Abstract
In male Drosophila, chemosensory cues control many aspects of social behavior. We found that males with a mutated Gustatory receptor 32a gene (Gr32a) show high courtship toward males and mated females, indicating that GR32a functions as a pheromone receptor for a male inhibitory pheromone. Notably, we discovered that tarsal Gr32a-expressing neurons were essential for courtship suppression and projected to the ventrolateral protocerebrum, implying direct communication of chemosensory neurons with a higher-order brain structure.
In the fruit fly Drosophila melanogaster, social behaviors are mediated through multiple sensory channels, including chemosensation, vision and audition1. For example, chemicals released by both sexes are thought to function as stimulatory and inhibitory pheromones that are necessary to direct courtship toward appropriate individuals. The only Drosophila pheromone receptors associated with specific roles during courtship are GR68a and OR67d/OR65a2–4. OR67d and OR65a are expressed in olfactory sensory neurons and recognize cis-vaccenyl acetate (cVa)4,5, a volatile male pheromone, and OR67d is required in males to suppress male-male courtship4. Gr68a is specifically expressed in male taste neurons in the forelegs and is necessary for efficient male courtship toward females2.
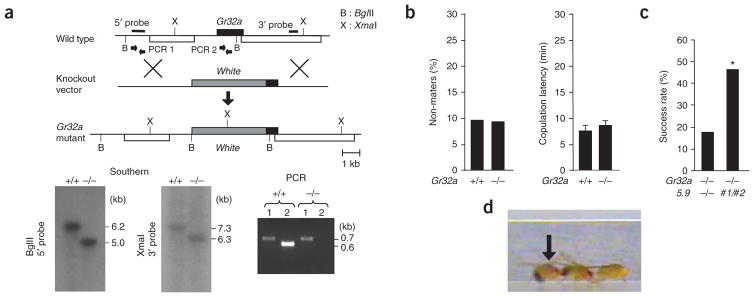
We generated a mutant fly strain by knocking out Gr32a, a close relative of Gr68a1,2, using ends-out homologous recombination6 (Supplementary Methods online). Precise replacement of the Gr32a coding sequence by the mini-white gene was confirmed by Southern analysis and PCR (Fig. 1a). Both Gr32a mutant males and females were healthy, fertile and without an obvious taste phenotype (Supplementary Fig. 1 online). Gr32a−/− and wild-type control males showed similar levels of courtship when paired individually with virgin female partners, suggesting that female pheromones were properly received in the absence of Gr32a (Fig. 1b). However, when two males competed for a single virgin female, the wild-type male outperformed the Gr32a−/− males by a ratio of 4 to 1, a phenotype that was rescued by two genomic Gr32a transgenes (Fig. 1c). Movie recordings from these courtship competition experiments showed that Gr32a−/− males frequently courted their competitor, in addition to the female (Fig. 1d and Supplementary Video 1 online). To further investigate this behavior, we carried out courtship experiments using passive, decapitated flies of either sex. Such flies are aggressively courted, but fail to display behavioral feedback that could confound precise measurement of sex appeal. Consistent with courtship experiments using intact females, decapitated, virgin females elicited the same courtship activity from Gr32a−/− and wild-type males (Fig. 2a). In contrast, courtship toward decapitated males increased only in Gr32a−/− males and included wing vibration and copulation attempts, behaviors that were not observed in wild-type males (Fig. 2b and Supplementary Table 1 online). Notably, the phenotype was rescued by two Gr32a transgenes (Fig. 2b and Supplementary Fig. 2 online).
Figure 1.
Knockout of the Gr32a gene and courtship analysis of mutant males. (a) Representation of the Gr32a locus before (top) and after (bottom) homologous recombination. Gels show Southern and PCR analysis from wild-type and Gr32a−/− DNA, respectively. B = BglII; X = XmaI. PCR1 and PCR2 indicate amplified fragments (black bars, 5′ and 3′ probe; white boxes, neighboring genes). (b) Gr32a−/− males mated with females as efficiently as wild-type males in single pair courtship assays (n > 30). Fraction of non-maters (left) and time to copulation (right) were the same in both type of males. Error bars represent s.e.m. (P > 0.05, χ2 test for non-maters, ANOVA for copulation latency). (c) Competition experiments (two males, one female). The bar shows the percentage of copulation success of either Gr32a−/− males (left) or Gr32a−/− males carrying two Gr32a_5.9 transgenes (right) competed with the wild-type males. (* P < 0.05, χ2 test for non-maters, ANOVA for copulation latency, n > 40). (d) Still frame from a movie showing a Gr32a−/− male (arrow) courting his competitor, who courts the female (see Supplementary Video 1).
