Abstract
Aims
This post hoc assessment evaluated the efficacy and safety of once‐daily, prandial glucagon‐like peptide‐1 receptor agonist lixisenatide in patients with type 2 diabetes (T2D) and normal renal function (estimated glomerular filtration rate ≥90 mL/min), or mild (60‐89 mL/min) or moderate (30‐59 mL/min) renal impairment.
Methods
Patients from 9 lixisenatide trials in the GetGoal clinical trial programme were categorized by baseline creatinine clearance: normal renal function (lixisenatide n = 2094, placebo n = 1150); renal impairment (mild: lixisenatide n = 637, placebo n = 414; moderate: lixisenatide n = 122, placebo n = 68). Meta‐analyses of placebo‐adjusted mean differences between baseline renal categories were performed for efficacy and safety outcomes.
Results
HbA1c, 2‐hour postprandial plasma glucose and fasting plasma glucose were comparably reduced in lixisenatide‐treated patients with normal renal function, and mild and moderate renal impairment. The most common adverse events (AEs) in all renal function categories were gastrointestinal (GI), predominantly nausea and vomiting. A 14% higher incidence of GI AEs and a 10% higher incidence of nausea and vomiting were seen with mild impairment vs normal function (P = .003 for both), but no significant differences were observed between the mild and moderate impairment categories (P = .99 and P = .57, respectively), or between the moderate impairment and normal categories (P = .16 and P = .65, respectively). Additionally, the incidence of hypoglycaemia was similar in all categories.
Conclusions
This study demonstrates that baseline renal status does not affect efficacy outcomes in lixisenatide‐ vs placebo‐treated patients, and that no lixisenatide dose adjustment is required for patients with T2D with mild or moderate renal impairment.
Keywords: GLP‐1, incretin therapy, meta‐analysis, type 2 diabetes
1. INTRODUCTION
Type 2 diabetes (T2D) is a progressive life‐threatening disease associated with altered glucose homoeostasis.1 The negative effects of the associated chronic hyperglycaemia may compromise organ and tissue function, including the cardiovascular system, eyes, nerves and kidneys.2
Owing to the systemic nature of the disease, clinical management of T2D is often complex and may be suboptimal, with associated co‐morbidities often confounding the issue.3 Of these co‐morbid conditions, chronic kidney disease is particularly common in T2D, with about one‐quarter of patients aged ≥60 years exhibiting moderate or severe impairment of renal function.4
A precise definition of the advantages and disadvantages of available therapeutic options in relation to the disease characteristics and co‐morbidities of the individual patient is needed to select the most appropriate treatment in this setting. In particular, it is essential to understand the pharmacokinetic profile, and specifically the mechanism of excretion, of any drug administered, and to adjust dosing accordingly.5
The most recent recommendations from the American Diabetes Association/European Association for the Study of Diabetes (ADA/EASD) suggest that first‐line therapy for T2D should be lifestyle modifications and metformin monotherapy.6 However, further guidance from the ADA states that the use of metformin is contraindicated in individuals with severe renal disease,7 with recent Food and Drug Administration (FDA) guidance advising that metformin may be used in patients with mild impairment and only in some with moderate impairment.8
In patients with T2D where first‐line metformin monotherapy does not provide sufficient glycaemic control or is not indicated, guidelines recommend the introduction of one of a number of different therapeutic options, including sulphonylureas, sodium–glucose co‐transporter‐2 (SGLT2) inhibitors, thiazolidinediones, dipeptidyl peptidase‐4 (DPP‐4) inhibitors, basal insulin or glucagon‐like peptide‐1 receptor agonists (GLP‐1 RAs).6 The extent of clinical experience with these drug classes in patients with renal disease varies, with many of these treatment options contraindicated or not recommended in patients with moderate or severe impairment9; specific concerns have been raised regarding the use of certain sulphonylureas and SGLT2 inhibitors in this population. With regard to GLP‐1 RAs, there is a relative lack of data investigating their use in patients with impaired renal function.10 The evidence to date indicates that exenatide is eliminated by renal mechanisms and should not be given to patients with severe impairment.10 Further data indicate that the glycaemic efficacy of liraglutide does not appear to be affected by moderate impairment.11 Elimination of lixisenatide, a once‐daily, injectable, prandial GLP‐1 RA, is presumed to occur via renal filtration, tubular reabsorption and metabolic catabolism; the resulting metabolites do not appear to stimulate the GLP‐1 receptor.12 Furthermore, clinical evidence suggests that the pharmacokinetic profile of lixisenatide is largely unaffected by mild or moderate renal impairment; however, only limited pharmacokinetic data are available.10
The efficacy and safety of lixisenatide have been investigated extensively across the GetGoal clinical trial programme, which included patients with T2D with or without renal impairment.13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Overall, these studies have demonstrated that lixisenatide is significantly more effective than placebo at reducing levels of glycated haemoglobin (HbA1c). Moreover, treatment with lixisenatide was associated with significant reductions in postprandial plasma glucose (PPG) and fasting plasma glucose (FPG) compared with placebo.
In order to investigate the potential effects of renal impairment on the efficacy and safety of lixisenatide in patients with T2D, we conducted a post hoc meta‐analysis based on trials reported previously from the comprehensive GetGoal clinical trial programme.13, 14, 15, 16, 18, 19, 20, 21, 22
2. MATERIALS AND METHODS
This meta‐analysis included data from nine GetGoal trials that had been published at the time of the analysis (October 2014). An overview of the designs of these trials, along with their primary results, is presented in Table 1. Five of the trials included assessed lixisenatide as an add‐on to oral antidiabetic drugs in patients with T2D over 24 weeks. Three of the trials compared lixisenatide with placebo when added to basal insulin over 24 weeks. One trial assessed lixisenatide as monotherapy over a period of 12 weeks. Furthermore, 2 of the aforementioned trials included in this analysis were conducted in a predominantly Asian population. All trials were approved by the Institutional Review Boards or ethics committees of the participating centres, and were conducted in accordance with the principles of the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice guidelines. All participants provided written informed consent.
