Abstract
We have recently demonstrated that repeated administrations of c-kitPOS cardiac progenitor cells (CPCs) have cumulative beneficial effects in rats with old myocardial infarction (MI), resulting in markedly greater improvement in left ventricular (LV) function compared with a single administration. To determine whether this paradigm applies to other species and cell types, mice with a 3-week- old MI received one or three doses of cardiac mesenchymal cells (CMCs), a novel cell type that we have recently described. CMCs or vehicle were infused percutaneously into the LV cavity, 14 days apart. Compared with vehicle-treated mice, the single-dose group exhibited improved LV ejection fraction (EF) after the 1st infusion (consisting of CMCs) but not after the 2nd and 3rd (vehicle). In contrast, in the multiple-dose group, LV EF improved after each CMC infusion, so that at the end of the study, LV EF averaged 35.5 ± 0.7% vs 32.7 ± 0.6% in the single-dose group (P < 0.05). The multiple-dose group also exhibited less collagen in the non-infarcted region vs the single-dose group. Engraftment and differentiation of CMCs were negligible in both groups, indicating paracrine effects. These results demonstrate that, in mice with ischemic cardiomyopathy, the beneficial effects of three doses of CMCs are significantly greater than those of one dose, supporting the concept that multiple treatments are necessary to properly evaluate the full therapeutic potential of cell therapy. Thus, the repeated-treatment paradigm is not limited to c-kit POS CPCs or to rats, but applies to other cell types and species. The generalizability of this concept dramatically augments its significance.
Keywords: Ischemic cardiomyopathy, Stem cells, Progenitor cells, Cell therapy
Introduction
Mounting evidence indicates that the beneficial effects of cell-based therapies are not caused by differentiation of transplanted cells into new cardiac myocytes but rather by paracrine actions [17, 25]. We have found that administration of c-kitPOS cardiac progenitors cells (CPCs) results in a sustained (up to 1 year) improvement in left ventricular (LV) function and structure despite the fact that almost all exogenous cells disappear within a few days of transplantation [6, 7, 14, 15, 19, 28, 30, 31, 33]. Similar findings have been reported by others with various cell types [1–4, 8, 13, 16, 17, 23–25, 32, 36]. In view of the fact that transplanted cells are rapidly cleared from the myocardium, we posit that administration of one dose of cells cannot be regarded as a valid test of the therapeutic efficacy of that product. Thus far, however, all clinical trials of cell therapy, and almost all preclinical studies, have examined the effects of one dose of cells.
In a recent study, we have proposed that the rapid disappearance of exogenous cells is a major factor limiting their efficacy and that repeated administration of cells can markedly increase their efficacy [33]. Indeed, we have found that, in rats with old myocardial infarction (MI), three administrations of c-kitPOS CPCs 35 days apart produce cumulative beneficial effects, such that the final outcome is markedly improved compared with a single-cell administration [33]. These findings imply that one dose of cells is insufficient to adequately evaluate their therapeutic utility. The notion that the full effects of a cell product require repeated administrations has major repercussions, for it implies that in the previous “negative” studies, the beneficial effects of cell therapy may have been overlooked and that future clinical and preclinical studies should adopt protocols based on repeated treatments. Thus, if this concept is applicable to other cell types and animal models, it could revolutionize the field of cell therapy.
At present, the evidence supporting this new paradigm is limited to administration of c-kitPOS CPCs in rats with chronic ischemic cardiomyopathy [33]. Before this concept can be generalized, it is necessary to verify its validity in other species and cell types. Accordingly, the goal of this study was to determine whether repeated cell treatments offer a therapeutic advantage over a single treatment in a different species (mice). A model of ischemic cardiomyopathy was used in which mice with a 3-week-old MI were given either one or three doses of cells. The rationale for studying a model of subacute/old MI was that the repeated-treatment paradigm is best evaluated in a setting of relatively stable post-MI LV dysfunction. Clinically, it is in this setting that multiple doses could be given to patients at regular intervals. To test whether the multiple-dose paradigm applies to a cell type other than c-kitPOS CPCs, we utilized cardiac mesenchymal cells (CMCs), a novel cardiac-derived cell type that we have recently found to possess reparative properties [35]. To perform repeated treatments with acceptable attrition of mice, we developed a protocol for percutaneous injection of CMCs into the LV cavity which enables repeated cell administrations with minimal mortality. Our results demonstrate that three CMC doses are superior to one dose, thereby corroborating the new paradigm of repeated cell treatments.
Methods
All animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (Department of Health and Human Services, Publication No. [NIH] 86–23) and with the guidelines of the Animal Care and Use Committee of the University of Louisville, School of Medicine (Louisville, KY, USA). The protocol was approved by the Institutional Animal Care and Use Committee of the University of Louisville (Permit Number: 14,034). All animals were maintained in micro-isolator cages under specific pathogen-free conditions in a room with a temperature of 24 °C, 55–65% relative humidity, and a 12-h light–dark cycle.
Isolation and culture of cardiac mesenchymal cells
Mouse CMCs were isolated from slowly adhering myocardial fractions as described [34, 35]. Briefly, myocardial cells were isolated from 12 to 15-week-old male C57BL/6-Tg(CAG-EGFP)1Osb/J mice (The Jackson Laboratory). Mice were euthanized by sodium pentobarbital injection (100 mg/kg i.p.). The hearts were excised, washed in room temperature PBS, minced into small pieces, and enzymatically dissociated with Collagenase II (5 μg/mL in PBS; Worthington) with gentle agitation for 45 min at 37 °C. After Collagenase II inactivation with DMEM/F12 medium containing 10% FBS, cells were centrifuged at 600×g for 10 min. The collected cell pellet was suspended in growth medium consisting of DMEM/F12 (Invitrogen), 10% FBS (Seradigm, VWR), bFGF (10 ng/ml), EGF (10 ng/ml), ITS (insulin/transferrin/selenium), glutamine, and Pen-Strep. A single-cell suspension was plated in the tissue culture flask. The non-adherent cells were removed 2 h after the initial plating, and the attached cells were gently washed with PBS and suspended in growth medium. Collected floating cells were plated in new tissue culture flasks and cultured for the next 2 h. The procedure was repeated three more times after 24, 48, and 72 h. A total of five fractions were collected from the myocardial digestion. The cell fractions that attached within 2 and 4 h (fractions 1′ and 2′, respectively) were collectively dubbed “rapidly adhering” (RA), whereas the cells that attached in 24, 48, and 72 h (fractions 3′, 4′, and 5′, respectively) were designated “slowly adhering” (SA). Cells isolated from SA fractions were further propagated in growth medium and used for experiments at passages 3–6.
Cytokine array
CMCs at passages 3–5 were seeded in T75 culture flasks and propagated in growth media till 80% confluence. Subsequently, the monolayer of cells was washed three times with PBS and suspended in basal media supplemented with 0.5% BSA (DMEM:F12). After 24 h of incubation, conditioned media was collected and centrifuged at 600×g for 10 min at 4 °C to remove dead cells and cell debris. Cytokine expression in the conditioned media was evaluated with the Proteome Profiler™ Mouse XL Cytokine Array (R&D) according to the manufacturer’s recommendations. The membranes were exposed with ECL substrate, and imaged with a Fuji LAS-3000 bioimaging analyzer. Densitometry was performed with ImageJ (1.37v).
