Abstract
BACKGROUND
The objective of this study was to evaluate the safety and efficacy of carbon‐ion radiotherapy (CIRT) in patients with hepatocellular carcinoma (HCC) with stepwise dose escalation and hypofractionation in 2 combined prospective trials.
METHODS
Sequential phase 1/2 (protocol 9603) and phase 2 (protocol 0004) trials were conducted for patients with histologically proven HCC. The phase 1 component of protocol 9603 was a dose‐escalation study; CIRT was delivered in 12, 8, or 4 fractions. After determination of the recommended dose, 2 phase 2 trials were performed in an expanded cohort, and the data were pooled to analyze toxicity, local control, and overall survival.
RESULTS
In the phase 1 component of protocol 9603, 69.6, 58.0, and 52.8 Gy (relative biological effectiveness [RBE]) in 12, 8, and 4 fractions, respectively, constituted the maximum tolerated doses, and 52.8 Gy (RBE) in 4 fractions was established as the recommended dose regimen for the 2 phase 2 studies. In 124 patients with a total of 133 lesions, few severe adverse effects occurred, and local‐control and overall survival rates at 1, 3, and 5 years were 94.7% and 90.3%, 91.4% and 50.0%, and 90.0% and 25.0%, respectively; this included 1‐, 3‐, and 5‐year local‐control rates of 97.8%, 95.5%, and 91.6%, respectively, in the phase 2 study. In a multivariate analysis, Child‐Pugh class B and the presence of a tumor thrombus were significant factors for mortality.
CONCLUSIONS
The safety and efficacy of CIRT in 12, 8, and 4 fractions were confirmed, with 52.8 Gy (RBE) in 4 fractions established as the recommended treatment course for eligible HCC patients. Cancer 2017;123:3955‐65. © 2017 The Authors. Cancer published by Wiley Periodicals, Inc. on behalf of American Cancer Society.
Keywords: adverse effect, carbon‐ion radiotherapy, hepatocellular carcinoma, local control, mortality, overall survival, prognostic factor, prospective study
Short abstract
Sequential phase 1/2 and phase 2 prospective trials including 133 lesions in 124 patients with histologically proven hepatocellular carcinoma have been performed to evaluate the safety and efficacy of carbon‐ion radiotherapy hypofractionation with 12, 8, and 4 fractions. Few severe adverse effects have been found, and the 3‐year local‐control rate is 91.4% for all lesions with a 3‐year local‐control rate of 95.5% in the phase 2 trial.
INTRODUCTION
Hepatocellular carcinoma (HCC) is generally a multifocal tumor found in the cirrhotic liver that potentially requires repeated therapy. Standard treatments for localized HCC include surgical resection, liver transplantation,1, 2 radiofrequency ablation (RFA),3, 4 and transcatheter arterial chemoembolization (TACE).5, 6, 7 Treatment eligibility is dependent on patient and tumor conditions. When patients are medically ineligible for or refuse these treatments, radiotherapy may be used instead.
Historically, external‐beam radiotherapy with curative intent has been difficult to perform in patients with HCC because of its inherent radiosensitivity and the potential for radiation‐induced liver disease (RILD).8 However, with improvements in treatment technology and targeting, such as computed tomography (CT)–based treatment planning with respiratory gating and fiducial markers, high doses may now be delivered to the diseased liver without gross deterioration of function. As such, stereotactic body radiotherapy (SBRT) with or without TACE9, 10 or proton‐beam radiotherapy11, 12, 13 has demonstrated favorable local‐control rates for HCC and few severe adverse effects.
The Hospital of the National Institute of Radiological Sciences (NIRS) began using carbon‐ion radiotherapy (CIRT) for HCC in 1995. The carbon‐ion beam and the proton beam have a high dose concentration, and CIRT can deliver significantly higher target conformity while sparing normal liver tissue in comparison with SBRT.14 In addition, the carbon‐ion beam has a higher biological effect because of inherently high linear energy transfer radiation; this distinguishes it from proton and X‐ray beams.15, 16 However, to date, there remains a lack of evidence for the safety and efficacy of CIRT for patients with HCC. Treatment safety and a favorable local‐control rate were demonstrated in the first prospective clinical phase 1/2 trial conducted, in which 15 fractions were used in a stepwise dose‐escalation study.17 This was followed by the current study conducted in patients with HCC; this constituted a prospective phase 1/2 clinical trial using 3 levels of hypofractionation (12, 8, and 4 fractions) and another phase 2 clinical trial using the same recommended dose regimen but with slight alterations in study eligibility.
The aims of this study were to evaluate the safety and efficacy of the carbon‐ion beam for HCC and to investigate the suitability of hypofractionation according to 2 prospective trials.
MATERIALS AND METHODS
Protocol and Eligibility Criteria
Protocols 9603 and 0004 were created by the protocol design committee of the Network Advisory Board for Heavy‐Ion Therapy at NIRS via prospective studies conducted from April 1997 to February 2001 and from April 2001 to February 2003, respectively.
The inclusion criteria for entry into the 9603 study were as follows: 1) biopsy‐proven HCC; 2) a measurable lesion; 3) recurrent or residual tumors after previous ineffective treatments or poor candidacy for other treatments; 4) stage II, IIIA, or IVA HCC without lymph node metastasis according to TNM Classification of Malignant Tumours (5th edition),18 which was determined by CT, magnetic resonance imaging (MRI), and/or ultrasonography; 5) a performance status of 0 to 2 on the Eastern Cooperative Oncology Group scale; and 6) an age ≤ 80 years. The exclusion criteria for this study were as follows: 1) target tumors that had been treated by other radiation therapies or by any other therapy within the past 2 months, 2) a hepatic disorder classified as Child‐Pugh class C,19 3) tumor thrombosis of the main portal vein and extrahepatic metastasis, 4) the presence of an untreatable esophageal or gastric varix, 5) the presence of active double cancers other than HCC, 6) an estimated life expectancy of less than 6 months, 7) the involvement of the digestive tract in the clinical target volume, and 8) a serious medical or psychological condition precluding the safe administration of the treatment. The eligibility criteria for the 0004 study were the same as those for the 9603 study except for the inclusion of patients with stage I HCC unaffected by or ineligible for other therapy and the exclusion of patients with a Child‐Pugh score of 9. In both protocols, there were no criteria concerning the tumor size or the number of lesions. When patients had other active HCC lesions in the liver considered eligible for CIRT, the aim was to include all lesions in a single target volume; if this was not possible, other therapies were used for the lesions that could not be treated by CIRT. In addition, if the minimum distance between the tumor and the gastrointestinal (GI) tract was less than 1 cm on pretreatment CT or MRI, we judged the case to be not eligible for CIRT.
