Abstract
The functionalization of carbon–hydrogen (C–H) bonds is one of the most attractive strategies for molecular construction in organic chemistry. The hydrogen atom is considered to be an ideal coupling handle, owing to its relative abundance in organic molecules and its availability for functionalization at almost any stage in a synthetic sequence1. Although many C–H functionalization reactions involve C(sp3)–C(sp2) coupling, there is a growing demand for C–H alkylation reactions, wherein sp3 C–H bonds are replaced with sp3 C–alkyl groups. Here we describe a polarity-match-based selective sp3 C–H alkylation via the combination of photoredox, nickel and hydrogen-atom transfer catalysis. This methodology simultaneously uses three catalytic cycles to achieve hydridic C–H bond abstraction (enabled by polarity matching), alkyl halide oxidative addition, and reductive elimination to enable alkyl–alkyl fragment coupling. The sp3 C–H alkylation is highly selective for the α-C–H of amines, ethers and sulphides, which are commonly found in pharmaceutically relevant architectures. This cross-coupling protocol should enable broad synthetic applications in de novo synthesis and late-stage functionalization chemistry.
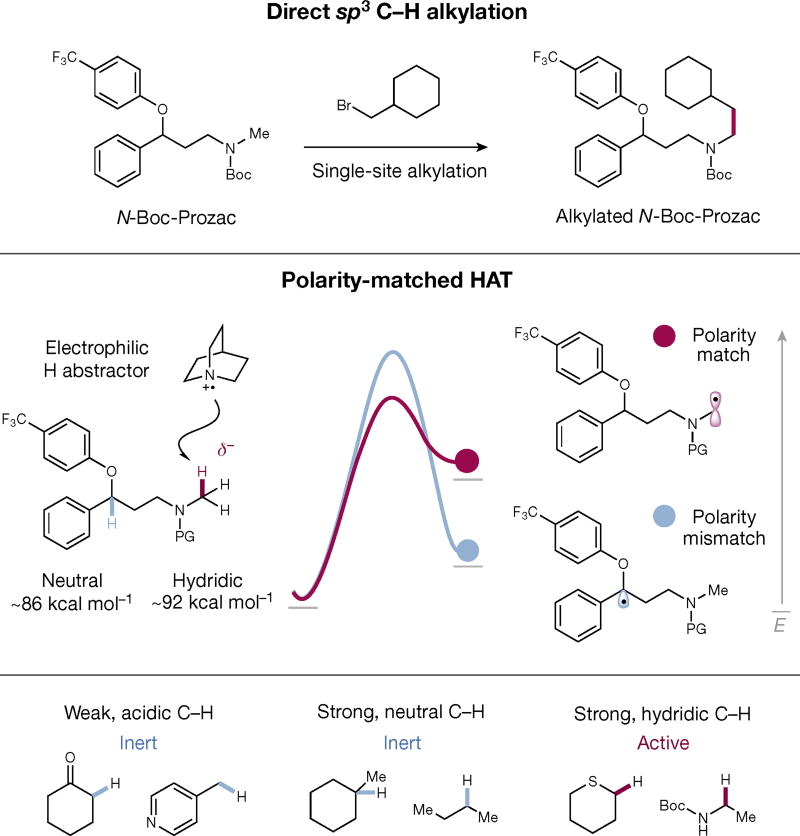
Perhaps the most highly sought after native group transformation in modern cross-coupling chemistry is the selective alkylation of a C–H bond2–5. From a structural-diversification standpoint, substitution of simple C–H bonds for alkyl groups is an important operation for organic synthesis, given the marked changes in chemical and biological properties that can arise from the incorporation of small aliphatic groups6. Whereas aryl or vinyl sp2 C–H alkylation has had considerable success7–10, there are few examples of generic aliphatic sp3 C–H alkylation. Several challenges are associated with native sp3 C–H bond functionalization, most notably regiocontrol, because organic molecules incorporate a diverse combination of methyl, methylene and methine groups. Recent approaches to this positional selectivity problem include the utilization of directing groups11–13 and metal–carbenoid complexes14–16. The requirement of specialized starting materials for these strategies can often limit reaction generality in terms of substrate availability and alkyl electrophile tolerance. With this in mind, we sought to develop a mild sp3 C–H nickel-catalysed alkylation, wherein the control of regioselectivity does not arise from directing groups, bond deactivation strategies or bond-strength considerations, but from the matching of electronic polarity in a C–H bond and a hydrogen-atom transfer (HAT) catalyst (Fig. 1). An aliphatic sp3 C–H direct alkylation would be beneficial to synthetic chemists involved in either de novo or late-stage functionalization strategies.