Mouse model of ischemic cardiomyopathy
The study was performed in C57BL/6J female mice (age 16–19 weeks, body weight 20–25 g), purchased from The Jackson Laboratory (Bar Harbor, ME, USA). The mouse model of myocardial ischemia and reperfusion has been described in detail [11, 12, 14, 20]. Briefly, mice were anesthetized with sodium pentobarbital (60 mg/kg i.p.) and ventilated using carefully selected parameters. The chest was opened through a midline sternotomy, and a non-traumatic balloon occluder was implanted around the mid-left anterior descending coronary artery using an 8–0 nylon suture. To prevent hypotension, blood from a donor mouse was given at serial times during surgery. Rectal temperature was carefully monitored and maintained between 36.8 and 37.2 °C throughout the experiment. Myocardial infarction (MI) was produced by a 60-min coronary occlusion followed by reperfusion. To exclude small infarctions, an echocardiogram was obtained 3 weeks after MI in all mice; animals with LV ejection fraction (EF) >35% were not used.
Echocardiography-guided intraventricular injection
To avoid multiple thoracotomies in the same mouse, we developed a percutaneous, echo-guided approach to inject cells or vehicle into the LV cavity. All injections were performed using a Vevo 2100 Imaging System (VisualSonics, Inc.) equipped with a 30-MHz transducer, a Vevo Image Station with Injection Mount, and micro-manipulation controls. Mice were anesthetized with isoflurane (3% for induction and 1.5% for maintenance). The anterior chest and abdomen were shaved and the animals were placed on the imaging table in the right lateral decubitus position with the left lateral side facing the injection mount. Body temperature was kept strictly at 37 ± 0.2 °C. With the imaging transducer aligned perpendicularly to the injection mount, the left ventricle was initially imaged in the parasternal plane and a good 2D long-axis view was procured by adjusting the angle and position of the imaging table. The transducer was then turned 90° clockwise; the LV was scanned in the 2D short-axis; and color Doppler views from apex to base were obtained to determine the optimal site for needle insertion, a site that did not include the infarct scar or a coronary artery. Under the guidance of a real-time B-mode view, a 0.5-inch 30-G injection needle attached to a 1.0-ml syringe was then carefully inserted from the left lateral side and advanced into the center of the LV cavity. CMCs [1 × 106 cells in 200 μl of phosphate-buffered saline (PBS)] or vehicle (200 μl of PBS) were infused at a rate of 33 μl/s for 1 min.
Pilot studies
Before initiating the protocol, we conducted a series of pilot studies to select a needle gauge, an infusion speed, and a dose of cells that would yield a myocardial retention of CMCs comparable to that observed in our previous studies using intramyocardial or intracoronary injection of 100,000 c-kitPOS CPCs, in which cell therapy was effective [19]. To select a rate of infusion and needle size that would not damage cells, 1 × 106 CMCs in 200 μl of PBS were infused through a 27-G or 30-G needle over 3, 10, or 30 s, and cell survival was measured after 24 h in culture. The selection of the cell dose (1 × 106) was based on our previous studies in rats [33], in which we found that when cells are injected via the intraventricular route, their number needs to be at least 10 times greater than that injected intracoronarily to achieve similar myocardial retention at 24 h. Since, in our previous study in mice, we injected1 × 105 cells intracoronarily or intramyocardially, and since this dose was effective [19], in this study, we injected 1 × 106 cells (a dose 10 times higher). Mice were subjected to a 60-min coronary occlusion followed by reperfusion; 3 weeks later, 1 × 106 CMCs in 200 μl of PBS were infused intraventricularly under echo guidance, and the retention of cells in the heart was measured at various times after injection using a highly sensitive and accurate real-time PCR method [14, 15].
Treatment protocol
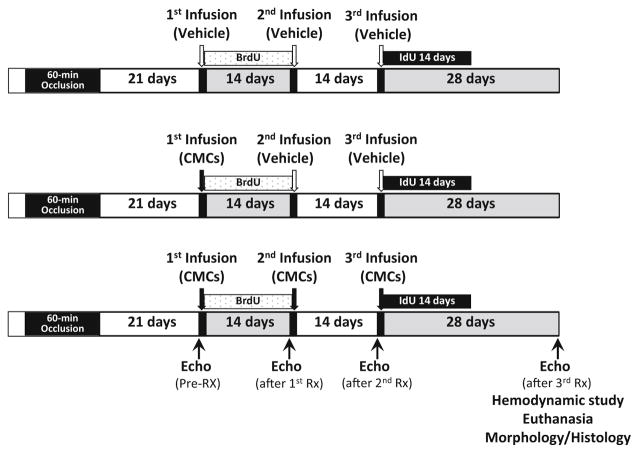
Three weeks after MI, after confirming that LV EF was ≤35%, mice were randomly allocated to three treatment groups: vehicle (control), single dose, or multiple doses (Fig. 1). The 1st treatment was given on the same day of group assignment, and consisted of either CMCs (1 × 106 cells in 200 μl of PBS) or vehicle (200 μl PBS) via echo-guided LV injection. The 2nd and 3rd treatments were given at 2-week intervals. The vehicle group received three injections of vehicle, the single-dose group received CMCs at the 1st injection and vehicle at the 2nd and 3rd injection, and the multiple-doses group received three injections of CMCs. To monitor formation of new cells, in all groups, 5-bromo-2′-deoxyuridine (BrdU, Sigma) was given after the 1st treatment (33 mg/kg/day s.c. for 14 days) and 5-iodo-2′-deoxyuridine (IdU, Sigma) for 14 days after the 3rd treatment (IdU was given in the drinking water at a final concentration of 0.1%). At 4 weeks after the 3rd treatment, mice underwent the final echocardiographic and hemodynamic studies, and were euthanized for histologic studies (Fig. 1).
Fig. 1.
Experimental protocol. Mice were subjected to a 60-min coronary artery occlusion followed by reperfusion to induce myocardial infarction (MI). Three weeks after MI, animals received the 1st treatment (vehicle or 1 × 106 CMCs) via echo-guided LV infusion, followed by a 2nd and 3rd treatment at 2-week intervals. BrdU was given right after the 1st treatment until the 2nd treatment; IdU was given for 2 weeks after the 3rd treatment
Echocardiographic studies
Echocardiographic studies were performed using the Vevo 2100 Imaging System (VisualSonics, Inc.) equipped with a 30-MHz transducer, as previously described [19, 22, 26, 29]. Serial echocardiograms were obtained 3 weeks after MI (before the 1st treatment [pre-treatment]), 2 weeks after the 1st treatment (immediately before the 2nd treatment), 2 weeks after the 2nd treatment (immediately before the 3rd treatment), and 4 weeks after the 3rd treatment (prior to hemodynamic studies and euthanasia). All echocardiographic studies were performed under isoflurane anesthesia (3% for induction and 1% for maintenance). Using a rectal temperature probe, body temperature was carefully maintained between 36.8 and 37.2 °C. The parasternal long-axis and parasternal short-axis views were used to obtain 2D-mode images for the measurement of LV mass, end-diastolic and end-systolic LV volume (LVEDV and LVESV), stroke volume (SV), EF, and fractional shortening (FS), as in our previous studies [9, 19]. Digital images were analyzed off-line by blinded observers using the Vevo 2100 workstation software. Measurements were performed according to the American Society for Echocardiography. At least three measurements were taken and averaged for each parameter.
