scholar]; (c) Boddien A., Mellmann D., Gärtner F., Jackstell R., Junge H., Dyson P. J., Laurenczy G., Ludwig R., Beller M. Science. 2011;333:1733. doi: 10.1126/science.1206613. [DOI] [PubMed] [Google Scholar]
- (a) Inoue Y., Izumida H., Sasaki Y., Hashimoto H. Chem. Lett. 1976:863. [Google Scholar]; (b) Graf E., Leitner W. J. Chem. Soc., Chem. Commun. 1992:623. [Google Scholar]; (c) Tsai J. C., Nicholas K. M. J. Am. Chem. Soc. 1992;114:5117. [Google Scholar]; (d) Burgemeister T., Kastner F., Leitner W. Angew. Chem., Int. Ed. 1993;32:739. [Google Scholar]; (e) Angermund K., Baumann W., Dinjus E., Fornika R., Görls H., Kessler M., Kruger C., Leitner W., Lutz F. Chem.–Eur. J. 1997;3:755. [Google Scholar]; (f) Ezhova N. N., Kolesnichenko N. V., Bulygin A. V., Slivinskii E. V., Han S. Russ. Chem. Bull., Int. Ed. 2002;51:2165. [Google Scholar]; (g) Li Y.-N., He L.-N., Liu A.-H., Lang X.-D., Yang Z.-Z., Yu B., Luan C.-R. Green Chem. 2013;15:2825. [Google Scholar]
- (a) Jessop P. G., Ikariya T., Noyori R. Nature. 1994;368:231. [Google Scholar]; (b) Jessop P. G., Hsiao Y., Ikariya T., Noyori R. J. Am. Chem. Soc. 1996;118:344. [Google Scholar]; (c) Munshi P., Main A. D., Linehan J. C., Tai C. C., Jessop P. G. J. Am. Chem. Soc. 2002;124:7963. doi: 10.1021/ja0167856. [DOI] [PubMed] [Google Scholar]; (d) Hayashi H., Ogo S., Fukuzumi S. Chem. Commun. 2004:2714. doi: 10.1039/b411633j. [DOI] [PubMed] [Google Scholar]; (e) Yu Y.-M., Zhang Y.-P., Fei J.-H., Zheng X.-M. Chin. J. Chem. 2005;23:977. [Google Scholar]; (f) Urakawa A., Jutz F., Laurenczy G., Baiker A. Chem.–Eur. J. 2007;13:3886. doi: 10.1002/chem.200601339. [DOI] [PubMed] [Google Scholar]
- (a) Himeda Y., Onozawa-Komatsuzaki N., Sugihara H., Arakawa H., Kasuga K. Organometallics. 2004;23:1480. [Google Scholar]; (b) Himeda Y., Onozawa-Komatsuzaki N., Sugihara H., Kasuga K. Organometallics. 2007;26:702. [Google Scholar]; (c) Tanaka R., Yamashita M., Nozaki K. J. Am. Chem. Soc. 2009;131:14168. doi: 10.1021/ja903574e. [DOI] [PubMed] [Google Scholar]; (d) Sanz S., Benítez M., Peris E. Organometallics. 2010;29:275. [Google Scholar]; (e) Schmeier T. J., Dobereiner G. E., Crabtree R. H., Hazari N. J. Am. Chem. Soc. 2011;133:9274. doi: 10.1021/ja2035514. [DOI] [PubMed] [Google Scholar]
- (a) Federsel C., Boddien A., Jackstell R., Jennerjahn R., Dyson P. J., Scopelliti R., Laurenczy G., Beller M. Angew. Chem., Int. Ed. 2010;49:9777. doi: 10.1002/anie.201004263. [DOI] [PubMed] [Google Scholar]; (b) Langer R., Diskin-Posner Y., Leitus G., Shimon L. J. W., Ben-David Y., Milstein D. Angew. Chem., Int. Ed. 2011;50:9948. doi: 10.1002/anie.201104542. [DOI] [PubMed] [Google Scholar]; (c) Ziebart C., Federsel C., Anbarasan P., Jackstell R., Baumann W., Spannenberg A., Beller M. J. Am. Chem. Soc. 2012;134:20701. doi: 10.1021/ja307924a. [DOI] [PubMed] [Google Scholar]
- Jeletic M. S., Mock M. T., Appel A. M., Linehan J. C. J. Am. Chem. Soc. 2013;135:11533. doi: 10.1021/ja406601v. [DOI] [PubMed] [Google Scholar]