Figure 1. Selective sp3 C–H alkylations via polarity-matched hydrogen-atom transfer (HAT).
A general and direct alkylation of sp3 C–H remains an elusive transformation in organic synthesis (top). Weak C–H bonds are typically functionalized as a result of the stability of the resulting radical. However, using the polarity-matching effect in HAT, an electrophilic radical should undergo selective hydrogen abstraction at the most hydridic C–H, owing to a lower kinetic barrier (middle). This effect enables selective functionalization beyond the conventional bond-dissociation-energy-driven model (bottom).
Photoredox catalysis has emerged in recent years as an enabling platform for the rapid production under mild conditions of open-shell species that tolerate a wide range of functional groups17–20. Moreover, several studies have demonstrated that photoredox and transition-metal catalysis can be successfully merged (metallaphotoredox) to deliver many selective native-group functionalizations, including decarboxylative and deoxygenative couplings21,22. Photoredox catalysis was recently used to address the issue of native C–H functionalization by taking advantage of the polarity matching effect, a subtle yet important element in HAT catalysis23. Among several advantages, the use of polarity matching can enable hydrogen abstraction events that are not dictated by the thermodynamic driving force of the HAT step itself, or by the relative bond dissociation energies of the various C–H bonds that are available. As a result, strong C–H bonds can be homolytically cleaved in the presence of weaker (non-polarity-matched) C–H bonds if the strong C–H bonds involve ionic matching between the HAT catalyst and the hydrogen atom undergoing exchange. As an example, we have previously demonstrated the use of a quinuclidinium radical cation as a useful HAT catalyst for the selective alkylation of strong, hydridic alcohol α-C–H bonds in the presence of weak C–H bonds24. Moreover, we have used this polarity principle in a triple catalysis coupling to deliver a selective sp3 C–H arylation of strong α-amine C–H bonds in the presence of weak acidic or neutral systems25. With this in mind, we questioned whether a selective aliphatic C–H direct alkylation might be possible using the concept of polarity-matched HAT in combination with photoredox and nickel catalysis. Specifically, we hoped to exploit the inherent kinetic selectivity of polarity matching to access a new class of C–H functionalization that would enable the direct replacement of strong hydridic bonds with alkyl groups, while neutral or acidic bonds (of any given bond dissociation energy) would remain effectively inert.
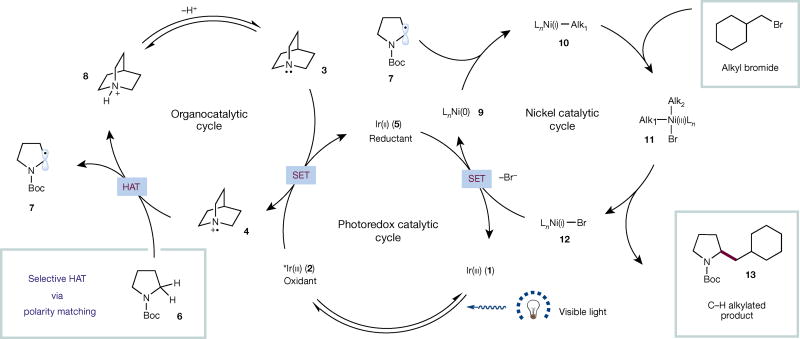
The proposed mechanism of our triple catalytic sp3 C–H alkylation protocol is outlined in Fig. 2. Excitation of photocatalyst Ir[dF(CF3) ppy]2(dtbbpy)PF6 (1) (dF(CF3)ppy = 2-(2,4-difluorophenyl)-5-(trifluoromethyl)pyridine; dtbbpy = 4,4′-di-tert-butyl-2,2′-bipyridyl) results in long-lived photoexcited Ir(iii) complex 2 (triplet excited-state lifetime τ = 2.3 µs)26. This highly oxidizing excited state (reduction potential versus the saturated calomel electrode in MeCN)26 can facilitate the oxidation of quinuclidine (3) (estimated oxidation potential Ep = + 1.1 V versus the saturated calomel electrode in MeCN)24 to yield cationic radical 4 and reduced Ir(ii) complex 5. At this stage, species 4 can engage in a HAT event with N-Boc pyrrolidine (6). The polarity-matching model predicts that the hydrogen abstraction event should be highly selective for the most hydridic C–H