Table 1.
Summary of clinical trials included in the analysis
| Trial | Drug treatments | Trial duration | Primary endpoint as published previously ΔHbA1c from baseline to study enda |
|---|---|---|---|
| GetGoal‐M13 (n = 680) | Lixisenatide morning or evening vs placebo asadd‐on to metformin | 24 weeks | −0.87 (morning) vs −0.75 (evening) vs −0.38% (placebo; P < .0001 for both arms vs placebo) |
| GetGoal‐F114 (n = 482) | Lixisenatide 1‐ or 2‐step dose increase vs placebo as add‐on to metformin | 24 weeks | −0.92 (1‐step) vs −0.83 (2‐step) vs −0.42% (placebo; P < .0001 for both arms vs placebo) |
| GetGoal‐Mono15 (n = 361) | Lixisenatide monotherapy 1‐ or 2‐step dose increase vs placebo | 12 weeks | −0.85 (1‐step) vs −0.73 (2‐step) vs −0.19% (placebo; P < .0001 for both arms vs placebo) |
| GetGoal‐P16 (n = 484) | Lixisenatide vs placebo as add‐on to pioglitazone ± metformin | 24 weeks | −0.90 (lixisenatide) vs −0.34% (placebo; P = .0001) |
| GetGoal‐L‐Asia18 (n = 311) | Lixisenatide vs placebo as add‐on to basal insulin ± sulphonylureas | 24 weeks | −0.77 (lixisenatide) vs +0.11% (placebo; P < .0001) |
| GetGoal‐M‐Asia19 (n = 390) | Lixisenatide vs placebo as add‐on to metformin ± sulphonylureas | 24 weeks | −0.83 (lixisenatide) vs −0.47% (placebo; P = .0004) |
| GetGoal‐L20 (n = 495) | Lixisenatide vs placebo as add‐on to basal insulin ± metformin | 24 weeks | −0.74 (lixisenatide) vs −0.38% (placebo; P = .0002) |
| GetGoal‐Duo121 (n = 446) | Lixisenatide vs placebo as add‐on to newly initiated basal insulin glargine + metformin ± thiazolidinediones | 24 weeks | −0.71 (lixisenatide) vs −0.40% (placebo; P < .0001) |
| GetGoal‐S22 (n = 859) | Lixisenatide vs placebo as add‐on to sulphonylureas ± metformin | 24 weeks | −0.85 (lixisenatide) vs −0.10% (placebo; P < .0001) |
Abbreviation: HbA1c, glycated haemoglobin.
P‐values are for the treatment group difference.
Safety and efficacy data from patients in the selected trials were pooled and stratified by each patient's baseline renal function. This was assessed by estimated glomerular filtration rates (eGFR) using baseline creatinine clearance levels based on the Cockcroft–Gault formula.24 For the purposes of the present analysis, renal function was categorized as: “normal” (eGFR ≥90 mL/min); “mild renal impairment” (60‐89 mL/min); “moderate renal impairment” (30‐59 mL/min); or “severe renal impairment” (<30 mL/min), as defined by the ADA.25 As the included trials had exclusion criteria based on renal function, there was a lack of patients with severe renal impairment in the present analysis.
2.1. Study endpoints
Efficacy endpoints were calculated as placebo‐adjusted mean change from baseline to week 24 with lixisenatide (week 12 for GetGoal‐Mono15) for: HbA1c, 2‐hour PPG, FPG, basal insulin dose (when appropriate) and body weight. Not all trials included in the present analysis reported every endpoint; the number of studies and sample sizes for each endpoint are shown in Table S1, and the endpoints evaluated in each study are shown in Table S2.
In the GetGoal trials that assessed 2‐hour PPG, measurements were recorded at baseline and week 24 by administration of a standardized 600‐kcal liquid breakfast meal challenge test [400 mL of Ensure Plus (Abbott Nutrition, Columbus, OH) 30 minutes after drug administration, with PPG measured 2 hours after the breakfast was administered].
Analyses of safety endpoints included the placebo‐adjusted rate of treatment‐emergent adverse events (AEs) by system and organ class (SOC) for SOCs identified across all of the renal function subgroups and by higher level term (HLT) for AEs by SOC that were significantly more frequent with lixisenatide.
2.2. Statistical analyses
Analyses of efficacy endpoints were performed using the pooled modified intent‐to‐treat (mITT) populations from the nine trials, which comprised all randomized participants who received at least one dose of study treatment and had both a baseline and at least one post‐baseline assessment for any of the primary or secondary efficacy variables. All of the studies included in the meta‐analysis employed the last observation carried forward method. If the end‐of‐study value was missing for any endpoint, the last available post‐baseline value was used. Differences in mean changes from baseline to study end between lixisenatide‐ and placebo‐treated patients (placebo‐adjusted changes) were compared for each of the five efficacy endpoints within each renal function category and between categories.
Analyses of safety outcomes were performed using the safety population, which comprised all patients who received at least one dose of study drug. AEs grouped by SOC were analysed using risk differences (placebo‐adjusted rates). Comparisons between renal function categories were based on differences in placebo‐adjusted rates. Where there were significant differences in the number of AEs between categories, further analyses using all available HLTs or preferred terms were carried out.