These studies were conducted in accordance with the ethical standards set forth by the Declaration of Helsinki.20 All patients satisfying the criteria were approved by the ethics committee and enrolled.
Study Design
Protocol 9603 (phase 1/2) and protocol 0004 (phase 2) were both nonrandomized, open‐label, single‐center studies of CIRT monotherapy. The primary endpoints were safety for protocol 9603 and local control for protocol 0004.
The phase 1 component of protocol 9603 was a dose‐escalation study consisting of 12, 8, or 4 fractions over 3, 2, or 1 weeks, respectively. This began with a dose escalation for the 12‐fraction arm, which was followed by dose escalation for the 8‐ and 4‐fractions arms sequentially. The prescription dose was started from 54, 48, and 48 Gy (RBE) for the 12‐, 8‐, and 4‐fraction arms, respectively. The dose per fraction was escalated, generally in 10% increments, and changes in fraction size and dose were set by the protocol committee, which was established as part of the Liver Cancer Working Group. The dose‐limiting toxicity was defined according to the dose causing acute RILD (ie, an elevated total bilirubin level > 4.0 mg/dL, an aspartate aminotransferase level > 800 IU/L, and/or a prothrombin time < 25%) within 3 months.
In the phase 1 study, in which 68 cases were enrolled, there were no dose‐limiting toxicities, but a 2‐point increase in the Child‐Pugh score was observed at 3 months in 1 case administered 69.6 Gy (relative biological effectiveness [RBE]) in 12 fractions, 58.0 Gy (RBE) in 8 fractions, and 52.8 Gy (RBE) in 4 fractions, which were determined to be the maximum tolerated doses (MTDs) for each respective fraction number. A grade 3 adverse effect was observed in 1 case, with a grade 3 skin reaction after treatment with 52.8 Gy (RBE) in 4 fractions, and local recurrence was seen in 1 case treated with 69.6 Gy (RBE) in 12 fractions among 3 MTDs by the end of the phase 1 study. As such, the recommended dose determined for the phase 2 component of protocol 9603 was the most hypofractionated dose of 52.8 Gy (RBE) in 4 fractions in 3 MTDs, which was extended to 14 additional cases. Another 44 cases were treated with the same dose regimen in protocol 0004.
Treatment
Irradiation fields were established with a 3‐dimensional planning system based on 5‐mm‐thick CT images. The gross tumor volume was defined as the macroscopic tumor and thrombi, and the clinical tumor volume was defined as the gross tumor volume plus 5 mm to account for microscopic invasion. The planning target volume was defined as the clinical tumor volume plus 5 mm, including internal and setup margins. The treatment policy required the entirety of the planning target volume to be covered by at least 95% of the prescribed dose. To reproduce the target position accurately, a low‐temperature thermoplastic sheet and a customized cradle were used. During treatment, patient‐machine alignment was achieved via the overlapping of the onboard image taken in a true lateral position with a kilovoltage X‐ray with the reconstructed 2‐dimensional image taken at planning CT; deviations in the skeletal anatomy, diaphragm, and inserted fiducial markers were minimized between the 2 images. Respiratory gating at the end of the expiratory phase was used for CT planning, positional verification on the treatment board, and irradiation.21 These treatment methods were the same for the 2 protocols.
Follow‐Up
Each patient was examined at least once a month for the first 6 months and once every 3 months thereafter. Blood tests and ultrasonography were performed every 3 months, and contrast‐enhanced CT and MRI were performed every 3 months for the first 6 months and every 6 months thereafter.
As for liver toxicity, the Child‐Pugh scores 3 and 6 months after CIRT were compared with the score before CIRT. Laboratory tests, including transaminase, alkaline phosphatase, total bilirubin, γ‐glutamyl transpeptidase, and albumin levels as well as white blood cell counts, hemoglobin levels, and platelet counts, were evaluated both before and after CIRT according to the National Cancer Institute's Common Toxicity Criteria (version 2.0).22 To evaluate RILD, fatigue, anicteric ascites, alkaline phosphatase levels, and transaminase levels were evaluated both 3 and 6 months after CIRT.
Other adverse effects were evaluated acutely, that is, within the first 3 months after CIRT was started (defined as the early phase), according to the National Cancer Institute's Common Toxicity Criteria (version 2.0).22 For late effects, that is, more than 3 months after CIRT was started (defined as the late phase), the late radiation morbidity scoring scheme from the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer23 was used. Each phase was evaluated according to the worst adverse‐effect grades observed during the follow‐up period.
Evaluation
Local failure was defined as either progressive disease according to the modified Response Evaluation Criteria in Solid Tumors24 or the new appearance of lesions within the target volume. Local control was defined as the absence of local failure. The local‐control duration was defined as the interval between the start of CIRT and the date of diagnosis of local failure or the date of last follow‐up. The survival time was defined as the interval between the start of CIRT and the date of death or the date of last follow‐up. Death from hepatic failure was defined as death caused by the progression of coexisting liver cirrhosis without progression of HCC. Patients who were enrolled multiple times for the irradiation of separate tumors were treated as separate cases for the purpose of this analysis. The cutoff date for analysis was September 2016.
Statistics
The phase 2 trial (protocol 0004) was designed to detect an increase in the 2‐year local‐control rate from 60% (based on conventional X‐ray radiotherapy data25) to 85% with a 1‐sided α value of 2.5% and a power of 80%. The number of cases required to detect this difference with normal approximation of the binominal distribution was 29. Taking dropout into consideration, we aimed for a total of 30 cases. On the other hand, because of the dose‐escalation nature of the phase 1 trial, the sample size was not set beforehand in protocol 9603.
Proportions were compared with the chi‐square test. The equality of population medians among groups was tested with a Kruskal‐Wallis 1‐way analysis of variance. Cumulative local‐control rates, with non‐HCC mortality accounted for as a competing risk, and overall survival rates were calculated with Gray's test26 and the Kaplan‐Meier method, respectively. Survival curves were compared with the log‐rank test. Univariate and multivariate analyses of predictive factors of local control, with non‐HCC mortality accounted for as a competing risk, and overall survival were calculated with the Fine and Gray model27 and the Cox proportional hazards model, respectively. A P value < .05 was considered significant. Gray's test and the Fine and Gray model were performed with EZR,28 and all other statistical analyses were performed with SPSS software (version 20.0; IBM Japan, Ltd, Tokyo, Japan).