bond, owing to the electrophilic nature of radical species 4, delivering exclusively carbon-centred radical 7. Concurrent with the formation of species 7, sequential reductions of precatalyst NiBr2•(dOMebpy) (dOMebpy = 4,4′-dimethoxy-2,2′-bipyridyl) by complex 5 yield active Ni(0) catalyst 9 (ref. 27). We hypothesized that complex 9 would be rapidly trapped by radical 7 to arrive at Ni(i)–alkyl intermediate 10 (ref. 28). In the presence of an alkyl bromide, oxidative addition29 readily occurs to yield the high-energy bis-alkyl Ni(iii) adduct 11, which proceeds to yield the desired coupled product 13 via reductive elimination. The nickel and photoredox catalytic cycles then simultaneously complete via a single-electron transfer event between Ni(i) intermediate 12 and Ir(ii) reductant 5. Finally, quinuclidine is regenerated via deprotonation of the quinuclidinium species 8. An alternative mechanism involving a formal oxidative addition of a Ni(0) species to the alkyl halide might also be operative.
Figure 2. Proposed mechanism for the triple catalytic selective sp3 C–H alkylation.
The photocatalyst is excited by visible light to produce a long-lived triplet excited state (2), which undergoes oxidation with quinuclidine 3 to yield quinuclidinium radical cation 4. This electrophilic radical species can participate in hydrogen-atom transfer (HAT) with amine 6, selectively abstracting the most hydridic C–H to yield the corresponding radical 7. The reactive carbon-centred radical intermediate reacts with Ni(0) species 9 to yield Ni(i) complex 10. This type of intermediate has been shown to undergo oxidative addition with alkyl bromides to yield Ni(iii) adduct 11. Reductive elimination gives the desired product 13. The photoredox and nickel catalytic cycles are turned over by single-electron transfer (SET) between the iridium and nickel species, while quinuclidine can be regenerated via deprotonation. Boc, tert-butoxycarbonyl.
We began our study of the proposed HAT-metallaphotoredox-mediated sp3 C–H alkylation by examining a wide range of nickel systems, and the loadings of quinuclidine and base. N-Boc pyrrolidine and cyclohexylmethyl bromide were coupled in good (58%) yield using 1 mol% photocatalyst Ir[dF(CF3)ppy]2-(dtbbpy)]PF6, 2 mol% NiBr2 catalyst (comprising NiBr2•6H2O and 4,4′-dimethoxy-2,2′-bipyridine), 10 mol% quinuclidine and 1.5 equiv. potassium carbonate under irradiation with 34-W blue-light-emitting diodes. The use of an N-acyl moiety not only prevents a deleterious N-alkylation pathway with the alkyl halide but also an unproductive oxidation of the amine by the photocatalyst. Moreover, installation of readily available acyl groups provides a flexible strategy for modulating the hydricity of the α-C–H amine. Critical to the success of this reaction was the use of catalytic quantities of quinuclidine, which limits the direct consumption of both the alkyl halide and this amine catalyst via SN2 alkylation. Indeed, formation of the alkyl–quinuclidinium cation was not observed under the optimized conditions. Furthermore, we observed exclusive alkylation at the most hydridic position, the α-amino hydrogen, providing validity for our polarity-matching model. Although we used an excess of N-Boc pyrrolidine (2:1 ratio with respect to cyclohexylmethyl bromide), the remaining nucleophile was recovered in excellent yield in all cases. We demonstrated the necessity of each catalyst in a series of control experiments, in which no product formation was observed when each component was individually omitted from the reaction mixture (see Supplementary Information).
