Abstract
Objective
To compare the presence of Staphylococcus aureus and pathogenic Gram-negative rods (GNR) in the anterior nares, posterior pharynx and three skin sites in community-based adults and nursing home-based adults before and after treatment with nasal mupirocin and topical chlorhexidine.
Methods
S. aureus-colonized adults were recruited from the community (n=26) and from nursing homes (n=8). Eligible participants were cultured for S. aureus and GNR during two study visits, then received intranasal mupirocin and topical chlorhexidine for 5 days, with a 2-month follow-up period.
Results
After decolonization, we found sustained decreases of S. aureus colonization in nose, throat and skin sites over 4–8 weeks in both populations. Intranasal mupirocin did not increase GNR colonization in nose or throat. Chlorhexidine did not decrease GNR colonization in skin sites.
Conclusions
Decolonization with mupirocin and chlorhexidine leads to a sustained effect on S. aureus colonization without affecting GNR colonization.
Keywords: Mupirocin, Chlorhexidine, Methicillin-Resistant Staphylococcus aureus, Microbiology, Humans, Nursing homes
1. Introduction
“Decolonization” is a rapidly growing strategy to prevent methicillin-resistant Staphylococcus aureus infections fueled by new healthcare policy initiatives, such as mandated surveillance cultures and public reporting of healthcare associated infections. (Medicare, ; Weber et al., 2007) Decolonization involves the application of targeted or non-targeted antimicrobials to the skin or mucosal surfaces. Given the role of our microbiota as a barrier to infection, decolonization regimens could have unintended negative consequences. For example, a Cochrane meta-analysis showed that decolonization with intranasal mupirocin, a Gram-positive antimicrobial agent, increases the risk of infections due to organisms other than S. aureus including Gram-negative rods. (van Rijen et al., 2008) Thus, interventions that shift infections from Gram-positive to Gram-negative pathogens could potentially have long-term negative consequences for patients.
In order to assess the effect of decolonization regimens on Gram-positive and Gram-negative pathogens over time, we conducted an interventional study. Our objective was to compare the presence of S. aureus and pathogenic Gram-negative rods (GNR) in community-based, S. aureus-colonized adults and nursing home-based, S. aureus-colonized adults before and after treatment with nasal mupirocin and topical chlorhexidine. We hypothesized that treatment with mupirocin and chlorhexidine would increase the presence of Gram-negative rods from the Enterobacteriaceae family, Acinetobacter baumannii and Pseudomonas aeruginosa within individuals particularly in the nursing home environment.
2. Material and methods
This was a single-center, interventional study in community-based, S. aureus-colonized adults and nursing home-based, S. aureus-colonized residents. Participants were microbiologically characterized over two study visits, then the nose and skin were decolonized for 5 days with a 2-month follow-up period in which participants were seen weekly for 4 weeks, and then once 4 weeks later (see x-axis of Figures 1–4). The two study visits prior to decolonization allowed each participant to serve as their own control. This convenience sample was recruited prospectively for this study from local VA primary care clinics and VA nursing homes and screened to document their eligibility and health status. Eligible participants were adults without: cancer treatment, HIV infection, immunosupressive medications, nasal steroids, antibiotic (including chlorhexidine or mupirocin) use or recent hospitalization within 3 months. The nursing homes did not use chlorhexidine bathing or mupirocin ointment as a part of infection control efforts. Non-invasive samples from the nose, throat and three skin sites: subclavian, femoral and perianal areas were collected by research staff for culture at the following intervals: 2 months to 2 weeks before intervention, same day intervention begins, weekly after intervention for 4 weeks, and 2 months after intervention. After visit 2, participants received a 5-day course of nasal mupirocin ointment and topical chlorhexidine. Mupirocin 2% nasal ointment was applied by participants or nursing home staff twice a day. 2% Chlorhexidine impregnated cloths were used after bathing each day using a standard protocol. Community based participants filled out a subject diary. Nursing home based participants had their regimen provided by and documented by nursing staff. The study was approved by the University of Maryland, Baltimore Institutional Review Board and the VA Research and Development Committee.
