Abstract
Background
We conducted a multi-institutional phase II study of capecitabine in combination with vinorelbine and trastuzumab in patients eligible to receive first- or second-line treatment for human epidermal growth factor receptor type 2 (HER2)– positive metastatic breast cancer (MBC).
Patients and Methods
The study was designed to test that the true confirmed response rate (CRR) was at most 45% vs a true CRR of at least 65%. Between March 2005 and June 2008, eligible patients received capecitabine, 825 mg/m2 orally on days 1–14; vinorelbine, 25 mg/m2 intravenously on days 1 and 8 every 3 weeks; and trastuzumab, 8 mg/kg intravenously on day 1 week 1 and 6 mg/kg every 3 weeks. The main outcome measure was CRR.
Results
Of 47 women accrued, 45 were evaluable. This design required at least 25 confirmed responses in the 45 evaluable patients for the treatment to be considered promising. Thirty women (67%) achieved a confirmed response (25 [56%] confirmed partial response; 5 [11%] confirmed complete responses). Median progression-free survival (PFS) was 11.3 months (95% confidence interval [CI], 8.4–16.7 months). Median overall survival was 28.5 months (95% CI, 24.8–36.4 months).
Conclusion
This triplet combination demonstrated promising activity in patients with HER2-positive MBC.
Keywords: capecitabine, combination chemotherapy, metastatic breast cancer, trastuzumab, vinorelbine
Introduction
Combinations of vinorelbine and capecitabine have shown promising antitumor activity in patients with metastatic breast cancer (MBC) previously treated with an anthracycline and a taxane (1–4). Human epidermal growth factor receptor type 2 (HER2) positivity considerably complicates the already problematic therapy of advanced MBC previously treated in the adjuvant setting with anthracyclines and taxanes. Each of the drugs has been demonstrated to have antitumor activity as single agents and in separate doublets for MBC (5–8). Each of these agents has different modes of action and toxicity profiles. As such, the potential of combining these agents for the treatment of HER2-overexpressing breast cancer has been pursued. Trastuzumab-containing regimens have significantly improved outcomes in patients with HER2-positive breast cancer (9,10). Efforts to improve the efficacy and tolerability of combination regimens with trastuzumab are important for patient care. Thus, we conducted a multi-institutional phase II study of a triplet combination—capecitabine in combination with vinorelbine and trastuzumab—in patients eligible to receive either first- or second-line treatment for HER2-positive MBC.
Patients and Methods
Eligibility
Inclusion Criteria
This prospective, interventional phase II study was conducted between March 2005 and June 2008 (North Central Cancer Treatment Group sites), with approval of the Mayo Clinic Institutional Review Board. The study’s ClinicalTrials.gov identifier is NCT00093808. Patients were eligible to enter the study if they had histologically confirmed HER2-positive invasive breast cancer with metastasis and were eligible to receive first- or second-line chemotherapy. Prior treatment with an anthracycline, a taxane, or trastuzumab was allowed as was prior hormonal therapy. Patients were required to be at least 18 years old with an Eastern Cooperative Oncology Group performance score of no more than 2. Patients were required to have adequate laboratory values, including hemoglobin of 8.0 g/dL or higher; absolute neutrophil count of 1.5×109/L or higher; platelet count of 100×109/L or higher; total bilirubin of no more than 3 times the upper limit of normal; reasonable aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, creatinine, and calcium levels. Baseline left ventricular ejection fraction was 50% or more (by multigated acquisition scan or echocardiography), and life expectancy was at least 12 weeks.
Exclusion Criteria
The following patients were excluded: pregnant or nursing women, women of child-bearing potential who were unwilling to use a dual method of contraception. Also excluded were patients who received more than 1 prior treatment with chemotherapy for metastatic disease; prior treatment with continuous fluorouracil infusion, capecitabine, or other fluoropyrimidine combinations; organ allografts requiring immunosuppressive therapy; radiotherapy to the axial skeleton, major surgery, or treatment in any investigational drug study 4 weeks or less before registration; or had a history of allergy or hypersensitivity to trastuzumab, capecitabine, vinorelbine, drug product excipients including polysorbate 80, chemically similar agents or prior unanticipated severe reaction to fluoropyrimidine therapy, or known hypersensitivity to fluorouracil or known dihydroxypyrimidine dehydrogenase deficiency; requirement for concurrent use of allopurinol, metronidazole, or the antiviral agent sorivudine (or chemically related analogues such as brivudine); central nervous system metastases, unless previously treated and stable for 3 months or longer; a history of uncontrolled seizures, central nervous system disorders, or psychiatric disability; a history of another malignancy within the preceding 5 years except a contralateral breast cancer; clinically significant (ie, active) cardiac disease; serious uncontrolled intercurrent infections or other serious uncontrolled concomitant disease; clinically significant malabsorption syndrome or inability to take oral medication; known, uncontrolled coagulopathy; or treatment with cimetidine within 2 weeks of registration.