Hemodynamic studies
At 4 weeks after the 3rd treatment, just before euthanasia, hemodynamic studies were performed as previously described [10, 19, 30]. Briefly, mice were anesthetized with sodium pentobarbital, intubated, and ventilated; rectal temperature was kept between 36.8 and 37.2 °C. A 1.0 French pressure–volume (PV) catheter (PVR-1035, Millar Instruments) was inserted into the left ventricle via the right carotid artery. The position of the catheter was carefully adjusted until typical and stable PV loop signals were acquired. After 30 min of stabilization, the PV signals were recorded continuously with an MPVS Single Segment Pressure–Volume unit (Millar Instruments) coupled with a Powerlab 16/30 converter (AD Instruments), stored, and displayed on a personal computer with the LabChart 7 software (AD Instruments). Inferior vena cava occlusions were performed with external compression to produce variably loaded beats for determination of the end-systolic PV relation and other derived constructs of LV performance. Parallel conductance from surrounding structures was calculated by a bolus injection of 5 μl of 30% NaCl through the jugular vein. All hemodynamic data analyses were performed off-line, using the LabChart 7 software, by an investigator blinded to the treatment.
Histological studies
The protocol for histologic analyses has been described [27, 30, 31, 33]. Briefly, at the end of the protocol, the heart was arrested in diastole by an i.v. injection of 0.15 ml of CdCl2(100 mM), excised, and perfused retrogradely at 60–80 mmHg (LVEDP = 8 mmHg) with heparinized PBS followed by 10% neutral buffered formalin solution for 15 min. The heart was fixed in formalin for 24 h, then sectioned into three transverse slices (~2 mm thick) from apex to base, and subjected to tissue processing and paraffin embedding. Slices were sectioned at 4-μm intervals and stained with Masson’s trichrome (Sigma), picrosirius red, or antibodies against EGFP, BrdU, and cell-type-specific markers. Images were acquired digitally and analyzed using NIH ImageJ (1.48v). From the Masson’s trichrome–stained images, morphometric parameters, including total LV area, risk region area, scar area, and LV wall thickness in the risk and non-infarcted regions, were measured in each section [27, 30, 31, 33]. Myocardial collagen content was quantitated on picrosirius red-stained heart images acquired under polarized light microscopy by determining collagen density (arbitrary unit) per mm2 of risk region or non-infarcted region with the NIH ImageJ software [27, 30, 31, 33]. Fluorescent stains and antibodies were used to identify specific cell markers and compartments. Imaging was performed using Zeiss 510 and Nikon TiE microscopes; digitally acquired images were analyzed using NIH ImageJ (1.46r) and NIS-Elements Ar software.
Immunohistochemistry
Immunohistochemistry was performed in formalin-fixed, paraffin-embedded, 4-μm-thick heart sections [5, 21, 37]. Cell proliferation was assessed by immunofluorescent staining of nuclei with specific antibodies for BrdU (Roche). Myocytes were stained with an anti-α-sarcomeric actin (α-SA) antibody (Sigma). Myocyte membranes were stained with Rhodamine-labeled wheat germ agglutinin (WGA) (Vector Labs) to facilitate the identification of individual myocytes for analysis of myocyte cross-sectional area and myocyte density. To determine vessel density, heart sections were stained with Fluorescein-labeled Griffonia simplicifolia Lectin I (GSL I) isolectin B4 (Vector Labs). Double immunofluorescent staining was conducted with specific anti-BrdU and anti- α-SA antibodies for evaluation of proliferating myocytes. Nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole).
Statistical analysis
Data are presented as mean ± SEM. All data were analyzed with one-way ANOVA for normally distributed data, or Kruskal–Wallis one-way analysis of variance on ranks for data that are not normally distributed, as appropriate, followed by unpaired Student’s t tests with the Bonferroni correction. A P value <0.05 was considered statistically significant. All statistical analyses were performed using the Sigma Stat software system [19, 20].
Results
Characterization of murine CMCs
Mouse CMCs isolated from slowly adhering fractions have previously been characterized in detail [35]. Briefly, CMCs observed under brightfield microscope have typical fibroblast-like characteristics, i.e., they adhere to plastic and acquire a spindle-shaped morphology. Flow cytometric analysis shows that CMCs express surface markers of mesenchymal lineage (CD90, CD29, CD73, CD105, and CD44) and lack expression of the hematopoietic marker CD45 and the pericyte marker CD146. Among other mesenchymal markers, CMCs express CD106, CD9, and CD13 but not CD271 and CD166. Expression of c-kit is low (<10%); most cells are positive for Sca-1 [35].
The results of the cytokine array confirmed that CMCs produce numerous factors potentially involved in angiogenesis, inflammation, and cell trafficking (Supplementary Fig. 1). Among them, angiopoietin-2, serpin E1, MMP-2, MMP-3, osteopondin, cystatin C, and IGFBP-6 were particularly abundant.
Exclusions, temperature, and heart rate
A total of 129 mice were enrolled in this study (Supplementary Table 1). Eighteen mice died within 48 h after MI, 18 were excluded because of prespecified criteria (EF > 35% at 3 weeks after MI), and 16 were used for the cell retention studies. The remaining 77 mice were allocated to one of the three treatment groups (vehicle, single dose, or multiple doses). Nineteen mice died after the 1st injection and one after the 2nd injection, with no further mortality after the 3rd injection. Therefore, a total of 57 mice were included in the final analysis: 20 in the vehicle group, 18 in the single-dose group, and 19 in the multiple-doses group (Supplementary Table 1).
By experimental design, rectal temperature was kept within a narrow, physiologic range (36.8–37.2 °C) in all groups during all procedures, including coronary occlusion/reperfusion, echo-guided injections, echocardiographic studies, and hemodynamic studies (Supplementary Table 2). Heart rate was also similar in all three groups (Supplementary Table 2).
Development of a method for repeated percutaneous cell injections
To perform this study, we have developed a method to deliver three doses of cells percutaneously via echo-guided LV injection in mice with old MI. The method was successful. Of 77 mice treated, 57 completed the three injections, with an overall successful rate of 74%. Most of the deaths (17 out of 20) occurred during the 1st injection in the first 35 mice—a phenomenon that reflected a “learning curve” for this procedure in our lab. Among the remaining 42 mice, only two died during the 1st injection and one died after the 2nd injection; no mice died after the 3rd injection (Supplementary Table 1).
A number of pilot studies were conducted to optimize the injection procedure. We examined whether the gauge of the needle and the speed of injection affect cell survival; we found that CMC survival is directly related to the size of the needle and inversely related to the speed of injection (Supplementary Table 3). Although a 27-G needle was associated with greater CMC survival, we elected to use a 30-G needle to minimize myocardial injury during injection. We selected injection duration of 30 s, which appeared to cause the least damage to the cells. With this combination (30 G needle and 30 s duration), cell survival was 95% (Supplemental Table 3). The pilot studies also revealed that the 2D short-axis view provided better resolution for locating an ideal injection site (avoiding the scar and the coronary arteries) and was the most helpful in guiding the injection. Finally, our measurements of myocardial cell retention (using a highly accurate PCR-based method [14, 15]) showed that after intraventricular injection of 1×106 CMCs, ~11,000 CMCs were present in the heart 24 h later—a retention rate at least equal to (or better than) that previously achieved [14, 15] with the administration of 1 × 105 CPCs via intramyocardial (~10,000 cells) or intracoronary (~5000 cells) injection (Fig. 2). Accordingly, the 1 × 106 dose was used in this study.
Fig. 2.