Figure 2.
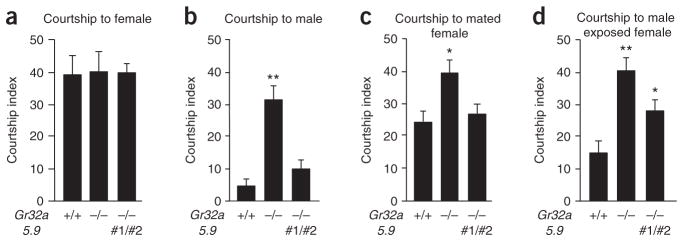
Gr32a mutant male shows high courtship activity toward males. (a–d) Courtship index of males toward decapitated females (a), males (b), mated females (c) or male-conditioned females (d). Male genotypes were wild type (left), Gr32a−/− (middle) or Gr32a−/− containing two Gr32a_5.9 transgenes (right). Error bars represent s.e.m. Statistical significance was measured by ANOVA (* P < 0.05, ** P < 0.0001, n > 30).
As Gr32a is expressed in both males and females, one might expect a role for this receptor in female mating behavior. We compared female receptivity of wild-type and Gr32a mutant females, but did not find any obvious differences (Supplementary Fig. 2). Further behavioral analyses need to be conducted to address a possible role for Gr32a in females.
Mated females are less attractive than mature virgin females, as they receive inhibitory male pheromones during copulation7,8. Although wild-type males showed a significant decrease in courtship toward mated females, Gr32a−/− males showed the same high courtship index toward both types of females (Fig. 2a, c), indicating that Gr32a is required for discrimination between virgin and mated females. To determine the source of the pheromones, we compared courtship levels that were elicited by mated females (contain both cuticular- and seminal fluid–derived pheromones) with those elicited by virgin, but male-conditioned, females (containing only cuticular pheromones; Fig. 2c, d). Male-conditioned females suppressed courtship in control males, but not in Gr32a−/− males. Increased courtship toward mated and male-conditioned females was fully and partially rescued by two Gr32a transgenes, respectively, indicating that cuticular pheromones are a major determinant of female mating status (Fig. 2c, d), but leaving open the possibility for compounds in seminal fluid as ligands for GR32a.
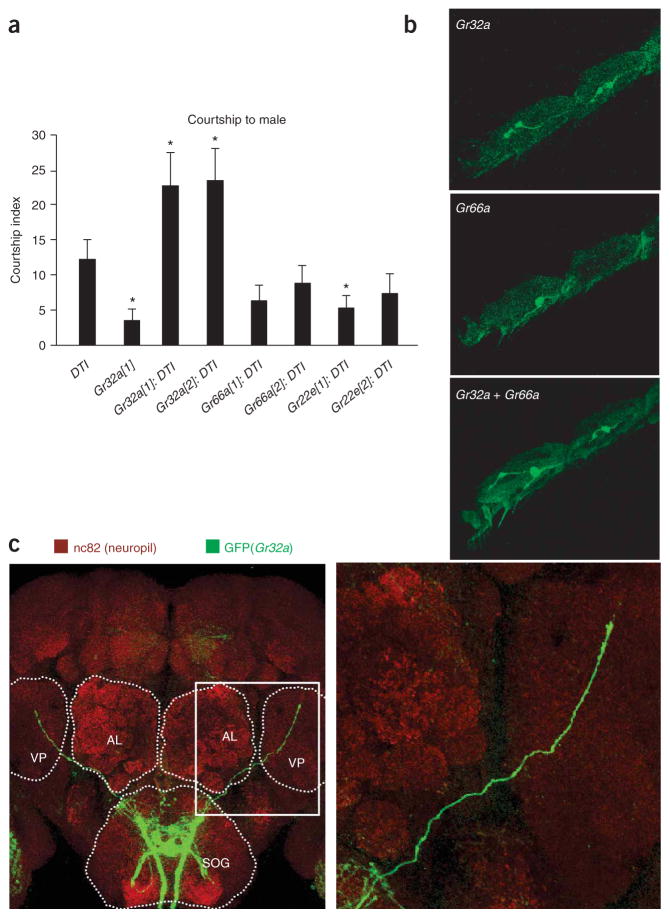
If GR32a is necessary for the detection of an inhibitory male pheromone, ablation of Gr32a-expressing neurons should lead to male-male courtship. Indeed, males with diphtheria toxin (DTI)-ablated Gr32a-expressing neurons showed similarly high levels of courtship as Gr32a−/− males (Figs. 3a and 2b and Supplementary Table 2 online). In contrast, males expressing DTI in Gr66a- or Gr22e-expressing neurons and UAS-DTI control males showed low male-male courtship. These observations imply that chemosensory neurons residing outside of the labial palps mediate Gr32a-dependent courtship suppression, as all labial palp neurons expressing Gr32a also express Gr66a and Gr22e9,10. Indeed, we found that Gr32a and Gr66a/Gr22e were expressed in distinct, nonoverlapping sets of gustatory receptor neurons in the foreleg (Fig. 3b and data not shown). A role for these neurons in courtship suppression is consistent with our observation of an increase in male-male courtship in Gr32a−/− males immediately after the tapping step (Supplementary Table 1). Finally, none of the ablations had an effect on male courtship toward females (Fig. 3a and Supplementary Fig. 2).