- For selected papers on the metal–ligand cooperative catalysis in hydrogenation, see: ; (a) Ohkuma T., Ooka H., Hashiguchi S., Ikariya T., Noyori R. J. Am. Chem. Soc. 1995;117:2675. [Google Scholar]; (b) Ikariya T., Blacker A. J. Acc. Chem. Res. 2007;40:1300. doi: 10.1021/ar700134q. [DOI] [PubMed] [Google Scholar]; (c) Gunanathan C., Milstein D. Acc. Chem. Res. 2011;44:588. doi: 10.1021/ar2000265. [DOI] [PubMed] [Google Scholar]; (d) Xie J.-B., Xie J.-H., Liu X.-Y., Zhang Q.-Q., Zhou Q.-L. Chem.–Asian J. 2011;6:899. doi: 10.1002/asia.201000716. [DOI] [PubMed] [Google Scholar]; (e) Xie J.-H., Liu X.-Y., Xie J.-B., Wang L.-X., Zhou Q.-L. Angew. Chem., Int. Ed. 2011;50:7329. doi: 10.1002/anie.201102710. [DOI] [PubMed] [Google Scholar]; (f) Han Z. B., Rong L. C., Wu J., Zhang L., Wang Z., Ding K. L. Angew. Chem., Int. Ed. 2012;51:13041. doi: 10.1002/anie.201207781. [DOI] [PubMed] [Google Scholar]
- For papers on the metal–ligand cooperative catalysis in hydrogenation reported by our group, see ref. 9d and e
- For the synthesis of ligands 2, ESI.
- For other iridium complexes of amino-phosphine ligands, see: Clarke Z. E., Maragh P. T., Dasgupta T. P., Gusev D. G., Lough A. J., Abdur-Rashid K., Organometallics, 2006, 25 , 4113 . [Google Scholar]
- For selected papers on the β-hydride shift of transition-metal complexes, see: ; (a) Cross R. J., in The Chemistry of the Metal Carbon Bond, ed. F. R. Hartley and S. Patai, John Wiley, New York, 1985, vol. 2, p. 559. [Google Scholar]; (b) Hartwig J. F. J. Am. Chem. Soc. 1996;118:7010. [Google Scholar]; (c) Ritter J. C. M., Bergman R. G. J. Am. Chem. Soc. 1998;120:6826. [Google Scholar]; (d) Scherer W., Wolstenholme D. J., Herz V., Eickerling G., Brück A., Benndorf P., Roesky P. W. Angew. Chem., Int. Ed. 2010;49:2242. doi: 10.1002/anie.200905463. [DOI] [PubMed] [Google Scholar]; (e) Gómez-Gallego M., Sierra M. A. Chem. Rev. 2011;111:4857. doi: 10.1021/cr100436k. [DOI] [PubMed] [Google Scholar]; (f) Schnerder S., Meiners J., Askevold B. Eur. J. Inorg. Chem. 2012:412. [Google Scholar]
- For selected papers on the ligand exchange of hydride by chloride on iridium, see: ; (a) Chetcuti P. A., Hawthorne M. F. J. Am. Chem. Soc. 1987;109:942. [Google Scholar]; (b) Dyson G., Zech A., Rawe B. W., Haddow M. F., Hamilton A., Owen G. R. Organometallics. 2011;30:5844. [Google Scholar]; (c) Ciganda R., Garralda M. A., Ibarlucea L., Mendicute-Fierro C., Torralba M. C., Torres M. R. Inorg. Chem. 2012;51:1760. doi: 10.1021/ic202065d. [DOI] [PubMed] [Google Scholar]
- For selected papers on the PNN ligands, see: ; (a) Doherty S., Knight J. G., Scanla T. H., Elsegood M. R. J., Clegg W. J. Organomet. Chem. 2002;650:231. [Google Scholar]; (b) Zotto A. D., Baratta W., Ballico M., Herdtweck E., Rigo P. Organometallics. 2007;26:5636. [Google Scholar]
- For selected papers on the PNO ligands, see: ; (a) Hu W.-Q., Sun X.-L., Wang C., Gao Y., Tang Y., Shi L.-P., Xia W., Sun J., Dai H.-L., Li X.-Q., Yao X.-L., Wang X.-R. Organometallics. 2004;23:1684. [Google Scholar]; (b) Oakes D. C. H., Kimberley B. S., Gibson V. C., Jones D. J., White A. J. P., Williams D. J. Chem. Commun. 2004:2174. doi: 10.1039/b409870f. [DOI] [PubMed] [Google Scholar]
- For other examples with a metal–ligand cooperative catalysis mechanism, see ref. 9
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.