Summary
Transmembrane adaptor PAG (Cbp) has been proposed to mediate membrane recruitment of Csk, a cytoplasmic protein tyrosine kinase playing a critical inhibitory role during T cell activation, by inactivating membrane-associated Src kinases. However, this model has not been validated by genetic evidence. Here we demonstrate that PAG-deficient mice display enhanced T cell activation responses in effector, but not in naive, T cells. PAG-deficient mice also have augmented T cell-dependent autoimmunity and greater resistance to T cell anergy. Interestingly, in absence of PAG, Csk becomes more associated with alternative partners, i.e. phosphatase PTPN22 and Dok adaptors. Combining PAG deficiency with PTPN22 or Dok adaptor deficiency further enhances effector T cell responses. Unlike PAG, Cbl ubiquitin ligases inhibit activation of naïve, but not of effector, T cells. Thus, Csk-associating PAG is a critical component of the inhibitory machinery controlling effector T cell activation, in cooperation with PTPN22 and Dok adaptors.
Introduction
T cell activation results from engagement of the T cell receptor (TCR) by antigen peptides and major histocompatibility complex (MHC) molecules displayed by antigen-presenting cells (APCs) (Weiss and Littman, 1994). TCR signaling is instigated by protein tyrosine phosphorylation, which is initiated by Src family enzymes, a group of intracellular protein tyrosine kinases (PTKs) associated with the plasma membrane inner leaflet. The Src kinases in T cells, Lck and Fyn, mediate this effect largely by phosphorylating immunoreceptor tyrosine-based activation motifs (ITAMs) in TCR-associated CD3 and ζ chains. ITAM tyrosine phosphorylation enables recruitment of another PTK, ZAP-70, which phosphorylates other proteins leading to effector functions.
The enzymatic activity of Src kinases is regulated by tyrosine phosphorylation (Veillette et al., 2002). Phosphorylation of a tyrosine (Y) in the kinase domain (Y394 in Lck; Y417 in Fyn) leads to activation. This is mediated by autophosphorylation, and reversed by protein tyrosine phosphatases (PTPs) such as PTPN22 and SHP-1. Defects in these phosphatases, in particular PTPN22, are linked to autoimmune diseases in humans (Rhee and Veillette, 2012; Stanford and Bottini, 2014). In contrast, phosphorylation of a carboxyl-terminal tyrosine (Y505 in Lck; Y528 in Fyn) inactivates Src kinases. This is mediated by another PTK, Csk, and is reversed by the transmembrane PTP CD45. Whereas Src kinases are membrane-bound, Csk is cytoplasmic. Thus, Csk needs to bind membrane-associated molecules to be near Src kinases.
A strong candidate for recruiting Csk to the plasma membrane is the transmembrane adaptor PAG, also known as Cbp (Brdicka et al., 2000; Hrdinka and Horejsi, 2014; Kawabuchi et al., 2000; Simeoni et al., 2008). Membrane-bound PAG is also targeted to lipid rafts, where large pools of Src kinases reside. PAG is prominently tyrosine phosphorylated in unstimulated T cells (Brdicka et al., 2000; Davidson et al., 2003). This phosphorylation enables binding to Csk, by way of tyrosine 314 (Y314) of PAG and the Src homology 2 (SH2) domain of Csk. Upon TCR stimulation, PAG is dephosphorylated and dissociates from Csk (Brdicka et al., 2000; Davidson et al., 2003; Torgersen et al., 2001). This event is presumed to alleviate the suppressive effect of Csk on Src kinases.
In support of the inhibitory role of Csk in TCR signaling, pharmacological inhibition of Csk in mature T cells augmented T cell activation (Manz et al., 2015). Conversely, Csk overexpression in a T cell line suppressed T cell activation (Chow et al., 1993; Manz et al., 2015). Furthermore, Csk-deficient (“knock-out”; KO) mice exhibited severe T cell developmental abnormalities (Schmedt et al., 1998; Tan et al., 2014). Although it had been presumed that PAG mediates membrane recruitment of Csk in T cells, two groups reported that PAG KO mice had no overt T cell phenotype (Dobenecker et al., 2005; Xu et al., 2005; Yang and Seed, 2003). This observation raised the possibility that PAG is not responsible for membrane recruitment of Csk, or, alternatively, that other Csk-binding molecules provide this function in the absence of PAG.
To resolve these matters, we further evaluated T cell functions of PAG-deficient mice. As reported (Dobenecker et al., 2005; Xu et al., 2005; Yang and Seed, 2003), PAG KO mice displayed no obvious alterations in T cell development, and had unaltered T cell activation responses when freshly isolated ("naive") T cells were analyzed. In contrast, these mice displayed increased activation responses in vitro and in vivo when previously activated ("effector") T cells were assessed. In the absence of PAG, Csk became more extensively associated with alternative Csk-interacting proteins, namely PTPN22 and Dok adaptors, which cooperated with PAG to suppress activation of previously activated T cells. Lastly, unlike mice lacking PAG, PTPN22 or Dok adaptors, mice genetically deficient for Cbl ubiquitin ligases had augmented activation responses in freshly isolated T cells, suggesting that a distinct set of inhibitory effectors, including the Cbl proteins, is controlling TCR signaling in naive T cells.
Results
PAG-deficient mice display augmented responses in previously activated, but not in freshly isolated, T cells
To elucidate the role of PAG in T cells, the impact of PAG deficiency was examined, using a previously described PAG KO mouse (Figure 1A) (Lindquist et al., 2011; Yang and Seed, 2003). As reported, PAG KO mice had no alterations in numbers of various subsets of thymocytes, compared to wild-type mice (Table S1) (Dobenecker et al., 2005; Xu et al., 2005). They also had no significant change in the numbers of all T cell subsets tested (Table S1). Levels of antibodies and germinal center B cells were also not affected (Table S2). Similar findings were made with young (1.5–3 months) and aged (9–13 months) mice (Tables S1 and S2).
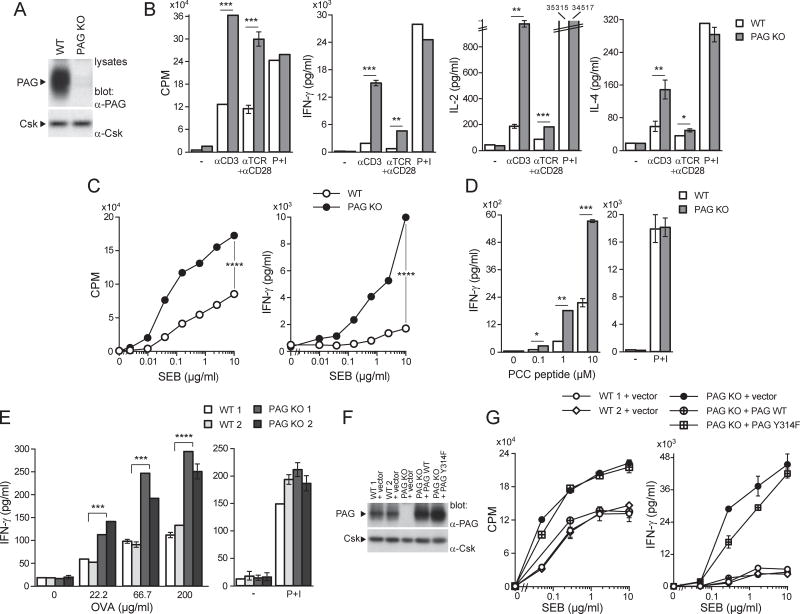
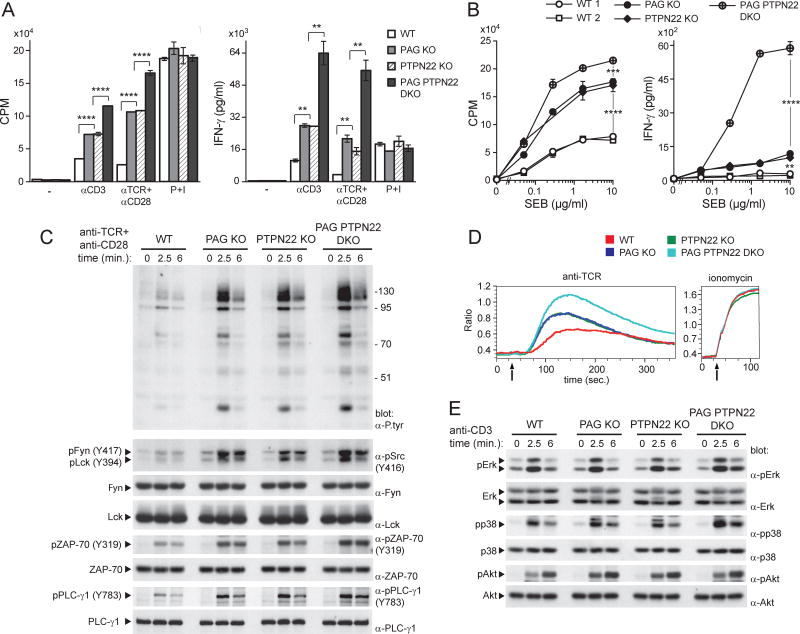
Figure 1. Enhanced responses of previously activated T cells in PAG-deficient mice.