Treatment Schedule
Treatment followed a 21-day cycle schedule. Capecitabine was administered orally twice daily at a dose of 825 mg/m2 on days 1–14, vinorelbine was administered intravenously (IV) at a dose of 25 mg/m2 on days 1 and 8 every 3 weeks, and trastuzumab was administered IV at a dose of 6 mg/kg on day 1 of every 3-week cycle (except cycle 1, when patients were given a loading dose of 8 mg/kg).
Response and Toxicity Criteria
Responses were assessed using the Response Evaluation Criteria in Solid Tumors (11). Measurable disease was defined as at least 1 lesion, the longest diameter (LD) of which could be accurately measured as 2.0 cm or larger by computed tomographic scan, magnetic resonance imaging, chest radiography, or physical examination. A subsequent scan was obtained 6 weeks or longer following initial documentation of an objective response of either complete response (CR) or partial response (PR). If this scan showed the same objective response, then the patient was said to have a confirmed response.
All measurable lesions up to a maximum of 10 lesions representative of all involved organs were identified as target lesions and recorded and measured at baseline. Target lesions were selected on the basis of their size and suitability for accurate repetitive measurements. No more than 5 lesions were required for any 1 organ.
Target lesions were evaluated as follows: CR, disappearance of all target lesions; PR, at least a 30% decrease in the sum of LDs of target lesions, taking as a reference the baseline sum of LDs; progression of disease, at least a 20% increase in the sum of LDs of target lesions, taking as a reference the smallest sum of LDs recorded since treatment started or the appearance of 1 or more new lesions; and stable disease, neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progression of disease, taking as a reference the smallest sum of LDs.
Statistical Design and Analysis
The primary end point was the proportion of confirmed responses (CRs or PRs). The study used a single-stage phase II clinical trial design to test the null hypothesis that the true confirmed response rate (CRR) is at most 45% vs a true CRR of at least 65%. This design had a significance level of 0.10 and 90% power. The regimen would be declared ineffective if no more than 24 of the first 45 evaluable patients had confirmed responses.
Secondary end points were survival time, time to progression (overall and split by prior regimen status), and adverse effects. The distributions of overall survival (OS) time (time from study entry to death), progression-free survival (PFS) time (time from study entry to disease progression or death), and documentation of response (time from first documentation of response until progression or death) were estimated using the Kaplan-Meier method. Toxicity data were summarized on all patients who received at least 1 dose of treatment. Simple descriptive statistics were used to summarize the adverse event profile and baseline characteristics.
Results
Patients
Overall 47 women were accrued to the study from March 2005 until June 2008, all of whom were eligible. One woman cancelled study participation before receiving any treatment (her data have not been included). Another was deemed a major protocol violation as she received capecitabine every day for the first 6 cycles by error. Her data were summarized in all analyses unless otherwise specified.
Patient demographics and clinical characteristics at study entry are listed in Table 1. The median age at study entry was 60.0 years (range, 40.0–77.0 years). Two-thirds of the women had a baseline performance score of 0. At baseline, 25 women (54.3%) were estrogen receptor positive, 17 (37.0%) were progesterone receptor positive, and 36 (78.3%) had visceral disease. The most common sites of metastases were lymph nodes (26/46 [56.5%]), lung (23/46 [50.0%]), liver (20/46 [43.5%]), and bone (19/46 [41.3%]). Fourteen patients (30.4%) had received prior chemotherapy, and 26 (56.5%) had received prior trastuzumab (13 [28.3%] for metastatic disease, 10 [21.7%] as adjuvant therapy, and 3 [6.5%] as neoadjuvant therapy) (Table 2).
Table 1.
Patient Demographic Data
| Patient Demographic Characteristics | Valuea |
|---|---|
| Age, y | |
| Median | 60.0 |
| Range | 40.0–77.0 |
| Female sex | 46 (100) |
| Performance score | |
| 0 | 31 (67.4) |
| 1 | 13 (28.3) |
| 2 | 2 (4.3) |
| ER | |
| Positive | 25 (54.3) |
| Negative | 21 (45.7) |
| PR | |
| Positive | 17 (37.0) |
| Negative | 29 (63.0) |
| Nottingham grade | |
| Well | 2 (4.3) |
| Moderate | 11 (23.9) |
| Poor | 23 (50.0) |
| Unknown | 10 (21.7) |
| Visceral involvement | |
| 0 | 10 (21.7) |
| 1 | 36 (78.3) |
| Baseline LVEF by MUGA/echo, % | |
| Median | 61.0 |
| Range | 50.1–80.0 |
| Normal left ventricular ejection function | 46 (100) |
Abbreviations: echo, echocardiography; ER, estrogen receptor; LVEF, left ventricular ejection fraction; MUGA, multigated acquisition scan; PR, protesterone receptor.
