Abstract
Drug and behavioral addictions have overlapping features, e.g., both manifest preference for larger, albeit costlier, reinforcement options in cost/benefit decision-making tasks. Our prior work revealed that the mixed-function serotonergic compound, mirtazapine, attenuates behaviors by rats motivated by abused drugs. To extend this work to behavioral addictions, here we determined if mirtazapine and/or ketanserin, another mixed-function serotonin-acting compound, can alter decision-making in rats that is independent of drug (or food)-motivated reward. Accordingly, we developed a novel variable-ratio task in rats wherein intracranial self-stimulation was used as the positive reinforcer. Using lever pressing for various levels of brain stimulation, the operant task provided choices between a small brain stimulation current delivered on a fixed-ratio schedule (i.e., a predictable reward) and a large brain stimulation delivered following an unpredictable number of responses (i.e., a variable-ratio schedule). This task allowed for demonstration of individualized preference and detection of shifts in motivational influences during a pharmacological treatment. Once baseline preference was established, we determined that pretreatment with mirtazapine or ketanserin significantly decreased preference for the large reinforcer presented after gambling-like schedules of reinforcement. When the rats were tested the next day without drug, preference for the unpredictable large reinforcer option was restored. These data demonstrate that mirtazapine and ketanserin can reduce preference for larger, costlier reinforcement options, and illustrate the potential for these drugs to alter behavior.
Keywords: intracranial self-stimulation, gambling, serotonin, cost/benefit decision-making, variable-ratio
INTRODUCTION
The ability to assess risk and to estimate costs and benefits associated with different choice options helps to determine advantageous courses of action (Baarendse et al., 2013;Orsini et al., 2015). Suboptimal/disadvantageous decision-making can have severe consequences, such as is observed in individuals who exhibit drug and/or behavioral addictions (Bechara et al., 2001;Rogers and Robbins, 2001;Ernst et al., 2003;Brand et al., 2005). Gambling disorders are examples of behavioral addiction that are highly relevant to modern society, yet there is no government-approved therapy for these disorders. We are interested in identifying efficacious pharmacotherapeutics for gambling disorders. To enhance translational value, we selected compounds already deemed safe for human use.
Serotonin (5-HT) is involved in normal executive function (Floresco and Jentsch, 2011), and dysregulation of this transmitter system is associated with risky decision-making (Nordin and Eklundh, 1999;Pallanti et al., 2006;Potenza et al., 2013), measures of impulsivity (Dalley and Roiser, 2012), and drug addiction (Muller and Homberg, 2015). Mirtazapine is an atypical antidepressant with a complex pharmacological profile that includes antagonism at 5-HT2A/2C receptors (De Boer, 1995;Wikstrom et al., 2002). Mirtazapine attenuates various behaviors motivated by abused drugs, including methamphetamine (Herrold et al., 2009;Voigt et al., 2011;Graves and Napier, 2011;Voigt and Napier, 2011) and morphine (Graves et al., 2012a) in rats, and reduces cocaine intake in humans (Graves et al., 2012b). We recently revealed that mirtazapine reduces the capacity of the dopamine D2/D3 receptor agonist, pramipexole, to induce risky decision-making by rats performing a probability discounting task (Holtz et al., 2016). The potential for mirtazapine to influence risky behaviors independent of drug-provoked effects has not been tested.
Ketanserin is another mixed-function antagonist with high affinity for 5-HT2A/2C receptors (Hoyer, 1988;Bonhaus et al., 1995). Ketanserin reduces behaviors by rats that are motivated by cocaine (Burmeister et al., 2004), nicotine (Levin et al., 2008) and methamphetamine (Bhatia et al., 2011), as well as neurophysiological effects of methamphetamine (McDaid et al., 2007). In studies of impulsivity, ketanserin reduces impulsive motor behavior (Passetti et al., 2003;Talpos et al., 2006;Fletcher et al., 2007). Thus, ketanserin, like mirtazapine, may be beneficial for the treatment of behavioral addiction.
