Abstract
OBJECTIVE:
Testing of methods to enhance the shear bond strength (SBS) between orthodontic metal brackets and amalgam by sandblasting and different primers.
MATERIALS AND METHODS:
Three hundred samples of amalgam restorations (KerrAlloy®) were prepared in self-cured acrylic blocks, polished, and divided into two groups: nonsandblasted and sandblasted. Each group was divided into five subgroups with different primers used in surface treatment methods, with a control group of bonded brackets on human mandibular incisors. Following the surface treatments, mandibular incisor brackets (Unitek®) were bonded on the amalgam with adhesive resin (Transbond XT®). The SBS of the samples was tested. The adhesive remnant index (ARI) and failure modes were then determined under a stereo-microscope. Two-way analysis of variance, Chi-square, and Kruskal–Wallis tests were performed to calculate the correlations between and among the SBS and ARI values, the failure modes, and surface roughness results.
RESULTS:
There were statistically significant differences of SBS among the different adhesive primers and sandblasting methods (P < 0.05). The sandblasted amalgam with Assure Plus® showed the highest SBS (P < 0.001). Samples mainly showed an ARI score = 1 and mix-mode failure. There was a statistically significant difference of surface roughness between nonsandblasted amalgam and sandblasted amalgam (P < 0.05), but no significant differences among priming agents (P > 0.05).
CONCLUSIONS:
Using adhesive primers with sandblasting together effectively enhances the SBS between orthodontic metal brackets and amalgam. The two primers with the ingredient methacryloxydecyl dihydrogen phosphate (MDP) monomer, Alloy Primer® and Assure Plus®, were the most effective. Including sandblasting in the treatment is essential to achieve the bonding strength required.
Keywords: Amalgam, orthodontic brackets, primers, shear bond strength, surface roughness
Introduction
Orthodontic treatment for both clinical and cosmetic purposes has long been a popular dental treatment for teenagers, but in recent times, middle-aged patients and seniors have also sought orthodontic treatment in greater numbers than previously. These patients, in earlier dental treatments, have commonly received amalgam or metal restorations. The amalgam restorations of various sizes and complexities are commonly found on mandibular molars, with treatments varying from small restorations on the buccal pit to restorations of the entire buccal surface of the tooth. At least one amalgam restoration was found in 50–85% of the population.[1,2] These restorations are regularly in clinically acceptable condition and there is no necessity to replace them when orthodontic treatments are sought. However, orthodontists frequently encounter bonding problems of orthodontic brackets on amalgam. Replacement of amalgam with resin composite is a common solution in small-sized restorations. Replacing an amalgam filling with resin composite may produce mercury vapor, which is toxic, during the amalgam removal and inevitably results in greater destruction of the tooth structure.[1]
Cementing an orthodontic band is preferred for a tooth which has a large amalgam restoration. Traditionally, orthodontic bands have been used, being anchored on teeth throughout the often-lengthy treatment period. The use of these devices often caused the accumulation of dental plaque, increasing the risk of dental caries, gingivitis, and periodontal disease, and often resulted in interdental spaces after de-banding. More recently, a buccal tube, using a dental adhesive, has become the preferred, and common, method for anchoring an orthodontic device to the teeth.
An acid-etching technique was introduced by Buonocore,[3] which creates micro-porosities on enamel surfaces, allowing the use of resin adhesives in multidisciplinary dentistry, including orthodontic treatment. Unfortunately, the bond strength of dental adhesives for bonding orthodontic devices to amalgam using this acid-etching technique provides a significantly lower bond strength (3.0–5.0 MPa) when compared with bonding directly to tooth substrates (6.0–18.0 MPa).[4] There have been many literatures published addressing this problem.[5,6,7,8,9,10,11,12,13,14,15,16,17] Adhesive systems have been developed which use chemical primers to prepare the substrate surface to improve the bonding strength of resin-based materials between amalgam and buccal tubes. However, these have not been shown to be completely effective.