Values are number (percentage) of patients (N=46) unless indicated otherwise. Percentages may not total 100% due to rounding.
Table 2.
Patients’ Prior Treatment History
| Prior Treatments | No. (%) of Patients (N=46)a |
|---|---|
| Prior chemotherapy | |
| Yes | 14 (30.4) |
| No | 32 (69.6) |
| Prior anthracycline | |
| Neoadjuvant | 10 (21.7) |
| Adjuvant | 30 (65.2) |
| Metastatic | 1 (2.2) |
| None | 5 (10.9) |
| Prior trastuzumab | |
| Neoadjuvant | 3 (6.5) |
| Adjuvant | 10 (21.7) |
| Metastatic | 13 (28.3) |
| None | 20 (43.5) |
| Prior taxane | |
| Neoadjuvant | 6 (13.0) |
| Adjuvant | 25 (54.3) |
| Metastatic | 9 (19.6) |
| None | 6 (13.0) |
Percentages may not total 100% due to rounding.
Follow-up
The median number of cycles administered was 8.5 (range, 1–48). All women have ended study participation. The reasons for study discontinuation were disease progression in 20 patients (43.5%), 10 (21.7%) had adverse events, 5 (10.8%) wanted to stop treatment, 3 (6.5%) had other medical problems (abdominal pain, cataracts, and recurrent ductal carcinoma in situ), 3 (6.5%) had CR (or near CR), 2 (4.4%) had alternate treatments, 1 (2.2%) died on study due to a gastrointestinal tract hemorrhage (not related to study drug or progression), and 2 (4.4%) stopped for other reasons (one was wintering in a location that is not near a North Central Cancer Treatment Group site and the other was afraid that her lesions had grown). Data were frozen in June 2010, and the latest follow-up data at that point revealed that 17 women (37.0%) remained alive, with a median follow-up time of 35.0 months (range, 20.7–54.1 months).
Efficacy
Thirty of the first 45 evaluable women (67%; 95% confidence interval [CI], 51%-80%) had confirmed responses, including 5 who had CRs. As this surpassed the predetermined threshold, this regimen may be considered to be effective in this patient population. Additionally, the woman who was deemed a major protocol violation had a confirmed CR, bringing the overall CRR to 31 of 46 (67%; 95% CI, 52%-80%), including 6 confirmed CRs. Additionally, 3 women had confirmed PRs that showed signs of CR immediately before ending study participation. The response was not independently adjudicated.
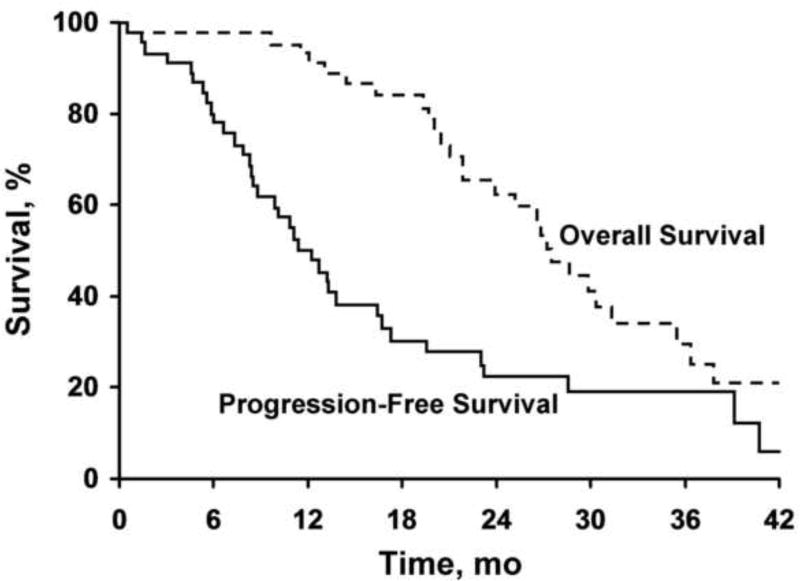
Of the 46 women who received capecitabine, 27 have died. The median overall survival (as estimated by the method of Kaplan-Meier) was 28.5 months (95% CI, 24.8–36.4 months). The median PFS was 11.3 months (95% CI, 8.4–16.7 months). The median duration of response (time from first documentation of response until progression or death) for the 31 responders was 13.2 months (95% CI, 8.6–23.2 months). Kaplan-Meier curves can be seen in the Figure.
Figure.