To evaluate the differences in assessed efficacy endpoints and safety outcomes across studies, study‐level meta‐analyses (random effects) were performed using placebo‐adjusted changes from baseline or placebo‐adjusted rates, respectively. Comparisons between renal categories were carried out using effect estimates calculated by study as the difference in placebo‐adjusted changes or placebo‐adjusted rates between categories. Effect estimates and their standard errors were entered as generic inverse variances in the Cochrane algorithm RevMan 5.2. Cochran's Q‐test and I 2 statistic were used to assess homogeneity between studies.
3. RESULTS
3.1. Patient demographics and clinical characteristics at baseline
The distribution of patients in the combined safety population by renal function category and a summary of patient characteristics at baseline is given in Table 2.
Table 2.
Baseline demographics by treatment (safety population)
| Characteristic | Placebo | Lixisenatide |
|---|---|---|
| (N = 1639) | (N = 2869) | |
| n (%) | n (%) | |
| Sex | ||
| Male | 811 (49.5) | 1362 (47.5) |
| Female | 828 (50.5) | 1507 (52.5) |
| Age | ||
| <65 years | 1286 (78.5) | 2352 (82.0) |
| ≥65 years | 353 (21.5) | 517 (18.0) |
| <75 years | 1600 (97.6) | 2805 (97.8) |
| ≥75 years | 39 (2.4) | 64 (2.2) |
| Race | ||
| White | 973 (59.4) | 1898 (66.2) |
| Black | 43 (2.6) | 73 (2.5) |
| Asian/Oriental | 601 (36.7) | 843 (29.4) |
| Other | 22 (1.3) | 55 (1.9) |
| HbA1ca | ||
| <8% | 744 (45.4) | 1277 (44.5) |
| ≥8% | 895 (54.6) | 1592 (55.5) |
| Body mass indexb | ||
| <30 kg/m2 | 832 (50.8) | 1376 (48.0) |
| ≥30 kg/m2 | 807 (49.2) | 1493 (52.0) |
| Renal function category | ||
| Total | 1636 | 2853 |
| NormalCC ≥90 mL/min | 1150 (70.3) | 2094 (73.4) |
| Mild impairmentCC 60 to 89 mL/min | 414 (25.3) | 637 (22.3) |
| Moderate impairmentCC 30 to 59 mL/min | 68 (4.2) | 122 (4.3) |
| Severe impairmentCC <30 mL/min | 4 (0.2) | 0 (0.0) |
Abbreviations: CC, creatinine clearance; HbA1c, glycated haemoglobin.
Only patients with assessment were included for any particular variable.
Screening.
Baseline.
The combined safety population from the nine GetGoal trials included in this analysis comprised 2869 patients who received lixisenatide and 1639 patients who received placebo; the mITT population comprised 2720 patients and 1577 patients who received lixisenatide and placebo, respectively. Within the safety population, 3244 patients were classified as having normal renal function, 1051 patients had a mild renal impairment and 190 patients a moderate renal impairment. No patient had severe renal impairment at baseline in the lixisenatide group.
The majority of patients were white and <65 years old, with approximately half of the patients having HbA1c ≥8% (64 mmol/mol) at baseline (Table 2). Baseline rates of microalbuminuria, an indicator of diabetic nephropathy, were generally low and similar across all studies and both treatment arms included in the analysis (P = .80 for heterogeneity).
3.2. Comparisons of changes in efficacy variables from baseline to study end within baseline renal function categories
Of the five efficacy variables evaluated in this analysis, placebo‐adjusted mean differences in HbA1c, 2‐hour PPG and FPG were significantly lower at study end in lixisenatide‐ than in placebo‐treated patients in all three renal function categories (Table 3).
Table 3.
Efficacy of lixisenatide compared with placebo by renal function category (mITT population)
| Endpoint placebo‐adjusted mean difference | Normal | Mild impairment | Moderate impairment |
|---|---|---|---|
| CC ≥90 mL/min | CC 60‐89 mL/min | CC 30‐59 mL/min | |
| HbA1c (%) | −0.52 (−0.65, −0.39) | −0.50 (−0.68, −0.31) | −0.85 (−1.09, −0.61) |
| P < .00001 | P < .00001 | P < .00001 | |
| 2‐hour PPG (mmol/L) | −4.78 (−5.90, −3.66) | −5.08 (−6.58, −3.58) | −6.81 (−10.81, −2.82) |
| P < .00001 | P < .00001 | P < .001 | |
| FPG (mmol/L) | −0.70 (−0.91, −0.50) | −0.48 (−0.74, −0.22) | −0.78 (−1.36, −0.20) |
| P < .00001 | P < .001 | P < .01 | |
| Basal insulin dose (U) | −2.53 (−3.84, −1.21) | −1.79 (−2.76, −0.83) | −0.70 (−2.22, 0.81) |
| P < .001 | P < .001 | NS | |
| Weight (kg) | −0.64 (−0.93, −0.34) | −0.59 (−0.90, −0.28) | −0.47 (−1.37, 0.42) |
| P < .0001 | P < .001 | NS |
Abbreviations: CC, creatinine clearance; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; NS, not significant; PPG, postprandial plasma glucose.
All data are presented as mean (95% CI).
Basal insulin dose data were retrieved from three GetGoal trials (Table S2). In both the normal renal function and the mild impairment categories, end‐of‐treatment basal insulin dose was significantly lower with lixisenatide than with placebo. However, in the moderate renal impairment category there was no significant difference between lixisenatide and placebo in basal insulin dose.