The purpose of our study was to develop a new method to increase the bond strength between orthodontic brackets and amalgam restorations reducing the risk of bond failure.
Materials and Methods
The study was approved by Naresuan University Institution Review Board: IRB No. 320/58.
Sample preparation
The extracted human mandibular incisors of above 20 years old patients for periodontal treatment were collected in 10% formaldehyde solution, no longer than 1 month. They must have the normal morphological feature, no cavities, and no restorations. Calculus and soft tissues were removed. All teeth were then submerged in fresh 10% formalin solution for 2 weeks and stored in 0.1% thymol solution. Thirty teeth were used as a control group to be bonded with brackets without sandblasting or primers. Three hundred amalgam samples (KerrAlloy®; Kerr, CA, USA) were prepared in box-like cavities of size 6 × 7 × 2 mm3 in self-cured acrylic blocks.[12] After the amalgams were completely set, they were polished with 1200-grit sand paper and then the amalgam samples were submerged in distilled water at 37°C for 24 h.[12]
The amalgam samples were divided into either a nonsandblasted or a sandblasted group. The surface of the latter group was treated by sandblasting with 50 μm aluminum oxide powder for 3 s, at a 10-mm distance and with 7 kg/cm2 air pressure[12] (Micro-abrasive sandblaster; Parkell Inc., New York, USA). Each group was divided into five subgroups with different surface treatment methods, as shown in Table 1. In group 1, mandibular incisor stainless steel brackets (Unitek®; 3M Unitek, CA, USA) were bonded with Transbond XT® primer and adhesive (Transbond XT®; 3M Unitek, CA, USA) on the enamel surfaces of lower mandibular incisors (control group). In groups 2–5, amalgam restorations were coated with different adhesive primers as shown in Table 1 [Monobond N® (MN), Metal primer® (MP), Alloy Primer® (AP), and Assure Plus® (As)] according to manufacturer's instructions.
Table 1.
Priming products used

The brackets were bonded immediately with Transbond XT® primer and adhesive on each group, except the As group where the brackets were bonded without Transbond XT® primer.
The light curing machine (Mini L.E.D. wavelength 420–480 nm; Acteon, Merignac, France) was held 3-mm from the bracket for 20 s on each interproximal side to cure the adhesive.
All bonded bracket samples were stored in distilled water at 37°C for 24 h followed by thermocycling at 5–50°C for 15 s in each bath and 10 s for traveling between two baths at room temperature[12] for 2000 cycles.
After being thermocycled, each bracket was outlined on a sample tooth with a permanent marker. When mounting brackets onto the tooth, the base of the bracket was set parallel to the direction of force to be applied in the bond shear tests. Using a Universal Testing Machine (Instron 8872; Instron Corp, Bucks, UK), 50-kg load force was applied at a crosshead speed of 1 mm/minute.
After debonding, the tooth surface, amalgam surface, and bracket base were observed under a stereomicroscope with ×25 magnification, and the percentage of surface area with adhesive remnants was determined for each by ImageJ software (Rasband, W.S. National Institutes of Health, Bethesda, MD, USA) and classified according to the adhesive remnant index (ARI) scores:
0 = no adhesive left on the samples (amalgam or tooth)
1 = <50% of the adhesive left on the samples
2 = >50% of the adhesive left on the samples
3 = 100% of the adhesive left on the samples.
The debonded surfaces were also categorized into three failure modes: cohesive failure, adhesive failure, and mixed-mode failure.
Determination of surface characteristics and surface roughness
After the surface treatment, the surfaces of the samples in each group were observed under scanning electron microscopy (SEM) at 500× and 1000× magnification. The surface roughness was analyzed by atomic force micrographs (AFM) (Flex-Axiom; Nanosurf, Liestal, Switzerland). The probe (Tab190AI-G; Budgetsensors, Sofia, Bulgaria) had a nominal spring constant of 48 N/m, 190 kHz resonance frequency, and 50 × 50 μm2 surface area.