RESULTS
Patient Characteristics
Between April 1997 and February 2003, 127 patients with histologically proven HCC were enrolled. Three patients were excluded from the analysis because their eligibility could not be verified after enrollment. Therefore, the total number of patients analyzed was 124; because 2 of these patients received a CIRT course in both protocols, the total number of cases analyzed was 126. In addition, 4 and 3 patients received 2 courses of CIRT in protocols 9603 and 0004, respectively. Therefore, 133 lesions were treated with CIRT in the current study. Figure 1 shows a flowchart of this study protocol, and Table 1 presents patient and tumor characteristics. The median follow‐up of the 124 patients was 27.1 months (range, 0.9‐154.8 months).
Figure 1.

Flow chart of this study. CIRT indicates carbon‐ion radiotherapy; CT, computed tomography; HCC, hepatocellular carcinoma; RBE, relative biological effectiveness.
Table 1.
Patient and Tumor Characteristics
| Protocol 9603 | Protocol 0004 | |||||
|---|---|---|---|---|---|---|
| Total | Phase 1 | Phase 1 | Phase 1/2 | Phase 2 | P a | |
| Fractions, No. | 12 | 8 | 4 | 4 | ||
| Cases, No. | 126 | 33 | 22 | 27 | 44 | |
| Age, median (range), year | 68 (37‐84) | 67 (45‐80) | 65 (44‐80) | 66 (37‐77) | 69 (46‐84) | .140 |
| Sex, No. (%) | ||||||
| Male | 90 (71) | 22 (67) | 15 (68) | 23 (85) | 30 (68) | .366 |
| Female | 36 (29) | 11 (33) | 7 (32) | 4 (15) | 14 (32) | |
| ECOG performance status, No. (%) | ||||||
| 0‐1 | 109 (87) | 30 (91) | 17 (74) | 23 (85) | 39 (89) | .534 |
| 2 | 17 (13) | 3 (9) | 5 (23) | 4 (15) | 5 (11) | |
| Type of chronic hepatitis, No. (%) | ||||||
| HBV | 17 (13) | 3 (9) | 4 (18) | 6 (22) | 4 (9) | .728 |
| HCV | 93 (74) | 25 (76) | 16 (73) | 18 (67) | 34 (77) | |
| Other | 16 (13) | 5 (15) | 2 (9) | 3 (11) | 6 (14) | |
| Prior treatment, No. (%) | ||||||
| None | 66 (52) | 16 (48) | 10 (45) | 12 (44) | 28 (64) | .316 |
| Recurrence after treatment | 60 (48) | 17 (52) | 12 (55) | 15 (56) | 16 (36) | |
| Child‐Pugh class, No. (%) | ||||||
| A | 97 (77) | 26 (79) | 19 (86) | 17 (63) | 35 (80) | .305 |
| B | 29 (23) | 7 (21) | 3 (14) | 10 (37) | 9 (20) | |
| ICG R15, No. (%)b | ||||||
| ≤20% | 48 (40) | 14 (42) | 9 (43) | 10 (40) | 15 (38) | .969 |
| >20% | 71 (60) | 19 (58) | 12 (57) | 15 (60) | 25 (62) | |
| Clinical stage, No. (%) | ||||||
| I | 10 (8) | 0 (0) | 0 (0) | 0 (0) | 10 (23) | <.001 |
| II | 61 (48) | 12 (36) | 11 (36) | 13 (48) | 25 (57) | |
| IIIA | 43 (34) | 16 (48) | 8 (37) | 11 (41) | 8 (18) | |
| IVA | 12 (10) | 5 (15) | 3 (14) | 3 (11) | 1 (2) | |
| BCLC stage, No. (%) | ||||||
| 0 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | .193 |
| A | 34 (27) | 11 (33) | 9 (41) | 5 (19) | 9 (20) | |
| B | 17 (13) | 7 (21) | 2 (9) | 2 (7) | 6 (14) | |
| C | 75 (60) | 15 (46) | 11 (50) | 20 (74) | 29 (66) | |
| D | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| AFP, No. (%) | ||||||
| <20 ng/mL | 49 (39) | 13 (42) | 9 (42) | 9 (36) | 18 (41) | .926 |
| ≥20 ng/mL | 77 (61) | 20 (58) | 13 (58) | 18 (64) | 26 (59) | |
| PIVKA‐II, No. (%) | ||||||
| <40 ng/mL | 48 (38) | 17 (53) | 8 (33) | 5 (18) | 16 (36) | .073 |
| ≥40 ng/mL | 78 (62) | 16 (47) | 14 (67) | 22 (82) | 28 (64) | |
| Total tumors, No. | 133 | 34 | 24 | 28 | 47 | |
| Maximum tumor size, median (range), mm | 40 (10‐120) | 36 (13‐72) | 26 (10‐120) | 47 (25‐120) | 37 (12‐86) | .002 |
| Maximum tumor size, No. (%) | ||||||
| ≤30 mm | 39 (29) | 7 (21) | 14 (58) | 4 (14) | 14 (30) | .004 |
| >30, ≤50 mm | 56 (42) | 18 (55) | 7 (29) | 10 (36) | 21 (45) | |
| >50 mm | 38 (29) | 9 (26) | 3 (13) | 14 (50) | 12 (25) | |
| Treatment duration (range), day | 8 (4‐22) | 21 (18‐22) | 14 (11‐15) | 4 (4‐8) | 4 (4‐8) | <.001 |
| Tumor thrombus, No. (%) | ||||||
| Present | 23 (17) | 5 (15) | 4 (17) | 11 (39) | 3 (6) | .003 |
| Absent | 110 (83) | 29 (85) | 20 (83) | 17 (61) | 44 (94) | |
| Tumor number, No. (%) | ||||||
| Single | 103 (77) | 23 (64) | 21 (73) | 24 (81) | 35 (74) | .308 |
| Multiple | 30 (23) | 11 (36) | 3 (27) | 4 (19) | 12 (26) | |
| Target volume, No. (%) | ||||||
| <158 mL | 66 (50) | 16 (47) | 15 (63) | 14 (50) | 21 (45) | .543 |
| ≥158 mL | 67 (50) | 18 (53) | 9 (37) | 14 (50) | 26 (55) | |
| Fields, No. (%) | ||||||
| 2 | 109 (82) | 17 (50) | 22 (92) | 27 (96) | 43 (91) | <.001 |
| 3 | 21 (16) | 14 (41) | 2 (8) | 1 (4) | 4 (9) | |
| 4 | 3 (2) | 3 (9) | 0 (0) | 0 (0) | 0 (0) | |
Abbreviations: AFP, α‐fetoprotein; BCLC, Barcelona Clinic Liver Cancer; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; ICG R15, indocyanine green retention rate at 15 minutes; PIVKA‐II, protein induced by vitamin K absence/antagonist II.