Based on this background, we sought to determine if mirtazapine and ketanserin would influence risky decision-making in rats using a task that approximated key features of gambling disorders, such as weighing cost versus benefit and the uncertainty of reward delivery, and was independent of drug-motivated reward. We also sought to avoid food-reinforcement protocols, for serotonin regulates feeding and satiety, and both mirtazapine and ketanserin alter these functions in humans (Risselada et al., 2010;Jeong and Bahk, 2014) and rats (Pratt et al., 2016). Accordingly, we implemented a modified choice task developed by Johnson et al. (Johnson et al., 2011;Johnson et al., 2012) using intracranial self-stimulation (ICSS) as the positive reinforcer. This task utilizes gambling-like schedules of reinforcement to model features of human cost/benefit decision-making including the choice between the unpredictable occurrences of a high effort/large reinforcer option, and the predictable occurrence of a low effort/small reinforcer option. In this paradigm, the response cost placed on obtaining the large reinforcer is the exertion of greater physical effort (i.e., increased number of lever presses) necessary to obtain the reward; the average number of responses to obtain brain stimulation reinforcement on the high effort/large reinforcer schedule was always greater than the number or responses required on the low effort/small reinforcer alternative. The first feature was modeled by a variable-ratio (VR) schedule of ICSS-mediated reinforcement, which was contrasted to the second feature wherein ICSS was available using a predictable, fixed-ratio (FR) schedule. Using this task, we tested the effects of mirtazapine and ketanserin on preference for the VR option of reinforcement.
METHODS AND MATERIALS
Animals
Male Sprague-Dawley rats (n=31) were purchased from Envigo Laboratories (Indianapolis, IN) weighing 250–274g upon arrival. They were housed in pairs under a 12hr light/dark cycle (lights on at 7AM) in an environmentally controlled facility with food and water available ad libitum. Procedures were in accordance with those established in the Guide for the Care and Use of Laboratory Animals (National Research Council, Washington DC) as approved by the Rush University Medical Center Institutional Animal Care and Use Committee.
Test Drugs
Mirtazapine (isolated from tablet form by Plantex, Hackensack, NJ; a division of Teva Pharmaceuticals Ltd., North Wales, PA) was dissolved in 1N HCl, diluted with sterile H2O, and the pH was adjusted to ~6.3–6.8 using 1N NaOH. Mirtazapine was administered intraperitoneally (ip) as 5.0mg/ml/kg. This dose was selected based on our extensive prior studies showing that it is sufficient to reduce several forms of methamphetamine- (Graves and Napier, 2011;Herrold et al., 2009;McDaid et al., 2007;Voigt et al., 2011;Voigt and Napier, 2011), and morphine- (Graves et al., 2012a) motivated behaviors in rats without increasing latency to lever press in cue reactivity paradigm or altering coordinated motor function on a rotarod (Graves and Napier, 2011). Ketanserin tartrate (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile H2O and administered ip at doses of 1.0, 2.5, or 5.0mg/ml/kg (as the free base). Ketanserin vehicle (sterile H2O) was administered as 1mL/kg, ip. The doses used for both mirtazapine and ketanserin convert to a “Human Equivalency Dose” that provides efficacious treatment in humans (Fawcett and Barkin, 1998b;Fawcett and Barkin, 1998a;Liechti et al., 2000;Graham et al., 2002). Mirtazapine and ketanserin were tested in separate groups of rats.
Stimulating Electrode Implantation
Rats were anesthetized with isoflurane and placed into a stereotaxic frame (David Kopf, Tujunga, CA) with the nose piece set at −3.3mm. A midline scalp incision was made, and a burr hole was drilled through the skull at the following coordinates (from Bregma): −2.8mm AP and −1.8mm ML. A bipolar stimulating electrode (MS303/3-B/SPC; Plastics One, Roanoke, VA) was lowered 8.4mm from the top of the skull into the lateral hypothalamus. Electrodes were secured to the skull using stainless steel screws and dental acrylic, and the incision was sutured. Rats were returned to the home cage following recovery from anesthesia; one week later, operant training began. Three rats were removed from the study due to loss of headpiece during training or lack of task acquisition due to electrode placement outside of the lateral hypothalamus.