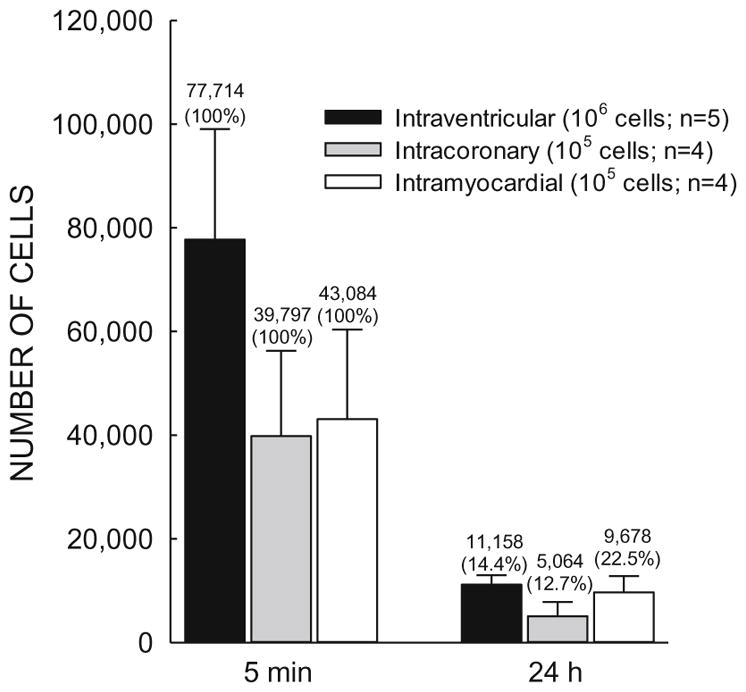
Myocardial retention of cells 24 h after injection. Mice underwent a 60-min coronary occlusion; three weeks later, 1×106 CMCs were injected percutaneously into the LV cavity under echo guidance. 5 min and 24 h after injection, the number of CMCs remaining in the left ventricle (solid black bars) was measured with a PCR-based method and compared with the number of c-kitPOS CPCs measured in the previous studies in the mouse model [14, 15] after intracoronary (dotted bars) or intramyocardial (open bars) injection of 1 ×105 c-kitPOS CPCs. The number of cells present in the myocardium at 5 min after injection is taken as 100% (initial value); the number present at 24 h is expressed as % of the initial (5-min) value. The values pertaining to intracoronary and intramyocardial injections are reproduced from previous publications [14, 15]. Data are mean ± SEM
Impact of multiple CMC administrations on LV function
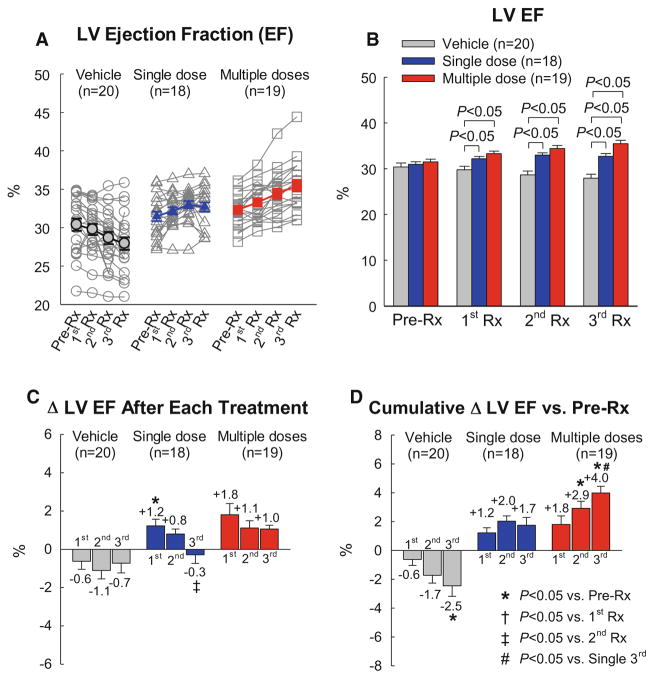
The results obtained by echocardiography are depicted in Fig. 3. As expected, the vehicle group showed a progressive deterioration in LV function, with average EF decreasing from 30.4 to 27.6% in the 8-week interval between the 1st treatment and euthanasia 56 days later (Fig. 3). In the single-dose group, the 1st treatment (consisting of CMCs) produced a significant increase in EF, which persisted to the end of the study; however, the 2nd and 3rd treatments (vehicle) effected no further improvement. In contrast, in the multiple-dose group, each treatment produced an increase in EF of at least 1% (Fig. 3c); as a result of this stepwise improvement, in the multiple-dose group, the total cumulative increase in EF from pre-treatment values was 1.8 ± 0.6% (absolute units) after the 1st treatment, 2.9 ± 0.5% after the 2nd treatment, and 4.0 ± 0.5% after the 3rd treatment (Fig. 3d), so that at the end of the protocol, LV EF (35.5 ± 0.8%) was significantly greater compared not only with the vehicle group (27.9 ± 0.9%) but also with the single-dose group (33.3 ± 0.6%, P < 0.05) (Fig. 3b). The total cumulative increase in EF from pre-treatment values was significantly greater in the multiple-dose vs. the single-dose group (4.0 ± 0.4 EF units vs. 1.7 ± 0.5 EF units, respectively, P < 0.001).
Fig. 3.
Echocardiographic assessment of LV EF. EF was measured in the vehicle, single-dose, and multiple-dose groups at 3 weeks after myocardial infarction [just before the 1st treatment (Pre-Rx)], 14 days after the 1st treatment (1st Rx), 14 days after the 2nd treatment (2nd Rx), and 28 days after the 3rd treatment (3rd Rx). a Values of LV EF in individual mice. b Mean values of LV EF. c Changes in LV EF (absolute units) after the 1st, 2nd, and 3rd treatments compared with the respective pre-treatment values. d Cumulative changes in LV EF (absolute units) after the 1st, 2nd, and 3rd treatments vs. the values measured at 3 weeks after myocardial infarction [just before the 1st treatment Pre-Rx)]. Data are mean ± SEM).
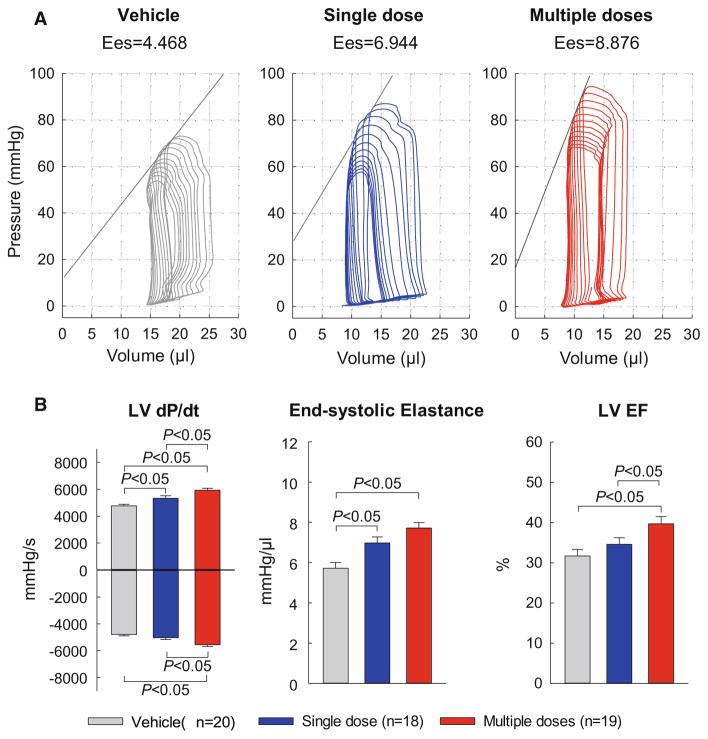
The echocardiographic results were corroborated by the hemodynamic studies performed just before euthanasia (Fig. 4). Compared with the vehicle group, a significant improvement in hemodynamic parameters was noted both in the single-dose and in the multiple-dose groups; however, the improvement was more robust in the latter. Thus, mice receiving a single dose of CMCs exhibited a significant increase in LV dP/dtmax and end-systolic elastance, but not LV dP/dtmin or LV EF. In contrast, mice receiving three doses of CMCs exhibited a significant increase in both LV dP/dtmax and LV dP/dtmin, in end-systolic elastance, and in LV EF (Fig. 4). Furthermore, the effects of multiple treatments were superior to those of a single treatment, as demonstrated by the fact that LV dP/dtmax, LV dP/dtmin, and EF were significantly greater in the multiple-dose group vs. the single-dose group (Fig. 4). In summary, two independent methods of functional assessment (echocardiography and hemodynamic studies) consistently demonstrated that the functional benefits of multiple administrations of CMCs are superior to those of a single administration.