Age, tumor size, and treatment duration were analyzed with the Kruskal‐Wallis test. Other variables were analyzed with the chi‐square test.
Seven cases did not undergo the indocyanine green test because of an allergy.
Toxicity
Treatment was completed for all 126 cases with 124 patients. A total of 124 patients were evaluated for liver toxicity at 3 months after CIRT. Three of these patients died, and 8 received additional treatments elsewhere from 3 to 6 months after CIRT; therefore, 113 patients were evaluated for liver toxicity at 6 months after CIRT. An evaluation of nonhepatic adverse effects was performed for all 124 patients, and Table 2 lists the noted adverse effects observed.
Table 2.
Adverse Effects: Liver, Hematology, Skin, Gastrointestinal Tract, Chest Wall Pain, Radiation Pneumonitis, and Pleural Effusion
| Protocol 9603 | Protocol 0004 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 2 | Total, No. (%) | |||||||||
| Fractions, No. | 12 | 8 | 4 | 4 | 4 | |||||||
| Total dose (RBE), Gy | 54 | 60 | 66 | 69.6a | 48 | 52.8 | 58a | 48 | 52.8a | 52.8 | 52.8 | |
| Patients, No. | 3 | 8 | 16 | 6 | 5 | 13 | 4 | 6 | 7 | 14 | 42b | 124 |
| Liver | ||||||||||||
| Change in Child‐Pugh score: 3 mo/6 moc | ||||||||||||
| +1 point | 1/1 | 3/2 | 6/2 | 2/2 | 1/0 | 2/3 | 1/1 | 1/1 | 3/2 | 2/1 | 14/10 | 36/25 (29/22) |
| +2 points | 0/0 | 0/1 | 0/0 | 1/0 | 0/1 | 0/0 | 1/1 | 0/2 | 1/0 | 0/0 | 1/1 | 4/6 (3/5) |
| +≥3 points | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/0 | 1/0 (1/0) |
| Laboratory test grade: before CIRT/3 mo/6 moc, d | ||||||||||||
| Grade 3 | 1/0/0 | 0/0/2 | 2/2/3 | 1/1/0 | 0/0/0 | 1/0/1 | 1/2/1 | 0/0/1 | 2/1/1 | 1/1/0 | 1/1/3 | 10/8/12 (8/6/10) |
| Hematology: before CIRT/3 mo | ||||||||||||
| White blood cells, grade 3 | 0/0 | 0/1 | 0/1 | 0/0 | 0/0 | 1/0 | 0/0 | 0/1 | 0/0 | 0/0 | 0/0 | 1/3 (1/2) |
| Hemoglobin, grade 3 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 0/0 | 1/1 (1/1) |
| Platelets, grade 3 | 0/0 | 2/1 | 3/3 | 1/1 | 0/0 | 0/0 | 0/0 | 2/2 | 1/1 | 0/1 | 3/4 | 12/13 (10/10) |
| Skin: early/latee | ||||||||||||
| Grade 2 | 0/0 | 1/2 | 0/1 | 0/0 | 0/0 | 2/1 | 0/0 | 1/0 | 1/0 | 2/1 | 2/5 | 9/10 (7/8) |
| Grade 3 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 1/1 | 2/0 | 0/2 | 3/3 (2/2) |
| Gastrointestinal tract: early/latee | ||||||||||||
| Grade 2 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 2/1 | 0/0 | 0/0 | 0/0 | 0/0 | 2/1 (2/1) |
| Grade 3 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 | 0/0 (0/0) |
| Chest wall pain: latee | ||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 (1) |
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Radiation pneumonitis: latee | ||||||||||||
| Grade 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 2 (1) |
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0) |
| Pleural effusion: latee | ||||||||||||
| Grade 2 | 0 | 1 | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 6 (5) |
| Grade 3 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 (1) |
Abbreviations: CIRT, carbon‐ion radiotherapy; RBE, relative biologically effectiveness.
There were no grade 4 or 5 adverse effects.
Maximum tolerated dose.
Two patients who overlapped with protocol 9603 were excluded from the analysis of adverse effects.
Six months after CIRT, 113 patients were evaluated for liver toxicity.
Highest grade determined by laboratory tests, including transaminase, alkaline phosphatase, total bilirubin, γ‐glutamyl transpeptidase, and albumin levels.
Early and late indicate the evaluation of adverse effects within 3 months and after 3 months, respectively.
As for liver toxicity, a 3‐point or greater increase in the Child‐Pugh score was seen for 1 patient who experienced acute progression and HCC rupture outside the planning target volume at 3 months and then died 5 months after CIRT. There were no other 3‐point or greater score increases observed. Of the 95 patients classified as Child‐Pugh class A before CIRT, 9 and 7 patients progressed to Child‐Pugh class B at 3 and 6 months after CIRT, respectively. As for RILD, an increase in anicteric ascites was observed in 3 and 4 patients at 3 and 6 months after CIRT, respectively, and all cases of ascites were controllable. Neither grade 2 or higher severe fatigue nor a grade 3 elevation of alkaline phosphatase levels was observed at 3 or 6 months. In addition, a grade 3 elevation of transaminase levels was observed in 2 patients each at 3 and 6 months after CIRT.
With respect to hematologic adverse effects, there were very few changes in grade 3 effects from the period before CIRT to 3 months after CIRT. During the early phase, although grade 3 adverse skin reactions were observed in 3 patients (4%) who were treated with 52.8 Gy (RBE) in 4 fractions in protocol 9603, there were no other grade 3 adverse effects.
The late‐phase grade 2 and 3 reactions in the skin and GI tract as well as chest wall pain, radiation pneumonitis, and pleural effusions are listed in Table 2. Of the 63 patients who were treated with 52.8 Gy (RBE) in 4 fractions, late grade 3 adverse reactions in the skin were observed in 3 (5%). A late nonskin grade 3 adverse effect was observed in a patient treated with 52.8 Gy (RBE) in 8 fractions. This patient had an initial Child‐Pugh score of 8 and suffered a pleural effusion that required hospitalization for intermittent pleural cavity paracentesis at 7 months after CIRT. No case of CIRT‐associated common bile duct or portal vein stenosis was noted in the current study.