End‐of‐treatment body weight was significantly lower compared with baseline in both the normal renal function and the mild impairment categories. Body weight loss was also observed in the moderate renal impairment category, but this did not reach statistical significance.
3.3. Meta‐analysis of changes in efficacy variables between renal function categories
Comparisons of normal vs mild renal function and mild vs moderate renal function were performed to examine the incremental effect of increasing renal impairment on efficacy measures, while the direct comparison of normal vs moderate renal function demonstrates the cumulative effect.
When between‐category changes for normal vs mild renal impairment and mild vs moderate renal impairment were assessed across the studies, there was no significant difference in HbA1c reductions at study end for patients with normal renal function vs those with mild renal impairment (P = .58), between patients with mild and moderate renal impairment (P = .36), or between patients with normal and moderate renal impairment (P = .20; Figure 1a).
Figure 1.
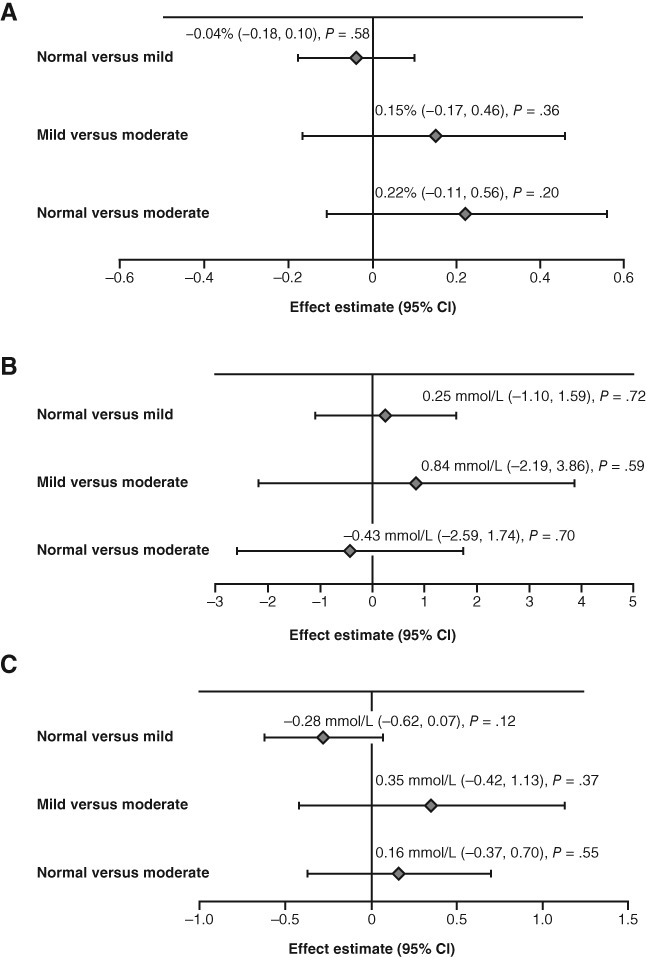
Placebo‐adjusted meta‐analysis of change in A, HbA1c; B, 2‐hour PPG and C, FPG from baseline to week 24 (week 12 for GetGoal‐Mono) between renal function categories in patients receiving lixisenatide. Normal renal function: eGFR ≥90 mL/min; mild impairment: eGFR 60‐89 mL/min; moderate impairment: eGFR 30‐59 mL/min. CI, confidence interval; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; PPG, postprandial plasma glucose
No difference was observed in placebo‐adjusted change in 2‐hour PPG at week 24 between patients with normal function compared with those with mild renal impairment (P = .72), between patients with mild and moderate renal impairment (P = .59), or between patients with normal function and those with moderate renal impairment (P = .70; Figure 1b).
Similarly, the difference in placebo‐adjusted FPG reductions from baseline was not significant for patients with normal renal function vs those with mild renal impairment (P = .12), between patients with mild and moderate renal impairment (P = .37), or between patients with normal function and those with moderate renal impairment (P = .55; Figure 1c).
Data for these three subgroup analyses, demonstrating the cumulative effect of increasing renal impairment (i.e. the normal vs moderate renal function comparisons) in each original study population can be seen in Figure S1.
There was no significant placebo‐adjusted difference in insulin dose change from baseline between the normal renal function and the mild impairment categories [−0.82 U; 95% confidence interval (CI) −2.65, 1.03; P = .39], or between the mild and moderate renal impairment categories (−3.20 U; 95% CI −8.31, 1.92; P = .22).
Finally, no significant difference was seen in placebo‐adjusted body weight change from baseline between the normal renal function and the mild impairment categories (−0.05 kg; 95% CI −0.47, 0.38; P = .82), or between the mild and moderate renal impairment categories (−0.19 kg; 95% CI −1.11, 0.73; P = .69).
3.4. Meta‐analysis of safety outcomes
Adverse events were analysed using SOCs that were identified across all of the renal function categories. There were 14 SOCs that met this criterion: gastrointestinal (GI) disorders; nervous system disorders; general disorders and administration‐site conditions; musculoskeletal and connective tissue disorders; skin and subcutaneous tissues disorders; metabolism and nutrition disorders; psychiatric disorders; cardiac disorders; injury, poisoning and procedural disorders; renal and urinary disorders; eye disorder investigations; respiratory, thoracic and mediastinal disorders; and infections and infestations. There were no significant differences between the normal and mild impairment or between the normal and moderate impairment renal function categories, with the exception of the GI disorders and metabolism and nutrition disorders (Table 4). The most common AEs in all renal function categories were GI‐related, predominantly nausea and vomiting.