Fig. 4.
Hemodynamic assessment of LV function. Hemodynamic studies were performed with a Millar conductance catheter at 28 days after the 3rd treatment, just before euthanasia.
a Representative pressure–volume loops recorded during preload manipulation by brief inferior vena cava occlusions.
b Quantitative analysis of hemodynamic variables. Data are mean ± SEM
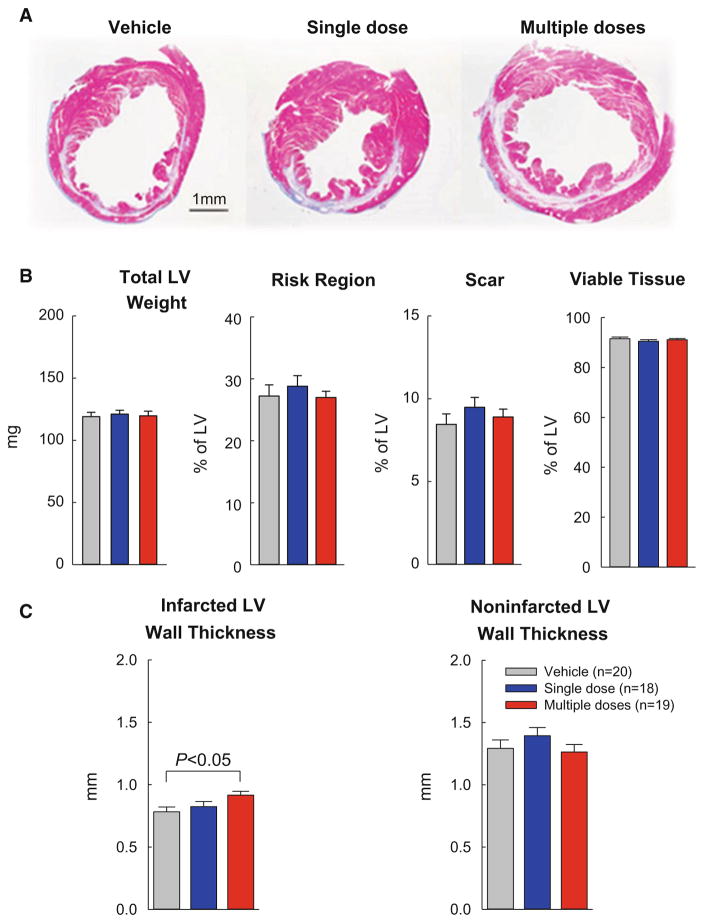
Morphometric analysis
In each heart, a detailed quantitative analysis was performed on three sections (one from each of three slices); the results are summarized in Fig. 5. There were no significant differences among the three groups with respect to LV weight, size of the risk region, amount of scar tissue, or amount of viable myocardium (Fig. 5b). Unlike the single-dose group, however, the multiple-dose group exhibited a significant increase in the thickness of the infarcted wall, although the thickness of the non-infarcted wall did not differ (Fig. 5c).
Fig. 5.
Morphometric analysis. A. Representative Masson trichrome-stained myocardial sections. Scar tissue and viable myocardium are identified in white/blue and red, respectively. b, c Quantitative analysis of LV morphometric parameters. The risk region comprises both the border zones and the scarred (infarcted) region. Data are mean ± SEM
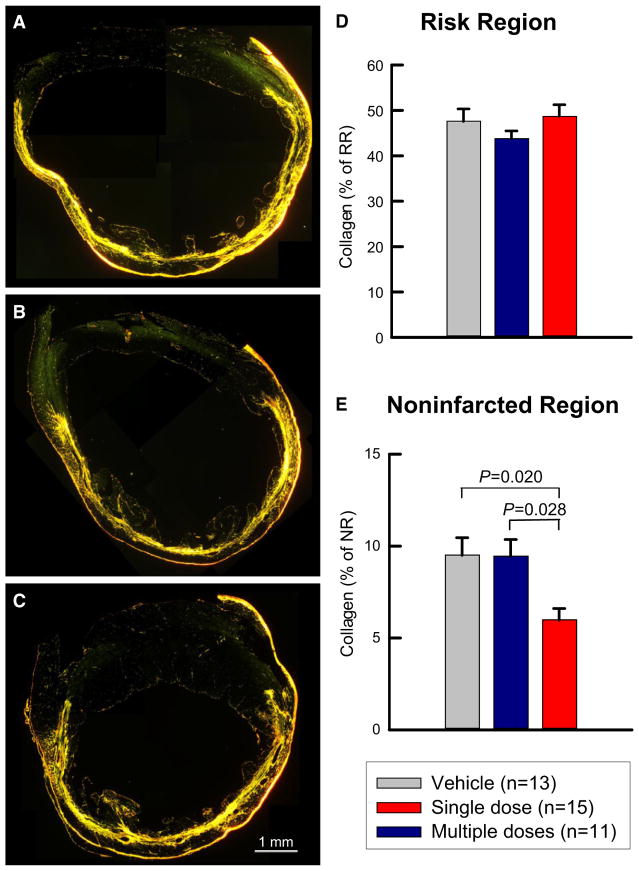
Impact of multiple CMC administrations on myocardial fibrosis
Quantitative analysis of collagen content was performed using picrosirius red staining and polarized light microscopy of LV sections (Fig. 6). In the risk region (which is the sum of the infarcted and border regions), collagen content did not differ among the three groups (Fig. 6d). In contrast, in the non-infarcted region, collagen content was significantly less in the multiple-dose group compared with the single-dose group (−37%; P < 0.05; Fig. 6e). This reduced collagen deposition in the myocardium may have contributed, at least in part, to the benefits of CMC therapy.
Fig. 6.