Kaplan-Meier survival graph of patients in the study.
Toxicities
Toxicity was defined as an adverse event considered possibly, probably, or definitely related to treatment. Of the 46 patients who received at least 1 dose of treatment, 28 (60.9%) had at least 1 severe (grade 3+) hematologic adverse event and 28 (60.9%) had at least 1 severe nonhematologic adverse event. Common toxicities included neutropenia (39/46 [84.8%], any grade); anemia (11/46 [23.9%], all grade 2); thrombocytopenia (9/46 [19.6%], all grades 1 and 2); bone pain (13/46 [28.2%], mostly grades 1 and 2); hand-foot syndrome (24/46 [52.2%], mostly grades 1 and 2, with grade 3 in 10.9%); and diarrhea (27/46 [58.7%], with grades 3 and 4 in 8.7%). No alopecia was noted with this regimen. One patient died from gastrointestinal tract hemorrhage, but it was deemed not related to study treatment. Table 3 details toxicities.
Table 3.
Specific Toxicitiesa
| Toxicity | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Total |
|---|---|---|---|---|---|
| Anemia | 0 (0) | 11 (23.9) | 0 (0) | 0 (0) | 11 (23.9) |
| Leukopenia | 0 (0) | 6 (13.0) | 4 (8.7) | 1 (2.2) | 11 (23.9) |
| Neutropenia | 1 (2.2) | 10 (21.7) | 19 (41.3) | 9 (19.6) | 39 (84.8) |
| Thrombocytopenia | 6 (13.0) | 3 (6.6) | 0 (0) | 0 (0) | 9 (19.6) |
| Neuromotor disorder | 3 (6.5) | 2 (4.3) | 1 (2.2) | 0 (0) | 6 (13.0) |
| Neurosensory disorder | 21 (45.7) | 7 (15.2) | 2 (4.3) | 0 (0) | 30 (65.2) |
| Abdominal pain | 0 (0) | 4 (8.7) | 1 (2.2) | 0 (0) | 5 (10.9) |
| Bone pain | 6 (13.0) | 6 (13.0) | 1 (2.2) | 0 (0) | 13 (28.2) |
| Myalgia | 11 (23.9) | 8 (17.3) | 2 (4.3) | 0 (0) | 21 (45.7) |
| Dyspnea | 0 (0) | 3 (6.6) | 2 (4.3) | 0 (0) | 5 (10.9) |
| Creatinine elevation | 6 (13.0) | 1 (2.2) | 0 (0) | 0 (0) | 7 (15.2) |
| Proteinuria | 0 (0) | 1 (2.2) | 0 (0) | 0 (0) | 1 (2.2) |
| Fatigue | 20 (43.5) | 16 (34.8) | 7 (15.2) | 0 (0) | 43 (93.5) |
| Rashes, desquamation | 4 (8.7) | 2 (4.3) | 1 (2.2) | 0 (0) | 7 (15.2) |
| Injection site reaction | 5 (11.0) | 1 (2.0) | 0 (0) | 0 (0) | 6 (13.0) |
| Hand-foot syndrome | 12 (26.1) | 7 (15.2) | 5 (10.9) | 0 (0) | 24 (52.2) |
| Anorexia | 0 (0) | 7 (15.2) | 0 (0) | 0 (0) | 7 (15.2) |
| Constipation | 0 (0) | 4 (8.7) | 0 (0) | 0 (0) | 4 (8.7) |
| Dehydration | 0 (0) | 2 (4.3) | 3 (6.6) | 0 (0) | 5 (10.9) |
| Diarrhea | 15 (32.6) | 8 (17.4) | 3 (6.5) | 1 (2.2) | 27 (58.7) |
| Nausea | 0 (0) | 4 (8.7) | 3 (6.6) | 0 (0) | 7 (15.2) |
| Oral cavity mucositis | 0 (0) | 7 (15.2) | 1 (2.2) | 0 (0) | 8 (17.4) |
| Vomiting | 0 (0) | 2 (4.3) | 3 (6.6) | 0 (0) | 5 (10.9) |
Values are number (percentage) of patients.
Discussion
Outcomes in patients with HER2-positive MBC have improved dramatically on treatment with trastuzumab-containing regimens (5,6,12). This multi-institutional phase II study of a triplet combination—capecitabine, vinorelbine, trastuzumab—studied patients eligible to receive either first- or second-line treatment for HER2-positive MBC.
Capecitabine is the standard drug approved by the US Food and Drug Administration for treatment of MBC that had progressed after treatment with a taxane and an anthracycline. Several phase II studies of doublets with trastuzumab in combination with paclitaxel, docetaxel, or vinorelbine reported response rates of 50% to 75%. Other phase II studies have documented the activity of vinorelbine combinations in the treatment of advanced breast cancer with response rates of 60% to 74% (13–16).