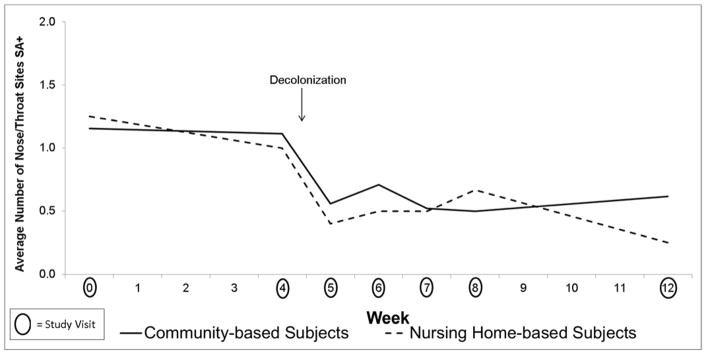
Figure 1.
Effect of Mupirocin on S. aureus Colonization in the Nose and Throat in Community (n=26) vs Nursing Home (n=8) Subjects
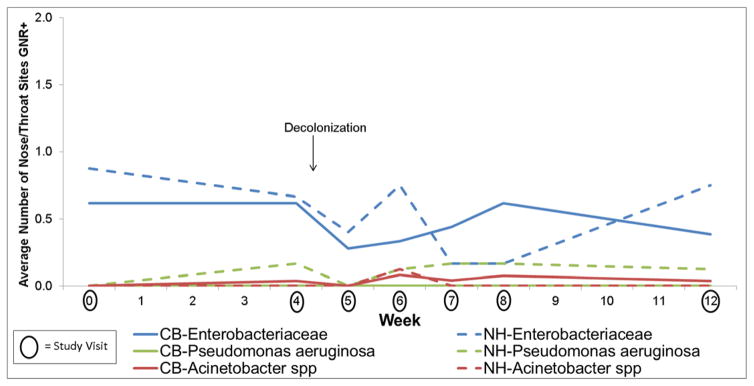
Figure 4.
Effect of Chlorhexidine on Pathogenic Gram-Negative Rod (GNR) Colonization on the Skin in Community (n=26) vs Nursing Home (n=8) Subjects
Enriched samples in Tryptic soy broth with 6.5% NaCl and CHROMagar Staph aureus (Becton Dickenson; Sparks, MD) were used for the detection of S. aureus. Mauve colonies growing on CHROMagar Staph aureus were considered positive for S. aureus. Any suspicious colony morphology was confirmed by Gram staining and latex agglutination (Staphaurex: Remel, Lenexa, KS) for the detection of clumping factor and protein A. Methicillin resistance was determined by oxacillin screen agar and antibiotic susceptibilities performed following CLSI guidelines. (CLSI, 2011)
Gram-negative rods were enriched in brain heart infusion broth and plated to MacConkey and Rambachrom Acinetobacter. All organisms were identified using Vitek compact (BioMerieux; Durham, NC).
All data were entered into study-specific centralized relational databases. Quality control was performed every 3 months via logic checks on the entirety of the databases and comparison of source documentation to the database values for 10% of the participants. The associations between dwelling and resident characteristics were measured using the chi square test or Fisher’s exact test for categorical variables, or the Student t test for continuous variables. The associations between dwelling and bacterial colonization pre intervention were measured using the chi square test or Fisher’s exact test.
Comparisons of the prevalence of body sites positive for S. aureus or Gram-negative rods at weeks post decolonization with the prevalence immediately pre decolonization were made. We combined the nose and throat sites and the three skin sites because intranasal mupirocin impacts the bacterial communities of nose and throat sites whereas topical chlorhexidine affect the skin flora. We used a GEE model for repeated measures to account for within patient correlation. Interactions were examined to assess whether the impact of the decolonization regimen was different between CB and NH patients; no significant differences were observed. The model used a binomial distribution error structure with a log link to estimate prevalence ratios for comparisons of weeks, using model contrasts. Results are presented as ratios post/pre, and no adjustments were made for multiple tests. All statistical tests were two-tailed and p values <0.05 were considered statistically significant. All statistical analyses were conducted with Stata 12.1 software (Stata Corporation, College Station, TX) and SAS 9.3 (SAS Institute, Cary NC).