Myocardial collagen content. a–c Representative images of LV sections stained with picrosirius red. Images were acquired with polarized light microscopy in the vehicle (a), single-dose (b), and multiple-dose (c) groups. d, e Quantitative analysis of collagen content expressed as a percentage of the risk or non-infarcted region. Data are mean ± SEM
Analysis of cell engraftment and proliferation
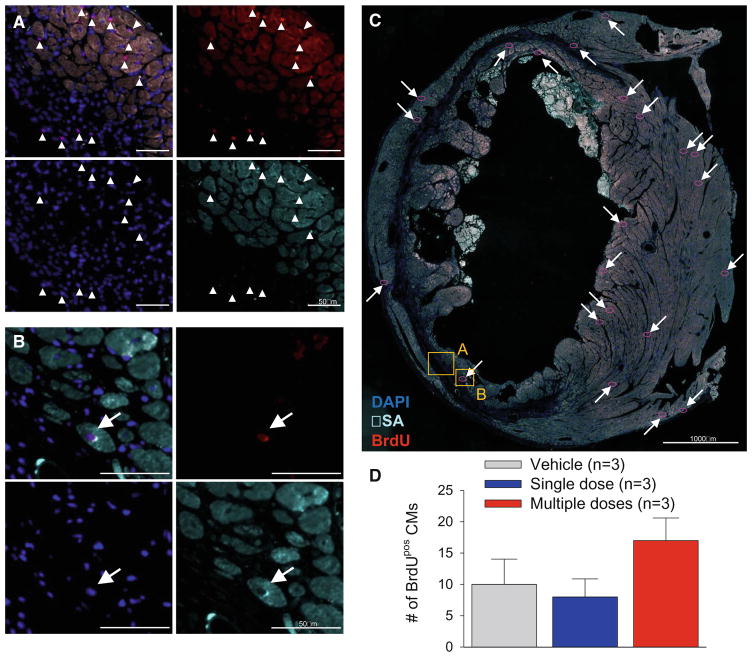
In this study, EGFPPOS male CMCs were transplanted into female mice. Consistent with our observations in multiple previous investigations [6, 7, 14, 15, 19, 28, 30, 31, 33], we found vary rare transplanted cells at the end of the study (56 days after the 1st treatment) (data not shown). We also assessed the possibility that CMC administration may promote formation of new cardiac myocytes from endogenous precursors. To this end, mice received BrdU for 14 days after the 1st treatment. As shown in Fig. 7, the number of BrdUPOS cardiac myocytes was extremely small (averaging <20/cardiac section) and did not differ significantly among groups, indicating that the beneficial effects of CMCs cannot be ascribed to the generation of new myocytes.
Fig. 7.
Analysis of BrdUPOS myocytes. Mice were given BrdU for 14 days starting immediately after the 1st treatment. Representative examples are shown of cardiac sections stained for BrdU and alpha-sarcomeric actin from a mouse that received three doses of CMCs. a Magnified field with arrowheads pointing to BrdUPOS non-myocytes within the risk region of the left ventricle. b Magnified field with arrow pointing to a BrdUPOS cardiomyocyte. c Overview image of the entire LV section, with arrows pointing to BrdUPOS cardiomyocytes (highlighted with red- circles) and boxes denoting the areas of the magnified images. This heart had the highest number of BrdUPOS cardiomyocytes among all hearts examined. d Quantitative analysis of the number of BrdUPOS cardiac myocytes (CMs; cells double positive for BrdU and alpha-sarcomeric actin) in a 4μm section of entire the left ventricle. Data are mean ± SEM
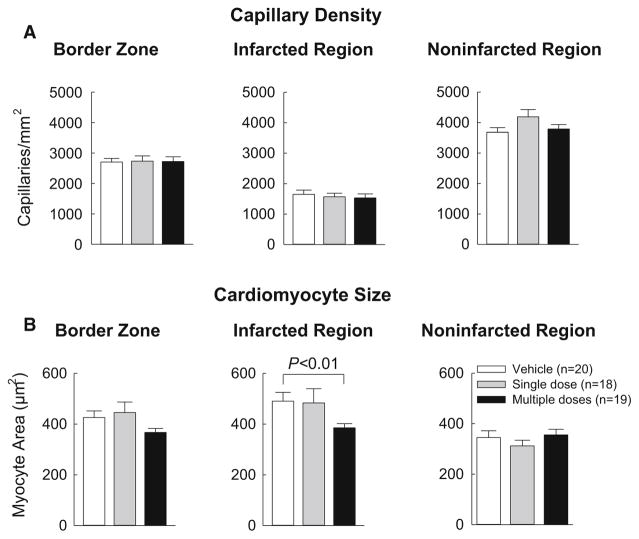
Capillary density and myocyte cross-sectional area
Capillary density was assessed by staining tissue sections with FITC-conjugated isolectin B4. In all three groups, capillary density was significantly lower in the infarcted region than in the non-infarcted and border regions, but no differences were noted among the three groups (Fig. 8a). Myocyte cross-sectional area was assessed by immunostaining myocytes with an anti-α-sarcomeric actin antibody and co-staining cell membranes with Rhodamine-labeled wheat germ agglutinin (WGA) to facilitate counting individual myocytes. Although in the infarcted region, myocyte cross-sectional area was significantly smaller in the multiple-dose group, it did not differ among groups in the border region or in the non-infarcted region (Fig. 8b).
Fig. 8.
Analysis of capillary density and myocyte cross-sectional area. a Capillary density was determined by assessing the number of isolectin-positive vessels within a defined area of the ventricle. Myocyte cross-sectional area was assessed by immunostaining of cardiac myocytes with an anti-α-sarcomeric actin antibody and co-staining with Rhodamine-labeled WGA to facilitate identification of cell membranes. Data are mean ±SEM
Discussion
The salient findings of this study can be summarized as follows: (1) in mice with a subacute (3-week-old) healed MI, administration of one dose of CMCs (1 × 106 cells infused percutaneously into the LV cavity) produces an inconsistent improvement in parameters of LV function, whereby some measurements (LV EF determined echocardiographically, LV dP/dtmax, and LV end-systolic elastance) increase significantly, but others (LV EF determined with pressure–volume catheter studies, LV dP/dtmin) do not; (2) in contrast, three doses of CMCs, given at 2-week intervals, result in a consistent improvement in parameters of LV function, with all measurements (LV EF determined echocardiographically, LV EF determined by pressure–volume studies, LV dP/dtmax, LV dP/dtmin, and LV end-systolic elastance) exhibiting a significant increase; (3) the improvement in LV function (EF, dP/dtmax, and dP/dtmin) is significantly greater after three than after one CMC doses; (4) three doses of CMCs affect a significant reduction in collagen content in the non-infarcted region and a significant increase in wall thickness in the infarcted region, whereas one dose of CMCs fails to do so; v) neither the effects of one CMC dose nor those of three CMC doses can be explained by engraftment and differentiation of exogenous cells (which was negligible), indicating a paracrine effect. Taken together, these results demonstrate that, in this mouse model of ischemic cardiomyopathy, the beneficial effects of three doses of CMCs are superior to those of one dose.
We have previously demonstrated that repeated administrations of c-kitPOS CPCs in rats produce a cumulative beneficial effect on LV function and structure [33]. The importance of this study stems from the fact that this is the first test of the repeated-treatment paradigm in a murine model of chronic ischemic cardiomyopathy and the first study to utilize CMCs as a therapeutic product to examine this paradigm. Our results are consistent with, and corroborate, those previously obtained in rats using c-kitPOS CPCs [33]. The finding that three doses were more effective than one dose in a different species (mice) and with a different cell type (CMCs) implies that the repeated-treatment paradigm is not limited to c-kitPOS CPCs or to rats, but is generalizable and applies to other cell types and other species as well. The generalizability of this concept dramatically augments its significance.
When one compares this investigation with our previous study in rats [33], the important point is that the present conclusions are in qualitative agreement with our previous findings. The quantitative differences in outcome (e.g., the magnitude of the changes in LV EF, LV dP/dtmax, LV dP/dtmin, collagen content, etc.) are less important, because they likely reflect the many differences between the two studies. The animal models are different (mice with a 3-week-old MI vs. rats with a 1-month-old MI). The interval between treatments was shorter in this study (14 vs. 35 days in the rat study). The cell type was also different (CMCs vs. c-kitPOS CPCs), and the optimal protocol for repeated use of CMCs remains unclear. This initial experience in mice leaves many unanswered questions, which must be addressed before the multiple-dose paradigm can be fully characterized and validated. For example, the choice of the cell dose (1 × 106) was rather arbitrary; whether this number of cells is adequate to examine the efficacy of CMCs, particularly when given by the intraventricular route, is unknown. The optimal interval between doses is unknown and may be longer than 14 days. The number of doses studied herein (three) may not be sufficient to achieve maximal therapeutic effects; additional doses may result in further improvement in LV performance. Further studies will be necessary to elucidate these issues.