(A). Purified CD4+ T cells from wild-type (WT) or PAG knock-out (KO) mice were activated in vitro with anti-CD3 plus anti-CD28 and expanded in IL-2. Expression of PAG was verified by immunoblotting of total cell lysates. (B). As in (A) except that cells were re-stimulated with anti-CD3 (0.05 µg/ml), anti-TCR (0.05 µg/ml) plus anti-CD28 (1 µg/ml) or P+I. Thymidine incorporation (expressed hereafter as CPM) and production of IFN-γ, IL-2 or IL-4 were monitored. Means with standard deviations (SD) of triplicate values are shown. (C). Same as in (B), except that cells were re-stimulated with the indicated concentrations of SEB and APCs. (D). Purified CD4+ T cells from TCR AND transgenic mice were first activated in vitro with anti-CD3 plus anti-CD28 and then re-stimulated with the indicated concentrations of PCC peptide and APCs, or with P+I. (E). Mice (two mice in each group) were immunized with OVA and adjuvant. After 9 days, CD4+ T cells were isolated and re-stimulated with the indicated concentrations of OVA and WT splenocytes, or P+I. After 4–5 days (for OVA re-stimulation) or 2 days (for P+I), production of IFN-γ was monitored, as detailed in (B). (F,G). CD4+ T cells activated in vitro with anti-CD3 plus anti-CD28 were infected with retroviruses encoding green fluorescent protein (GFP) alone, or in combination with WT PAG or PAG Y314F. After sorting of GFP-positive cells, expression of PAG was verified by immunoblotting of total cell lysates (F). Responsiveness to SEB was also examined, as detailed in (C) (G). *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. Representative of n > 25 (A), n = 4 (B), n = 6 (C), n = 4 (D), n = 3 (E) and n = 2 (F,G). Related to Figure S1.
To study functional responses, we focused on CD4+ T cells. Freshly isolated (“ex vivo”) PAG KO T cells stimulated with antibodies against CD3 (anti-CD3) alone, or anti-CD3 plus anti-CD28, showed normal proliferation and cytokine production, compared to wild-type T cells (Figure S1A). In striking contrast, previously activated PAG KO T cells, i.e. cells that have encountered a previous stimulation, demonstrated increased thymidine incorporation, as well as augmented secretion of interferon (IFN)-γ, interleukin (IL)-2 and IL-4, in comparison to wild-type T cells (Figure 1B). These effects were not seen with phorbol myristate acetate (PMA) and ionomycin (P+I), which trigger activation by bypassing proximal TCR signaling (Figure 1B).
The superantigen staphylococcal enterotoxin B (SEB), in the presence of APCs, triggers activation of Vβ8.2+ CD4+ T cells. Whereas freshly isolated PAG KO T cells did not have altered responses to SEB and APCs (Figure S1B), previously activated PAG KO T cells showed significantly augmented proliferation and IFN-γ production, compared to wild-type T cells (Figure 1C). No difference in abundance of Vβ8.2+ CD4+ T cells existed between wild-type and PAG KO mice (Table S1). Likewise, when PAG KO mice were bred with class II MHC-restricted pigeon cytochrome C (PCC)-specific TCR transgenic mice, freshly isolated PAG KO T cells did not show altered ability to respond to PCC peptide and APCs (Figure S1C). However, previously activated PAG KO T cells displayed increased IFN-γ secretion and, to a lesser extent, thymidine incorporation, in comparison to wild-type T cells (Figure 1D; Figure S1D).
The effect of PAG deficiency was also ascertained in an ex vivo antigen re-stimulation assay. Mice were immunized in the foot pad with ovalbumin (OVA) in the presence of adjuvant. After 9 days, CD4+ T cells from draining lymph nodes were re-stimulated in vitro with OVA and APCs. T cells from PAG KO mice displayed markedly increased OVA-induced proliferation and IFN-γ production (Figure 1E; Figure S1E).
To confirm that the increased responses of PAG KO T cells were due to PAG deficiency, PAG expression was reconstituted using retrovirus-mediated gene transfer of either wild-type PAG or a PAG mutant unable to bind Csk [PAG tyrosine 314-to-phenylalanine 314 (Y314F)]. Levels of expression of the two proteins were similar (Figure 1F). Wild-type PAG, but not PAG Y314F, corrected the enhanced SEB-induced proliferation and IFN-γ production of PAG KO T cells (Figure 1G; Figure S1F).
Hence, loss of PAG in CD4+ T cells augmented antigen receptor-mediated responses in previously activated, but not in freshly isolated, T cells. This effect was corrected by wild-type PAG, but not by a PAG mutant unable to bind Csk.
Increased TCR signaling in previously activated PAG-deficient T cells
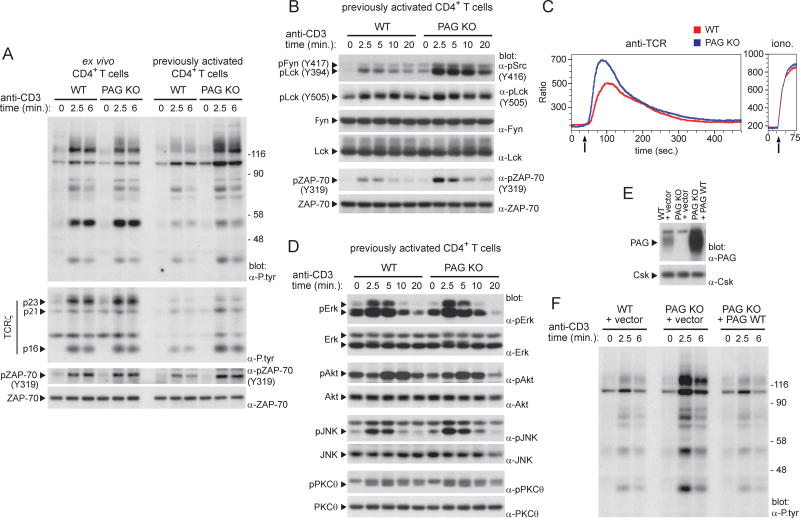
The impact of PAG deficiency on TCR signaling was assessed. Freshly isolated PAG KO T cells showed no alteration of TCR-triggered protein tyrosine phosphorylation, compared to wild-type T cells (Figure 2A). This was observed whether cells were triggered with anti-CD3 or anti-TCR (data not shown). There was also no change in activation of Erk, Akt and calcium fluxes (Figure S2A,B). In contrast, previously activated PAG KO T cells demonstrated prominently enhanced TCR-induced protein tyrosine phosphorylation, compared to wild-type T cells (Figure 2A). This effect involved all TCR-regulated substrates, including ITAMs and ZAP-70, as well as Lck and Fyn, which showed augmented phosphorylation at their activating tyrosine (Y394 for Lck and Y417 for Fyn) (Figure 2A,B; Figure S2C). The identity of Lck and Fyn in the phospho-Src kinase immunoblots was confirmed by immunodepletion (Figure S2D). Surprisingly, little or no appreciable effect was seen on phosphorylation of the inhibitory tyrosine of Lck, Y505 (Figure 2B). Previously activated PAG KO T cells also showed increased TCR-triggered calcium fluxes (Figure 2C). Nevertheless, they exhibited minimal or no increase in activation of Erk, Akt, c-Jun N-terminal kinase (JNK), PKC-θ, p38 kinase, and inhibitor of κBα (IκBα) (Figure 2D; Figure S2E). A quantitation of these findings is shown in Figure S2F.