Figure 3.
Expression of Gr32a in tarsal neurons and their role in male-male courtship suppression. (a) Courtship index of males (6–10 d old) with DTI-ablated neurons toward decapitated males. DTI was expressed in distinct neurons (indicated by Gal4 drivers; 1 and 2 indicate different lines). Error bars represent s.e.m. Statistical significance was measured by ANOVA (* P < 0.05, ** P < 0.0001, n > 30). (b) Visualization of Gr32a- and Gr66a-expressing neurons in a fore tarsi; mCD8::GFP reporter was expressed by Gr32a (top), Gr66a (middle) or both drivers (bottom). (c) Projection pattern of Gr32a-expressing neurons in brain. Axons (antibody to GFP, green) and the neuropil (antibody to nc82, red) were visualized by performing antibody staining on whole brains. Outlined square is enlarged on the right. AL, antennal lobe; VP, ventro-lateral protocerebrum.
All Gr32a-expressing neurons have been reported to project to a small area in the subesophageal ganglion (SOG)9,10. However, we occasionally observed axons of Gr32a-expressing neurons projecting into the ventrolateral protocerebrum. Thus, we reexamined neuronal projections using a more sensitive reporter (UAS-mCD8::gfp) and a stronger Gr32a-Gal4VP16 driver (Fig. 3c and Supplementary Fig. 3 online). This analysis revealed that GFP-labeled afferents exited the SOG anterior into the ventrolateral protocerebrum (Fig. 3c). These projections strongly correlated with the presence of the forelegs, but axons from the mid- and hindlegs may also be found in this afferent (Supplementary Table 3 online). We noted that individual animals varied in route and endpoint of this afferent (Supplementary Fig. 3 and Supplementary Table 4 online), implying that either the location of these target cells varies or that the actual targets represent only a small subset of potential targets in the protocerebrum. We did not observe any Gr32a projection differences between sexes, albeit their targets may well show sex-specific properties (see below). To the best of our knowledge, Gr32a-expressing neurons represent the first instance of chemosensory neurons relaying sensory input directly to a higher-order brain structure.
Even though cellular expression of Gr32a has been determined indirectly using the Gal/UAS expression system, three lines of evidence suggest that this expression profile reflects largely endogenous Gr32a expression. First, several driver lines showed identical expression patterns9 (Supplementary Fig. 3). Second, neuronal ablation by Gr32a-Gal4 driving diphtheria toxin mimicked the Gr32a−/− phenotype. And third, expression of a Gr32a cDNA using the Gr32a-Gal4 driver suppressed male-male courtship in Gr32a−/− males (Supplementary Fig. 2). Thus, although we cannot positively affirm that the Gr32a-Gal4 driver is active in every single neuron expressing endogenous Gr32a RNA, these experiments demonstrate that the Gr32a-Gal4 driver is expressed in the cells that provide essential, endogenous Gr32a function.
