Abstract
Background
Proinflammatory cytokine IL‐6 and anti‐inflammatory cytokine IL‐10 are expressed in herniated intervertebral disks of Kazakhs, but their significance is not yet understood.
Methods
Specimens of herniated lumbar disk were collected from 30 patients with single disk herniation during lumbar discectomy. As a control, 10 specimens were collected postmortem from the cadavers of individuals who did not die from spinal disease. The clinical symptoms in all cases were evaluated preoperation by the JOA index. The expressions of IL‐6 and IL‐10 in the degenerative disk cells of each specimen were detected using immunohistochemical staining. The amounts of IL‐6 and IL‐10 in each intervertebral disk were assessed by enzyme‐linked immunosorbent assay.
Results
The expression levels of IL‐6 and IL‐10 were significantly higher in the patient group than in the control group. Statistical analysis showed differences between ethnic Kazakh and Han patients in both the expression of IL‐6 and JOA scores. Immunohistochemical staining showed that in the patient group, IL‐6 was found in 28 patients and IL‐10 was found in 27 patients.
Conclusion
The high expression of cytokines, such as IL‐6 and IL‐10, has an important relationship with disk degeneration. Of course, age is another factor in cell apoptosis of degenerative disks. More research is necessary to discover how ethnicity and heredity impact the levels of expressed cytokines.
Keywords: cytokine, ethnicity, intervertebral disk herniation
1. Background
Lower back pain puts a heavy burden on the economy, not to mention the spirits of the people who have lumbar disk degeneration in modern society. However, the exact mechanism for lumbar disk degeneration is unknown.1, 2, 3, 4 Now, most scholars believe that disk degeneration is not only related to mechanical factors, such as biomechanical changes in the intervertebral disk, but also related to biochemical factors. We detected the expression levels of proinflammatory cytokine IL‐6 and anti‐inflammatory cytokine IL‐10 in herniated disks in an attempt to discover the relationship between disk degeneration and its associated symptoms.2, 3, 5, 6
2. Materials and Methods
2.1. Patients and controls
Cases were selected from August 2006 to August 2007 of single segment herniated disks. All 30 patient cases were hospitalized patients treated by our department. The 18 Han and 12 Kazakh patients consisted of 16 men and 14 women, aged 23‐71 years, with a mean age of 44.5 years. The individual intervertebral herniated disks obtained during lumbar discectomy consisted of the following: 2 of L3‐L4, 15 of L4‐L5, and 13 of L5‐S1. All patients had a history of typical lower back pain, without acute or chronic infections, diabetes, etc., and their JOA scores were assessed preoperatively by the same surgeon. During surgery, it was confirmed that there were 25 cases of prominent type disk herniation and five cases of prolapse, with no evidence of any bulging cases among the total 30 cases. The control group consisted of lumbar intervertebral disk tissue extracted from the cadavers of 10 recently deceased individuals who had died from nonvertebral diseases. These individuals were six men and four women, aged 30‐68 years, with a mean age of 46.6 years. Their cadavers provided specimens of L4‐5 or L5‐S1 nucleus pulpous and were checked during surgical tissue removal to have no evidence of intervertebral disk herniation.
2.2. Experimental methods
After surgical removal of tissue from the body, all disk tissue from both the patient and control groups was washed repeatedly with phosphate‐buffered saline (0.01% PBS).4, 6, 7, 8, 9 After this washing to remove any contamination, such as blood, each specimen was divided into two samples and placed in liquid nitrogen for preservation. After specimen collection was completed, the remainder of the experimental process was carried out by a laboratory technician.
For each of the 40 specimens, part of one sample was made into paraffin‐embedded sections, used for HE staining, and the remaining sections of the paraffin block were used for immunohistochemistry. The main methods and processes of immunohistochemistry were as follows: after cutting each of the paraffin‐embedded tissue sections to 4‐μm thick, they were placed in an oven at 60°C and baked for 2 hours. The samples were then put through xylene dewaxing and graded alcohol dehydration, placed in 3% H2O2 for 10 minutes, and then under the microwave 0.01 mol/L citrate antigen retrieval solution repair for 10 minutes. Following this, the samples were placed in a solution of 0.01% PBS wash to which the primary antibody (IL‐6 and IL‐10 antibody) was added. After 1 hour shaking in a 37°C humidified incubator chamber, the samples were placed in a 0.01% PBS wash with added secondary antibody (universal IgG antibody—HRP polymer) and stored in a 4°C refrigerator overnight. The next day, the samples were exposed for 5 minutes to a 0.01% PBS rinse containing freshly prepared DAB color and VIS color to stop the reaction, then thoroughly washed with distilled water. They were washed again with hematoxylin, dried with neutral resin amines, and mounted. After this, PBS was used to replace the primary antibody to make it negative for microscopic examination.