Testing Apparatus
Rats were tested in operant chambers (30.5cm × 24.1cm × 21.0cm; Med-Associates, St. Albans, VT), enclosed in ventilated, sound-attenuating boxes. Each chamber was fitted with two retractable levers on one wall with a stimulus light above each lever. On the opposite wall, a single 100mA house light was located in the top center. Intracranial stimulation was delivered by constant current stimulators (PHM-152/2 dual programmable ICSS stimulator) via bipolar leads connected to 2-channel commutators (Plastics One) mounted above the chamber. All experimental data were recorded by a PC connected to the operant chambers via an interface.
Behavioral Testing Protocol
The ICSS testing protocol was modified from those previously established in our laboratory using probability discounting (Rokosik and Napier, 2011;Rokosik and Napier, 2012;Holtz et al., 2016) and delay discounting (Tedford et al., 2015) tasks.
Phase 1: Shaping/Fixed-Ratio 1 (FR1)
Rats were trained to associate a reinforcing electrical stimulation of the brain with pressing a lever using a forepaw. Rats were primed with 100µA at 100Hz, then “guided” towards the extended lever with subsequent stimulation. Current parameters were incremented as needed to train the rats to associate the stimulation with pressing the lever, then we verified that the rats would consistently self-initiate lever pressing and maintain a stable lever pressing on both levers on a FR1 schedule of reinforcement (to ensure that a lever bias did not develop).
Phase 2: Fixed-Ratio 3 (FR3)
This phase verified that the current parameters determined in Phase 1 were sufficient to maintain a stable FR3 schedule of reinforcement within a session. To do so, rats were placed in the operant chamber with one lever (either the right or left) extended for 10min, and they were trained to press the lever three times in order to obtain brain stimulation. Rats were trained on both levers to ensure that a lever bias did not develop.
Phase 3: Choice Fixed-Ratio Task
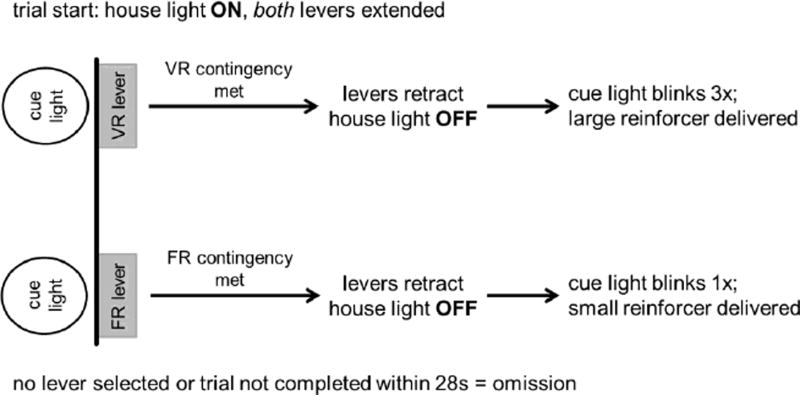
This phase verified that the rats could discriminate between the small and large reinforcers. The stimulation current values for small and large reinforcements were determined from a previously published current frequency vs. lever pressing response curve (Tedford et al., 2015); the small reinforcer was set at 50Hz, a value slightly above the threshold for stimulation current levels that supported responding. The large reinforcer was 100Hz, a value that is slightly below the current level that supports maximal responding (Tedford et al., 2015). The choice test consisted of 3 fixed blocks, each with 10 forced and 10 choice trials. Each lever was assigned distinct stimulation values on a FR3 schedule of reinforcement. In the forced trials, rats learned the lever contingencies, as only one lever was extended at a time. As illustrated in Fig 1, in the choice trials, both levers were extended at the same time, and the rat had to choose between the two. To enhance discrimination between the reinforcers, upon meeting the FR3 contingency, a light above the lever that delivered the small reinforcer was illuminated for 0.5s. For the large reinforcer, the cue light above the alternate lever flashed 3 times in 0.5s intervals. Failure to complete the number of lever presses required to obtain a reinforcer in the 28s time frame resulted in an omission. Data collected during this phase were summarized as number of selections of the large reinforcer / total number of completed choices, and termed ‘percent selection large reinforcer’.