Statistical analysis
Shear bond strength (SBS) data were analyzed by two-way analysis of variance (ANOVA) with least square difference post-hoc test at P < 0.05. The ARI data and failure modes were analyzed by Chi-square test at P < 0.05. Root mean squared roughness (Rq) was analyzed by the Kruskal–Wallis and the Mann–Whitney U-tests at P < 0.05.
Results
Shear bond strength
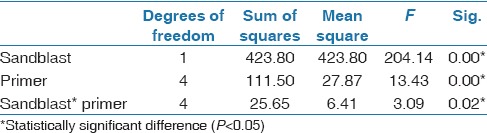
SBS showed statistically significant differences among all variables: sample types, sandblast technique, and types of primers. SBS of sandblasted groups were significantly higher than nonsandblasted groups (P < 0.05). Types of primers also had significant influence on SBS (P < 0.05). SBS had a significant effect on the interaction between the sandblast technique and the types of primers on SBS on amalgam surfaces (P = 0.02) [Table 2].
Table 2.
Analysis of variance for SBS (two-way ANOVA)
As illustrated in Table 3, the groups where the brackets were bonded to teeth (24.59 MPa) had significantly higher SBS than all groups in which the brackets were bonded to amalgam (P < 0.0001). Bonding to a tooth is obviously preferable to bonding to amalgam.
Table 3.
Standard descriptive statistics of SBS (MPa)
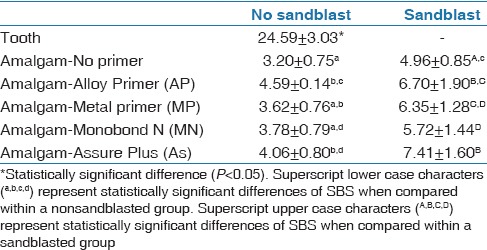
Preparing the surface of the amalgam samples by sandblasting (4.96 MPa) provided a significantly higher SBS than the amalgam samples which had not been sandblasted (3.20 MPa). Sandblasting is an effective preparation method.
In all cases sandblasting prior to bonding with a primer provided a stronger bond, which was significantly different to the primer only (P < 0.04) and sandblasted only (P < 0.0001) samples. That is, sandblasting with primer is better than primer or sandblasting only. The table also illustrates that there is a difference between the sandblasted only and the AP primer group with no sandblasting, but this was not statistically significantly different.
In the nonsandblasted group, the AP group had the highest SBS (4.59 MPa) which was not significantly different from the MP (3.62 MPa) and the As (4.06 MPa) groups (P > 0.05). In the sandblasted primer group, the As group had the highest SBS (7.41 MPa), which was not statistically different from the AP group (6.70 MPa). The SBS of the MP and MN groups was significantly lower than that of the As group. This demonstrates that specific types of primers strengthen the SBS of metal brackets bonded on sandblasted amalgam.
Adhesive remnant index scores and failure modes
Both ARI scores and failure modes showed statistically significant differences between tooth and amalgam samples (P < 0.05) and between nonsandblasted and sandblasted amalgam groups (P < 0.05) [Figures 1 and 2, Table 4].
Figure 1.
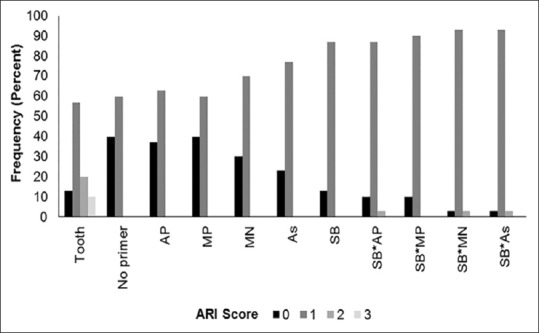
Frequency of ARI scores
Figure 2.