Table 4.
Placebo‐adjusted meta‐analysis (fixed effect) of selected AEsa between renal function categories (safety population)
| Parameter | Normal vs mild | Mild vs moderate | Normal vs moderate | |||
|---|---|---|---|---|---|---|
| Estimate | P‐value | Estimate | P‐value | Estimate | P‐value | |
| GI disorders (SOC) | −0.14 | .003 | 0.00 | .99 | −0.10 | .16 |
| Nausea/vomiting (HLT) | −0.1 | .003 | 0.04 | .57 | −0.03 | .65 |
| Metabolism and nutrition disorders (SOC) | −0.08 | .005 | 0.15 | .03 | 0.11 | .09 |
| Hypoglycaemia (HLT) | −0.02 | .26 | 0.04 | .53 | 0.03 | .63 |
| Appetite disorders (HLT) | −0.03 | .009 | 0.01 | .79 | −0.03 | .47 |
| Decreased appetite (PT) | −0.03 | .004 | 0.03 | .47 | −0.02 | .63 |
Abbreviations: GI, gastrointestinal; HLT, higher level term; PT, preferred term; SOC, system and organ class (MedDRA).
Fourteen SOCs were identified as common for all three renal impairment categories. Those showing significant placebo‐adjusted risk differences are shown here.
There was a lower incidence of any GI disorder AE (SOC), and a lower incidence of nausea and vomiting (HLT), in the normal renal function vs mild renal impairment category (P = .003 for both), but no difference was found for either of these endpoints between the mild and moderate impairment categories (P = .99 and P = .57, respectively), or between the moderate impairment and normal categories (P = .16 and P = .65, respectively). The incidences of any metabolism/nutrition disorder AE (SOC) and of decreased appetite (HLT) were significantly lower in the normal function vs the mild impairment category (P = .005 and P = .004, respectively). The difference in the incidence of any metabolism/nutrition disorder AE between the mild and moderate renal impairment categories was also significant (P = .03), but no other significant difference between renal function groups was found for either of these safety endpoints (Table 4).
Importantly, with respect to hypoglycaemia, there was no significant placebo‐adjusted difference in the incidence rate between the normal renal function and the mild impairment categories (−0.02; 95% CI −0.06, 0.02; P = .26), between the mild and the moderate renal impairment categories (0.04; 95% CI −0.08, 0.15; P = .53), or between the normal renal function and the moderate impairment categories (−0.03; 95% CI −0.08, 0.14).
No significant difference was reported for placebo‐adjusted change from baseline in heart rate between the normal renal function and the mild impairment categories [−1.13 beats per min (bpm); 95% CI −2.54, 0.28; P = .12], or between the mild and moderate renal impairment categories (1.73 bpm; 95% CI −2.72, 6.17; P = .45). Similarly, there was no significant placebo‐adjusted difference between the change from baseline in systolic blood pressure (SBP) in the normal renal function and mild impairment categories (−0.99 mm Hg; 95% CI −3.82, 1.84; P = .49), or between the mild and moderate renal impairment categories (−7.41 mm Hg; 95% CI −16.30, 1.47; P = .10). Although there was no significant placebo‐adjusted difference between the change from baseline in diastolic blood pressure (DBP) in the normal renal function and mild impairment categories (−0.18 mm Hg; 95% CI −1.56, 1.20; P = .80), a significant difference was seen between the mild and moderate renal impairment categories (−5.11 mm Hg; 95% CI −8.93, −1.29; P < .01).
4. DISCUSSION
In patients with T2D, good glycaemic control is necessary to prevent the micro‐ and macrovascular complications associated with the disease. Establishing this control in patients with co‐morbid renal impairment is complex for a number of reasons, including restrictions in the use of certain treatment options based on their pharmacokinetic profiles. In this clinical setting it is of critical importance to understand whether the efficacy or safety of a potential treatment is affected by impaired renal function so that an appropriate decision can be made regarding drug treatment and dosing regimen.