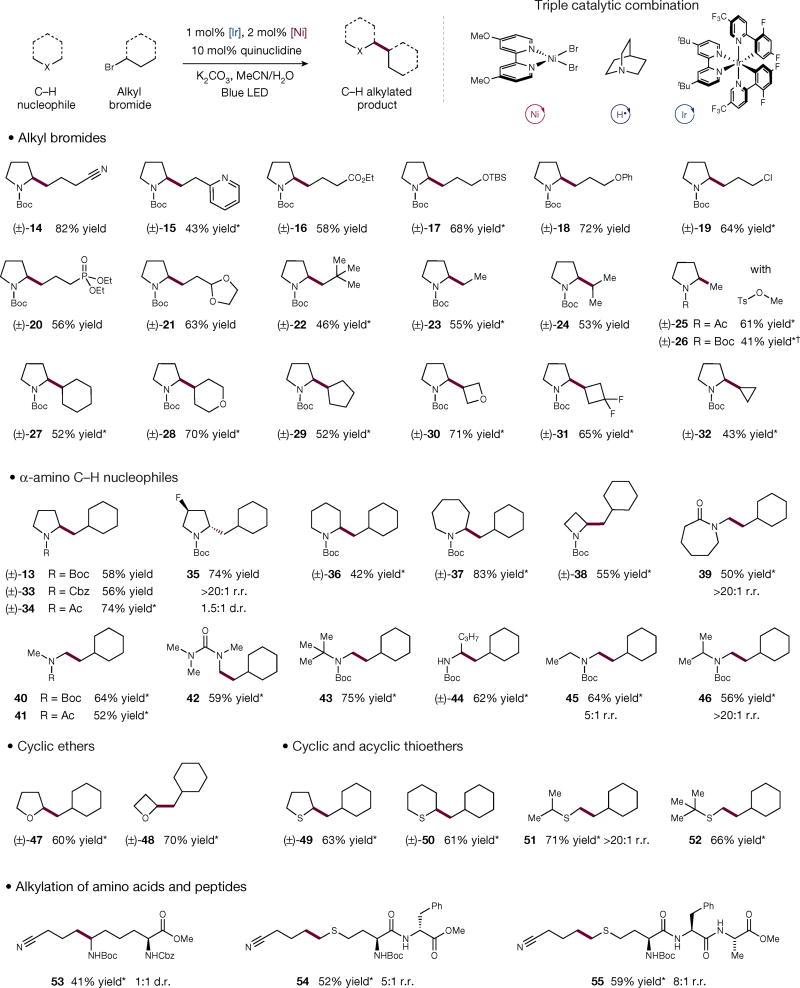
With the optimized conditions in hand, we investigated the scope of the sp3 C–H alkylation protocol. With respect to the alkyl bromide coupling partner, we observed good to excellent yield with a wide range of acyclic halides. As shown in Fig. 3, alkyl fragments carrying commonly used functional groups such as nitriles, heteroaromatics, esters, ethers, phosphonates and acetals were all used in good yields (14–21, 43%–82% yield). Notably, when 1-bromo-3-chloropropane was subjected to the same reaction conditions, coupling occurred selectively at the bromide-bearing carbon, yielding chloro-amine 19 in 64% yield. Sterically demanding alkyl fragments, such as neopentyl, were also tolerated, providing the desired product 22 in 46% yield. Substitution of an sp3 C–H for a methyl, ethyl or isopropyl group could be carried out with ease in good yield (23–26, 41%–61% yield). Given that the potency and specificity of a drug lead molecule can be enhanced via substitution of C–H for a methyl group (the “magic methyl effect”6), we expect this direct methylation protocol to be immediately useful to medicinal chemists. In addition to acyclic fragments, our light-mediated sp3 C–H alkylation can also be used to enable secondary–secondary bond formation between disparate cyclic systems. Accessing such synthons has been considered a long-standing challenge in cross-coupling methodology; we found that three- to six-membered ring alkyl bromides were competent coupling partners, providing the corresponding adducts in reasonable to good yields (27–32, 43%–71% yield).
Figure 3. The scope of the alkyl bromide coupling partner in the light-enabled selective sp3 C–H alkylation.
A wide variety of alkyl bromides can be used in this triple catalytic protocol. Broad ranges of functional groups are well tolerated. Secondary–secondary couplings work in good yield. Cyclic and acyclic amines, as well as ethers and thioethers, are well tolerated in this protocol. Hydridic C–H of less sterically hindered carbon is selectively alkylated. In complex molecules, the most hydridic C–H is functionalized, while other neutral or acidic C–H bonds are left completely intact. Me, methyl; Et, ethyl; Ts, tosyl; Ac, acetyl; TBS, tert-butyldimethylsilyl; Ph, phenyl; tBu, tert-butyl; Cbz, carboxybenzyl; d.r., diastereomeric ratio; r.r., regiomeric ratio. All reactions were replicated at least three times for consistency. * See Supplementary Information for experimental details. †Yield determined by gas chromatography analysis versus biphenyl as the internal standard.