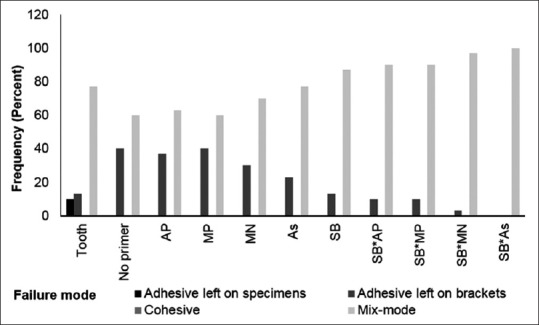
Frequency of failure modes
Table 4.
Comparison between group of ARI scores and failure modes (Chi-square test)
Scanning electron microscope analysis
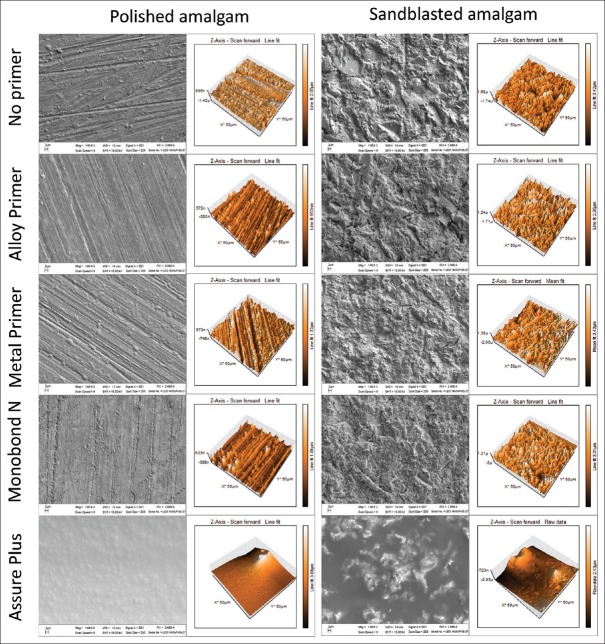
The SEM analysis of the polished amalgam showed scratch lines on the amalgam surfaces. The treated and no treated enamel surfaces with 37% phosphoric acid were used as controls [Figure 3]. For the sandblasted amalgam, the surfaces of samples were much rougher. When primers were applied on both the polished amalgam and sandblasted amalgam, the surface appearances were unchanged, except for the surfaces primed with As. For all the samples primed with As, the surfaces looked smooth and seemed to have film covering the whole surface [Figure 4].
Figure 3.
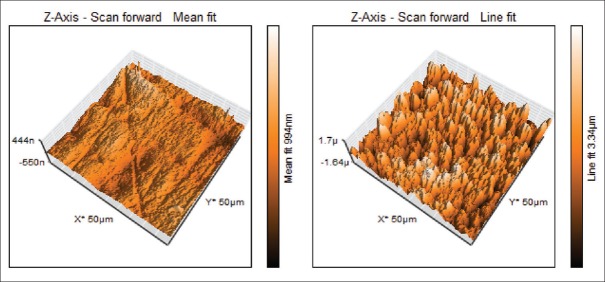
Three-dimensional AFM of human incisor. Unetched enamel (left) and etched enamel with 37% phosphoric acid (right)
Figure 4.
Scanning electron micrographs (×1000 magnifications) and 3D AFM of treated amalgam surfaces. The polished amalgam surfaces followed by each type of primers (left) and the sandblasted amalgam surfaces followed by different types of primers (right)
Atomic force micrographs and root mean square roughness
Figure 4 shows the surface characteristics of the polished amalgam and the sandblasted amalgam, with and without primers, as shown in three-dimensional (3D) AFM.
The mean average surface roughness of the amalgam and the tooth samples are shown in Table 5.
Table 5.