Local Control
The 1‐, 3‐, and 5‐year local‐control rates for all 133 lesions were 94.7% (95% confidence interval [CI], 89.9%‐97.6%), 91.4% (95% CI, 85.7%‐95.5%), and 90.0% (95% CI, 83.5%‐94.6%), respectively. The 86 lesions evaluated in protocol 9603 and the 47 lesions evaluated in protocol 0004 demonstrated 1‐, 3‐, and 5‐year local‐control rates of 92.9% (95% CI, 86.1%‐97.1%) and 97.8% (95% CI, 89.9%‐99.8%), 89.1% (95% CI, 81.3%‐94.7%) and 95.5% (95% CI, 86.5%‐99.2%), and 89.1% (95% CI, 81.3%‐94.7%) and 91.6% (95% CI, 78.7%‐98.1%), respectively. Figure 2 shows the Kaplan‐Meier curves for the local‐control rates of all 133 lesions in the protocols. The local‐failure rates for each dose regimen and the local‐control rates for each dose fraction size in the 2 protocols are presented in Table 3. Table 4 shows the predictive factors for local failure; none of the variables examined were significant in the univariate analysis.
Figure 2.
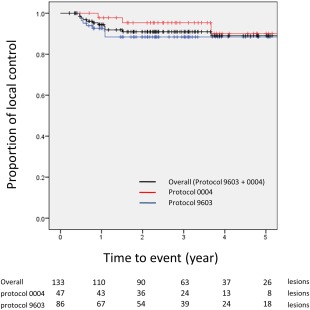
Kaplan‐Meier curves for the local‐control rates of all 133 lesions in the 2 protocols. No cases of local failure occurred after 5 years.
Table 3.
Dose Regimens for 133 Lesions and Local‐Control Rates
| Protocol 9603 | Protocol 0004 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 | Phase 2 | Phase 2 | |||||||||||
| Fractions, No. | 12 | 8 | 4 | 4 | 4 | Total | |||||||
| Total dose (RBE), Gy | 54 | 60 | 66 | 69.6a | 48 | 52.8 | 58a | 48 | 52.8a | 52.8 | 52.8 | ||
| Fraction dose, Gy | 4.5 | 5 | 5.5 | 5.8 | 6 | 6.6 | 7.25 | 12 | 13.2 | 13.2 | 13.2 | ||
| Lesions, No. | 3 | 9 | 16 | 6 | 5 | 15 | 4 | 6 | 8 | 14 | 47 | 133 | |
| Local failure, No. (%) | 0 (0) | 1 (11) | 2 (13) | 1 (17) | 1 (20) | 1 (7) | 0 (0) | 1 (17) | 0 (0) | 2 (14) | 3 (6) | 12 (9) | |
| Local‐control rate, % (95% CI)b | 1 y | 97.1 (86.8‐99.8) | 91.5 (75.7‐97.6) | 88.9 (75.7‐97.3) | 97.8 (89.9‐99.8) | 94.7 (89.9‐97.6) | |||||||
| 3 y | 87.3 (73.0‐96.1) | 91.5 (75.7‐97.6) | 88.9 (75.7‐97.3) | 95.5 (86.5‐99.2) | 91.4 (85.7‐95.5) | ||||||||
| 5 y | 87.3 (73.0‐96.1) | 91.5 (75.7‐97.6) | 88.9 (75.7‐97.3) | 91.6 (78.7‐98.1) | 90.0 (83.5‐94.6) | ||||||||
Abbreviations: CI, confidence interval; RBE, relative biologically effectiveness
Maximum tolerated dose.
Gray's test.
Table 4.
Predictive Factors for Local Failure in Univariate Analyses
| Factor | HR | 95% CI | P | |
|---|---|---|---|---|
| Age | <68 y | Reference | ||
| ≥68 y | 0.589 | 0.188‐1.841 | .360 | |
| Sex | Male | Reference | ||
| Female | 2.881 | 0.931‐8.910 | .066 | |
| ECOG performance status | 0/1 | Reference | ||
| 2 | 0.601 | 0.078‐4.629 | .630 | |
| Type of chronic hepatitis | HCV | Reference | ||
| HBV or other | 1.471 | 0.448‐4.834 | .520 | |
| Prior treatment | None | Reference | ||
| Recurrence after treatment | 0.495 | 0.152‐1.618 | .240 | |
| Child‐Pugh class | A | Reference | ||
| B | 1.073 | 0.294‐3.917 | .920 | |
| ICG R15 | ≤20% | Reference | ||
| >20% | 1.376 | 0.411‐4.611 | .610 | |
| Clinical stage | I/II | Reference | ||
| IIIA/IVA | 1.376 | 0.446‐4.245 | .580 | |
| Maximum tumor size | ≤30 mm | Reference | ||
| >30 mm, ≤50 mm | 4.850 | 0.622‐37.800 | .130 | |
| >50 mm | 4.329 | 0.495‐37.890 | .190 | |
| Tumors in target volume | Single | Reference | ||
| Multiple | 0.665 | 0.150‐2.937 | .590 | |
| Tumor thrombus | Absent | Reference | ||
| Present | 1.920 | 0.527‐7.002 | .320 | |
| Target volume | <158 mL | Reference | ||
| ≥158 mL | 1.431 | 0.460‐4.448 | .540 | |
| Fractions, No. | 4 | Reference | ||
| 8 | 0.741 | 0.135‐4.077 | .730 | |
| 12 | 0.670 | 0.192‐2.339 | .530 | |
| Fields, No. | 2 | Reference | ||
| 3/4 | 2.400 | 0.732‐7.871 | .150 | |
| Protocol | 9603 | Reference | ||
| 0004 | 0.569 | 0.159‐2.013 | .380 | |
Abbreviations: CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; ICG R15, indocyanine green retention rate at 15 minutes.
Recurrence and Mortality
Regional and distant failures, defined as the occurrence of lesions in the liver outside the planning target volume and as the occurrence of metastatic lesions outside the liver, respectively, were noted in 95 cases (77%) and 32 cases (26%), respectively, at the end of follow‐up.
At the last follow‐up, 6 patients were alive, and 118 patients were dead. For the 118 patients who died, mortality was attributed to progressive HCC (n = 84 [71%]), other cancers (n = 5 [4%]), hepatic failure (n = 4 [3%]), pneumonitis (n = 3 [3%]), accidental death (n = 2), respiratory failure (n = 2), acute myocardial infarction (n = 2), aortic dissection (n = 2), chronic renal failure (n = 2), obstructive jaundice due to gallstones (n = 1), intracranial hemorrhage (n = 1), or brain infarction (n = 1); the cause of death was unknown for 9 patients. The 4 patients who died of hepatic failure had other lesions detected outside the irradiated area of the liver before the completion of CIRT and, therefore, received additional local therapies immediately after CIRT. The changes in the Child‐Pugh score from the period before CIRT to 6 months after CIRT for these 4 patients were from 8 to 10, from 7 to 9, from 7 to 7, and from 7 to 7, and these patients died 12.2, 23.8, 33.2, and 54.7 months after the completion of CIRT, respectively.