Next, we examined the generality of the alkylation protocol with regard to the nucleophile component. The transformation was tolerant of a wide range of substituents on the adjacent nitrogen atom, with carbamate, amide and urea groups being amenable to this coupling protocol (13, 33, 34 and 42, 56%–74% yield). Moreover, when an electron-withdrawing group, such as fluorine, was added to the pyrrolidine ring, exclusive functionalization was observed away from the electron-withdrawing substituent (35, 74% yield). This remarkable selectivity can be rationalized by the inductive properties of fluorine, which reduces the hydridicity of the nearby hydrogen atoms without compromising the reactivity of the distal hydrogen atoms. Furthermore, cyclic amines of different sizes, ranging from azetidine to azepane, afforded the desired alkylated adduct in useful to excellent efficiencies (36–38, 42%–83% yield). Complete regioselectivity was observed for abstraction and alkylation at the exocyclic α-amino methyl group in the presence of the endocyclic hydridic methylene (39, 50% yield). We found that acyclic amines could also be used to provide alkylated products (40–46, 52%–75% yield). Interestingly, for compounds in which multiple hydridic C–H bonds are present, the sterically less hindered site undergoes preferential alkylation; for example, a 5:1 regioselectivity was observed between a methyl and methylene group (45, 64% yield). However, when the two α-carbons are further differentiated, the regioselectivity can be increased to >20:1, as in the case of methyl versus methine (46, 56% yield). We reason that the observed selectivity is attributed to the effect of the steric component in the HAT event. This result illustrates that comparisons of bond dissociation energies are not sufficient for determining the regiochemical outcomes of these radical-based couplings.
Given these results, we postulated that other heteroatoms that can imbue a hydridic nature to their neighbouring α-C–H bonds might be susceptible to this approach. We found that ethers were competent substrates, with both tetrahydrofuran and oxetane affording alkylated products in good yield (47 and 48, 60% and 70% yield, respectively). In additional, owing to the electron-donating nature of the sulfur atom, the corresponding α-hydrogens underwent selective functionalization (49–52, 61%–71% yield).
The inherent value of our aliphatic sp3 C–H alkylation is further demonstrated by its applicability to biorelevant substrates. When a protected form of lysine was subjected to this protocol, functionalization was observed exclusively on the amine of the pendent side chain, leaving the protic α-amino, α-carbonyl hydrogen untouched (53, 41% yield). Furthermore, peptides containing methionine residues exhibit the corresponding chemoselectivity, in which alkylation occurs exclusively adjacent to the sulfur atom (54 and 55, 52% and 59% yield, respectively).
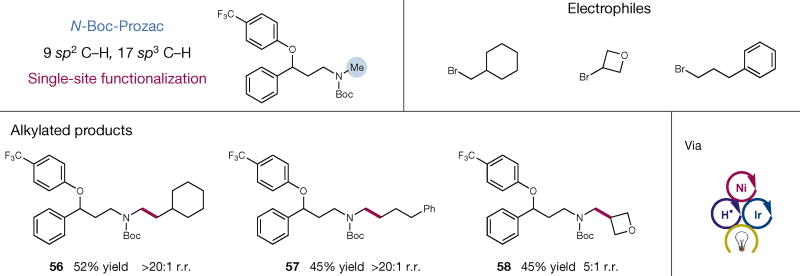
Finally, we applied the triple catalytic C–H alkylation to late-stage functionalization of medicinal agents. Using N-Boc-Prozac as a representative drug molecule for diversification (Fig. 4), three different alkylation derivatives were readily accessed using sp3 C–H coupling (56–58, 45%–52% yield). Good to excellent regioselectivity was observed in all cases. Given that most known therapeutic agents contain heteroatoms, we expect our methodology to provide a new approach to late-stage functionalization and studies of structure–activity relationships in modern drug discovery30.