The other samples from each of the 40 specimens were used to measure the IL‐6, IL‐10 proteins by enzyme‐linked immunosorbent assay (ELISA) technique.
The main methods and processes were as follows: 40 specimens were weighed, 200 mg was removed from each for the test, cell lysate RIPA 2 mL and PMSF 20 μL were added, homogenized, centrifuged at 2800 cm/s2 for 10 minutes at 4°C, supernatant was selected and used for uniform detection, and carried out according to the experimental procedure kit instructions. ELISA kits were purchased from Hysen‐Technology Industrial Co. (Xiamen City, China), cell lysates were purchased from Solarbio Company (Beijing Solarbio Science & Technology Co. Ltd agent, Beijing City, China).
2.3. Statistical analysis
The t test was used to assess the difference in IL‐6 and IL‐10 levels for the control group and the patient group, as well as the IL‐6 levels in the Kazak and Han patients. Multivariate analysis assessed the variance between patients’ JOA scores.
3. Results
- The difference in IL‐10 and IL‐6 levels in the lumbar disk tissue of the patient group and the control group was statistically significant (P<.01), with a significantly higher level in the patient group (Figures 1 and 2).
Figure 1.
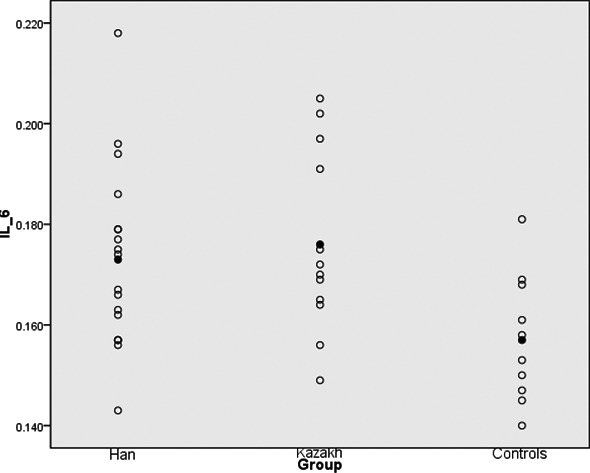 The IL‐6 level of two groups, both higher than that of the control group (P<.01). Mean values are marked with a black point
The IL‐6 level of two groups, both higher than that of the control group (P<.01). Mean values are marked with a black pointFigure 2.
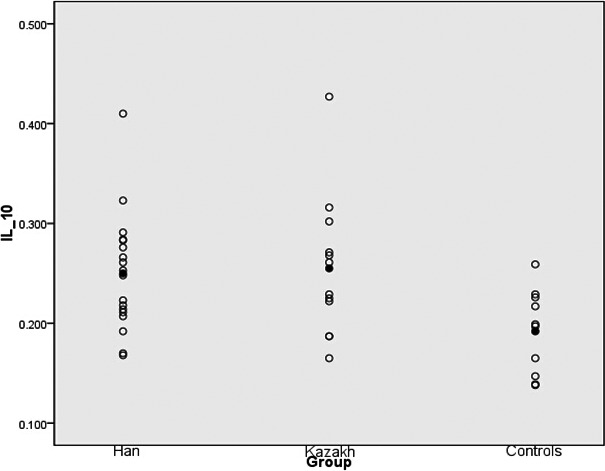 The IL‐10 level of two groups, both higher than that of the control group (P<.01). Mean values are marked with a black point
The IL‐10 level of two groups, both higher than that of the control group (P<.01). Mean values are marked with a black point Using multivariate analysis of variance, we can conclude that the levels of IL‐6 in Kazakh patients and JOA score were significantly higher, statistically so (P<.01), compared with the Han patients. We also found the F value of .962 between two groups, the mean values are 12.222 of Hans and 10.666 of Kazakh
- Immunohistochemical staining found 28 cases of positive expression of IL‐6 in the 30 patient cases of herniated lumbar disk tissue and 27 cases of positive IL‐10 expression, including 24 cases that were positively expressing both of these cytokines. In the microscope field, cells expressing IL‐6 and IL‐10 proteins were mainly concentrated in the inflammatory granulation tissue around the herniated lumbar disk. The nucleus pulposus and annulus fibrosus were found scattered. The cytoplasmic color was brown and the background was light blue. The positive cells were mainly fibroblasts, chondrocytes, and lymphocytes (Figures 3 and 4).