Figure 2. Increased TCR signaling in previously activated PAG-deficient T cells.
(A). Ex vivo or previously activated CD4+ T cells from WT or PAG KO mice were stimulated for the indicated times with anti-CD3. Protein tyrosine phosphorylation was then detected by immunoblotting of total cell lysates with anti (α)-phosphotyrosine (P.tyr). An 8% gel was used to detect overall protein tyrosine phosphorylation (first panel). A 15% gel was used to detect phosphorylated ITAM-containing ζ chains (p23, p21, p16) (second panel). Phosphorylated ZAP-70 and total ZAP-70 were also detected (third and fourth panels) by immunoblotting with phospho-specific anti-ZAP-70, and anti-ZAP-70, respectively. The positions of prestained molecular mass markers are shown on the right. (B). Phosphorylation of protein tyrosine kinases in previously activated CD4+ T cells was assessed as detailed for (A), using phospho-specific antibodies. (C). After loading with Indo-1, previously activated T cells were stimulated with anti-TCR (left) or ionomycin (iono; right). Changes in intracellular calcium were assessed by measuring the ratio between calcium-bound Indo-1 and calcium-free Indo-1, using flow cytometry. Arrow indicates when the stimulus was added. (D). Same as (B), except that cell lysates were probed by immunoblotting with the indicated antibodies. (E,F). Previously activated CD4+ T cells were infected with retroviruses encoding GFP alone, or in combination with WT PAG. After sorting GFP-positive cells, expression of PAG was verified by immunoblotting of total cell lysates (E). Anti-CD3-induced protein tyrosine phosphorylation was also examined (F). Representative of n = 3 (A), n = 4 (B), n = 2 (C), n = 3 (D), n = 2 (E,F) Related to Figure S2.
To ensure that the increased TCR-evoked signals in PAG KO mice were due to PAG deficiency, wild-type PAG was re-introduced using retroviral infection (Figure 2E). Re-expression of PAG, but not of empty vector, corrected the augmented TCR-triggered protein tyrosine phosphorylation of PAG KO cells (Figure 2F).
Combined, these findings showed that PAG deficiency globally enhanced TCR signaling, including activation of Src kinases. As was the case for functional responses, these effects were observed in previously activated but not in freshly isolated, T cells.
PAG-deficient mice have enhanced susceptibility to experimental autoimmune encephalomyelitis
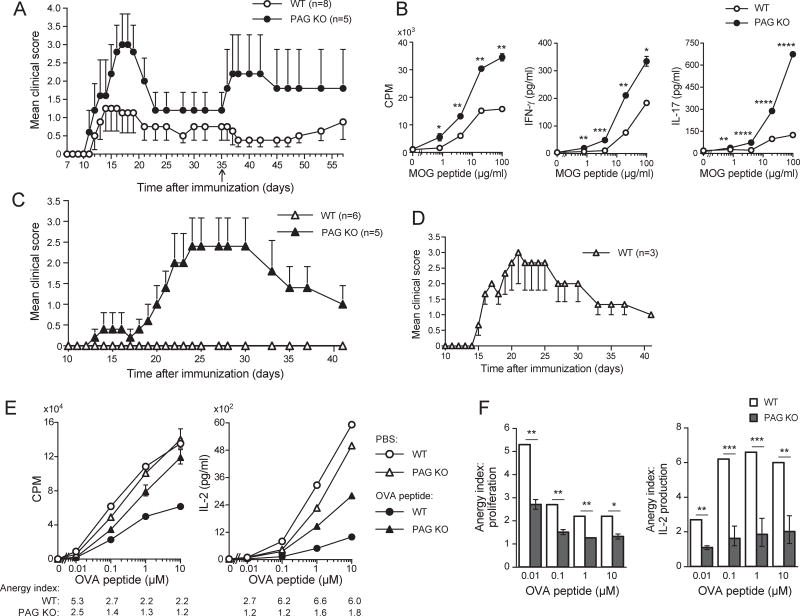
To validate in vivo the notion that PAG controls activation of previously activated cells, susceptibility to experimental autoimmune encephalomyelitis (EAE), a model of T cell-dependent autoimmunity, was examined. In this model, mice are immunized with a myelin oligodendrocyte glycoprotein (MOG)-derived peptide. MOG-specific effector CD4+ T cells subsequently migrate to the nervous system, where they are re-activated by endogenous MOG. PAG KO mice were much more susceptible to EAE than wild-type mice (Figure 3A). Moreover, after a boost immunization, given at a time when clinical signs of EAE have resolved, PAG KO mice developed the disease again, while wild-type mice were tolerant (Figure 3A).
Figure 3. Increased susceptibility to EAE and resistance to anergy in mice lacking PAG.
(A). WT or PAG KO mice (5–8/group) were immunized with MOG peptide in the presence of adjuvant, followed by pertussis toxin. Then, they were followed for neurological deficits. A boost immunization was also given at day 35 (arrow). Means of clinical scores with one-sided standard errors of means (SEM) are depicted. p = 0.002. (B). CD4+ T cells were purified from spleen of MOG-immunized mice. They were then re-stimulated in vitro with the indicated concentrations of MOG peptide and WT splenocytes. Proliferation and cytokine production were analyzed. Means with SD are shown. (C). As in (B), except that T cells were transferred into RAG-1 KO mice (5–6/group), and induction of EAE was monitored as detailed in Experimental Procedures. Means of clinical scores with one-sided SEM are depicted. (D). Same as (C), except that three times greater numbers of T cells from WT mice were transferred. (E,F). OVA-specific OT-II TCR transgenic mice were injected intravenously (I.V.) twice with soluble OVA peptide or PBS alone. After 10 days, splenic CD4+ CD44hi Vα2+ T cells were isolated by cell sorting, and re-stimulated with the indicated concentrations of OVA peptide and APCs. Proliferation and IL-2 secretion were monitored. The anergy index is the ratio of thymidine incorporation or IL-2 secretion between PBS-injected and OVA peptide-injected mice. Means with SD of triplicate values are depicted (E). Anergy indices are represented graphically (F). *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. Representative of n = 2 (A), n = 2 (B), n = 2 (C), n = 2 (D), n = 2 (E,F). Related to Figure S3.
To confirm that the increased susceptibility to EAE was due to functional differences in CD4+ T cells, an ex vivo antigen re-stimulation assay was performed. Re-stimulation of PAG KO CD4+ T cells with MOG peptide in the presence of APCs, resulted in greater thymidine incorporation, as well as augmented secretion of IFN-γ and IL-17, two cytokines which have a key pathogenic role in EAE (Figure 3B; Figure S3A). Adoptive transfer experiments were also performed. CD4+ T cells were purified from MOG-immunized mice, transferred into RAG-1 KO mice and their ability to induce EAE was monitored. Mice injected with PAG KO T cells displayed a greater severity of EAE than mice injected with wild-type T cells (Figure 3C). The poor induction of EAE by wild-type T cells was not due to lack of functionality, as EAE was induced when 3 times more wild-type T cells were transferred (Figure 3D).
Therefore, loss of PAG increased sensitivity of mice to EAE. Moreover, it decreased tolerance to EAE. These effects were T cell-intrinsic.
Increased resistance to T cell anergy in PAG-deficient mice
Our previous studies showed that T cells from transgenic mice overexpressing PAG had decreased resistance to anergy, compared to wild-type T cells (Davidson et al., 2007). However, a role of PAG in anergy had not been supported by earlier studies of PAG KO mice (Dobenecker et al., 2005; Xu et al., 2005). To clarify the role of PAG in anergy, PAG KO mice were tested in two models of T cell anergy. First, effector CD4+ T cells, generated by stimulation with anti-CD3 plus anti-CD28, were anergized in vitro by treatment with anti-CD3 alone. Anergy induction was assessed by monitoring proliferation and IL-2 secretion after re-stimulation with anti-TCR plus anti-CD28. In wild-type T cells, anti-CD3 treatment suppressed subsequent T cell responses, as reflected by an “anergy index” above 1.0. However, the opposite occurred in PAG KO T cells; anti-CD3 enhanced subsequent T cell responses, resulting in an anergy index below 1.0 (Figure S3B,C).