To monitor formation of new myocytes from exogenous cells, we administered male (Y-chromosomePOS) EGFPPOS cells to female mice. Analysis of recipient hearts for Y-chromosomePOS and/or EGFPPOS cells showed that the number of transplanted cells surviving until the end of the experiment (56 days after the 1st treatment) was very low, and clearly insufficient to account for the improvement in cardiac function. This means that CMCs acted by releasing factors that produced persistent changes in the host myocardium. Among these factors, extracellular vesicles (EVs) may play an important role [18]. We propose that repeated administrations of CMCs (or other cells) are superior to a single administration, because they are associated with repetitive bursts of EV release that have a cumulative beneficial effect on LV function and structure. The precise mechanism(s), whereby paracrine factors, such as EVs, alleviate LV dysfunction and remodeling, remains to be identified. We did not find evidence of significant formation of new (BrdUPOS) myocytes either from exogenous or endogenous cells (Fig. 7) (although BrdU was given only for 14 days; Fig. 1), of increased angiogenesis (assessed from capillary density (Fig. 8a), or of reduced hypertrophy (assessed by myocyte cross-sectional area; Fig. 8b). Administration of three CMC doses was associated with a reduction in fibrosis in the non-infarcted region (Fig. 6); however, the fact that LV function improved in the single-dose group despite unchanged levels of fibrosis (Fig. 6) implies that other mechanisms must also be operative. As illustrated in Supplementary Fig. 1, we found that CMCs are a rich source of cytokines potentially involved in angiogenesis, inflammation, and cell trafficking. Further experiments will be necessary to elucidate the role of these cytokines in post-MI myocardial repair.
In addition to the finding that multiple CMC doses are superior to a single dose, this study describes a method for repeated cell administrations in mice that does not require repeated thoracotomies, thereby avoiding the prohibitive mortality associated with multiple surgeries in the same animal. After an initial learning phase, mortality in mice receiving three doses of CMCs or vehicle was almost nil (Supplementary Table 1). Using our PCR-based, quantitative method for measuring the number of transplanted cells, we found that echo-guided intraventricular delivery of 1 × 106 CMCs resulted in retention of ~11,100 cells at 24 h, a number equivalent to, or greater than, that previously observed [14, 15] after administration of 1 × 105 c-kitPOS CPCs by the intramyocardial or intracoronary route (Fig. 2). We also found that cell delivery by the percutaneous intraventricular route was reproducible and consistent. This relatively non-invasive method for cell delivery should be useful in future studies of repeated cell therapy.
In summary, this is the first study of repeated administrations of CMCs in a murine model of ischemic cardiomyopathy. Our results demonstrate that the salubrious effects of three doses of CMCs on LV function are significantly greater than those of one dose, thereby supporting the concept that multiple treatments are necessary to evaluate the full therapeutic potential of these cells. That the conclusions previously obtained in rats given kitPOS CPCs [33] are now reproduced in mice given CMCs supports the generalizability of the repeated-treatment paradigm to different species and different cell products. If this concept applies to larger animal models and to humans, it will have major implications for the design and interpretation of future preclinical and clinical studies, and implications that may fundamentally transform the field of cell therapy.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants P20 GM103492, P01 HL078825 (to RB and MW), and UM1 HL113530 (to RB), and an AHA Scientist Development Grant 13SDG14560005 (to MW).
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00395-017-0606-5) contains supplementary material, which is available to authorized users.
References
- 1.Aicher A, Brenner W, Zuhayra M, Badorff C, Massoudi S, Assmus B, Eckey T, Henze E, Zeiher AM, Dimmeler S. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation. 2003;107:2134–2139. doi: 10.1161/01.CIR.0000062649.63838.C9. [DOI] [PubMed] [Google Scholar]
- 2.Al Kindi A, Ge Y, Shum-Tim D, Chiu RC. Cellular cardiomyoplasty: routes of cell delivery and retention. Front Biosci. 2008;13:2421–2434. doi: 10.2741/2855. [DOI] [PubMed] [Google Scholar]
- 3.Barbash IM, Chouraqui P, Baron J, Feinberg MS, Etzion S, Tessone A, Miller L, Guetta E, Zipori D, Kedes LH, Kloner RA, Leor J. Systemic delivery of bone marrow-derived mesenchymal stem cells to the infarcted myocardium: feasibility, cell migration, and body distribution. Circulation. 2003;108:863–868. doi: 10.1161/01.CIR.0000084828.50310.6A. [DOI] [PubMed] [Google Scholar]
- 4.Blocklet D, Toungouz M, Berkenboom G, Lambermont M, Unger P, Preumont N, Stoupel E, Egrise D, Degaute JP, Goldman M, Goldman S. Myocardial homing of nonmobilized peripheral-blood CD34/cells after intracoronary injection. Stem Cells. 2006;24:333–336. doi: 10.1634/stemcells.2005-0201. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Patel BS, Jeroudi MO, Li XY, Triana JF, Lai EK, McCay PB. Iron-mediated radical reactions upon reperfusion contribute to myocardial “stunning”. Am J Physiol. 1990;259:H1901–H1911. doi: 10.1152/ajpheart.1990.259.6.H1901. [DOI] [PubMed] [Google Scholar]
- 6.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation. 2013;128:122–131. doi: 10.1161/CIRCULATIONAHA.112.001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai C, Guo Y, Teng L, Nong Y, Tan M, Book MJ, Zhu X, Wang XL, Du J, Wu WJ, Xie W, Hong KU, Li Q, Bolli R. Preconditioning human cardiac stem cells with an HO-1 inducer exerts beneficial effects after cell transplantation in the infarcted murine heart. Stem Cells. 2015;33:3596–3607. doi: 10.1002/stem.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng K, Li TS, Malliaras K, Davis DR, Zhang Y, Marban E. Magnetic targeting enhances engraftment and functional benefit of iron-labeled cardiosphere-derived cells in myocardial infarction. Circ Res. 2010;106:1570–1581. doi: 10.1161/CIRCRESAHA.109.212589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawn B, Guo Y, Rezazadeh A, Huang Y, Stein AB, Hunt G, Tiwari S, Varma J, Gu Y, Prabhu SD, Kajstura J, Anversa P, Ildstad ST, Bolli R. Postinfarct cytokine therapy regenerates cardiac tissue and improves left ventricular function. Circ Res. 2006;98:1098–1105. doi: 10.1161/01.RES.0000218454.76784.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman MD, Erikson JM, Mao Y, Korcarz CE, Lang RM, Freeman GL. Validation of a mouse conductance system to determine LV volume: comparison to echocardiography and crystals. Am J Physiol Heart Circ Physiol. 2000;279:H1698–H1707. doi: 10.1152/ajpheart.2000.279.4.H1698. [DOI] [PubMed] [Google Scholar]