The median survival time (MST) of the 124 patients was 35.4 months (range, 4.1‐185.1 months), and the 1‐, 3‐, and 5‐year overall survival rates were 90.3% (95% CI, 83.6%‐94.4%), 50.0% (95% CI, 40.9%‐58.4%), and 25.0% (95% CI, 17.8%‐32.9%), respectively. The 1‐, 3‐, and 5‐year overall survival rates for the 82 cases in protocol 9603 (MST, 33.2 months; range, 4.1‐185.1 months) and for the 44 cases in protocol 0004 (MST, 38.3 months; range, 5.7‐159.3 months) were 89.2% (95% CI, 80.0%‐94.1%) and 93.2% (95% CI, 80.0%‐97.7%), 46.3% (95% CI, 35.3%‐56.7%) and 56.8% (95% CI, 41.0%‐69.9%), and 25.6% (95% CI, 16.8%‐35.4%) and 25.0% (95% CI, 13.5%‐38.4%), respectively (Fig. 3). Predictive factors for overall mortality were evaluated (Table 5), and Child‐Pugh class B and the presence of a tumor thrombus were found to be significant factors for mortality in both univariate and multivariate analyses.
Figure 3.
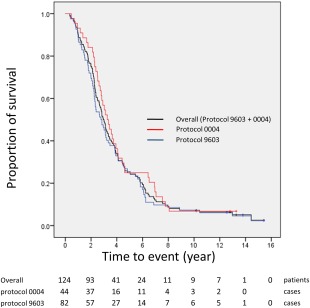
Kaplan‐Meier curves for the overall survival rates of all 124 patients in the 2 protocols.
Table 5.
Predictive Factors for Overall Mortality in Univariate and Multivariate Analyses
| Factor | Univariate Analyses | Multivariate Analyses | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | <68 y | Reference | |||||
| ≥68 y | 0.942 | 0.655‐1.354 | .942 | 0.922 | 0.611‐1.393 | .700 | |
| Sex | Male | Reference | |||||
| Female | 1.132 | 0.755‐1.697 | .549 | 1.160 | 0.717‐1.875 | .546 | |
| ECOG performance status | 0/1 | Reference | |||||
| 2 | 1.348 | 0.789‐2.301 | .274 | 1.027 | 0.573‐1.843 | .928 | |
| Type of chronic hepatitis | HCV | Reference | |||||
| HBV or other | 1.111 | 0.727‐1.697 | .628 | 1.588 | 0.982‐2.570 | .060 | |
| Prior treatment | None | Reference | |||||
| Recurrence after treatment | 1.225 | 0.851‐1.764 | .276 | 1.041 | 0.679‐1.596 | .855 | |
| Child‐Pugh class | A | Reference | |||||
| B | 2.029 | 1.320‐3.121 | .001 | 2.670 | 1.461‐4.878 | .001 | |
| ICG R15 | ≤20% | Reference | |||||
| >20% | 1.577 | 1.079‐2.306 | .019 | 1.311 | 0.853‐2.015 | .217 | |
| Clinical stage | I/II | Reference | |||||
| IIIA/IVA | 1.154 | 0.802‐1.660 | .441 | 0.974 | 0.639‐1.483 | .901 | |
| Maximum tumor size | ≤30 mm | Reference | |||||
| >30 mm, ≤50 mm | 0.879 | 0.560‐1.379 | .574 | 0.981 | 0.583‐1.652 | .945 | |
| >50 mm | 0.998 | 0.616‐1.617 | .992 | 0.964 | 0.562‐1.653 | .894 | |
| Tumor thrombus | Absent | Reference | |||||
| Present | 1.747 | 1.097‐2.784 | .019 | 1.798 | 1.005‐3.217 | .048 | |
| AFP, PIVKA‐II | Both normal | Reference | |||||
| Other | 1.846 | 1.126‐3.028 | .015 | 1.761 | 0.991‐3.219 | .054 | |
| Protocol | 9603 | Reference | |||||
| 0004 | 0.828 | 0.563‐1.218 | .337 | 0.870 | 0.557‐1.360 | .541 | |
Abbreviations: AFP, α‐fetoprotein; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; HBV, hepatitis B virus; HCV, hepatitis C virus; HR, hazard ratio; ICG R15, indocyanine green retention rate at 15 minutes; PIVKA‐II, protein induced by vitamin K absence/antagonist II.
Bolded values are significant.
DISCUSSION
In the phase 1 component of protocol 9603, 52.8 Gy (RBE) in 4 fractions was concluded to be the recommended dose for CIRT. Combined results from the 2 prospective trials demonstrated few severe adverse effects, and the 1‐, 3‐, and 5‐year local‐control rates for all 133 lesions were 94.7%, 91.4%, and 90.0%, respectively. In particular, in the phase 2 protocol 0004, with hypofractionation of 52.8 Gy (RBE) in 4 fractions, high 1‐,3‐, and 5‐year local‐control rates of 97.8%, 95.5%, and 91.6%, respectively, were achieved. To the best of our knowledge, there have been no prospective hypofractionation trials of CIRT for HCC until now.
As for adverse effects, except for 1 patient who experienced HCC rupture outside the planning target volume, no 3‐point or greater increases in the Child‐Pugh score were observed in the current study. Our previous dose‐escalation study using 15 fractions also indicated no 3‐point or greater increases in the Child‐Pugh score at any time point evaluated and no grade 3 or higher adverse effects during the late phase.17 In addition, the incidence rates of RILD at 3 and 6 months after CIRT were considered to be low in the current study. In a study comparing CIRT and proton therapy for HCC, Komatsu et al29 observed no severe adverse effects, including effects in the liver for patients treated with CIRT. These results suggest that the high dose‐concentrating ability of the carbon‐ion beam offsets the higher biological effect of high linear energy transfer radiation on healthy tissue damage, and this allows the benefits of high linear energy transfer radiation to affect primarily the target.
However, 4 patients died of hepatic failure in this study. These 4 patients were Child‐Pugh class B with other active HCCs, although it was difficult to conclude whether the cause of death was radiation‐induced liver failure, tumor progression, side effects caused by other therapies, or the natural course of their liver disease. In fact, this study revealed that patients classified as Child‐Pugh class B had a significantly higher mortality rate than those classified as Child‐Pugh class A; this finding is similar to reports evaluating SBRT9, 30 and proton therapy.12, 31 This suggests that patients with poor liver function, such as those who have Child‐Pugh class B or worse disease, and especially those with other active HCCs should be treated with caution when they are undergoing radiotherapy, including CIRT.