Vinorelbine and Capecitabine Combination Therapy
Intravenous vinorelbine combined with continuous infusion of fluorouracil in first- and second-line treatment regimens has been studied. As first-line treatment, this combination has demonstrated high response rates from 61% to 64% and a median overall survival of 22 months (14). However, the route of infusion of fluorouracil decreases patient comfort by using a central line or peripheral access system ports. The development of the oral fluoropyrimidine, capecitabine has stimulated interest in combining IV vinorelbine and capecitabine.
Several phase I trials have been performed. In a study by Nole et al (17), the maximum tolerated dose was not reached after the ninth level of escalated dose (IV vinorelbine, 22.5 mg/m2 on days 1 and 3, and capecitabine, 1,250 mg/m2 twice daily on days 1–14). However, the regimen’s efficacy was observed at all dose levels (odds ratio, 49%). The second phase I trial was done by Welt et al (18) in which the dose-limiting toxicities were nausea, vomiting, and diarrhea. The response rate was 52%, with a manageable toxicity level. The recommended doses for phase II trials were 25 mg/m2 on days 1 and 8 for IV vinorelbine and 1,000 mg/m2 twice daily on days 1–14 every 3 weeks for capecitabine.
Several phase II studies have been published on the use of vinorelbine and capecitabine, mainly as second-line chemotherapy for MBC. Intravenous vinorelbine was administered at a dose of 25 mg/m2 on days 1 and 8, while the capecitabine dosage varied between 825 mg/m2 twice daily to 1,250 mg/m2 twice daily on days 1–14 every 3 weeks. The response rate as second-line therapy was higher than 50%, and the toxicity was mild: hand-foot syndrome was related to the capecitabine dosage. Only 1 phase II trial with this combination was done as first-line chemotherapy (19), in which capecitabine was administered at a dose of 825 mg/m2 twice daily on days 1–14 and IV vinorelbine at a dose of 25 mg/m2 on days 1 and 8 every 3 weeks. Thirty patients with MBC were treated with this combination; 67% of patients achieved an objective response and 20% had stable disease. The toxicity profile was good: only 2 patients experienced grade 3/4 neutropenia, and no patient had grade 3 hand-foot syndrome.
Vinorelbine and Trastuzumab Combination Therapy
One phase II study evaluated this regimen as first- and second-line treatment in patients with MBC with both drugs given on a weekly schedule. Vinorelbine was given at the dose of 25 mg/m2 weekly, and trastuzumab was given at 4 mg/kg on loading, then at 2 mg/kg weekly. Among 40 evaluable patients, 75% achieved an objective response, with median time to progression (TTP) of 7 months. The safety profile was without severe cardiac toxicity (5). In addition, Jahanzeb et al (6) has reported significant results with this combination as first-line therapy. Similar doses of trastuzumab were tested, but IV vinorelbine was administered at the dose of 30 mg/m2 per week. A response rate of 78% was achieved among 37 evaluable patients. Time to progression was 17 months. This combination was well tolerated.
In this study, we combined trastuzumab, capecitabine, and vinorelbine. This regimen produced a response rate of 67% (4 [9%] CRs and 26 [58%] PRs). We did meet our statistical end point for the study, which was 65% and above. Compared with other doublets, the triple-drug protocol showed a reasonable response rate.
Table 4 compares studies using capecitabine and combinations in patients receiving first- and second-line treatment. The range of TTP was 5.6 to 8.2 months, and overall survival was between 17 and 28 months (5,20–22). Table 5 compares the results of 2 phase III studies with the results with this study of patients with MBC. Lapatinib is the first dual inhibitor of the EGFR and HER2 tyrosine kinases approved by the US Food and Drug Administration. A phase III trial comparing lapatinib plus capecitabine with capecitabine alone showed that the combination had an improved overall response rate (22% vs 14%) and a significantly increased median TTP (8.4 vs 4.4 months) in patients with pretreated advanced HER2-overexpressing disease (23). Another trial of single-agent lapatinib in trastuzumab-refractory advanced breast cancer showed a 12.8% response rate and a median TTP of 15.3 weeks (24). Thus, lapatinib is an important and effective therapy for HER2-overexpressing MBCs that have progressed on trastuzumab. Similar to trastuzumab, the median duration of response to lapatinib was less than 1 year.
Table 4.