One of the Gr32a phenotypes that we have described here is reminiscent of the phenotype recently reported for a mutation in the olfactory receptor gene Or67d4. Or67d−/− males show an increase in male-male courtship, although they are still attracted to females. Hence, these two receptors appear to mediate general courtship suppression toward other males. However, there are clear differences between Or67d−/− and Gr32a−/− males. First, the two receptors mediate their role in courtship suppression along different sensory pathways. OR67d, which detects the volatile chemical cVA, is expressed in olfactory sensory neurons that project to a single glomerulus in the antennal lobe4, whereas GR32a-expressing neurons mediate sensory input via gustatory pathways to the SOG and higher brain centers in the ventrolateral protocerebrum. Second, we observed that Gr32a−/− males, which have a functional Or67d gene and hence should respond normally to cVA, courted virgin and mated females equally well (Fig. 2a, c), suggesting that cVA/OR67d are not involved in courtship suppression toward mated females. However, to unequivocally determine whether Or67d has a role in courtship suppression toward mated females, respective mutant animals will have to be subjected to the assays presented here. Finally, OR67d4, but not GR32a (Supplementary Fig. 2), appears to be required in females for efficient mating.
How are pheromone cues from Gr32a-expressing neurons integrated into the neural courtship circuitry? Neuroanatomical studies have revealed that the ventrolateral protocerebrum, to which Gr32a-expressing neurons project, also receives input from visual, auditory and possibly olfactory channels11–13. Thus, we propose that different sensory courtship cues are integrated in this brain region. Notably, several neuronal cell clusters expressing male forms of the FRUITLESS protein (FRUM)14 are located near the ventrolateral protocerebrum, even though immunohistochemical studies have not yet revealed direct contact between FRUM- and Gr32a-expressing neurons (Supplementary Fig. 3).
In this study, we found that Gr32a is important for suppressing courtship toward males and mated females. Thus, GR32a is required for discriminating between the two sexes and between females of different mating status. We suggest that the latter role is especially relevant because the physical difference between mated and virgin females is restricted to chemosensory signals and mated females are ‘low-efficiency’ mating targets that show extensive rejection behavior.
Supplementary Material
Acknowledgments
We would like to thank J. Daniels for assistance with behavioral experiments and H. Matsunami for comments on the manuscript. We acknowledge N. Thorne for initially making the observation that Gr32a neurons project beyond the SOG. We also would like to thank L. Vosshall for providing fly strains. This work was supported by grants from the US National Institutes of Health (01DC005606 and 01DC009014) to H.A. T.M. was supported by a long-term fellowship of the International Human Frontier Science Program Organization.
Footnotes
Note: Supplementary information is available on the Nature Neuroscience website.
AUTHOR CONTRIBUTIONS
T.M. and H.A. conceived the experiments. T.M. conducted all of the experiments. T.M. and H.A. wrote the paper.
References
- 1.Amrein H. Curr Opin Neurobiol. 2004;14:435–442. doi: 10.1016/j.conb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 2.Bray S, Amrein H. Neuron. 2003;39:1019–1029. doi: 10.1016/s0896-6273(03)00542-7. [DOI] [PubMed] [Google Scholar]
- 3.Ejima A, et al. Curr Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurtovic A, Widmer A, Dickson BJ. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 5.van der Goes van Naters W, Carlson JR. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rong YS, Golic KG. Genetics. 2001;157:1307–1312. doi: 10.1093/genetics/157.3.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scott D. Proc Natl Acad Sci USA. 1986;83:8429–8433. doi: 10.1073/pnas.83.21.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolfner MF. Insect Biochem Mol Biol. 1997;27:179–192. doi: 10.1016/s0965-1748(96)00084-7. [DOI] [PubMed] [Google Scholar]
- 9.Thorne N, Chromey C, Bray S, Amrein H. Curr Biol. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Singhvi A, Kong P, Scott K. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Jefferis GS, et al. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamikouchi A, Shimada T, Ito K. J Comp Neurol. 2006;499:317–356. doi: 10.1002/cne.21075. [DOI] [PubMed] [Google Scholar]
- 13.Strausfeld NJ. Atlas of an Insect Brain. Springer; New York: 1976. [Google Scholar]
- 14.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.