Figure 3.
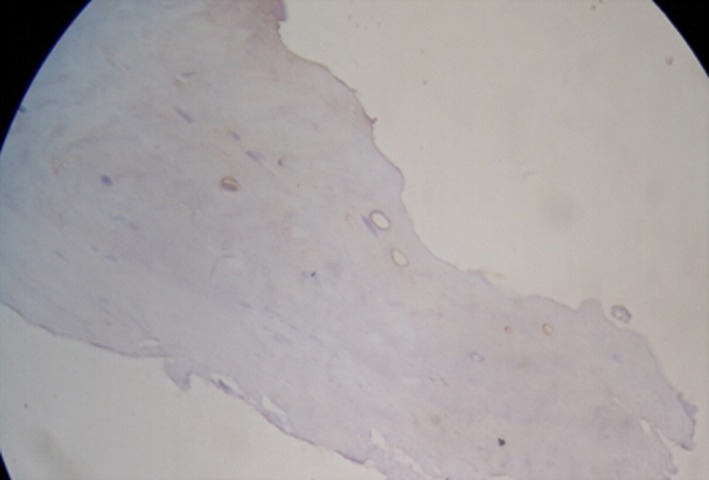 Expression of IL‐6
Expression of IL‐6Figure 4.
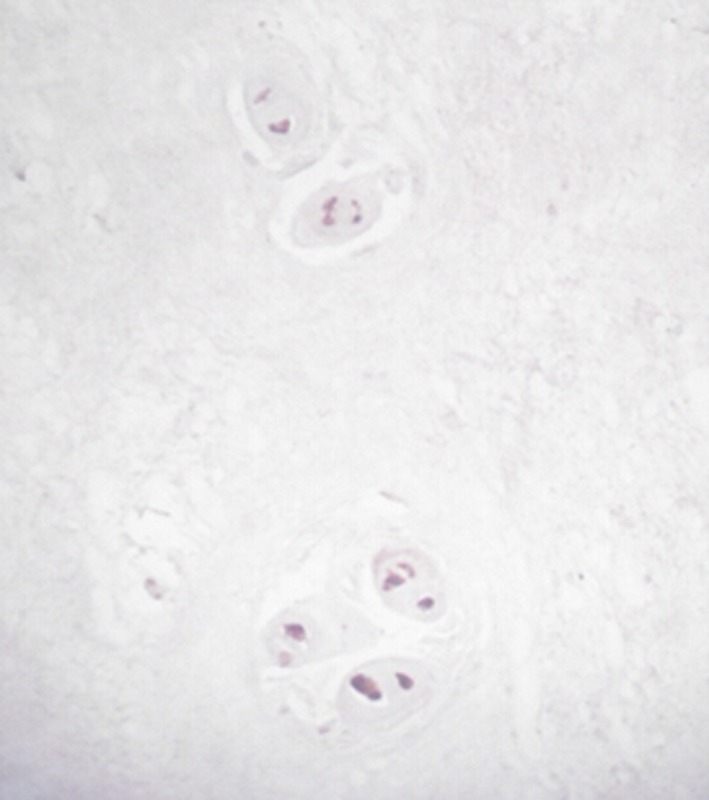 Expression of IL‐10
Expression of IL‐10
4. Discussion
In recent years, with the development of imaging techniques, the degree of lumbar disk herniation patients with pressure on the nerve root causing clinical symptoms is not always consistent.5, 7, 9, 10, 11 Therefore, recent studies indicate that pressure on the nerve root not only produces more than just a simple mechanical problem but also a fairly complex pathophysiological process, which involves biochemical substances such as cytokines which may be an additional source of pain.10, 11
The role of IL‐6 and other inflammatory cytokines in degenerative joint disease has been confirmed as a cause of joint damage and has a similar effect in causing inflammation in lumbar disk degeneration. Takahashi found IL‐6‐positive cells in herniated lumbar disk tissue, but lacked a control group for study. IL‐10 is considered to be an important anti‐inflammatory factor. Published studies suggest the anti‐inflammatory effect of IL‐10 occurred during the inflammatory response when proinflammatory factors reached a certain level, suggesting that the role of IL‐10 is immunomodulatory. In this study, both normal and protruding lumbar disk tissues were used for testing, resulting in a statistically significant difference in IL‐6 and IL‐10 levels between the patient group and the control group. By immunohistochemical staining, 30 cases of herniated lumbar disk tissue, 28 cases of IL‐6 expression, and 27 cases of IL‐10 expression, with 26 cases having positive expression of two cytokines were found. In the control group, of the 10 specimens, there were three cases of positive expression of IL‐6. In this study, with a control group in the study design, the results suggest that the lumbar disk tissue's relatively high secretion of anti‐inflammatory cytokines (IL‐10) coincides with the high level of proinflammatory cytokine (IL‐6), suggesting protruding lumbar disk tissue can also stimulate the production of anti‐inflammatory cytokine. The average level of IL‐10 in the patient group with a JOA score of less than 13 points is 0.271, while the average for those with a JOA score of more than 13 points was 0.232, indicating that patients with severe clinical symptoms in vivo have high levels of anti‐inflammatory cytokine (IL‐10). Overseas studies have found the presence of a large number of human degenerative lumbar disk chondrocyte apoptosis tissue in degenerative lumbar disk, with the apoptosis rate being as high 61.3±24.5%, while the normal level is 15.5±6.8%. The study also suggested that a herniated disk may also stimulate a variety of inflammatory cytokines, thereby increasing disk degeneration and apoptosis. Other studies found normal lumbar disks undergoing age‐related degeneration. In this study, there were only three cases with high levels of cytokines whose ages were younger than 51 years of age, so we cannot ignore the role of age and other factors in the degeneration of the intervertebral disk.