The most recent combined ADA/EASD 2015 position statement continues to recommend metformin as first‐line therapy in T2D6; however, subsequent ADA guidance includes severe renal impairment as a contraindication for use,7 with additional guidance from the FDA stating that metformin may be used in patients with mild impairment and only some with moderate impairment.8 Concerns regarding the use of metformin in patients with renal insufficiency are due to lactic acid accumulation, and although recent evidence suggests that cautious use of the treatment in mild‐to‐moderate renal impairment may be appropriate, dosage reductions and careful monitoring of kidney function are recommended.26
In cases where the use of metformin is not appropriate, current treatment guidelines recommend a number of alternative therapeutic options, including sulphonylureas, SGLT2 inhibitors, thiazolidinediones, DPP‐4 inhibitors and GLP‐1 RAs; however, many of these commonly used options are also contraindicated or require dose adjustments. For example, the risk of hypoglycaemia is increased due to the accumulation of sulphonylureas and/or their active metabolites, and their long duration of action27; dose reductions are required for glipizide despite evidence that the kidneys only have minimal involvement in the metabolism of the drug.28 The DPP‐4 inhibitors sitagliptin, vildagliptin and saxagliptin all require dose reductions in patients with renal impairment, with only linagliptin not requiring dose adjustment due to minimal renal excretion of the drug.28 Furthermore, although the SGLT2 inhibitors dapagliflozin, canagliflozin and empagliflozin do not increase the risk of hypoglycaemia, there is evidence that they increase the risk of hypovolemic side‐effects and that their glycaemic efficacy is diminished in moderate‐to‐severe renal impairment.28 In addition, alpha glucosidase inhibitors are not generally recommended for people with renal impairment due to potential accumulation and lack of safety information.28, 29
The ADA/EASD treatment guidelines have designated GLP‐1 RAs as a therapeutic option in T2D owing to their proven efficacy in improving glycaemic control.6 Interestingly, preclinical evidence indicates that GLP‐1 may play a role in the regulation of renal function, and thus it has been hypothesized that treatment with certain GLP‐1 RAs may even aid in improving diabetic nephropathy.30, 31 As clearance of exenatide is via the renal route, it is not recommended in patients with severe impairment.10 In contrast, despite minor increases in the plasma concentrations of albiglutide and dulaglutide, and increased rates of hypoglycaemia and GI side‐effects,32 the FDA has approved both treatments for use in patients with renal impairment without dose reduction. In Europe, however, their use in patients with severe impairment is not recommended until further data are available.28 Further data relating to the use of GLP‐1 RAs in patients with renal disease are provided by a recent study of liraglutide in patients with moderate impairment.11 In this population, liraglutide did not appear to impact renal function and demonstrated improved glycaemic control compared with placebo. In addition, there was no increase in hypoglycaemia incidence with liraglutide; however, a greater number of withdrawals due to GI events was observed.11
This meta‐analysis of nine lixisenatide trials demonstrates that mild or moderate renal impairment does not have a statistically significant effect on efficacy outcomes in patients with T2D treated with lixisenatide. Across the trials included, clinical outcomes were generally consistent between the normal renal function and the mild and moderate renal impairment categories. Basal insulin dose reduction and body weight loss did not reach statistical significance in the moderate renal impairment category; this could be attributable to a change in exposure to lixisenatide in this group. There was a trend toward a significant reduction in body weight in the moderate renal impairment group, but it should be noted that the sample size in this category was relatively small and that post hoc analyses may not always be powered sufficiently for the assessment of all outcomes. None of the five placebo‐adjusted efficacy parameters tested (HbA1c, 2‐hour PPG, FPG, basal insulin dose, weight) was found to be significantly different between renal function categories.
In the current analysis of lixisenatide trials, there was no significant difference in the incidence of GI AEs between patients with mild or moderate renal impairment; however, in patients with mild renal impairment, there was an increased risk (P = .003) of experiencing any GI AE compared with patients with normal renal function. It is important to note that the incidence of hypoglycaemia was similar in all categories. These findings indicate that lixisenatide dose adjustment is not required in patients with T2D with mild or moderate renal impairment. This is in contrast to other anti‐hyperglycaemic agents, such as SGLT2s and DPP‐4 inhibitors, where in cases of moderate renal disease, efficacy tends to be diminished and safety concerns are a possibility.28, 33 Furthermore, no significant changes were seen in placebo‐adjusted difference in heart rate change from baseline or SBP between the normal renal function and the mild impairment categories. In contrast, a significant difference was seen between the change from baseline in DBP between the mild and moderate renal impairment categories. This difference was driven primarily by a change in a single trial (GetGoal‐M‐Asia: −23.4 mm Hg; 95% CI −37.6, −9.1).
A further consideration regarding the choice of treatment in this patient population is the potential for anti‐hyperglycaemic drugs to negatively affect renal function. There are conflicting data available for liraglutide depending on duration of treatment. Evidence from a 26‐week study suggests that liraglutide has no effect on renal function11; however, findings from a 1‐year study with a small sample size indicate that the drug may elicit a significant reversible decline in GFR, suggestive of a reversible metabolic or haemodynamic effect, without accompanying structural changes in renal physiology.34 These conflicting results may indicate that there is considerable variation in pharmacodynamic and pharmacokinetic profiles in patients with renal impairment, and that these factors need to be carefully considered prior to initiation of any anti‐hyperglycaemic treatment.
This analysis was a post hoc evaluation of multiple trials. One limitation of this approach is that the nine trials included were of varying design, specifically with regard to duration and treatment regimen (i.e. monotherapy or add‐on); however, this may also be viewed as a strength of this analysis as it permitted evaluation of a broad range of patients. It should be noted that patient numbers in the moderate category were relatively low, prohibiting some specific statistical comparisons. In addition, effects of treatment on diabetic nephropathy were not investigated in these trials; these clinical data are needed for all of the GLP‐1 RAs. It should also be noted that considering the lifelong nature of T2D, the duration of follow‐up in the trials included in this analysis was relatively short. While rates of AEs with lixisenatide in this analysis were placebo‐adjusted, rates of AEs in patients who received placebo in the different renal function groups were not analysed. The lower incidence of GI‐disorder AEs seen in patients in the normal renal function vs mild impairment categories may also have been observed in patients who received placebo because of the symptoms of reduced kidney function; the absence of these placebo data creates an inherent bias against lixisenatide in the AE analysis.
In summary, the clinical management of patients with T2D and impaired renal function can be challenging; however, the results of this analysis support that no lixisenatide dose adjustment is required for patients with T2D with mild or moderate renal impairment.
ORCID
Markolf Hanefeld http://orcid.org/0000-0001-7323-1203
Lawrence A. Leiter http://orcid.org/0000-0002-1040-6229
Supporting information
Table S1. Patient distribution (sample sizes) by renal impairment stage and endpoint (mITT population).
Table S2. Endpoints evaluated in each of the included trials.
Figure S1. Placebo‐adjusted analysis by original study population of change in A, HbA1c; B, FPG and C, PPG from baseline to week 24 (week 12 for GetGoal‐Mono) between renal function categories (normal function versus moderate impairment).