Severe skin reactions are possible with CIRT, and caution is warranted. Of the 18 patients with grade 2 or 3 skin adverse effects during the early or late phase, 17 were treated with 2 fields in the current study. CIRT can enable a favorable dose distribution to the target volume with 2 fields; 109 of 133 lesions (82%) were treated with 2 fields in this study. However, an adjustment of the field number or angle may minimize high dose delivery to the skin or subcutaneous tissue.
Meanwhile, no severe adverse effects in the GI tract were observed in this study. Our institutional treatment policy prohibiting high dose delivery to the GI tract, including the requirement of tumors being at least 1 cm away from the GI tract or the irradiation field fractions being differentially balanced to offset radiation delivery to the GI tract, may have contributed to preservation of the GI tract.
Chest wall pain due to a rib fracture requiring pain killers was observed in 2 patients enrolled in protocol 0004 (52.8 Gy [RBE] in 4 fractions). The tumors of the 2 patients were relatively large in size (56 and 72 mm, respectively), and both were located close to the chest wall. Hypofractionation, tumor location, and tumor size might have influenced the chest wall pain.
There were no cases of CIRT‐related common bile duct or portal vein stenosis in the current study. Irradiation of the hepatic portal region was avoided because patients with tumor thrombosis of the main portal vein were ineligible. Because the results of the current study do not ensure the safety of CIRT to the porta hepatis, CIRT as well as other types of radiotherapy such as proton and photon radiotherapy should be conducted carefully in this region.
Recently, for the purpose of dose reduction to organs at risk, scanning irradiation with respiratory gating has been started at NIRS.32, 33
The current study showed a favorable local‐control rate in patients with HCC similar to the findings of previous studies on particle therapy,11, 12, 13, 29, 31 and there were no significant differences in local failure according to tumor characteristics such as the size or stage before CIRT; this is similar to what has been reported previously.29 This study revealed no significant differences in local failure between the 38 cases with a tumor size > 50 mm (3‐ and 5‐year local‐control rates of 88.5% and 88.5%, respectively), who might have been ineligible for liver transplantation, and the 39 cases with a tumor size ≤ 30 mm (3‐ and 5‐year local‐control rates of 97.1% and 91.0%, respectively). Moreover, even for the 58 cases with both a pre‐CIRT indocyanine green retention rate at 15 minutes > 20% and a tumor size > 30 mm, which limited their eligibility for surgery and RFA, respectively, the 3‐ and 5‐year local‐control rates were 89.3% and 85.8%, respectively. In addition, our previous report indicated no difference in clinical outcomes according to proximity to the porta hepatis.34 These results suggest that CIRT is efficacious for local control, regardless of the tumor characteristics.
The overall survival rate in the current study was not favorable despite good local control. One reason may be that the patients who enrolled in this study generally had recurrent or locally advanced HCC; they included patients with a tumor thrombus or impaired liver function (Child‐Pugh class B), which significantly decreased the overall survival rate. Because CIRT is a local therapy, it may not contribute significantly to survival, especially for patients with recurrent liver lesions or extrahepatic metastases after CIRT. Another reason for the unfavorable overall survival is that liver transplantation and RFA were not routinely performed during the protocol time frame (1998‐2003) in Japan. Among the 64 patients with recurrent lesions before CIRT, only 3 received RFA; moreover, no patients were treated by transplantation after CIRT in the current study, even though these treatments are currently regarded as essential for HCC.35 Nevertheless, the 3‐year overall survival rate (50.0%) and MST (35.4 months) achieved with CIRT in the current study were not inferior to those achieved with TACE (3‐year overall survival rate, 29%; MST, 28.7 months)36 or sorafenib treatment (MST, 10.7 months),37 which are recommended for patients with Barcelona Clinic Liver Cancer stage B or C disease, respectively.35
There were limitations to this study. First, these prospective studies were conducted at a single institution; a multi‐institutional, prospective study is needed. Second, there were some discrepancies in the treatment strategies and diagnostic accuracy because more than 13 years had passed since the completion of this protocol treatment. Since these protocols, CIRT delivered in 2 fractions has been conducted at NIRS for patients with HCC, and clinical outcomes are forthcoming.
In conclusion, CIRT appears to be safe and effective even for patients with recurrent or locally advanced HCC. The safety and efficacy of CIRT hypofractionation with 15, 12, 8, and ultimately 4 fractions were confirmed, and 52.8 Gy (RBE) delivered in 4 fractions was established as the recommended CIRT treatment course for eligible HCC patients.
FUNDING SUPPORT
This work was supported by the Research Project for Heavy Ions at the National Institute of Radiological Sciences (China, Japan).
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosure.
AUTHOR CONTRIBUTIONS
Goro Kasuya: Study design, data assembly, statistical analyses and interpretation, writing of the manuscript, and approval of the final manuscript. Hirotoshi Kato: Study design, data assembly, statistical analyses and interpretation, writing of the manuscript, and approval of the final manuscript. Shigeo Yasuda: Study design, data assembly, statistical analyses and interpretation, writing of the manuscript, and approval of the final manuscript. Hiroshi Tsuji: Study design, data assembly, statistical analyses and interpretation, writing of the manuscript, and approval of the final manuscript. Shigeru Yamada: Study design, data assembly, statistical analyses and interpretation, writing of the manuscript, and approval of the final manuscript. Yasuo Haruyama: Statistical analyses and interpretation and approval of the final manuscript. Gen Kobashi: Statistical analyses and interpretation and approval of the final manuscript. Daniel K. Ebner: Data interpretation, writing of the manuscript, and approval of the final manuscript. Naomi Nagatake Okada: Data interpretation, writing of the manuscript, and approval of the final manuscript. Hirokazu Makishima: Data interpretation, writing of the manuscript, and approval of the final manuscript. Masaru Miyazaki: Data interpretation, writing of the manuscript, and approval of the final manuscript. Tadashi Kamada: Treatment of the patients, revision of the manuscript, and approval of the final manuscript. Hirohiko Tsujii: Treatment of the patients, revision of the manuscript, and approval of the final manuscript.
We express our deep appreciation to the late Dr. Masao Ohto, the principal investigator of the Liver Cancer Working Group, as well as the members of the group.