Second, anergy was induced in vivo by injecting soluble OVA peptide into OVA-specific OT-II TCR transgenic mice. CD4+ T cells were subsequently isolated, and tested by re-stimulation with OVA peptide and APCs. Injection of soluble OVA peptide suppressed subsequent T cell responses in wild-type mice. This effect was much weaker in PAG KO mice, as reflected by the lower anergy indices (Figure 3E,F).
Thus, PAG KO mice displayed increased resistance to anergy in the two experimental models tested.
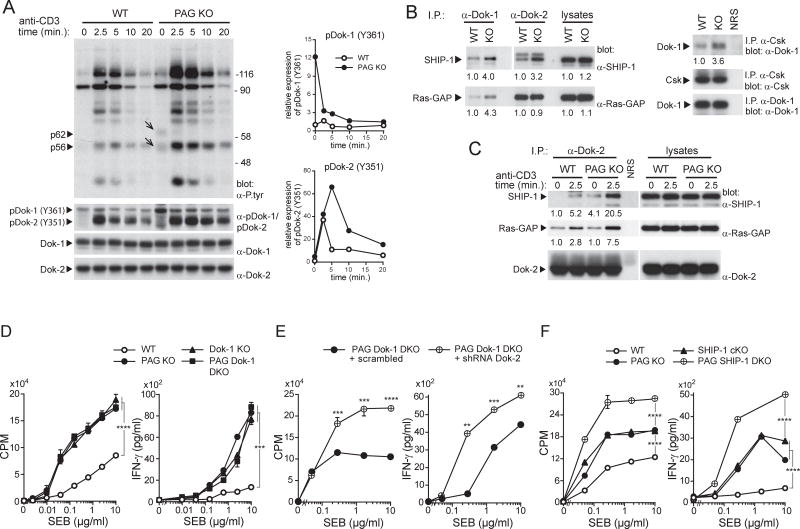
Increased association of Csk with PTPN22 in PAG-deficient T cells
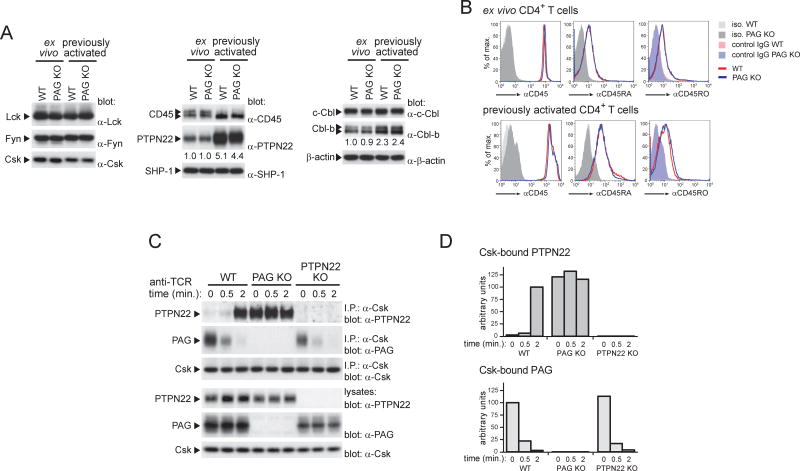
The finding that the alterations of T cell functions in PAG KO mice were milder than those in mice lacking Csk (Schmedt et al., 1998) suggested that compensatory mechanisms might be limiting TCR signaling in PAG KO mice. To address this idea, we tested the impact of PAG deficiency on expression of various TCR signaling molecules. PAG KO T cells displayed no change in expression of Lck, Fyn, Csk, CD45, PTPN22, SHP-1 or ubiquitin ligases c-Cbl and Cbl-b, compared to wild-type T cells both in freshly isolated and in previously activated T cells (Figure 4A,B). Of note, however, previously activated T cells displayed 4- to 5-fold higher levels of PTPN22 and 2.0- to 2.5-fold higher levels of Cbl-b, than freshly isolated T cells (Figure 4A). Moreover, in protein gels, CD45 migrated as a doublet in freshly isolated T cells, but as a single species in previously activated T cells, likely reflecting variations in CD45 isoforms (Figure 4A,B) (Rhee and Veillette, 2012; Zikherman and Weiss, 2008).
Figure 4. Increased association of Csk with PTPN22 in PAG-deficient T cells.
(A,B). The expression levels of various regulators of TCR signaling in ex vivo or previously activated CD4+ T cells from WT or PAG KO mice were analyzed by immunoblotting of total cell lysates with the indicated antibodies. Relative levels of expression are shown below each panel (A). Expression of CD45 was also assessed by flow cytometry, using antibodies recognizing all CD45 isoforms, CD45RA or CD45RO; iso.: isotype control (B). (C,D). The association of Csk with PTPN22 or PAG was assessed in previously activated CD4+ T cells, in the absence or the presence of stimulation by anti-TCR, using immunoprecipitation (C). A quantitation of the extent of Csk-PTPN22 and Csk-PAG association is shown (D). T cells from PTPN22 KO mice were analyzed as control. Representative of n = 2–6, depending on protein (A), n = 2 (B) and n = 2 (C).
We examined the association of Csk with PTPN22, which cooperates with Csk in inhibition of TCR signaling (Rhee and Veillette, 2012). In wild-type T cells, Csk was minimally associated with PTPN22 prior to TCR triggering (Figure 4C,D). This interaction was markedly increased by TCR stimulation. Conversely, Csk was strongly associated with PAG prior to TCR stimulation and this association was decreased by TCR stimulation (Figure 4C,D) as previously reported (Brdicka et al., 2000; Davidson et al., 2003). In PAG KO T cells, however, the association of Csk with PTPN22 was markedly enhanced (more than 10-fold) in unstimulated cells, compared to wild-type T cells (Figure 4C,D). This association was not affected by TCR stimulation.
Thus, in PAG-deficient T cells, expression of various components of the TCR signaling machinery was not altered. Nonetheless, the Csk-PTPN22 interaction was constitutively augmented.
PTPN22 cooperates with PAG to suppress effector T cell responses
To assess whether PTPN22 might attenuate the impact of PAG deficiency in T cells, mice lacking both PAG and PTPN22 (PAG PTPN22 DKO) were generated and analyzed in parallel with mice lacking PAG or PTPN22 alone (Figure S4A). Contrary to PAG KO or PTPN22 KO T cells, freshly isolated PAG PTPN22 DKO T cells displayed a small increase in proliferation and IFN-γ production in response to anti-CD3, anti-TCR plus anti-CD28 or SEB, when compared to wild-type T cells (Figure S4B,C). A small increase in TCR-triggered protein tyrosine phosphorylation was also observed (Figure S4D).
More strikingly, however, when previously activated T cells were studied, PAG PTPN22 DKO cells exhibited markedly enhanced proliferation and IFN-γ secretion in response to these stimuli, compared to PAG KO or PTPN22 KO cells (Figure 5A,B). They also exhibited greater TCR-triggered protein tyrosine phosphorylation and calcium fluxes (Figure 5C,D; Figure S4E). There was little or no effect on Erk, p38 and Akt (Figure 5E; Figure S4E). In keeping with an earlier report (Hasegawa et al., 2004), previously activated PTPN22 KO T cells, but not freshly isolated PTPN22 KO T cells, also displayed enhanced TCR-triggered protein tyrosine phosphorylation, calcium fluxes and effector responses, compared to wild-type T cells (Figure 5A–D). These changes were analogous to those seen in PAG KO T cells.
Figure 5. PTPN22 cooperates with PAG to suppress the responses of previously activated T cells.