ACKNOWLEDGEMENTS
The authors would like to thank Drs Anu Ambos and Marcos Tambascia for their contribution to the interpretation of the study. Editorial support for this manuscript was provided by Andy Shepherd and Alex Mitchell of Caudex (Oxford, UK), funded by Sanofi.
Conflict of interest
M. H. has served on an advisory panel and as an author for Bristol‐Myers Squibb, GlaxoSmithKline, Sanofi and Takeda; and has served on a speakers’ bureau and as an author for Bayer Health Care, Eli Lilly, GlaxoSmithKline, Roche, Sanofi and Takeda. J. M. A. has served on an advisory panel and as an author for AstraZeneca, Bristol‐Myers Squibb, Novo Nordisk and Sanofi; and as a speaker for Sanofi. L. A. L. has received research funding from, has provided CME on behalf of, and/or has served as an adviser to AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Pfizer, Sanofi, Servier and Takeda. G. M. has received research support from and served as an author for MSD; has served on a speakers’ bureau and as an author for Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Novo Nordisk and Sanofi; and has served on an advisory panel for Boehringer Ingelheim, Eli Lilly and Sanofi. E. N. is an employee of Artech Information Systems, under contract with Sanofi as a clinical data associate. M. S. has served on an advisory panel and as an author for Novo Nordisk; has received research support from and served as an author for Novartis; and has served on speakers’ bureau and as an author for AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly, MSD, Novartis, Novo Nordisk and Servier. W. S. is an employee of Sanofi. R. G.‐H. has served on an advisory panel and as an author for Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk and Sanofi.
Author contributions
M. H. contributed to the design of the study, data analysis and drafting of the manuscript. J. M. A., L. A. L., G. M., M. S. and R. G.‐H. contributed to data analysis and interpretation. E. N. and W. S. contributed to the design of the study, data acquisition and analysis, and drafting of the manuscript. All authors provided critical revisions, approved the final version, and are accountable for the accuracy and integrity of the data.
Financial disclosure
This study was sponsored by Sanofi.
Hanefeld M, Arteaga JM, Leiter LA, Marchesini G, Nikonova E, Shestakova M, Stager W and Gómez‐Huelgas R. Efficacy and safety of lixisenatide in patients with type 2 diabetes and renal impairment. Diabetes Obes Metab. 2017;19:1594–1601. https://doi.org/10.1111/dom.12986
Present address E. Nikonova, Eisai, Inc., Woodcliff Lake, New Jersey.
Funding information This study was sponsored by Sanofi.
REFERENCES
- 1. Roder PV, Wu B, Liu Y, Han W. Pancreatic regulation of glucose homeostasis. Exp Mol Med. 2016;48:e219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gilbert RE. Endothelial loss and repair in the vascular complications of diabetes: pathogenetic mechanisms and therapeutic implications. Circ J. 2013;77:849‐856. [DOI] [PubMed] [Google Scholar]
- 3. Molitch ME. Current state of type 2 diabetes management. Am J Manag Care. 2013;19:S136‐S142. [PubMed] [Google Scholar]
- 4. Huang ES, Liu JY, Moffet HH, John PM, Karter AJ. Glycemic control, complications, and death in older diabetic patients: the diabetes and aging study. Diabetes Care. 2011;34:1329‐1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacCallum L. Optimal medication dosing in patients with diabetes mellitus and chronic kidney disease. Can J Diabetes. 2014;38:334‐343. [DOI] [PubMed] [Google Scholar]
- 6. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetologia. 2015;58:429‐442. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . Standards of medical care in diabetes – 2016. Diabetes Care. 2016;39(suppl 1):S1‐S112. [DOI] [PubMed] [Google Scholar]
- 8. Food and Drug Administration . FDA Drug Safety Communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. 2016. http://www.fda.gov/Drugs/DrugSafety/ucm493244.htm. Accessed July 7 2016.
- 9. Gallwitz B. Management of patients with type 2 diabetes and mild/moderate renal impairment: profile of linagliptin. Ther Clin Risk Manag. 2015;11:799‐805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Scheen AJ. Pharmacokinetics and clinical use of incretin‐based therapies in patients with chronic kidney disease and type 2 diabetes. Clin Pharmacokinet. 2015;54:1‐21. [DOI] [PubMed] [Google Scholar]
- 11. Davies MJ, Bain SC, Atkin SL, et al. Efficacy and safety of liraglutide versus placebo as add‐on to glucose‐lowering therapy in patients with type 2 diabetes and moderate renal impairment (LIRA‐RENAL): a randomized clinical trial. Diabetes Care. 2016;39:222‐230. [DOI] [PubMed] [Google Scholar]
- 12. Christensen M, Miossec P, Larsen BD, Werner U, Knop FK. The design and discovery of lixisenatide for the treatment of type 2 diabetes mellitus. Expert Opin Drug Discov. 2014;9:1223‐1251. [DOI] [PubMed] [Google Scholar]
- 13. Ahrén B, Leguizamo DA, Miossec P, Saubadu S, Aronson R. Efficacy and safety of lixisenatide once‐daily morning or evening injections in type 2 diabetes inadequately controlled on metformin (GetGoal‐M). Diabetes Care. 2013;36:2543‐2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bolli GB, Munteanu M, Dotsenko S, et al. Efficacy and safety of lixisenatide once daily versus placebo in patients with type 2 diabetes insufficiently controlled on metformin (GetGoal‐F1). Diabet Med. 2014;31:176‐184. [DOI] [PubMed] [Google Scholar]