Figure 1.
Flow chart showing contingencies for the free-choice trials in the variable-ratio task. Definitions: VR, variable ratio; FR, fixed-ratio. Free-choice trials allow the rat to make a selection between the VR lever, which delivers the large reinforcer (100Hz) immediately after the required number of presses is completed and the FR lever that delivers the small reinforcer (50Hz) immediately after 3 presses. If no selection is made within 28s, the levers are withdrawn, and a new trial begins 2s later.
Phase 4: Forced Variable-Ratio 10 (VR10)
Rats were tested in a VR10 schedule of reinforcement to ensure that sufficient lever pressing rates were exhibited on an unpredictable schedule of reinforcement. In each 20min session, rats were placed in the operant chamber with either the left or right lever extended. To illustrate the protocol, on a VR10 schedule, rats had to press 1, 5, 10, 15 or 19 times to receive the large reinforcer. The VR requirement for each trial was randomized.
Phase 5a. Choice Variable-Ratio Task: Testing with Mirtazapine
Given that we have seen mirtazapine reduce numerous drug and ICSS reward-motivated behaviors, we recapitulated 5mg/kg mirtazapine here as a means to demonstrate proof-of-concept for pharmacological interventions of the current task. To do so, rats were tested in multiple sessions with various VR schedules wherein for each session they had the option to choose between the FR3 and the VR (which ranged from VR6 to VR18). The assignment of the VR and FR levers was counterbalanced across rats, and the VR requirement in each trial was determined randomly with replacement. The VR schedules used are illustrated in Table 1.
Table 1.
Illustration of the VR schedules of reinforcement.
| VR Schedule of Reinforcement |
Number of Lever Presses to Receive Large Reinforcer |
|---|---|
| VR6 | 1, 3, 6, 9, or 11 |
| VR8 | 1, 3, 8, 13, or 15 |
| VR10 | 1, 5, 10, 15, or 19 |
| VR12 | 1, 7, 12, 17, or 23 |
| VR15 | 1, 8, 15, 22, or 29 |
| VR18 | 1, 9, 18, 27, or 35 |
Similar to the Phase 3, each VR schedule of reinforcement consisted of 3 fixed blocks, each with 10 forced and 10 choice trials; each trial length totaled to 28s. Selection of the FR3 lever resulted in delivery of the small reinforcer after three lever presses; the cue light above the lever was illuminated for 0.5s. Selection of the VR lever delivered the large reinforcer immediately after a variable number of lever presses, and the cue light above the lever flashed three times in 0.5s intervals. Data collected during this phase were summarized as number of selections / total number of choice trials. Failure to initiate lever pressing or to complete the number of lever presses required to obtain a reinforcer in the 28s time frame resulted in an omission, summarized as number of omissions / total number of choice trials.
Rats (n=12) began the task on the VR6 schedule of reinforcement and had to meet a stability criterion prior to drug testing. Stability was defined as <10% variability between 2 consecutive sessions. Based on our initial studies with this task in untreated animals, we determined that variability (±2SD) in task performance fell within 10%. Therefore, we identified this as the criteria for stability for the remainder of the study. Once stable baseline was achieved in the VR6 schedule, the effect of mirtazapine on preference for gambling-like schedules of reinforcement was determined. To do so, mirtazapine was administered 30min before the task, and a change in preference for the VR lever by >15% was required to be considered effective (Holtz et al., 2016). When mirtazapine induced this change, rats were tested the next day in the absence of the drug to assure that responding normalized. If mirtazapine had no effect in the VR6 (i.e., <15% change from baseline preference), rats were tested in a higher VR schedule of reinforcement, and the testing cycle was repeated.