Figure 4. Application of direct sp3 C–H alkylation in late-stage functionalization of pharmaceutical compounds.
N-Boc-Prozac was readily alkylated selectively at the methyl position with simple electrophiles to quickly gain access to a small library of alkylated products via polarity-match-based selective C–H alkylation.
Supplementary Material
Acknowledgments
This research was supported by the NIH National Institute of General Medical Sciences (R01 GM078201-05) and gifts from Merck, Bristol-Myers Squibb, Eli Lilly and Johnson & Johnson. The authors thank T. Liu for assistance in preparing this manuscript.
Footnotes
Online Content Methods, along with any additional Extended Data display items and Source Data, are available in the online version of the paper; references unique to these sections appear only in the online paper.
Data Availability The data that support the findings of this study are available from the corresponding author on reasonable request.
Supplementary Information is available in the online version of the paper.
Author Contributions C.L., Y.L., R.W.E. and X.L. performed and analysed the experiments. C.L., Y.L., R.W.E., X. L. and D.W.C.M. designed the experiments. C.L., Y.L., R.W.E., X.L. and D.W.C.M. prepared the manuscript.
The authors declare no competing financial interests.
References
- 1.Davies HML, Morton D. Recent advances in C–H functionalization. J. Org. Chem. 2016;81:343–350. doi: 10.1021/acs.joc.5b02818. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Engle KM, Wang D-H, Yu J-Q. Palladium(II)-catalyzed C–H activation/C–C cross-coupling reactions: versatility and practicality. Angew. Chem. Int. Ed. 2009;48:5094–5115. doi: 10.1002/anie.200806273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann L. Metal-catalyzed direct alkylations of (hetero)arenes via C–H bond cleavages with unactivated alkyl halides. Chem. Commun. 2010;46:4866–4877. doi: 10.1039/c0cc00778a. [DOI] [PubMed] [Google Scholar]
- 4.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem. Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colby DA, Tsai AS, Bergman RG, Ellman JA. Rhodium catalyzed chelation-assisted C–H bond functionalization reactions. Acc. Chem. Res. 2012;45:814–825. doi: 10.1021/ar200190g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schönherr H, Cernak T. Profound methyl effects in drug discovery and a call for new C–H methylation reactions. Angew. Chem. Int. Ed. 2013;52:12256–12267. doi: 10.1002/anie.201303207. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y-H, Shi B-F, Yu J-Q. Palladium(II)-catalyzed ortho alkylation of benzoic acids with alkyl halides. Angew. Chem. Int. Ed. 2009;48:6097–6100. doi: 10.1002/anie.200902262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aihara Y, Chatani N. Nickel-catalyzed direct alkylation of C–H bonds in benzamides and acrylamides with functionalized alkyl halides via bidentate-chelation assistance. J. Am. Chem. Soc. 2013;135:5308–5311. doi: 10.1021/ja401344e. [DOI] [PubMed] [Google Scholar]
- 9.Ackermann L, Novák P, Vicente R, Hofmann N. Ruthenium-catalyzed regioselective direct alkylation of arenes with unactivated alkyl halides through C–H bond cleavage. Angew. Chem. Int. Ed. 2009;48:6045–6048. doi: 10.1002/anie.200902458. [DOI] [PubMed] [Google Scholar]
- 10.Ilies L, Matsubara T, Ichikawa S, Asako S, Nakamura E. Iron-catalyzed directed alkylation of aromatic and olefinic carboxamides with primary and secondary alkyl tosylates, mesylates, and halides. J. Am. Chem. Soc. 2014;136:13126–13129. doi: 10.1021/ja5066015. [DOI] [PubMed] [Google Scholar]
- 11.Shabashov D, Daugulis O. Auxiliary-assisted palladium-catalyzed arylation and alkylation of sp2 and sp3 carbon–hydrogen bonds. J. Am. Chem. Soc. 2010;132:3965–3972. doi: 10.1021/ja910900p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang S-Y, Li Q, He G, Nack WA, Chen G. Stereoselective synthesis of β-alkylated α-amino acids via palladium-catalyzed alkylation of unactivated methylene C(sp3)–H bonds with primary alkyl halides. J. Am. Chem. Soc. 2013;135:12135–12141. doi: 10.1021/ja406484v. [DOI] [PubMed] [Google Scholar]