- 15. Fonseca VA, Alvarado‐Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE; EFC6018 GetGoal‐Mono Study Investigators. Efficacy and safety of the once‐daily GLP‐1 receptor agonist lixisenatide in monotherapy: a randomized, double‐blind, placebo‐controlled trial in patients with type 2 diabetes (GetGoal‐Mono). Diabetes Care. 2012;35:1225‐1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pinget M, Goldenberg R, Niemoeller E, Muehlen‐Bartmer I, Guo H, Aronson R. Efficacy and safety of lixisenatide once daily versus placebo in type 2 diabetes insufficiently controlled on pioglitazone (GetGoal‐P). Diabetes Obes Metab. 2013;15:1000‐1007. [DOI] [PubMed] [Google Scholar]
- 17. Rosenstock J, Raccah D, Koranyi L, et al. Efficacy and safety of lixisenatide once daily versus exenatide twice daily in type 2 diabetes inadequately controlled on metformin: a 24‐week, randomized, open‐label, active‐controlled study (GetGoal‐X). Diabetes Care. 2013;36:2945‐2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Seino Y, Min KW, Niemoeller E, Takami A, EFC10887 GETGOAL‐L Asia Study Investigators . Randomized, double‐blind, placebo‐controlled trial of the once‐daily GLP‐1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal‐L‐Asia). Diabetes Obes Metab. 2012;14:910‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu Pan C, Han P, Liu X, et al. Lixisenatide treatment improves glycaemic control in Asian patients with type 2 diabetes mellitus inadequately controlled on metformin with or without sulfonylurea: a randomized, double‐blind, placebo‐controlled, 24‐week trial (GetGoal‐M‐Asia). Diabetes Metab Res Rev. 2014;30:726‐735. [DOI] [PubMed] [Google Scholar]
- 20. Riddle MC, Aronson R, Home P, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled by established basal insulin: a 24‐week, randomized, placebo‐controlled comparison (GetGoal‐L). Diabetes Care. 2013;36:2489‐2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riddle MC, Forst T, Aronson R, et al. Adding once‐daily lixisenatide for type 2 diabetes inadequately controlled with newly initiated and continuously titrated basal insulin glargine: a 24‐week, randomized, placebo‐controlled study (GetGoal‐Duo 1). Diabetes Care. 2013;36:2497‐2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenstock J, Hanefeld M, Shamanna P, et al. Beneficial effects of once‐daily lixisenatide on overall and postprandial glycemic levels without significant excess of hypoglycemia in type 2 diabetes inadequately controlled on a sulfonylurea with or without metformin (GetGoal‐S). J Diabetes Complications. 2014;28:386‐392. [DOI] [PubMed] [Google Scholar]
- 23. Rosenstock J, Guerci B, Hanefeld M, et al; GetGoal Duo‐2 Trial Investigators. Prandial options to advance basal insulin glargine therapy: testing lixisenatide plus basal insulin versus insulin glulisine either as basal‐plus or basal‐bolus in type 2 diabetes: the GetGoal Duo‐2 trial. Diabetes Care. 2016;39:1318‐1328. [DOI] [PubMed] [Google Scholar]
- 24. Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31‐41. [DOI] [PubMed] [Google Scholar]
- 25. American Diabetes Association . Standards of medical care in diabetes – 2013. Diabetes Care. 2013;36(suppl 1):S11‐S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inzucchi SE, Lipska KJ, Mayo H, Bailey CJ, McGuire DK. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668‐2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arnouts P, Bolignano D, Nistor I, et al. Glucose‐lowering drugs in patients with chronic kidney disease: a narrative review on pharmacokinetic properties. Nephrol Dial Transplant. 2014;29:1284‐1300. [DOI] [PubMed] [Google Scholar]
- 28. Alsahli M, Gerich JE. Hypoglycemia in patients with diabetes and renal disease. J Clin Med. 2015;4:948‐964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weng J, Soegondo S, Schnell O, et al. Efficacy of acarbose in different geographical regions of the world: analysis of a real‐life database. Diabetes Metab Res Rev. 2015;31:155‐167. [DOI] [PubMed] [Google Scholar]
- 30. Farah LX, Valentini V, Pessoa TD, Malnic G, McDonough AA, Girardi AC. The physiological role of glucagon‐like peptide‐1 in the regulation of renal function. Am J Physiol Renal Physiol. 2016;310:F123‐F127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Filippatos TD, Elisaf MS. Effects of glucagon‐like peptide‐1 receptor agonists on renal function. World J Diabetes. 2013;4:190‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Young MA, Wald JA, Matthews JE, Yang F, Reinhardt RR. Effect of renal impairment on the pharmacokinetics, efficacy, and safety of albiglutide. Postgrad Med. 2014;126:35‐46. [DOI] [PubMed] [Google Scholar]
- 33. Scheen AJ. Pharmacokinetics, pharmacodynamics and clinical use of SGLT2 inhibitors in patients with type 2 diabetes mellitus and chronic kidney disease. Clin Pharmacokinet. 2015;54:691‐708. [DOI] [PubMed] [Google Scholar]
- 34. von Scholten BJ, Hansen TW, Goetze JP, Persson F, Rossing P. Glucagon‐like peptide 1 receptor agonist (GLP‐1 RA): long‐term effect on kidney function in patients with type 2 diabetes. J Diabetes Complications. 2015;29:670‐674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Patient distribution (sample sizes) by renal impairment stage and endpoint (mITT population).
Table S2. Endpoints evaluated in each of the included trials.
Figure S1. Placebo‐adjusted analysis by original study population of change in A, HbA1c; B, FPG and C, PPG from baseline to week 24 (week 12 for GetGoal‐Mono) between renal function categories (normal function versus moderate impairment).