3. Results
We screened 77 adults who lived in the community to detect 36 (47%) with S. aureus colonization at one or more body sites from September 2012 to April 2014. 28 of 36 had two pre-intervention study visits; 26 of 36 completed the intervention and at least 3 post intervention study visits. We screened 21 adults who lived in nursing homes to detect 9 (43%) with S. aureus colonization at one or more body sites. 7 of 9 had two pre-intervention study visits; 6 of 9 completed the intervention and at least 3 post intervention study visits. Baseline characteristics of our participants with at least one study visit pre and post intervention are shown in Table 1. Of note, none of the participants had antibiotics or hospitalizations in the past 3 months, chronic sinus conditions or active skin conditions.
Table 1.
Baseline characteristics of participants with at least one study visit pre and post intervention
| Characteristic | Community-based Participants (n=26) | Nursing Home-based Participants (n=8) | P value |
|---|---|---|---|
|
| |||
| Age (years) (mean ± SD) | 47 ± 18 | 71 ± 13 | <0.01 |
|
| |||
| Male, n (%) | 15 (58) | 8 (100) | 0.03 |
|
| |||
| Race, n (%) | |||
| American Indian or Alaskan Native | 0 (0) | 1 (13) | 0.24 |
| Asian | 1 (4) | 0 (0) | 1.00 |
| African American | 16 (62) | 1 (13) | 0.04 |
| White | 9 (35) | 6 (75) | 0.10 |
|
| |||
| Body Mass Index (mean ± SD) | 26 ± 4 | 29 ± 3 | 0.04 |
|
| |||
| Diabetes, n (%) | 1 (4) | 2 (25) | 0.13 |
|
| |||
| Currently smoke, n (%) | 10 (38) | 0 (0) | 0.07 |
|
| |||
| S. aureus colonized, n (%) | |||
|
| |||
| Nose | 16 (62) | 5 (63) | 1.00 |
|
| |||
| Throat | 19 (73) | 6 (75) | 1.00 |
|
| |||
| Subclavian skin | 8 (31) | 0 (0) | 0.15 |
|
| |||
| Femoral skin | 9 (35) | 1 (13) | 0.39 |
|
| |||
| Perianal skin | 13 (50) | 1 (13) | 0.10 |
|
| |||
| Any skin site* | 16 (62) | 1 (13) | 0.04 |
|
| |||
| Any body site | 26 (100) | 8 (100) | 1.00 |
|
| |||
| Enterobacteriaceae colonized†, n (%) | |||
|
| |||
| Nose | 14 (54) | 7 (88) | 0.12 |
|
| |||
| Throat | 6 (23) | 4 (50) | 0.20 |
|
| |||
| Subclavian skin | 2 (8) | 4 (50) | 0.02 |
|
| |||
| Femoral skin | 6 (23) | 6 (75) | 0.01 |
|
| |||
| Perianal skin | 22 (85) | 7 (88) | 1.00 |
|
| |||
| Any body site | 21 (75) | 8 (100) | 0.31 |
|
| |||
| Pseudomonas aeruginosa colonized, n (%) | |||
|
| |||
| Nose | 0 (0) | 0 (0) | - |
|
| |||
| Throat | 0 (0) | 1 (13) | 0.24 |
|
| |||
| Subclavian skin | 0 (0) | 0 (0) | - |
|
| |||
| Femoral skin | 0 (0) | 2 (25) | 0.05 |
|
| |||
| Perianal skin | 0 (0) | 0 (0) | - |
|
| |||
| Any body site | 0 (0) | 3 (38) | <0.01 |
|
| |||
| Acinetobacter spp colonized, n (%) | |||
|
| |||
| Nose | 0 (0) | 0 (0) | - |
|
| |||
| Throat | 1 (4) | 0 (0) | 1.00 |
|
| |||
| Subclavian skin | 2 (8) | 0 (0) | 1.00 |
|
| |||
| Femoral skin | 1 (4) | 0 (0) | 1.00 |
|
| |||
| Perianal skin | 2 (8) | 1 (13) | 1.00 |
|
| |||
| Any body site | 4 (15) | 1 (13) | 1.00 |
Subclavian, femoral or perianal skin
Participants could be colonized with more than one Enterobacteriaceae. Analysis is per participant. Of the 29 participants colonized with at least one GNR at any body site at enrollment, 11 (38%) have 1 GNR, 14 (48%) have 2 GNRs, and 4 (14%) have 3 GNRs.