Phase 5b. Choice Variable-Ratio Task: Testing with Ketanserin
To test ketanserin, we streamlined the testing process used for mirtazapine in order to reduce the number of phases and allow for a within-subjects dose-response assessment. Phase 4, testing in the VR10, was eliminated from the testing protocol and rats (n=16) were moved directly to the VR task after completing Phase 3. We first determined the highest VR schedule in which each rat was willing to work for the large reinforcer. To do so, rats began testing on the VR6 schedule of reinforcement, and they advanced to the next VR schedule once a stable baseline was obtained in the VR6 schedule. This testing cycle continued until preference switched from the VR to the FR3 (i.e., >50% preference for the FR3 lever). Rats were moved back to the previous VR schedule in which the VR was preferred (the range was VR8 to VR18). Once stable baseline was achieved, rats began testing the next day in the ketanserin dose-response. Rats were pretreated with vehicle (1ml/kg) or ketanserin (1.0, 2.5 or 5.0mg/kg) 30min before the operant task. The following day, the rats were tested in the absence of drug to determine if shifts in preference for the VR were due to drug treatment. Rats were allowed to re-establish a stable baseline, and they were then tested with another dose of ketanserin (in a random order). This testing cycle continued until three doses of ketanserin were tested, with a minimum of three days between each drug test.
Statistical Analyses
Data are presented as mean+SEM. A paired t-test or one-way repeated measures ANOVA with a post hoc Newman-Keuls were used as appropriate. Data were analyzed using Prism software version 5 (Graphpad, La Jolla, CA, USA). Criterion for inclusion in data analysis was that rats had to complete more than 50% of the trials in each choice block of the VR task.
RESULTS
Development of the ICSS-Mediated Variable-Ratio Task
Phase 1: Shaping/FR1 Schedule of Reinforcement
In this phase, rats learned to associate the press of the lever with electrical stimulation, to initiate lever pressing at the start of the sessions, and demonstrate steady lever pressing on the left and right lever (at least 8 presses/min for 2 consecutive sessions). The average lever presses/min for the left and right lever was 20.0±1.9 and 23±2.8, respectively. The response rate was not different between levers, (t(14)=1.5, p=0.17) indicating that there was no lever bias. The range of current intensities used was 100–280µA.
Phase 2: Training on FR3 Schedule of Reinforcement
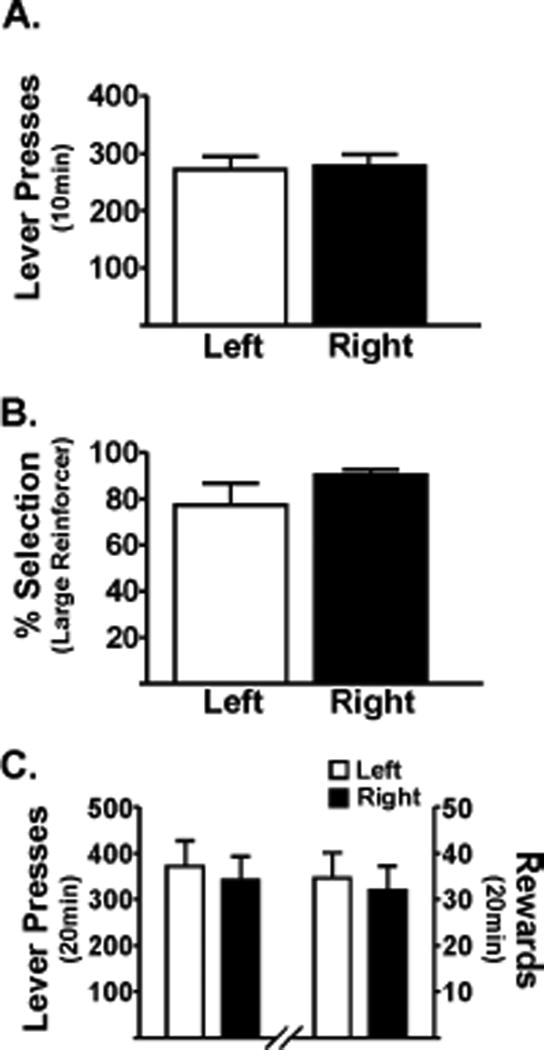
In this phase, rats had to demonstrate steady lever pressing on a FR3 schedule on both the left and right lever. All rats met these criteria within four sessions. The rate of responding on either lever was not different (t(14)=0.35; p=0.73; Fig 2A), suggesting no lever bias developed.