Surface roughness analysis (Mann-Whitney U-test)
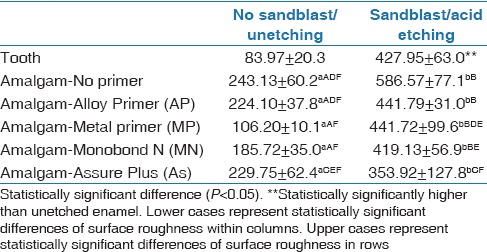
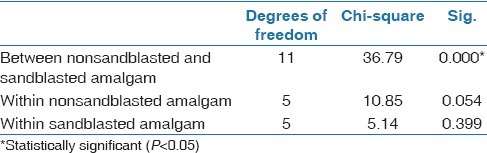
The surface roughness of the amalgam showed statistically significant differences between the sandblasted and nonsandblasted groups (P < 0.001) except after being coated with Assure Plus (P = 0.117) (data not shown). However, there was no statistically significant difference in the surface roughness within both the sandblasted and nonsandblasted amalgam groups [Table 6].
Table 6.
Surface roughness analysis (Kruskal-Wallis test)
Discussion
Bonding orthodontic brackets on amalgam restorations is challenging for orthodontists. Additional surface treatments have been introduced to enhance the bond strength on different substrates by various mechanisms; mechanical creation of surface roughness (sandblasting, grinding with dental burs, or using a laser) and a chemical surface treatment (using metal primers or intermediate resin).[5,6,7,8,9,10,11,12,13,14,15,16,17] When comparing all techniques for their ability to promote surface roughness, the sandblasting technique has the highest potential to increase the bond strength.[5,10]
In 2015, the International Standards Organization (ISO)[18] recommended the protocol for thermocycling: 500 cycles, 5–50°C, 20 s immersion time, 5–10 s traveling time at room temperature.[12,14] However, in our study, we performed thermocycling of 2000 cycles, 5–50°C, 15 s immersion time, and 10 s traveling time. The figure of 2000 cycles is closer to intraoral situations in real life.[18] Furthermore, the 500 cycles recommended in the ISO standard correspond to intraoral temperature variations over a period of <2 months,[19] which is a too short time period for our testing. Perhaps because of the large number of thermocycles in our study, the SBS results which were achieved were lower than that in previous studies.[5,6,7,8,9,10,11,12,13,14,15,16,17]
We selected Transbond XT® as it is commonly used in SBS evaluation in orthodontics due to its high SBS.[8,20,21,22,23,24] Furthermore, it has the benefit of rapid polymerization under a light-cure system, which allows a more accurate bracket position.[25]
The amalgams coated with primers had lower surface roughness, but they showed higher SBS than that of the nonprimer-coated groups, both nonsandblasted and sandblasted. This implies that the primers are effective in promoting chemical bonds between the amalgam and the adhesive resin.
In the sandblasted group, the rough surface of the amalgam samples (both with and without primers applied) showed under SEM and AFM.[26,27] However, when the amalgam was coated with Assure Plus, the surfaces of this group looked smoothly covered with film in the same way as the nonsandblasted group. When compared with other sandblasted groups, the Assure Plus coated group had higher SBS, even though the surface roughness was similar. Assure Plus, therefore, seems to provide better chemical bonds with amalgam that do other primers.
We found that the SBS of the amalgam samples enhanced with the primer still had a lower SBS than the tooth samples of the control group. However, all the primers enhanced the bond strength between brackets and amalgam. These findings agree with the published research.[4,9,10,11,12,14]
The highest SBS of the amalgam samples without sandblasting was shown in the groups bonded with Alloy Primer® (4.59 MPa), which contains 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP). This is a popular monomer used as a component in resin cement, including amalgam bonds for operative dentistry.[17] 10-MDP is a phosphate functional monomer which is effective in chemically bonding to non-noble metals. Apparently, amalgam is this type of metal.