Other studies have found that disk degeneration has familial aggregation. For example, a 5‐year follow‐up study on 75 pairs of male monozygotic twins found that genetic factors played a clear role in the etiology of disk degeneration.1, 6, 12, 13, 14, 15, 16 Some even pointed out that the cause of 74% of disk degeneration can be explained by genetic factors, and the literature has confirmed a much higher level of incidence for Caucasians than for people of color.14, 15, 16 In this study, using multivariate variance for analysis, we found that Kazakh patients with high expression levels of IL‐6 and a high JOA score were statistically significant as compared with Han Chinese patients. This study confirms this trend of racial and genetic differences, as the Kazakhs are the descendants of two sources, Caucasian and Mongoloid, while Hans are essentially Mongoloid in descent.16 Of course, other factors such as climate may have an influence on health. North of the Tianshan Mountains, Kazakhs live mainly above 40°′ north latitude, with winters as long as 5‐6 months. There remains much room for further study as the differences between Kazakh and Han genetics, living environments, cultures, and many other factors are beyond the scope of this study. This study only scratches the surface of examining the role of ethnicity and genetic factors in intervertebral disk degeneration. Disk degeneration and herniation is a complex process, and its mechanism is unknown. Further elucidation of the role of cytokines in intervertebral disk degeneration will undoubtedly provide clues for the prevention and treatment of degenerative disk disease.14, 17
Authors’ contributions
Miao Xiaogang designed the study. Kaken Habaxi attended and performed all the operations for specimen collection, Quanshan Hou and Liping Zhang did the preoperation and postoperation tasks, and took JOA scores, followed up with patients, and performed analysis and interpretation of data.
Abbreviations
- ELISA
enzyme‐linked immunosorbent assay
- IL‐10
interleukin‐10
- IL‐6
interleukin‐6
- JOA
Japanese Orthopaedic Association Scores
Acknowledgments
We acknowledge the help of Professor Miao Xiaogang and Kaken Habaxi in designing this article and attending the operations.
As no individual patient's identity is revealed by the photographs, verbal consent for publication was obtained from the patient or their relative, either telephonic or on a follow‐up visit.