(A). Previously activated CD4+ T cells from WT, PAG KO, PTPN22 KO or PAG PTPN22 DKO mice were stimulated with the indicated antibodies or P+I, as in Figure 1A. Proliferation and IFN-γ production were monitored. Means with SD of triplicates are shown. (B). Same as in (A), except that cells were re-stimulated with the indicated concentrations of SEB and APCs. (C). Previously activated CD4+ T cells from the indicated mice were stimulated for the indicated times with anti-TCR plus anti-CD28. Phosphorylation was then detected by immunoblotting of total cell lysates with antibodies against P.tyr (first panel), activated Src kinases (second panel), activated ZAP-70 (fifth panel) or tyrosine phosphorylated phospholipase C (PLC)-γ (seventh panel). The positions of molecular mass markers are shown on the right. (D). Calcium fluxes in previously activated T cells were analyzed as detailed for Figure 2C. (E). Same as in (C), except that total cell lysates were probed by immunoblotting with the indicated antibodies. **p<0.01; ***p<0.001; ****p<0.0001. Representative of n = 2 (A), n = 2 (B), n = 3 (C), n = 2 (D) and n = 3 (E). Related to Figure S4.
Therefore, loss of PTPN22 accentuated the impact of PAG deficiency in previously activated CD4+ T cells, seemingly by further enhancing overall TCR-triggered protein tyrosine phosphorylation.
Augmented tyrosine phosphorylation of Dok adaptors in PAG-deficient T cells
We noted that previously activated PAG KO T cells exhibited augmented tyrosine phosphorylation of two proteins, p62 and p56, compared to wild-type T cells (Figure 6A). These proteins might represent Dok-1 and Dok-2, two inhibitory adaptor molecules associating with Csk, and with other inhibitory effectors such as lipid phosphatase SHIP-1 and Ras-GTPase activating protein (Ras-GAP) (Veillette et al., 2002). Immunoblotting with phospho-specific antibodies jointly recognizing Dok-1 and Dok-2 showed that Dok-1 and Dok-2 were indeed hyperphosphorylated in PAG KO T cells (Figure 6A). For Dok-1, the increase was prominent in unstimulated T cells and was attenuated by TCR stimulation. For Dok-2, the enhancement was minimal in unstimulated T cells and was accentuated by TCR stimulation (Figure 6A). These findings were confirmed by immunoprecipitation (Figure S5A).
Figure 6. Dok family adaptors cooperate with PAG to suppress responses of previously activated T cells.
(A) Previously activated CD4+ T cells from WT or PAG KO mice were stimulated with anti-CD3. Overall protein tyrosine phosphorylation, including tyrosine phosphorylation of two proteins of 62 (p62) and 56 (p56) kDa (shown by arrows), is depicted in the first panel (left). Tyrosine phosphorylation of Dok-1 and Dok-2 was examined by immunoblotting of total cell lysates with a phospho-specific antibody recognizing Dok-1 and Dok-2 (second panel). The positions of molecular mass markers are shown on the right. A quantitation of relative tyrosine phosphorylation of Dok-1 and Dok-2 is depicted on the right. (B,C). Lysates of unstimulated previously activated CD4+ T cells from WT or PAG KO mice were immunoprecipitated with anti-Dok-1, anti-Dok-2 or normal rabbit serum (NRS) (left) and probed by immunoblotting with anti-SHIP-1 or anti-Ras-GAP antibodies. Alternatively, lysates were immunoprecipitated with antibodies against Csk and probed by immunoblotting with anti-Dok-1 (right). Quantitation of extent of association is shown below each panel. (C). Same as (B), except that cells were stimulated or not with anti-CD3. Association of Dok-2 with SHIP-1 and Ras-GAP was analyzed. (D). Previously activated CD4+ T cells from WT, PAG KO, Dok-1 KO or PAG Dok-1 DKO mice were stimulated with SEB and APCs. Proliferation and IFN-γ production were monitored. Means of triplicates with SD are shown. (E). Same as (D), except that PAG Dok-1 DKO cells were transduced with scrambled or Dok-2-specific shRNAs. (F). Same as (D), except that previously activated T cells from WT, PAG KO, SHIP-1 KO or PAG SHIP-1 DKO mice were studied. **p<0.01; ***p<0.001; ****p<0.0001. Representative of n = 3 (A), n = 4 (B), n = 4 (C), n = 3 (D), n = 3 (E) and n = 2 (F). Related to Figures S5 and S6.
Immunoblotting of Dok-1 or Dok-2 immunoprecipitates with anti-SHIP-1 or anti-Ras-GAP antibodies revealed that Dok-1 and Dok-2 were more extensively associated with SHIP-1 and Ras-GAP in PAG KO T cells, compared to wild-type T cells (Figure 6B). For Dok-2, this effect was enhanced by TCR stimulation (Figure 6C). In addition, Dok-1 was more associated with Csk in PAG KO T cells (Figure 6B).
Therefore, loss of PAG resulted in enhanced tyrosine phosphorylation of Dok-1 and Dok-2, as well as augmented association of these adaptors with Csk, SHIP-1 and Ras-GAP, three known inhibitory molecules.
Dok adaptors and SHIP-1 cooperate with PAG to inhibit effector T cell responses
To test whether the Dok adaptors might restrict the influence of PAG deficiency, mice lacking both PAG and Dok-1 (PAG Dok-1 DKO) were generated and analyzed together with mice lacking PAG or Dok-1 alone (Figure S5B). Like PAG KO T cells, Dok-1 KO T cells displayed enhanced T cell responses in previously activated T cells, but not in freshly isolated T cells (Figure 6D; Figure S5C,D). However, PAG Dok-1 DKO T cells did not show greater increases in T cell responses than PAG KO or Dok-1 KO T cells (Figure 6D; Figure S5C,D). To probe the involvement of Dok-2, PAG KO T cells or PAG Dok-1 DKO T cells were infected with retroviruses encoding small hairpin (sh) RNAs against Dok-2. This resulted in a ~80% reduction of Dok-2 expression (Figure S6A). Compared to previously activated PAG Dok-1 DKO T cells transduced with scrambled shRNAs, those transduced with Dok-2 shRNAs demonstrated significantly augmented SEB-induced proliferation and IFN-γ production (Figure 6E). A small increase in TCR-triggered protein tyrosine phosphorylation was also seen (Figure S6B). These effects were not seen in Dok-2 shRNAs-transduced PAG KO T cells (Figure S6C,D). These observations implied that loss of both Dok-1 and Dok-2, but not of either adaptor alone, increased the impact of PAG deficiency in previously activated T cells.
Given that SHIP-1 is a prime binding partner of members of the Dok family (Veillette et al., 2002), we tested the possibility that SHIP-1 was involved in the compensation effected by the Dok adaptors in PAG KO T cells by analyzing PAG SHIP-1 DKO T cells (Figure S6E). Previously activated PAG SHIP-1 DKO T cells showed greater SEB-triggered proliferation and cytokine production than PAG KO or SHIP-1 KO T cells (Figure 6F). TCR-triggered calcium fluxes, a known target of SHIP-1-mediated inhibition, were also enhanced (Figure S6F).
Hence, combined loss of Dok-1 and Dok-2 further enhanced the responses of previously activated PAG KO T cells. A similar effect was seen with loss of SHIP-1, a known effector of Dok-mediated inhibition.