Published Studies of Capecitabine, Vinorelbine, and Trastuzumab Combinations
| Chemotherapy Regimen |
Source | No. of Patients |
TTP, mo | OS, mo | RR, % | CR/PR, % |
|---|---|---|---|---|---|---|
| Cap and T (2nd line and beyond) | Bartsch et al (20) | 40 | 8 | 24 | 20 | 2.5/17.5 |
| Cap (1st and 2nd line) | von Minckwitz et al (21) | 78 | 5.6 | 20.4 | 27 | NR |
| Cap and T (1st and 2nd line) | von Minckwitz et al (21) | 78 | 8.2 | 25.5 | 48 | NR |
| Cap and T (2nd or 3rd line) | Schaller et al (22) | 27 | 6.7 | 28 | 45 | 15/30 |
| V and T (1st and 2nd line) | Burstein et al (5) | 40 | 8.2 | 17 2nd line | 75 | 8/68 |
Abbreviations: Cap, capecitabine; CR, complete response; NR, not reported; OS, overall survival; PR, partial response; RR, response rate; T, trastuzumab; TTP, time to progression; V, vinorelbine.
Table 5.
Comparison of Present Study With Phase III Studies of Capecitabine With Trastuzumab or Lapatinib
| Treatment Regimen | Source | PFS, mo | RR, % | OS, mo |
|---|---|---|---|---|
| Cap | Geyer et al (23) | 4.4 | 15 | NR |
| Cap and lapatinib | Geyer et al (23) | 8.4 | 22 | NR |
| Cap | von Minckwitz et al (21) | 5.6 | 27 | 20.4 |
| Cap and T | von Minckwitz et al (21) | 8.4 | 48 | 24 |
| Cap, T, and V | Present study | 11.3 | 67 | 27 |
Abbreviations: Cap, capecitabine; NR, not reported; OS, overall survival; PFS, progression-free survival; RR, response rate; T, trastuzumab; V, vinorelbine.
In a phase III study using capecitabine and trastuzumab for first- and second-line treatment, the response rate was 48% with overall survival of 24 months (23). Although cross-comparison of trials is not optimal, we thought that our trial in patients with MBC showed results that are hypothesis generating and promising. What was quite interesting is that this regimen, unlike other combinations, did provide a median PFS of 11.3 months (95% CI, 8.4–23.2 months) and a 27.2-month median OS (95% CI, 26.6–35.5 months).
Data on several other interesting triplet regimens have been reported. Chan et al (25), in a study of HER2-positive patients with MBC receiving first-line treatment with oral vinorelbine, oral capecitabine, and IV trastuzumab had a response rate of 81% with CR of 13% and PR of 58%. Infante et al (26), in a phase II study of weekly docetaxel, vinorelbine, and trastuzumab as first-line treatment, had a response rate of 68% (41/60 patients; 95% CI, 55%-80%) with CR of 27% and PR of 42%. Median PFS was 12 months (95% CI, 9.1–16.3 months), and median OS was 40.8 months (95% CI, 25 months-not reached). Neutropenia 72% (grade 4) was the most common hematologic toxicity.
In our study, a subset analysis showed PFS was 19 months and response rate was 75% in patients who received first-line treatment and 8.3 months with a response rate of 61.5% in those who had second-line treatment. These results are comparable if not better than other phase II studies.
Table 6 summarizes outcomes in patients who did and did not receive prior treatment with trastuzumab. Twenty-six patients received prior trastuzumab: median OS was 25.2 months (95% CI, 20.0–27.2 months). There are 16 responders (61.5% [95% CI, 40.6%-79.8%]) in this cohort. Twenty patients were trastuzumab naïve; median OS was 37.8 months (95% CI, 28.4 months-upper limit not available), median TTP was 17.0 months (95% CI, 11.0–28.5 months). There were 15 responders (75.0% [95% CI, 50.9%-91.3%]) in this cohort.
Table 6.
Subset Analysis of Patients According to Their Prior Exposure to Trastuzumab and Outcomes
| Parameter | Prior Trastuzumab | No Trastuzumab |
|---|---|---|
| No. of patients | 26 | 20 |
| Median OS | 26.6 mo | 37.8 mo |
| 1-y survival | 92.3% (95% CI, 82.6%-100%) | 90.0% (95% CI, 77.8%-100%) |
| Median TTP | 8.6 mo | 17.0 mo |
| No. (%) of responders | 16 (61.5%) | 15 (75%) |
Abbreviations: CI, confidence interval; NA, not available; OS, overall survival; TTP, time to progression.
Conclusions
This vinorelbine, capecitabine, and trastuzumab regimen provides a reasonable response rate, TTP, and OS as first- and second-line treatment in patients with HER2-positive MBC. This regimen is a reasonable choice for patients with visceral disease requiring rapid tumor burden reduction and can be considered in women who are anxious about alopecia. The study has shown that alopecia is not a side effect. This drug combination would be a reasonable option for patients who have rapidly progressive visceral disease but are not candidates for clinical trials of pertuzumab, trastuzumab emtansine, and other novel drugs. Further studies, including a phase III trial of this regimen compared with lapatinib and capecitabine in patients with advanced stages of HER2-positive breast cancer, are worthy of consideration.