Participant S. aureus colonization status by body site and dwelling are shown in Table 1. There was no difference in colonization at the anterior nares or throat; however, 62% of community-based participants were colonized on at least one skin site before decolonization as compared to 13% of nursing home-based participants (p=0.04, Fisher’s exact). Thirty-two percent of participants were colonized with methicillin-resistant Staphylococcus aureus (MRSA) at baseline. There were no significant differences between nursing home and community participants; 35% of community participants had MRSA and 50% of nursing home participants had MRSA (p=0.68, Fisher’s exact). In addition, there were no significant differences in body sites colonized. There were also significant differences in colonization with pathogenic Gram-negative rods in the Enterobacteriaceae family and P. aeruginosa with nursing home residents more likely to be colonized with both at multiple body sites (Table 1). The three most common organisms from the Enterobacteriaceae family found at baseline were Escherichia coli, Proteus mirabilis and Klebsiella pneumoniae.
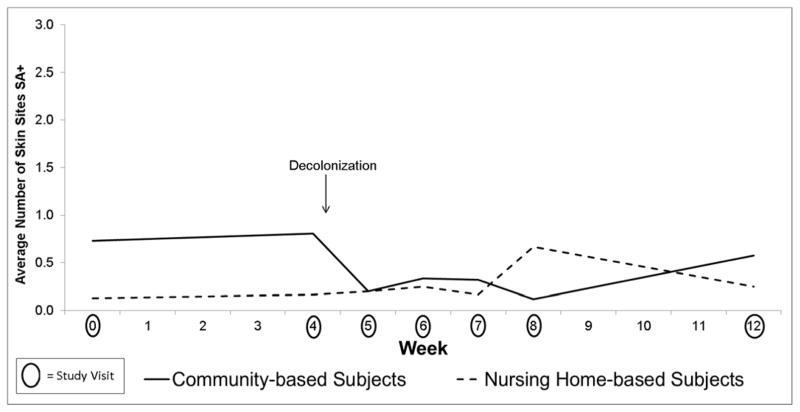
Table 2 combines data from community and nursing home-based participants because there were no differences in the effect of decolonization between them. There was a decrease in S. aureus colonization at all body sites after intranasal mupirocin and topical chlorhexidine in both study populations (Figures 1–2). The effect was sustained out to 8 weeks for S. aureus colonization at the nose and throat and out to 4 weeks for S. aureus colonization at the skin sites as shown in Table 2.
Table 2.
Comparison of the prevalence of body sites positive for S. aureus or Gram-negative rod (GNR) after decolonization with intranasal mupirocin and topical chlorhexidine
| Weeks post decolonization* | 1 week: Week 5 vs 4 | 2 weeks: Week 6 vs 4 | 3 weeks: Week 7 vs 4 | 4 weeks: Week 8 vs 4 | 8 weeks: Week 12 vs 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogen | Body site | PR | p value | PR | p value | PR | p value | PR | p value | PR | p value |
| S. aureus | Nasal/throat | 0.43 | <0.01 | 0.54 | <0.01 | 0.42 | <0.01 | 0.39 | <0.01 | 0.56 | <0.01 |
| Skin | 0.27 | <0.01 | 0.54 | 0.10 | 0.48 | 0.10 | 0.19 | 0.01 | 0.89 | 0.71 | |
| GNR | Nasal/throat | 0.61 | 0.06 | 0.56 | 0.02 | 0.71 | 0.15 | 0.88 | 0.61 | 0.68 | 0.18 |
| Skin | 1.15 | 0.32 | 1.13 | 0.34 | 0.87 | 0.40 | 1.05 | 0.78 | 1.1 | 0.62 | |
PR= prevalence ratio
Indicates the number of weeks after decolonization that the samples were obtained. For example, at 1 week post decolonization the samples collected at the Week 5 study visit were compared to the samples collected at the Week 4 study visit. The Week 4 study visit occurred immediately before decolonization. The Week 5 study visit occurred very soon after decolonization.