There was no statistically significant difference between the Metal primer® and theMonobond N® (in Asia). The active composition in Metal primer® is 4-META, which provides chemical bonding to non-noble metals by phosphate functional monomer. Monobond N® is a universal primer, since its components are silane, phosphate functional monomer and sulfide functional monomer, which perform chemical bonds between resin and silica, non-noble metals or noble metals. However, the stability of the chemical bond provided from 4-META was less thanfrom10-MDP when exposed to fluid over a period of time.[28] This might explain the higher SBS from Alloy Primer® compared with that from Metal primer® and Monobond N®.
Furthermore, the present study also showed no statistical difference in the SBS between the Assure Plus® and the other primers in the non-sandblasted groups. The composition of Assure Plus® is different from other primers. Assure Plus® comprises HEMA, MDP and Bis-GMA, which is a large polymer with molecular weight of 512 g/mol. Other primers in this study contain methylmethacrylate which has a molecular weight of 100 g/mol. This might create the Assure Plus® viscosity which resulted in the film-like characteristic covering of the amalgam surfaces. HEMA is an hydrophilic material, and it is able to decrease the surface tension of materials.[29] In 2009, the effectiveness of a suitable amount of HEMA in glass ionomer cement (GI) was observed with SBS on both precious alloys and non-precious alloys. The SBS continuously increased with the increase in the amount of HEMA. The peak of the SBS occurred with HEMA at 40% for non-precious metal and 30% for precious metals.[30] Our observations were that the SBSs of the sandblasted amalgam were high with both Assure Plus® and Alloy Primer®, with little difference between them. Both primers have MDP as a functional monomer. MDP manifests chemical interaction with hydroxyapatite and has chemical bonds with phosphate groups on nonprecious metal.[31,32] Previous studies found that the MDP bonds have more hydrolytic stability than other functional monomers.[28,33] These observations might explain the high SBS in Assure Plus® and Alloy Primer® in the sandblasted groups.
When comparing SBS between the sandblasted and the nonsandblasted groups for each primer, SBS of the sandblasted groups significantly increased in all types of primers, clearly indicating that the sandblasting technique enhances SBS of resin on amalgam surfaces.
Several studies reported that bonding failure occurred most often between the amalgam surfaces and the adhesive when using conventional orthodontic bonding techniques: ARI score = 0.[8,11,12,13,14,16] In the present study, adhesive failure most often occurred in mixed-mode failures: ARI score = 1. The number of samples that experienced ARI failure mode = 0, increased in the nonsandblasted group. This indicates that sandblasting and adhesive primers do enhance bond strength, which is supported by the ARI score = 2 in the sandblasted groups, which showed the highest SBS. This demonstrates that the ARI score is positively correlated with the SBS.
Reynolds's in vitro study suggested that the SBS for clinical success in orthodontic treatment should be between 5.9 and 7.9 MPa.[33] The present study showed that the highest SBS of nonsandblasted amalgam samples was the Alloy Primer® group (4.59 MPa), which was lower than the orthodontic clinical acceptable value. The range of SBS of sandblasted amalgam with primer coating, however, was 6.70–7.41 MPa, which are acceptable clinical values.
Conclusions
It was demonstrated that creating a rough amalgam surface by the sandblasting technique, and fortified by a primer, was a more effective technique for increasing the bond strength of orthodontic brackets on dental amalgam than other techniques. It is suggested that this technique is also effective for bonding orthodontic brackets on other metal restorations, such as inlays, onlays, or crowns.
Financial support and sponsorship
The student's research grant from the Graduate School of Naresuan University.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Many thanks to Mr. Roy Morien of the Naresuan University Language Centre for his editing assistance and advice on English expression in this document.