Figure 2.
Rats press for the positive reinforcer, independent of lever presentation. (A) Lever-pressing in Phase 2. Presses during training for FR3 when only one lever is extended (either left or right). Rats (n=15) self-stimulated equally, independent of which lever was extended. (B) Lever-pressing during Phase 3. Both levers were extended, and the rats selected the preferred reinforcer. Data shown verify that rats (n=14) preferred the large reinforcer when associated with either the right or left lever. (C) Rats (n=11) exerted high physical effort to obtain the large reinforcer in a forced VR10. A similar number of reinforcers were delivered, regardless of the lever assignment. On the left y-axis is the number of lever presses averaged across two consecutive sessions. On the right y-axis is the average number of large reinforcers received. Paired t-test, p>0.05.
Phase 3: Choice Test
In this phase, rats had to discriminate between the brain stimulation reinforcer values associated with the small and large reinforcer options on the FR3 schedule of reinforcement. When given the choice, rats reliably selected the large reinforcer over the small reinforcer approximately 80% of the time, independent of the lever that was assigned to the large reinforcer (t(13)=0.36; p=0.73; Fig 2B).
Phase 4: Variable Ratio-10 (VR10) Schedule of Reinforcement
In this phase, rats were tested with a VR10 schedule of reinforcement associated with the large reinforcer to demonstrate that rats would work on an unpredictable, gambling-like schedule of reinforcement. In a 20min session, rats pressed the VR lever an average of 375 times, corresponding to approximately 33 rewards received. As in Phase 3, rats were tested on alternating levers, and no lever bias was observed (t(10) = 0.76; p=0.47; Fig 2C).
Variable-Ratio Tasks for Mirtazapine and Ketanserin
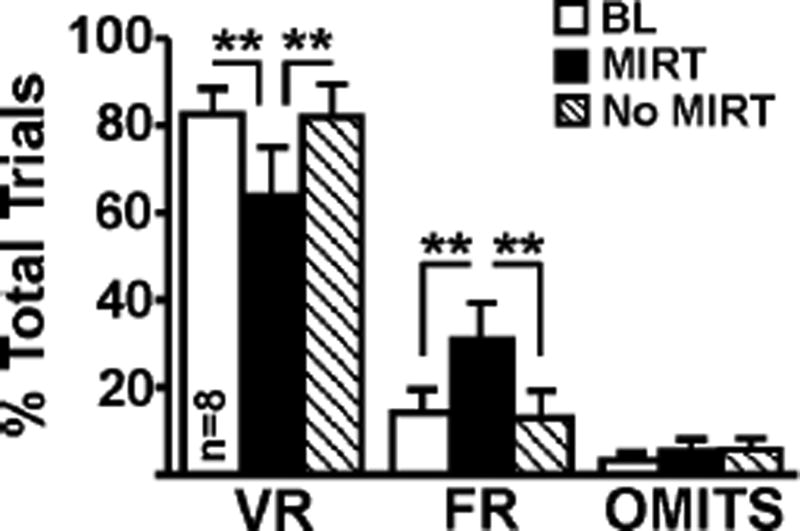
The goal of the VR task was to assess preference for a large reinforcer delivered on unpredictable schedules of reinforcement, and to determine if compounds that target 5HT2A/2C receptors shifted preference to a small reinforcer (FR3) alternative. Four rats were excluded from mirtazapine data analysis due to high omissions (see Methods). At baseline, rats demonstrated ~85% preference for the VR lever. Mirtazapine pretreatment attenuated preference for the VR lever by ~20% (F2,7=8.74, p=0.003; Fig 3), and shifted preference to the FR3 schedule of reinforcement (F2,7=10.84, p=0.001). Mirtazapine did not increase the number of omitted trials (F(2,7)=0.47, p=0.63). When tested one day later, in the absence of mirtazapine, preference for the VR lever was restored
Figure 3.