Figure 2.
Effect of Chlorhexidine on S. aureus Colonization on the Skin in Community (n=26) vs Nursing Home (n=8) Subjects
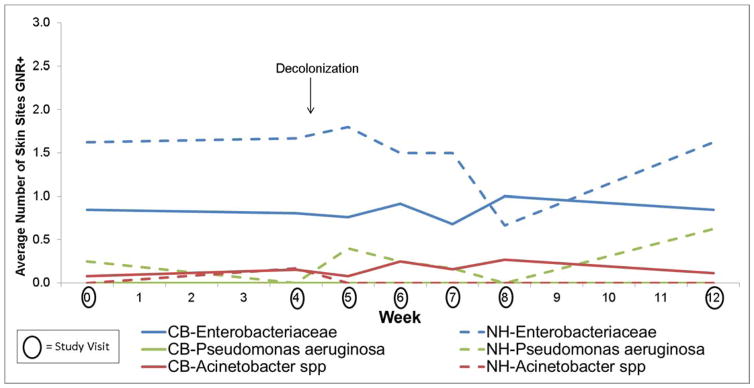
No increase in pathogenic Gram-negative rod colonization was observed in the nose and throat with mupirocin in either community-based or nursing home-based study participants (Figure 3 and Table 2), providing evidence against our hypotheses. Indeed, there was a decrease in Gram-negative rod colonization in the nose and throat post decolonization. In contrast to the effect of decolonization on S. aureus colonization, there were no changes in pathogenic Gram-negative rod colonization of the skin with the use of chlorhexidine for either community-based or nursing home based study participants (Figure 4 and Table 2). Of note, Escherichia coli persisted after the use of chlorohexidine, predominately at the perianal skin. Additionally, Klebsiella pneumoniae persisted and in some cases newly appeared immediately after the use of chlorohexidine. The small sample size precludes the examination of trends for other Gram-negative rods.
Figure 3.
Effect of Mupirocin on Pathogenic Gram-Negative Rod (GNR) Colonization in the Nose and Throat in Community (n=26) vs Nursing Home (n=8) Subjects
4. Discussion
At baseline, we found that there was more S. aureus colonization on skin sites in community based participants than in nursing home based participants. This could reflect differences in living environment and host with the community based participants being younger and more likely to have USA300 S. aureus. USA300 is more likely to colonize and infect the perineal and groin skin. (Diep et al., 2008) In contrast, there was more Gram-negative rod colonization on skin sites in nursing home based participants than community based participants. This could be due to differences in hygiene between the two study populations; community based participants were able to shower daily whereas nursing homes based participants often received a full bath or shower two or three times a week. It also provides a potential mechanism for why nursing home residents are at greater risk for Gram-negative infections particularly pneumonia and urinary tract infections. (Garibaldi, 1999)
After decolonization, we found sustained decreases in S. aureus colonization in nose, throat and skin sites in both populations. This is consistent with past studies of mupirocin in community dwelling adults (Ammerlaan et al., 2009; Harbarth et al., 1999; Perl et al., 2002) and nursing home residents. (Mody et al., 2003) Importantly intranasal mupirocin did not increase GNR colonization in the nose or throat in either of our populations. Of note, chlorhexidine did not decrease Gram-negative rod colonization at skin sites in either population at 1 week indicating that its effect on GNR is more time limited than its effect on S. aureus. Popovich and colleagues found that chlorhexidine skin concentrations fell below effective levels after 1–3 days after chlorhexidine bathing was discontinued which could explain this finding. (Popovich et al., 2012)
Our study is limited by a relatively small sample size particularly of nursing home residents. It is possible that larger samples might detect an increase in Gram-negative rod colonization that we were unable to detect. Despite this, to our knowledge, this is the first report of the ecologic effect of decolonization regimen in patient populations.