- 13.Zhu R-Y, He J, Wang X-C, Yu J-Q. Ligand-promoted alkylation of C(sp3)–H and C(sp2)–H bonds. J. Am. Chem. Soc. 2014;136:13194–13197. doi: 10.1021/ja508165a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams J, et al. Rhodium acetate catalyzes the addition of carbenoids α- to ether oxygens. Tetrahedr. Lett. 1989;30:1749–1752. [Google Scholar]
- 15.Davies HML, Venkataramani C, Hansen T, Hopper DW. New strategic reactions for organic synthesis: catalytic asymmetric C–H activation α to nitrogen as a surrogate for the Mannich reaction. J. Am. Chem. Soc. 2003;125:6462–6468. doi: 10.1021/ja0290072. [DOI] [PubMed] [Google Scholar]
- 16.Doyle MP, Duffy R, Ratnikov M, Zhou L. Catalytic carbene insertion into C–H bonds. Chem. Rev. 2010;110:704–724. doi: 10.1021/cr900239n. [DOI] [PubMed] [Google Scholar]
- 17.Prier CK, Rankic DA, MacMillan DWC. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 2013;113:5322–5363. doi: 10.1021/cr300503r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw MH, Twilton J, MacMillan DWC. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016;81:6898–6926. doi: 10.1021/acs.joc.6b01449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kärkäs MD, Porco JA, Stephenson CRJ. Photochemical approaches to complex chemotypes: applications in natural product synthesis. Chem. Rev. 2016;116:9683–9747. doi: 10.1021/acs.chemrev.5b00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiRocco DA, et al. Late-stage functionalization of biologically active heterocycles through photoredox catalysis. Angew. Chem. Int. Ed. 2014;53:4802–4806. doi: 10.1002/anie.201402023. [DOI] [PubMed] [Google Scholar]
- 21.Zuo Z, et al. Merging photoredox with nickel catalysis: coupling of α-carboxyl sp3-carbons with aryl halides. Science. 2014;345:437–440. doi: 10.1126/science.1255525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, MacMillan DWC. Alcohols as latent coupling fragments for metallaphotoredox catalysis: sp3–sp2 cross-coupling of oxalates with aryl halides. J. Am. Chem. Soc. 2016;138:13862–13865. doi: 10.1021/jacs.6b09533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts BP. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 1999;28:25–35. [Google Scholar]
- 24.Jeffrey JL, Terrett JA, MacMillan DWC. O–H hydrogen bonding promotes H-atom transfer from α C–H bonds for C-alkylation of alcohols. Science. 2015;349:1532–1536. doi: 10.1126/science.aac8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw MH, Shurtleff VW, Terrett JA, Cuthbertson JD, MacMillan DWC. Native functionality in triple catalytic cross-coupling: sp3 C–H bonds as latent nucleophiles. Science. 2016;352:1304–1308. doi: 10.1126/science.aaf6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowry MS, et al. Single-layer electroluminescent devices and photoinduced hydrogen production from an ionic iridium(III) complex. Chem. Mater. 2005;17:5712–5719. [Google Scholar]
- 27.Durandetti M, Devaud M, Périchon J. Investigation of the reductive coupling of aryl halides and/or ethyl chloroacetate electrocatalyzed by the precursor NiX (bpy) with X = C, Br, or MeSO4 and bpy = 2,2′-dipyridyl. New J. Chem. 1996;20:659–667. [Google Scholar]
- 28.Gutierrez O, Tellis JC, Primer DN, Molander GA, Kozlowski MC. Nickel-catalyzed cross-coupling of photoredox-generated radicals: uncovering a general manifold for stereoconvergence in nickel-catalyzed cross-couplings. J. Am. Chem. Soc. 2015;137:4896–4899. doi: 10.1021/ja513079r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung MS, Sheong FK, Marder TB, Lin Z. Computational insight into nickel-catalyzed carbon–carbon versus carbon–boron coupling reactions of primary, secondary, and tertiary alkyl bromides. Chem. Eur. J. 2015;21:7480–7488. doi: 10.1002/chem.201500110. [DOI] [PubMed] [Google Scholar]
- 30.Cernak T, Dykstra KD, Tyagarajan S, Vachal P, Krska SW. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 2016;45:546–576. doi: 10.1039/c5cs00628g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.