Clinical Practice Points.
Multiple treatment options are available for patients with metastatic HER2-positive breast cancer.
The combination treatment described in this study is another option for patients. Due to its toxicity profile, this combination should be reserved for patients who have aggressive disease, are symptomatic, and need quick cytoreduction of their tumor.
Further studies with this combination may be limited due to the fact that newer options with less toxicity, for example, pertuzumab and trastuzumab emtansine, will be available soon.
Acknowledgments
Funding
Supported in part by grant 25224 from the National Institutes of Health. Financial support was provided by Genentech, a member of the Roche Group.
Abbreviations
- CI
confidence interval
- CR
complete response
- CRR
confirmed response rate
- HER2
human epidermal growth factor receptor type 2
- IV
intravenous, intravenously
- LD
longest diameter
- MBC
metastatic breast cancer
- OS
overall survival
- PFS
progression-free survival
- PR
partial response
- TTP
time to progression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None.
References
- 1.Livingston RB, Ellis GK, Gralow JR, Williams MA, White R, McGuirt C, et al. Dose-intensive vinorelbine with concurrent granulocyte colony-stimulating factor support in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1997 Apr;15(4):1395–400. doi: 10.1200/JCO.1997.15.4.1395. [DOI] [PubMed] [Google Scholar]
- 2.Fumoleau P, Delgado FM, Delozier T, Monnier A, Gil Delgado MA, Kerbrat P, et al. Phase II trial of weekly intravenous vinorelbine in first-line advanced breast cancer chemotherapy. J Clin Oncol. 1993 Jul;11(7):1245–52. doi: 10.1200/JCO.1993.11.7.1245. [DOI] [PubMed] [Google Scholar]
- 3.Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, et al. Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol. 1999 Feb;17(2):485–93. doi: 10.1200/JCO.1999.17.2.485. [DOI] [PubMed] [Google Scholar]
- 4.O’Shaughnessy J, Miles D, Vukelja S, Moiseyenko V, Ayoub JP, Cervantes G, et al. Superior survival with capecitabine plus docetaxel combination therapy in anthracycline-pretreated patients with advanced breast cancer: phase III trial results. J Clin Oncol. 2002 Jun 15;20(12):2812–23. doi: 10.1200/JCO.2002.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Burstein HJ, Kuter I, Campos SM, Gelman RS, Tribou L, Parker LM, et al. Clinical activity of trastuzumab and vinorelbine in women with HER2-overexpressing metastatic breast cancer. J Clin Oncol. 2001 May 15;19(10):2722–30. doi: 10.1200/JCO.2001.19.10.2722. [DOI] [PubMed] [Google Scholar]
- 6.Jahanzeb M, Mortimer JE, Yunus F, Irwin DH, Speyer J, Koletsky AJ, et al. Phase II trial of weekly vinorelbine and trastuzumab as first-line therapy in patients with HER2(+) metastatic breast cancer. Oncologist. 2002;7(5):410–7. doi: 10.1634/theoncologist.7-5-410. [DOI] [PubMed] [Google Scholar]
- 7.Chan A, Untch M, Petruzelka L, Martin M, Guillem Porta V, Gil M, et al. Multinational phase II study of navelbine (N) and herceptin (H) as first-line therapy for HER2-overexpressing metastatic breast cancer (HER2+ MBC) [abstract] Br Cancer Res Treat. 2002;76(Suppl 1):S112. [Google Scholar]
- 8.Pegram MD, Konecny GE, O’Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004 May 19;96(10):739–49. doi: 10.1093/jnci/djh131. [DOI] [PubMed] [Google Scholar]
- 9.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005 Jul 1;23(19):4265–74. doi: 10.1200/JCO.2005.04.173. Epub 2005 May 23. [DOI] [PubMed] [Google Scholar]
- 10.Esteva FJ, Valero V, Booser D, Guerra LT, Murray JL, Pusztai L, et al. Phase II study of weekly docetaxel and trastuzumab for patients with HER-2-overexpressing metastatic breast cancer. J Clin Oncol. 2002 Apr 1;20(7):1800–8. doi: 10.1200/JCO.2002.07.058. [DOI] [PubMed] [Google Scholar]