- 11.Guo Y, Jones WK, Xuan YT, Tang XL, Bao W, Wu WJ, Han H, Laubach VE, Ping P, Yang Z, Qiu Y, Bolli R. The late phase of ischemic preconditioning is abrogated by targeted disruption of the inducible NO synthase gene. Proc Natl Acad Sci USA. 1999;96:11507–11512. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–H1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann M, Wollert KC, Meyer GP, Menke A, Arseniev L, Hertenstein B, Ganser A, Knapp WH, Drexler H. Monitoring of bone marrow cell homing into the infarcted human myocardium. Circulation. 2005;111:2198–2202. doi: 10.1161/01.CIR.0000163546.27639.AA. [DOI] [PubMed] [Google Scholar]
- 14.Hong KU, Guo Y, Li QH, Cao P, Al-Maqtari T, Vajravelu BN, Du J, Book MJ, Zhu X, Nong Y, Bhatnagar A, Bolli R. c-kit+ Cardiac stem cells alleviate post-myocardial infarction left ventricular dysfunction despite poor engraftment and negligible retention in the recipient heart. PLoS One. 2014;9:e96725. doi: 10.1371/journal.pone.0096725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong KU, Li QH, Guo Y, Patton NS, Moktar A, Bhatnagar A, Bolli R. A highly sensitive and accurate method to quantify absolute numbers of c-kit+ cardiac stem cells following transplantation in mice. Basic Res Cardiol. 2013;108:346. doi: 10.1007/s00395-013-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou D, Youssef EA, Brinton TJ, Zhang P, Rogers P, Price ET, Yeung AC, Johnstone BH, Yock PG, March KL. Radio-labeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 2005;112:I150–I156. doi: 10.1161/CIRCULATIONAHA.104.526749. [DOI] [PubMed] [Google Scholar]
- 17.Keith MC, Bolli R. “String theory” of c-kit(pos) cardiac cells: a new paradigm regarding the nature of these cells that may reconcile apparently discrepant results. Circ Res. 2015;116:1216–1230. doi: 10.1161/CIRCRESAHA.116.305557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishore R, Khan M. More than tiny sacks: stem cell exosomes as cell-free modality for cardiac repair. Circ Res. 2016;118:330–343. doi: 10.1161/CIRCRESAHA.115.307654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Guo Y, Ou Q, Chen N, Wu WJ, Yuan F, O’Brien E, Wang T, Luo L, Hunt GN, Zhu X, Bolli R. Intracoronary administration of cardiac stem cells in mice: a new, improved technique for cell therapy in murine models. Basic Res Cardiol. 2011;106:849–864. doi: 10.1007/s00395-011-0180-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q, Guo Y, Ou Q, Cui C, Wu WJ, Tan W, Zhu X, Lanceta LB, Sanganalmath SK, Dawn B, Shinmura K, Rokosh GD, Wang S, Bolli R. Gene transfer of inducible nitric oxide synthase affords cardioprotection by upregulating heme oxygenase-1 via a nuclear factor-{kappa}B-dependent pathway. Circulation. 2009;120:1222–1230. doi: 10.1161/CIRCULATIONAHA.108.778688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li RC, Ping P, Zhang J, Wead WB, Cao X, Gao J, Zheng Y, Huang S, Han J, Bolli R. PKCepsilon modulates NF-kappaB and AP-1 via mitogen-activated protein kinases in adult rabbit cardiomyocytes. Am J Physiol Heart Circ Physiol. 2000;279:H1679–H1689. doi: 10.1152/ajpheart.2000.279.4.H1679. [DOI] [PubMed] [Google Scholar]
- 22.Li XY, McCay PB, Zughaib M, Jeroudi MO, Triana JF, Bolli R. Demonstration of free radical generation in the “stunned” myocardium in the conscious dog and identification of major differences between conscious and open-chest dogs. J Clin Invest. 1993;92:1025–1041. doi: 10.1172/JCI116608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panda NC, Zuckerman ST, Mesubi OO, Rosenbaum DS, Penn MS, Donahue JK, Alsberg E, Laurita KR. Improved conduction and increased cell retention in healed MI using mesenchymal stem cells suspended in alginate hydrogel. J Interv Card Electrophysiol. 2014;41:117–127. doi: 10.1007/s10840-014-9940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takano H, Bolli R, Black RG, Jr, Kodani E, Tang XL, Yang Z, Bhattacharya S, Auchampach JA. A(1) or A(3) adenosine receptors induce late preconditioning against infarction in conscious rabbits by different mechanisms. Circ Res. 2001;88:520–528. doi: 10.1161/01.res.88.5.520. [DOI] [PubMed] [Google Scholar]
- 27.Tang XL, Li Q, Rokosh G, Sanganalmath S, Chen N, Ou Q, Stowers H, Hunt G, Bolli R. Long-term outcome of administration of c-kitPOS cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res. 2016;118:1091–1105. doi: 10.1161/CIRCRESAHA.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang XL, Li Q, Rokosh G, Sanganalmath SK, Chen N, Ou Q, Stowers H, Hunt G, Bolli R. Long-term outcome of administration of c-kit(POS) cardiac progenitor cells after acute myocardial infarction: transplanted cells do not become cardiomyocytes, but structural and functional improvement and proliferation of endogenous cells persist for at least one year. Circ Res. 2016;118:1091–1105. doi: 10.1161/CIRCRESAHA.115.307647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang XL, Qiu Y, Park SW, Sun JZ, Kalya A, Bolli R. Time course of late preconditioning against myocardial stunning in conscious pigs. Circ Res. 1996;79:424–434. doi: 10.1161/01.res.79.3.424. [DOI] [PubMed] [Google Scholar]
- 30.Tang XL, Rokosh G, Sanganalmath SK, Tokita Y, Keith MC, Shirk G, Stowers H, Hunt GN, Wu W, Dawn B, Bolli R. Effects of intracoronary infusion of escalating doses of cardiac stem cells in rats with acute myocardial infarction. Circ Heart Fail. 2015;8:757–765. doi: 10.1161/CIRCHEARTFAILURE.115.002210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terrovitis J, Lautamaki R, Bonios M, Fox J, Engles JM, Yu J, Leppo MK, Pomper MG, Wahl RL, Seidel J, Tsui BM, Bengel FM, Abraham MR, Marban E. Noninvasive quantification and optimization of acute cell retention by in vivo positron emission tomography after intramyocardial cardiac-derived stem cell delivery. J Am Coll Cardiol. 2009;54:1619–1626. doi: 10.1016/j.jacc.2009.04.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokita Y, Tang XL, Li Q, Wysoczynski M, Hong KU, Nakamura S, Wu WJ, Xie W, Li D, Hunt G, Ou Q, Stowers H, Bolli R. Repeated administrations of cardiac progenitor cells are markedly more effective than a single administration: a new paradigm in cell therapy. Circ Res. 2016;119:635–651. doi: 10.1161/CIRCRESAHA.116.308937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wysoczynski M, Dassanayaka S, Zafir A, Ghafghazi S, Long BW, Noble C, DeMartino AM, Brittian KR, Bolli R, Jones SP. A new method to stabilize c-kit expression in reparative cardiac mesenchymal cells. Front Cell Dev Biol. 2016;4:78. doi: 10.3389/fcell.2016.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wysoczynski M, Guo Y, Moore J, Muthusamy M, Li Q, Nasr M, Li H, Nong Y, Wu W, Tomlin A, Zhu X, Hunt G, Gumpert A, Book M, Khan A, Tang XL, Bolli R. A new population of cardiac mesenchymal cells isolated on the basis of adherence: phenotype and reparative properties. J Am Coll Cardiol. 2016 doi: 10.1016/j.jacc.2017.01.048. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J, Du KT, Fang Q, Gu Y, Mihardja SS, Sievers RE, Wu JC, Lee RJ. The use of human mesenchymal stem cells encapsulated in RGD modified alginate microspheres in the repair of myocardial infarction in the rat. Biomaterials. 2010;31:7012–7020. doi: 10.1016/j.biomaterials.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 37.Zhu WX, Myers ML, Hartley CJ, Roberts R, Bolli R. Validation of a single crystal for measurement of transmural and epicardial thickening. Am J Physiol. 1986;251:H1045–H1055. doi: 10.1152/ajpheart.1986.251.5.H1045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.