References
- 1.Eley BM, Cox SW. Mercury from dental amalgam fillings in patients. Br Dent J. 1987;163:221–6. doi: 10.1038/sj.bdj.4806247. [DOI] [PubMed] [Google Scholar]
- 2.Jones DW. The enigma of amalgam in dentistry. J Can Dent Assoc. 1993;59:155–60. [PubMed] [Google Scholar]
- 3.Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849–53. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 4.Powers JM, Kim HB, Turnur DS. Orthodontic adhesives and bond strength testing. Semin Orthod. 1997;3:147–56. doi: 10.1016/s1073-8746(97)80065-5. [DOI] [PubMed] [Google Scholar]
- 5.Nergiz I, Schmage P, Herrmann W, Ozcan M. Effect of alloy type and surface conditioning on roughness and bond strength of metal brackets. Am J Orthod Dentofacial Orthop. 2004;125:42–50. doi: 10.1016/s0889-5406(03)00507-9. [DOI] [PubMed] [Google Scholar]
- 6.Gianini M, Paulilo LA, Ambrosano GM. Effect of surface roughness on amalgam repair using adhesive systems. Braz Dent J. 2002;13:179–83. doi: 10.1590/s0103-64402002000300007. [DOI] [PubMed] [Google Scholar]
- 7.Jost-Brinkmann PG, Drost C, Cans S. In-vitro study of the adhesive strengths of brackets on metals, ceramic and composite. Part 1: Bonding to precious metals and amalgam. J Orofac Orthop. 1996;57:76–87. doi: 10.1007/BF02190481. [DOI] [PubMed] [Google Scholar]
- 8.Kuntharaporn P, Winarakwong L, Charoenying H. A comparison of shear bond strength between orthodontic brackets bonded to tooth surfaces and orthodontic brackets bonded to enamel surfaces with amalgam. J Thai Associated Orthod. 2008;7:15–23. [Google Scholar]
- 9.Zachrisson BU, Buyukyilmaz T. Recent advances in bonding to gold, amalgam, and porcelain. J Clin Orthod. 1993;27:661–75. [Google Scholar]
- 10.Zachrisson BU, Buyukyilmaz T, Zachrisson YO. Improving orthodontic bonding to silver amalgam. Angle Orthod. 1995;65:35–42. doi: 10.1043/0003-3219(1995)065<0035:IOBTSA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Gross MW, Foley TF, Mamandras AH. Direct bonding to Adlloy-treated amalgam. Am J Orthod Dentofacial Orthop. 1997;112:252–8. doi: 10.1016/S0889-5406(97)70252-X. [DOI] [PubMed] [Google Scholar]
- 12.Sperber RL, Watson PA, Rossouw P, Sectakof PA. Adhesion of bonded orthodontic attachments to dental amalgam: In vitro study. Am J Orthod Dentofacial Orthop. 1999;116:506–13. doi: 10.1016/s0889-5406(99)70180-0. [DOI] [PubMed] [Google Scholar]
- 13.Oskoee PA, Kachoei M, Rikhtegaran S, Fathalizadeh F, Navimipour EJ. Effect of surface treatment with sandblasting and Er, Cr:YSGG laser on bonding of stainless steel orthodontic brackets to silver amalgam. Med Oral Patol Oral Cir Bucal. 1012;17:292–6. doi: 10.4317/medoral.17473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Germec D, Cakan U, Ozdemir FI, Aun T, Cakan M. Shear bond strength of brackets bonded to amalgam with different intermediate resins and adhesive. Eur J Orthod. 2009;31:207–12. doi: 10.1093/ejo/cjn086. [DOI] [PubMed] [Google Scholar]
- 15.Fonseca RG, de Almeida JG, Haneda IG, Adabo GL. Effect of metal primer on bond strength of resin cements to base metal. J Prosthet Dent. 2009;101:262–8. doi: 10.1016/S0022-3913(09)60050-0. [DOI] [PubMed] [Google Scholar]
- 16.Yetkiner E, Özcan M. Adhesive strength of metal brackets on existing composite, amalgam and restoration-enamel complex following air-abrasion protocols. Int J Adhes Adhes. 2014;54:200–5. [Google Scholar]