- 11.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000 Feb 2;92(3):205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 12.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001 Mar 15;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 13.Spielmann M, Dorval T, Turpin F, Antoine E, Jouve M, Maylevin F, et al. Phase II trial of vinorelbine/doxorubicin as first-line therapy of advanced breast cancer. J Clin Oncol. 1994 Sep;12(9):1764–70. doi: 10.1200/JCO.1994.12.9.1764. [DOI] [PubMed] [Google Scholar]
- 14.Dieras V, Extra JM, Bellissant E, Espie M, Morvan F, Pierga JY, et al. Efficacy and tolerance of vinorelbine and fluorouracil combination as first-line chemotherapy of advanced breast cancer: results of a phase II study using a sequential group method. J Clin Oncol. 1996 Dec;14(12):3097–104. doi: 10.1200/JCO.1996.14.12.3097. [DOI] [PubMed] [Google Scholar]
- 15.Romero Acuna L, Langhi M, Perez J, Romero Acuna J, Machiavelli M, Lacava J, et al. Vinorelbine and paclitaxel as first-line chemotherapy in metastatic breast cancer. J Clin Oncol. 1999 Jan;17(1):74–81. doi: 10.1200/JCO.1999.17.1.74. [DOI] [PubMed] [Google Scholar]
- 16.Fumoleau P, Fety R, Delecroix V, Perrocheau G, Azli N. Docetaxel combined with vinorelbine: phase I results and new study designs. Oncology (Williston Park) 1997 Jun;11(6 Suppl 6):29–31. [PubMed] [Google Scholar]
- 17.Nole F, Catania C, Mandala M, Zampino MG, Munzone E, Feretti G, et al. Phase I study of vinorelbine (V) and capecitabine (C) in advanced breast cancer (ABC) [abstract] Br Cancer Res Treat. 2000 Nov;64(1):125. [Google Scholar]
- 18.Welt A, Von Minckwitz G, Borquez D, Oberhoff C, Kaufmann M, Seeber S, et al. Extended phase I study of capecitabine in combination with vinorelbine in pretreated patients with metastatic breast cancer [abstract] Br Cancer Res Treat. 2002;76(Suppl 1):S90. [Google Scholar]
- 19.Ghosn M, Kattan J, Farhat F, Gasmi J. Navelbine (N) capecitabine (C) combination (Navcap): the new first line chemotherapy regimen for metastatic breast cancer (MBC): results of phase II trial [abstract] Br Cancer Res Treat. 2002;76(Suppl 1):S133. [Google Scholar]
- 20.Bartsch R, Wenzel C, Altorjai G, Pluschnig U, Rudas M, Mader RM, et al. Capecitabine and trastuzumab in heavily pretreated metastatic breast cancer. J Clin Oncol. 2007 Sep 1;25(25):3853–8. doi: 10.1200/JCO.2007.11.9776. Epub 2007 Aug 6. [DOI] [PubMed] [Google Scholar]
- 21.von Minckwitz G, du Bois A, Schmidt M, Maass N, Cufer T, de Jongh FE, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a German breast group 26/breast international group 03-05 study. J Clin Oncol. 2009 Apr 20;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. Epub 2009 Mar 16. [DOI] [PubMed] [Google Scholar]
- 22.Schaller G, Fuchs I, Gonsch T, Weber J, Kleine-Tebbe A, Klare P, et al. Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol. 2007 Aug 1;25(22):3246–50. doi: 10.1200/JCO.2006.09.6826. Epub 2007 Jun 18. [DOI] [PubMed] [Google Scholar]
- 23.Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006 Dec 28;355(26):2733–43. doi: 10.1056/NEJMoa064320. Erratum in: N Engl J Med. 2007 Apr 5;356(14):1487. [DOI] [PubMed] [Google Scholar]
- 24.Blackwell KL, Pegram MD, Tan-Chiu E, Schwartzberg LS, Arbushites MC, Maltzman JD, et al. Single-agent lapatinib for HER2-overexpressing advanced or metastatic breast cancer that progressed on first- or second-line trastuzumab-containing regimens. Ann Oncol. 2009 Jun;20(6):1026–31. doi: 10.1093/annonc/mdn759. Epub 2009 Jan 29. [DOI] [PubMed] [Google Scholar]
- 25.Chan A, Ganju V, Becquart D, Conte P, Petruzelka L, Aubert D, et al. Efficacy of oral vinorelbine (NVBo), capecitabine (X) and trastuzumab (H) triple combination (NVBoXH) in HER-2 positive metastatic breast cancer (MBC): first results of an international phase II trial [abstract] J Clin Oncol. 2007;25(Suppl):45s. [Google Scholar]
- 26.Infante JR, Yardley DA, Burris HA, 3rd, Greco FA, Farley CP, Webb C, et al. Phase II trial of weekly docetaxel, vinorelbine, and trastuzumab in the first-line treatment of patients with HER2-positive metastatic breast cancer. Clin Breast Cancer. 2009 Feb;9(1):23–8. doi: 10.3816/CBC.2009.n.004. [DOI] [PubMed] [Google Scholar]