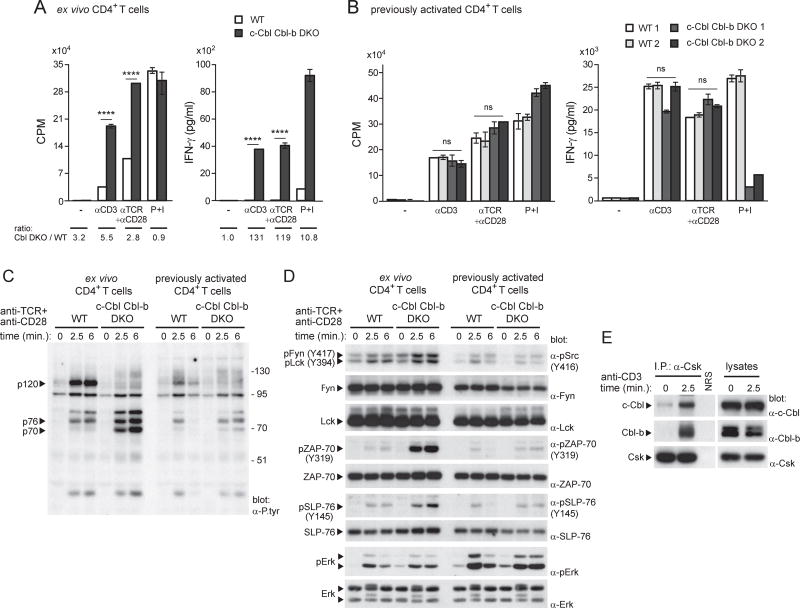
Loss of Cbl ubiquitin ligases enhances responses in freshly isolated T cells
The finding that PAG deficiency had no appreciable effect on responses in freshly isolated T cells (Dobenecker et al., 2005; Xu et al., 2005) (this report) implied that alternative mechanisms were responsible for inhibiting TCR signaling in these cells. Although many candidates such as other PTPs and various ubiquitin ligases may be involved, we focused on the Cbl ubiquitin ligases, which cause degradation of tyrosine phosphorylated substrates, in particular ZAP-70 (Huang and Gu, 2008; Thien and Langdon, 1998; Veillette et al., 2002; Zhang et al., 2014). To test this idea, T cell responses were examined in mice lacking c-Cbl and Cbl-b, the two major Cbl proteins expressed in T cells (Figure S7A). Unlike PAG KO mice, c-Cbl Cbl-b DKO mice showed a marked enhancement of TCR-evoked proliferation and IFN-γ secretion in freshly isolated T cells, compared to wild-type mice (Figure 7A). Similar results were obtained with naive CD4+ T cells purified by cell sorting (Figure S7B). This was important, because c-Cbl Cbl-b DKO mice had a marked increase in the proportion of effector-memory T cells at steady state (Figure S7C). Significantly and in contrast, little or no difference in TCR-evoked proliferation and IFN-γ secretion was seen in previously activated c-Cbl Cbl-b DKO T cells, compared to wild-type T cells (Figure 7B).
Figure 7. Cbl ubiquitin ligases inhibit responses of freshly isolated T cells.
(A). Freshly isolated CD4+ T cells from WT or c-Cbl Cbl-b DKO mice were activated in vitro with anti-CD3 (3µg/ml), anti-TCR (3µg/ml), plus anti-CD28 (1µg/ml), or P+I. Proliferation and IFN-γ production were monitored. Means with SD of triplicates are shown. Ratio of values observed in c-Cbl Cbl-b DKO (Cbl DKO) T cells, compared to WT T cells, are shown at the bottom. (B). Same as in (A), except that previously activated T cells were analyzed with anti-CD3 (0.1µg/ml), anti-TCR (0.1µg/ml), plus anti-CD28 (1µg/ml). Two mice of each genotype were studied. (C). Freshly isolated or previously activated CD4+ T cells from WT or c-Cbl Cbl-b DKO mice were stimulated with anti-TCR plus anti-CD28. Overall protein tyrosine phosphorylation was analyzed by anti-P.tyr immunoblotting. The positions of the substrates showing marked changes in tyrosine phosphorylation between WT and c-Cbl Cbl-b DKO are indicated on the left and that of molecular mass markers on the right. (D). Same as in (C), except that phosphorylation of specific substrates was analyzed using phospho-specific antibodies. (E). WT T cells were stimulated or not with anti-CD3. After lysis, Csk was immunoprecipitated and probed by immunoblotting with antibodies against c-Cbl or Cbl-b. NRS: normal rabbit serum. ****p<0.0001; ns: not significant. Representative of n = 2 (A), n = 2 (B), n = 2 (C), n = 2 (D) and n = 2 (E). Related to Figure S7.
Freshly isolated, but not previously activated, c-Cbl Cbl-b DKO T cells also displayed a pronounced increase in TCR-triggered protein tyrosine phosphorylation (Figure 7C; Figure S7D). Unlike PAG KO T cells, this effect was restricted to a subset of TCR-regulated substrates, especially p70 and p76. These substrates likely represented ZAP-70 and SLP-76, a known ZAP-70 substrate, as revealed by immunoblotting with phospho-specific antibodies (Figure 7D; Figure S7D).
Interestingly, freshly isolated c-Cbl Cbl-b DKO T cells also displayed increased phosphorylation of Fyn and, to a lesser extent, Lck at their activating tyrosine (Figure 7D; Figure S7D), raising the possibility that the Cbl proteins were also inhibiting, directly or indirectly, the function of Src kinases. Indeed, a previous study has shown that c-Cbl induced degradation of activated Fyn in T cells (Andoniou et al., 2000). However, as Cbl proteins can behave not only as ubiquitin ligases but also as adaptors (Huang and Gu, 2008; Thien and Langdon, 1998; Veillette et al., 2002; Zhang et al., 2014), it was possible that the Cbl proteins were inhibiting Fyn also by recruiting Csk. In keeping with this possibility, Csk co-immunoprecipitated with both c-Cbl and Cbl-b (Figure 7E). The extent of this association was much greater in TCR-stimulated T cells than in unstimulated T cells.
Therefore, unlike PAG deficiency, loss of Cbl proteins caused increased T cell activation responses in freshly isolated or naive, but not in previously activated, T cells. This effect correlated with augmented tyrosine phosphorylation of selected TCR-regulated substrates, in particular ZAP-70, SLP-76 and Fyn.
Discussion
In this report, we found that PAG has a prominent impact on secondary T cell responses. Previously activated, but not freshly isolated, CD4+ T cells from PAG-deficient mice had increased proliferation and cytokine production in response to anti-TCR complex antibodies, superantigen or antigen, compared to cells from wild-type mice. These alterations were corrected by re-expression of wild-type PAG, but not of a PAG mutant unable to interact with Csk (PAG Y314F), implying that they were likely due to compromised Csk recruitment. PAG KO mice also displayed augmented susceptibility to EAE. Thus, PAG is a critical negative regulator of T cell activation in vitro and in vivo. This role had not been appreciated in earlier studies of PAG KO mice, given that only freshly isolated T cells had been analyzed (Dobenecker et al., 2005; Xu et al., 2005).
While a role for PAG in anergy was not recognized in earlier studies of PAG KO mice (Dobenecker et al., 2005; Xu et al., 2005), our previous studies of transgenic mice overexpressing PAG suggested that PAG promotes T cell anergy (Davidson et al., 2007), possibly reflecting its ability to inhibit signaling by co-stimulatory receptors, which prevent anergy. Herein, we observed that loss of PAG markedly reduced anergy induced in vitro by anti-CD3, and in vivo by soluble OVA peptides. The reason why PAG KO mice showed no defect in the anergy models evaluated by the other groups remains to be elucidated.
Previously activated, but not freshly isolated, PAG KO T cells also displayed globally enhanced TCR-triggered protein tyrosine phosphorylation and calcium fluxes, compared to wild-type T cells. These effects were accompanied by augmented phosphorylation of the activating tyrosine of Src kinases Lck and Fyn and were corrected by re-expression of wild-type PAG. Therefore, the increased functional responses of PAG KO T cells were likely due to hyperactive Src kinases, the targets of Csk. However, PAG KO T cells showed little or no appreciable decrease in phosphorylation of the inhibitory tyrosine of Lck (Y505), the immediate target of Csk. Possibly the pool of TCR-activated Lck, which is regulated by PAG and Csk, is too small to be discriminated from the total pool of Lck. Alternatively, when activated by loss of Y505 phosphorylation in the absence of PAG or Csk, Lck molecules may rapidly re-phosphorylate at Y505.
Unlike protein tyrosine phosphorylation and calcium fluxes, PAG KO T cells had little or no enhancement of TCR-mediated activation of downstream effectors such as Erk, Akt, JNK, PKC-θ, p38 and IκBα. A similar observation was made with PTPN22 KO T cells (Hasegawa et al., 2004; Tan et al., 2014) (this report). In the absence of PAG or PTPN22, a rate-limiting step or negative feedback mechanism may be restricting coupling to these downstream effectors. Interestingly, uncoupling to the Erk pathway was also observed in T cells in which Csk was inactivated pharmacologically (Tan et al., 2014). It was proposed that the actin cytoskeleton physically restricted coupling of protein tyrosine phosphorylation to the Erk pathway. A related mechanism may be operational in PAG KO or PTPN22 KO T cells.