References
- 1. Linda P, Elina S, Line J, et al. Serum levels of the pro‐inflammatory interleukins 6(IL‐6) and ‐8 (IL‐8) in patients with lumbar radicular pain due to disc herniation: a 12‐month prospective study. Brain Behav Immun. 2014;46:132–136. [DOI] [PubMed] [Google Scholar]
- 2. Kohyama K, Saura R, Doilka M, et al. Intervertebral disc cell apoptosis by nitric oxide biological understanding of intervertebral degeneration. Kobe J Med Sci. 2000;46:283–295. [PubMed] [Google Scholar]
- 3. Battie MC, Videman T, Parent E. Lumbar disc degeneration. J Spine. 2004;29:2679–2690. [DOI] [PubMed] [Google Scholar]
- 4. Ala‐Kokko L. Genetic risk factors for lumbar disc disease. J Ann Med. 2002;34:42–47. [DOI] [PubMed] [Google Scholar]
- 5. Jordon J, Shawver Morgan T, Weinstein J, Konstantinou K. Herniated lumbar disc. J Clin Evid. 2006;15:1570–1586. [PubMed] [Google Scholar]
- 6. Lama P, Zehra U, Balkovec C, et al. Significance of cartilage endplate within herniated disc tissue. Eur Spine J. 2014;23:1869–1877. [DOI] [PubMed] [Google Scholar]
- 7. Zhu Z, Huang P, Chong Y, et al. Nucleus pulposus cells derived IGF‐1 and MCP‐1 enhance osteoclast genesis and vertebrae disruption in lumbar disc herniation. Int J Clin Exp Pathol. 2014;7:8520–8531. [PMC free article] [PubMed] [Google Scholar]
- 8. Takahasi H, Suguro T, Okazima Y, et al. Inflammatory cytokines in the herniated disc of the lumbar. J Spine. 1996;21:18–224. [DOI] [PubMed] [Google Scholar]
- 9. Oberholzer A, Oberholzer C, Moldawer LL. Interleukin‐10: acomplex role in the pathogenesis of sepsis syndrome and its potential as an anti‐inflammatory drug. J Crit Care Med. 2002;30(Suppl 1):S58–S63. [PubMed] [Google Scholar]
- 10. Willems N, Tellegen AR, Bergknut N, et al. Inflammatory profiles in canine intervertebral disc degeneration. BMC Vet Res. 2016;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber KT, Alipui DO, Sison CP, et al. Serum levels of the proinflammatory cytokine interleukin‐6 vary based on diagnoses in individuals with lumbar intervertebral disc diseases. Arthritis Res Ther. 2016;18:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sekiguchi M, Yonemoto K, Kakuma T, et al. Relationship between lumbar spinal stenosis and psychosocial factors: a multicenter cross‐sectional study (DISTO project). Eur Spine J. 2015;24:2288–2294. [DOI] [PubMed] [Google Scholar]
- 13. Krock E, Rosenzweig DH, Chabot‐Dore AJ, et al. Painful, degenerating intervertebral discs up‐regulate neurite sprouting and CGRP through nociceptive factors. J Cell Mol Med. 2014;18:1213–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pedersen LM, Schistad E, Jacobsen LM, et al. Serum levels of the pro‐inflammatory interleukins 6 (IL‐6) and ‐8 (IL‐8) in patients with lumbar radicular pain due to disc herniation: a 12‐month prospective study. Brain Behav Immun. 2015;46:132–136. [DOI] [PubMed] [Google Scholar]
- 15. Schistad EI, Espeland A, Pedersen LM, et al. Association between baseline IL‐6 and 1‐year recovery in lumbar radicular pain. Eur J Pain. 2014;18:1394–1401. [DOI] [PubMed] [Google Scholar]
- 16. Li N. Epidemiology study on hypertension in Kazakh from the pasture area of Fukang in Xinjiang. J Hypertens. 2012;30:e194. [Google Scholar]
- 17. Sainoh T, Orita S, Miyagi M, Sakuma Y, et al. Interleukin‐6 and interleukin‐6 receptor expression, localization, and involvement in pain‐sensing neuron activation in a mouse intervertebral disc injury model. J Orthop Res. 2015;33:1508–1514. [DOI] [PubMed] [Google Scholar]