- 17.Setcos JC, Staninec M, Wilson NH. The development of resin-bonding for amalgam restoration. Braz Dent J. 1999;7:328–32. doi: 10.1038/sj.bdj.4800102. [DOI] [PubMed] [Google Scholar]
- 18.ISO-Standards. ISO/TR 11405 Dental materials: Guidance of testing of adhesion on amalgam international standards organization. 2015:1–14. [Google Scholar]
- 19.Stewardson DA, Shortell AC, Marquis PA. The effect of clinically relevant thermocycling on the flexural properties of endodontic post materials. J Dent. 2010;38:437–42. doi: 10.1016/j.jdent.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 20.Owens SE, Jr, Miller BH. A comparison of shear bond strengths of three visible light-cured orthodontic adhesives. Angle Orthod. 200;;0:352–6. doi: 10.1043/0003-3219(2000)070<0352:ACOSBS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.Scougall-Vilchis RJ, Zárate-Díaz C, Kusakabe S, Yamamoto K. Bond strengths of different orthodontic adhesives after enamel conditioning with the same self-etching primer. Aust Orthod J. 2010;26:84–9. [PubMed] [Google Scholar]
- 22.Suwanwitid P, Sirichumpun C. Comparison of the shear bond strength of metal brackets photo-activated by LED-curing devices with different light intensities. Chulalongkorn University Dental Journal. 2014;37:259–66. [Google Scholar]
- 23.Öztürk B, Malkoç S, Koyutürk AE, Çatalbaş B, Özer F. Influence of different tooth types on the bond strength of two orthodontic adhesive systems. Eur J Orthod. 2008;30:407–12. doi: 10.1093/ejo/cjn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan AH. Shear bond strength of precoated orthodontic brackets: An in vivo study. Clin Cosmet Investig Dent. 2010;2:41–5. [PMC free article] [PubMed] [Google Scholar]
- 25.Shukla C, Singh G, Jain U, Swamy K. Comparison of mean shear bond strength of light cure, self-cure composite resins, self-etching and moisture-insensitive primers: An in vitro study. J Indian Orthod Soc. 2012;48:254–7. [Google Scholar]
- 26.Russell P, Batchelor D, Thornton J. SEM and AFM: Complementary techniques for high resolution surface investigations. Veeco Instruments Inc; 2004. [Google Scholar]
- 27.Silikas N, Lennie AR, England K, Watts DC. AFM as a tool in dental research microscopy. Microanalysis. 2001:19–22. [Google Scholar]
- 28.Yoshida Y, Nagakane K, Fukuda R, Nakayama Y, Okazaki M, Shintani H, Inoue S, et al. Comparative study on adhesive performance of functional monomers. J Dent Res. 2004;83:454–8. doi: 10.1177/154405910408300604. [DOI] [PubMed] [Google Scholar]
- 29.Asmussen E, Peutzfeldt A. Surface energy characteristics of adhesive monomers. Dent Mater. 1998;14:21–8. doi: 10.1016/s0109-5641(98)00005-0. [DOI] [PubMed] [Google Scholar]
- 30.Lim HN, Kim SH, Yu B, Lee YK. Influence of HEMA content on the mechanical and bonding properties of experimental HEMA-added glass ionomer cements. J Appl Oral Sci. 2009;17:340–9. doi: 10.1590/S1678-77572009000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inoue S, Koshiro K, Yoshida Y, De Munck J, Nagakane K, Suzuki K, et al. Hydrolytic stability of self-etch adhesives bonded to dentin. J Dent Res. 2005;84:1160–4. doi: 10.1177/154405910508401213. [DOI] [PubMed] [Google Scholar]
- 32.Fukegawa D, Hayakawa S, Yoshida Y, Suzuki K, Osaka A, Van Meerbeek B. Chemical interaction of phosphoric acid ester with hydroxyapatite. J Dent Res. 2006;85:941–4. doi: 10.1177/154405910608501014. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds IR. A review of direct orthodontic bonding. Br J Orthod. 1975;2:171–8. [Google Scholar]