Compared to wild-type T cells, PAG KO T cells displayed increased association of Csk with its partner PTPN22. This increase might occur because loss of PAG releases a pool of Csk that becomes available to bind PTPN22. Although PAG and PTPN22 interact with distinct domains of Csk (Veillette et al., 2002), PAG may restrict the accessibility of Csk through steric hindrance, physical competition, conformational modification or spatial sequestration. There may also be a hierarchy in binding of Csk to its partners, based on their abundance, phosphorylation state or affinity. Indeed, PAG KO T cells exhibited enhanced tyrosine phosphorylation of Dok-1 and Dok-2 and increased association of these adaptors with Csk, and also with SHIP-1 and Ras-GAP. Our observations suggested that PTPN22 and Dok adaptors cooperate with PAG to restrict responses of previously activated T cells. This notion was firmly established by analyses of mice lacking PAG in combination with PTPN22 or Dok adaptors.
What is the purpose of having multiple Csk partners to control activation of previously activated T cells? We postulate that this network enables recruitment of Csk to multiple cellular locales, where Src kinases are positioned, and may be needed for efficient control of T cell activation. PAG may recruit Csk to lipid rafts, while PTPN22 may bring it to tyrosine phosphorylated ITAMs and ZAP-70, which are targets of PTPN22 (Cloutier and Veillette, 1996). Likewise, Dok adaptors may recruit Csk to areas of activation of phosphatidylinositol 3’ kinase, which triggers recruitment of Dok adaptors by way of their pleckstrin homology domain (Veillette et al., 2002).
Unlike previously activated PAG KO T cells, there was no increase in T cell responses in freshly isolated PAG KO T cells. A similar phenomenon was observed in PTPN22 KO and Dok-1 KO T cells (Hasegawa et al., 2004) (this report). In the case of PTPN22, only stimulation by weak agonists revealed an impact of PTPN22 deficiency in freshly isolated T cells (Salmond et al., 2014). Hence, we presume that inhibitors other than PAG, PTPN22 and Dok adaptors are suppressing activation of naive T cells. These inhibitors could provide alternative mechanisms to recruit Csk to the membrane or could even suppress T cell activation independently of Csk.
One candidate for controlling TCR-mediated responses in freshly isolated T cells is the Cbl proteins. Indeed, we observed that c-Cbl Cbl-b DKO mice displayed enhanced activation responses in freshly isolated, but not in previously activated, T cells. This function may relate to the ability of Cbl proteins to cause degradation of key effectors of TCR signaling, such as ZAP-70 and Fyn, or to act as adaptors recruiting other inhibitory molecules, including Csk (Huang and Gu, 2008; Thien and Langdon, 1998; Veillette et al., 2002; Zhang et al., 2014). In support of the latter idea, we found that Csk co-immunoprecipitated with the Cbl proteins, in particular in TCR-stimulated T cells. In addition to Cbl proteins, it is likely that other Csk-interacting proteins also contribute to inhibition of naive T cells. These notions are compatible with previous studies in which Csk was acutely inhibited in freshly isolated T cells (Tan et al., 2014). Unlike Cbl deficiency, inactivation of Csk resulted in a more global enhancement of protein tyrosine phosphorylation. Furthermore, it is possible that the network of Csk binding partners might differ between freshly isolated and previously activated T cells.
In summary, our data provided evidence that PAG is a critical negative regulator of TCR signaling and T cell activation in effector T cells. This function seemingly relates to the ability of PAG to recruit Csk to the plasma membrane, where Src kinases are located. In this function, PAG cooperates with other Csk-interacting proteins, namely PTPN22 and Dok adaptors. In contrast, PAG has no appreciable role in naive T cells, likely due to the ability of other negative regulators, including the Cbl proteins, to control TCR signaling in these cells. The involvement of PAG in the control of effector T cell activation may be crucial to enable faster suppression of effector T cell responses, compared to naïve T cell responses. Given their role in the control of effector T cell activation, it is also attractive to speculate that not only PTPN22, as reported (Bottini and Peterson, 2014), but also PAG and Dok adaptors, may be implicated in the pathogenesis of disorders involving repeated or sustained T cell responses in humans, such as autoimmunity.
Experimental procedures
Mice
C57BL/6J, B10.BR and RAG-1 KO (Rag1−/−) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). PAG KO (Pag1−/−) mice, PTPN22 KO (Ptpn22−/−) mice, Dok-1 KO (Dok1−/−) mice, mice carrying a conditional allele of SHIP-1 (Inpp5dfl/fl), and mice lacking c-Cbl and Cbl-b in T cells (Cblfl/fl; Cd4-Cre; Cblb−/−) have been described elsewhere (Davidson et al., 2003; Kitaura et al., 2007; Lindquist et al., 2011; Tarasenko et al., 2007; Yang and Seed, 2003). Mice expressing the Cre recombinase under the control of the CD4 promoter (Cd4-Cre) were from Taconic (Hudson, NY). Transgenic mice expressing class II MHC-restricted TCR OT-II or AND were also reported (Barnden et al., 1998; Kaye et al., 1989). All mice were maintained in the C57BL/6 background. Littermates were used as controls in all experiments. Animal experimentation was done in accordance to the Canadian Council of Animal Care and approved by the IRCM Animal Care Committee.
Cells
Freshly isolated CD4+ T cells were obtained from spleen using a StemCell Purification Kit (Stem Cell Technology Inc., Vancouver, BC, Canada) or a Dynabead CD4+ T cell enrichment kit (ThermoFisher Scientific, Waltham, MA). Cell purity was consistently greater than 90% (data not shown). In some experiments, naive CD4+ T cells were purified by cell sorting. Purity was greater than 99%. Previously activated CD4+ T cells were generated by stimulating purified cells with anti-CD3 (3 µg/ml) immobilized on plastic and soluble anti-CD28 (1 µg/ml) for 2 days, then expanding in IL-2 (50 units/ml) for 3 days.
T cell activation assays
CD4+ T cells were activated with anti-CD3, or anti-TCR with or without anti-CD28. For stimulation with superantigen, CD4+ T cells were incubated with the indicated concentrations of SEB (Sigma-Aldrich, St. Louis, MO), in the presence of irradiated splenocytes from C57BL/6 mice. For stimulation with antigenic peptides, T cells were activated with the indicated concentrations of OVA peptide (323–339) or PCC peptide (88–104), in the presence of irradiated splenocytes from C57BL/6 or B10.BR mice, respectively. As control, cells were activated with PMA (100 ng/ml) and ionomycin (1 mM). Proliferation was measured by [3H]-thymidine incorporation and cytokine production was assessed by ELISA (R&D Systems, Minneapolis, MN). All assays were done in triplicate.
Immunization with ovalbumin
Mice were immunized in the foot pad with OVA protein (100 µg; Sigma-Aldrich) in the presence of complete Freund adjuvant (CFA; Sigma-Aldrich). After 9 days, CD4+ T cells were isolated from popliteal lymph nodes and re-stimulated in vitro with the indicated concentrations of OVA and irradiated splenocytes, or with P+I. Proliferation and cytokine production were determined.
Biochemical assays
Cells were stimulated for the indicated times at 37°C with biotinylated anti-CD3 or anti-TCR, with or without anti-CD28, and avidin. After lysis in maltoside-containing buffer supplemented with protease and phosphatase inhibitors, lysates were processed for immunoprecipitation or immunoblotting (Davidson et al., 2003; Davidson et al., 2007; Davidson et al., 2010). Quantification of blots was performed with the ImageJ software.
EAE
Mice were injected sub-cutaneously with MOG peptide 35–55 in CFA plus Mycobacterium tuberculosis H37Ra (Difco Laboratories, Detroit, MI), followed 24 and 72 hrs later by injection of pertussis toxin as described (Davidson et al., 2010). In some experiments, a boost was administered at day 35. Mice were scored regularly for neurological deficits as follows: 0: no clinical signs; 1: loss of tail tonicity; 2: flaccid tail; 3: hind leg paralysis; 4: hind leg paralysis with hind body paresis; and 5: hind and fore leg paralysis. After 6 weeks, splenic CD4+ T cells were re-stimulated in vitro with MOG peptide plus irradiated splenocytes or with P+I. For adoptive transfer, CD4+ T cells from MOG-immunized mice were purified 10 days after immunization, and re-stimulated in vitro with MOG peptide plus irradiated splenocytes in the presence of IL-23 (10 ng/ml) and anti-IFN-γ (10 µg/ml) to expand MOG-specific TH17 cells. After 5 days, T cells were injected in RAG-1 KO mice. 24 hrs later, pertussis toxin was injected in the recipient mice. Mice were then monitored for EAE.