REFERENCES
- 1. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907‐1917. [DOI] [PubMed] [Google Scholar]
- 2. Borie F, Bouvier AM, Herrero A, et al. Treatment and prognosis of hepatocellular carcinoma: a population based study in France. J Surg Oncol. 2008;98:505‐509. [DOI] [PubMed] [Google Scholar]
- 3. Lu DS, Yu NC, Raman SS, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954‐960. [DOI] [PubMed] [Google Scholar]
- 4. Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L. Local recurrence after hepatic radiofrequency coagulation: multivariate meta‐analysis and review of contributing factors. Ann Surg. 2005;242:158‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huo T, Huang YH, Wu JC, et al. Comparison of transarterial chemoembolization and percutaneous acetic acid injection as the primary loco‐regional therapy for unresectable hepatocellular carcinoma: a prospective survey. Aliment Pharmacol Ther. 2004;19:1301‐1308. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi K, Ina H, Tezuka M, Okada Y, Irie T. Local therapeutic results of computed tomography–guided transcatheter arterial chemoembolization for hepatocellular carcinoma: results of 265 tumors in 79 patients. Cardiovasc Intervent Radiol. 2007;30:1144‐1155. [DOI] [PubMed] [Google Scholar]
- 7. Rou WS, Lee BS, Moon HS, Lee ES, Kim SH, Lee HY. Risk factors and therapeutic results of early local recurrence after transcatheter arterial chemoembolization. World J Gastroenterol. 2014;20:6995‐7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J Radiat Oncol Biol Phys. 1995;31:1237‐1248. [DOI] [PubMed] [Google Scholar]
- 9. Andolino DL, Johnson CS, Maluccio M, et al. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447‐e453. [DOI] [PubMed] [Google Scholar]
- 10. Takeda A, Sanuki N, Tsurugai Y, et al. Phase 2 study of stereotactic body radiotherapy and optional transarterial chemoembolization for solitary hepatocellular carcinoma not amenable to resection and radiofrequency ablation. Cancer. 2016;122:2041‐2049. [DOI] [PubMed] [Google Scholar]
- 11. Chiba T, Tokuuye K, Matsuzaki Y, et al. Proton beam therapy for hepatocellular carcinoma: a retrospective review of 162 patients. Clin Cancer Res. 2005;11:3799‐3805. [DOI] [PubMed] [Google Scholar]
- 12. Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer. 2009;115:5499‐5506. [DOI] [PubMed] [Google Scholar]
- 13. Bush DA, Kayali Z, Grove R, Slater JD. The safety and efficacy of high‐dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer. 2011;117:3053‐3059. [DOI] [PubMed] [Google Scholar]
- 14. Abe T, Saitoh J, Kobayashi D, et al. Dosimetric comparison of carbon ion radiotherapy and stereotactic body radiotherapy with photon beams for the treatment of hepatocellular carcinoma. Radiat Oncol. 2015;10:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanai T, Furusawa Y, Fukutsu K, et al. Irradiation of mixed beam and design of spread‐out Bragg peak for heavy‐ion radiotherapy. Radiat Res. 1997;147:78‐85. [PubMed] [Google Scholar]
- 16. Kanai T, Matsufuji N, Miyamoto T, Itsukaichi H, Eguchi‐Kasai K, Ohara H. Examination of GyE system for HIMAC carbon therapy. Int J Radiat Oncol Biol Phys. 2006;64:650‐656. [DOI] [PubMed] [Google Scholar]
- 17. Kato H, Tsujii H, Miyamoto T, et al. Results of the first prospective study of carbon ion radiotherapy for hepatocellular carcinoma with liver cirrhosis. Int J Radiat Oncol Biol Phys. 2004;59:1468‐1476. [DOI] [PubMed] [Google Scholar]
- 18. International Union Against Cancer . TNM Classification of Malignant Tumours. 5th ed New York, NY: Wiley‐Blackwell; 1997:74‐77. [Google Scholar]
- 19. Pugh RN, Murray‐Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646‐649. [DOI] [PubMed] [Google Scholar]
- 20. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2000;284:3043‐3045. [PubMed] [Google Scholar]
- 21. Minohara S, Kanai T, Endo M, Noda K, Kanazawa M. Respiratory gated irradiation system for heavy‐ion radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:1097‐1103. [DOI] [PubMed] [Google Scholar]
- 22. National Cancer Institute . Common Toxicity Criteria, Version 2.0. Bethesda, MD: National Cancer Institute; 1998. [PubMed] [Google Scholar]
- 23. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341‐1346. [DOI] [PubMed] [Google Scholar]
- 24. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 25. Yasuda S, Ito H, Yoshikawa M, et al. Radiotherapy for large hepatocellular carcinoma combined with transcatheter arterial embolization and percutaneous ethanol injection therapy. Int J Oncol. 1999;15:467‐473. [PubMed] [Google Scholar]
- 26. Gray RJ. A class of k‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141‐1154. [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496‐509. [Google Scholar]
- 28. Kanda Y. Investigation of the freely‐available easy‐to‐use software “EZR” (Eazy R) for medical statistics. Bone Marrow Transplant. 2013;48:452‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Komatsu S, Fukumoto T, Demizu Y, et al. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer. 2011;117:4890‐4904. [DOI] [PubMed] [Google Scholar]
- 30. Huang WY, Jen YM, Lee MS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355‐361. [DOI] [PubMed] [Google Scholar]
- 31. Mizumoto M, Okumura T, Hashimoto T, et al. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys. 2011;81:1039‐1045. [DOI] [PubMed] [Google Scholar]
- 32. Mori S, Zenklusen S, Inaniwa T, et al. Conformity and robustness of gated rescanned carbon ion pencil beam scanning of liver tumors at NIRS. Radiother Oncol. 2014;111:431‐436. [DOI] [PubMed] [Google Scholar]
- 33. Ebner DK, Tsuji H, Yasuda S, Yamamoto N, Mori S, Kamada T. Respiration‐gated fast‐rescanning carbon‐ion radiotherapy. Jpn J Clin Oncol. 2017;47:80‐83. [DOI] [PubMed] [Google Scholar]
- 34. Imada H, Kato H, Yasuda S, et al. Comparison of efficacy and toxicity of short‐course carbon ion radiotherapy for hepatocellular carcinoma depending on their proximity to the porta hepatis. Radiother Oncol. 2010;96:231‐235. [DOI] [PubMed] [Google Scholar]
- 35. Bruix J, Sherman M; American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020‐1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734‐1739. [DOI] [PubMed] [Google Scholar]
- 37. Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378‐390. [DOI] [PubMed] [Google Scholar]