Decolonization strategies may provide a simple and relatively low-cost solution to prevent infection and readmissions by reducing colonization with bacteria that can cause infection. Decolonization is an attractive option particularly in nursing homes where isolation, a mainstay of infection control in hospitals, does not provide a practical solution. For nursing homes, socialization and a home-like environment are key components of recovery and quality of life. Our results suggest that mupirocin and chlorhexidine are highly effective at decreasing S. aureus colonization for 4–8 weeks without accompanying increase in Gram negative rod colonization. In contrast, they were not effective at decreasing Gram negative rod colonization at 1 week. Overall mupirocin and chlorhexidine are attractive options for controlling S. aureus infections; however, they may not be effective at reducing Gram-negative infections.
Acknowledgments
This work supported by the University of Maryland Clinical Translational Science Institute and the University of Maryland General Clinical Research Center.
Funding Information
This work was supported by the United States (U.S.) Department of Veterans Affairs Clinical Sciences Research and Development Service [I01 CX000491-01A1]; and the National Institute of Allergy and Infectious Diseases [1R01AI087865-01A1]. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Footnotes
Conflicts of Interest
None.
References
- Ammerlaan HS, Kluytmans JA, Wertheim HF, Nouwen JL, Bonten MJ. Eradication of methicillin-resistant Staphylococcus aureus carriage: a systematic review. Clin Infect Dis. 2009;48:922–930. doi: 10.1086/597291. [DOI] [PubMed] [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-First Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2011. [Google Scholar]
- Diep BA, Chambers HF, Graber CJ, Szumowski JD, Miller LG, Han LL, Chen JH, Lin F, Lin J, Phan TH, Carleton HA, McDougal LK, Tenover FC, Cohen DE, Mayer KH, Sensabaugh GF, Perdreau-Remington F. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann Intern Med. 2008;148:249–257. doi: 10.7326/0003-4819-148-4-200802190-00204. [DOI] [PubMed] [Google Scholar]
- Garibaldi RA. Residential care and the elderly: the burden of infection. J Hosp Infect. 1999;43(Suppl):S9–18. doi: 10.1016/s0195-6701(99)90061-0. [DOI] [PubMed] [Google Scholar]
- Harbarth S, Dharan S, Liassine N, Herrault P, Auckenthaler R, Pittet D. Randomized, placebocontrolled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1412–146. doi: 10.1128/aac.43.6.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicare Hospital Compare: Healthcare-Associated Infections. Available at: https://www.medicare.gov/hospitalcompare/Data/Healthcare-Associated-Infections.html.
- Mody L, Kauffman CA, McNeil SA, Galecki AT, Bradley SF. Mupirocin-based decolonization of Staphylococcus aureus carriers in residents of 2 long-term care facilities: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003;37:1467–1474. doi: 10.1086/379325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl TM, Cullen JJ, Wenzel RP, Zimmerman MB, Pfaller MA, Sheppard D, Twombley J, French PP, Herwaldt LA. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871–187. doi: 10.1056/NEJMoa003069. [DOI] [PubMed] [Google Scholar]
- Popovich KJ, Lyles R, Hayes R, Hota B, Trick W, Weinstein RA, Hayden MK. Relationship between chlorhexidine gluconate skin concentration and microbial density on the skin of critically ill patients bathed daily with chlorhexidine gluconate. Infect Control Hosp Epidemiol. 2012;33:889–896. doi: 10.1086/667371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rijen M, Bonten M, Wenzel R, Kluytmans J. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev. 2008:CD006216. doi: 10.1002/14651858.CD006216.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber SG, Huang SS, Oriola S, Huskins WC, Noskin GA, Harriman K, Olmsted RN, Bonten M, Lundstrom T, Climo MW, Roghmann MC, Murphy CL, Karchmer TB Society for Healthcare Epidemiology of America, Association of Professionals in Infection Control and Epidemiology. Legislative mandates for use of active surveillance cultures to screen for methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci: position statement from the Joint SHEA and APIC Task Force. Infect Control Hosp Epidemiol. 2007;28:249–260. doi: 10.1086/512261. [DOI] [PubMed] [Google Scholar]