Statistical analyses
Prism 6 software was used for unpaired or paired Student’s t-tests (two-tailed), non-parametric Wilcoxon matched-pairs t-test, or one-way ANOVA followed by Tukey's or Dunnet's multiple comparison test when appropriate.
Supplementary Material
Acknowledgments
We thank the members of the Veillette lab for discussions. This work was supported by a grant from the Canadian Institutes of Health Research (CIHR; FRN #143338) to A.V. A.V. holds the Canada Research Chair in Signaling in the Immune System.
Footnotes
Accession Numbers
Flow cytometry data are deposited in Flow Repository Database (http://flowrepository.org/) with accession numbers FR-FCM-ZZVL and FR-FCM-ZZVR.
Authors’ contribution
Conceptualization: D.D., A.V.; Methodology: D.D., A.V., M.-C.Z.; Investigation and validation: D.D, M.-C.Z.; Writing: original draft: A.V., D.D.; Writing - review, editing: all authors; Funding: A.V.; Resources: A.V., P.P.P., S.B., R.X., B.S., X.L., H.G.; Supervision: A.V.
Competing financial interests
The authors declare no competing financial interest.
References
- Andoniou CE, Lill NL, Thien CB, Lupher ML, Jr, Ota S, Bowtell DD, Scaife RM, Langdon WY, Band H. The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation. Mol Cell Biol. 2000;20:851–867. doi: 10.1128/mcb.20.3.851-867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. doi: 10.1046/j.1440-1711.1998.00709.x. [DOI] [PubMed] [Google Scholar]
- Bottini N, Peterson EJ. Tyrosine phosphatase PTPN22: multifunctional regulator of immune signaling, development, and disease. Annu Rev Immunol. 2014;32:83–119. doi: 10.1146/annurev-immunol-032713-120249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brdicka T, Pavlistova D, Leo A, Bruyns E, Korinek V, Angelisova P, Scherer J, Shevchenko A, Hilgert I, Cerny J, et al. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow LM, Fournel M, Davidson D, Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993;365:156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- Cloutier JF, Veillette A. Association of inhibitory tyrosine protein kinase p50csk with protein tyrosine phosphatase PEP in T cells and other hemopoietic cells. EMBO J. 1996;15:4909–4918. [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Bakinowski M, Thomas ML, Horejsi V, Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol Cell Biol. 2003;23:2017–2028. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Schraven B, Veillette A. PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes. Mol Cell Biol. 2007;27:1960–1973. doi: 10.1128/MCB.01983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson D, Shi X, Zhong MC, Rhee I, Veillette A. The phosphatase PTP-PEST promotes secondary T cell responses by dephosphorylating the protein tyrosine kinase Pyk2. Immunity. 2010;33:167–180. doi: 10.1016/j.immuni.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Dobenecker MW, Schmedt C, Okada M, Tarakhovsky A. The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Mol Cell Biol. 2005;25:10533–10542. doi: 10.1128/MCB.25.23.10533-10542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303:685–689. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- Hrdinka M, Horejsi V. PAG--a multipurpose transmembrane adaptor protein. Oncogene. 2014;33:4881–4892. doi: 10.1038/onc.2013.485. [DOI] [PubMed] [Google Scholar]
- Huang F, Gu H. Negative regulation of lymphocyte development and function by the Cbl family of proteins. Immunol Rev. 2008;224:229–238. doi: 10.1111/j.1600-065X.2008.00655.x. [DOI] [PubMed] [Google Scholar]
- Kawabuchi M, Satomi Y, Takao T, Shimonishi Y, Nada S, Nagai K, Tarakhovsky A, Okada M. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- Kaye J, Hsu ML, Sauron ME, Jameson SC, Gascoigne NR, Hedrick SM. Selective development of CD4+ T cells in transgenic mice expressing a class II MHC-restricted antigen receptor. Nature. 1989;341:746–749. doi: 10.1038/341746a0. [DOI] [PubMed] [Google Scholar]
- Kitaura Y, Jang IK, Wang Y, Han YC, Inazu T, Cadera EJ, Schlissel M, Hardy RR, Gu H. Control of the B cell-intrinsic tolerance programs by ubiquitin ligases Cbl and Cbl-b. Immunity. 2007;26:567–578. doi: 10.1016/j.immuni.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Karitkina D, Langnaese K, Posevitz-Fejfar A, Schraven B, Xavier R, Seed B, Lindquist JA. Phosphoprotein associated with glycosphingolipid-enriched microdomains differentially modulates SRC kinase activity in brain maturation. PLoS One. 2011;6:e23978. doi: 10.1371/journal.pone.0023978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manz BN, Tan YX, Courtney AH, Rutaganira F, Palmer E, Shokat KM, Weiss A. Small molecule inhibition of Csk alters affinity recognition by T cells. Elife. 2015:4. doi: 10.7554/eLife.08088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee I, Veillette A. Protein tyrosine phosphatases in lymphocyte activation and autoimmunity. Nat Immunol. 2012;13:439–447. doi: 10.1038/ni.2246. [DOI] [PubMed] [Google Scholar]
- Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat Immunol. 2014;15:875–883. doi: 10.1038/ni.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmedt C, Saijo K, Niidome T, Kuhn R, Aizawa S, Tarakhovsky A. Csk controls antigen receptor-mediated development and selection of T-lineage cells. Nature. 1998;394:901–904. doi: 10.1038/29802. [DOI] [PubMed] [Google Scholar]
- Simeoni L, Lindquist JA, Smida M, Witte V, Arndt B, Schraven B. Control of lymphocyte development and activation by negative regulatory transmembrane adapter proteins. Immunol Rev. 2008;224:215–228. doi: 10.1111/j.1600-065X.2008.00656.x. [DOI] [PubMed] [Google Scholar]
- Stanford SM, Bottini N. PTPN22: the archetypal non-HLA autoimmunity gene. Nat Rev Rheumatol. 2014;10:602–611. doi: 10.1038/nrrheum.2014.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YX, Manz BN, Freedman TS, Zhang C, Shokat KM, Weiss A. Inhibition of the kinase Csk in thymocytes reveals a requirement for actin remodeling in the initiation of full TCR signaling. Nat Immunol. 2014;15:186–194. doi: 10.1038/ni.2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasenko T, Kole HK, Chi AW, Mentink-Kane MM, Wynn TA, Bolland S. T cell-specific deletion of the inositol phosphatase SHIP reveals its role in regulating Th1/Th2 and cytotoxic responses. Proc Natl Acad Sci U S A. 2007;104:11382–11387. doi: 10.1073/pnas.0704853104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thien CB, Langdon WY. c-Cbl: a regulator of T cell receptor-mediated signalling. Immunol Cell Biol. 1998;76:473–482. doi: 10.1046/j.1440-1711.1998.00768.x. [DOI] [PubMed] [Google Scholar]
- Torgersen KM, Vang T, Abrahamsen H, Yaqub S, Horejsi V, Schraven B, Rolstad B, Mustelin T, Tasken K. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J Biol Chem. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- Veillette A, Latour S, Davidson D. Negative regulation of immunoreceptor signaling. Annu Rev Immunol. 2002;20:669–707. doi: 10.1146/annurev.immunol.20.081501.130710. [DOI] [PubMed] [Google Scholar]
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Xu S, Huo J, Tan JE, Lam KP. Cbp deficiency alters Csk localization in lipid rafts but does not affect T-cell development. Mol Cell Biol. 2005;25:8486–8495. doi: 10.1128/MCB.25.19.8486-8495.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Seed B. Site-specific gene targeting in mouse embryonic stem cells with intact bacterial artificial chromosomes. Nat Biotechnol. 2003;21:447–451. doi: 10.1038/nbt803. [DOI] [PubMed] [Google Scholar]
- Zhang J, Liu Q, Langdon WY. Cbl-b: Roles in T Cell Tolerance, Proallergic T Cell Development, and Cancer Immunity. Inflamm Cell Signal. 2014;1 doi: 10.14800/ics.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikherman J, Weiss A. Alternative splicing of CD45: the tip of the iceberg. Immunity. 2008;29:839–841. doi: 10.1016/j.immuni.2008.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.