Pretreatment with mirtazapine decreased preference for the gambling-like lever by ~20%, switching preference to the FR lever. This switch suggests that the drug was able to attenuate preference for the lever associated with the large reinforcer. In the absence of mirtazapine (i.e., 24h post-injection), preference for the VR lever was restored. The range of the VR schedule was VR6–VR18. One-way repeated measures ANOVA with post hoc Newman-Keuls. **, p<0.01.
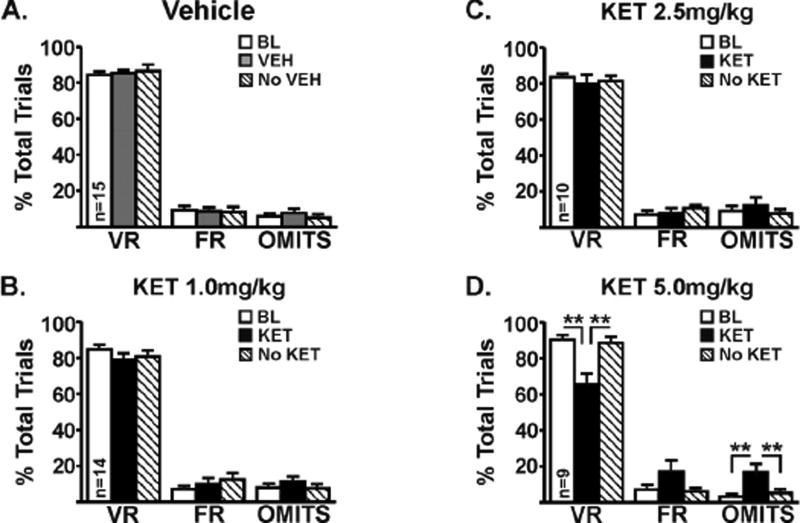
Using a within-subjects design in 16 rats, we tested three doses of ketanserin and the vehicle in random order. For the ketanserin dose-response, the number of rats that met criterion were as follows: vehicle (15 of 16 tested), 1.0mg/kg (14 of 16 tested), 2.5mg/kg (10 of 10 tested), and 5.0mg/kg (9 of 16 tested). To confirm the reliability of the behavior and that it is stable across time, we analyzed the baseline data from all rats prior to treatment with vehicle, 1mg/kg ketanserin and 5 mg/kg ketanserin; there was less than 10% change between sessions (range of means was 91–93%), which was not statistically significant (p=0.53). There was no effect of vehicle (F(2,14)=0.30, p=0.74; Fig 4A), 1.0mg/kg ketanserin (F(2,13)=1.2, p=0.30; Fig 4B), or 2.5mg/kg ketanserin (F(2,9)=0.45, p=0.65; Fig 4C) on preference for the VR lever. In contrast, there was a significant ketanserin effect at 5.0mg/kg wherein preference for the VR lever was decreased by ~25% (F(2,8)=14.34, p=0.0003; Fig 4D) and preference for the FR3 lever was increased (F(2,8)=3.79, p=0.04). Unlike mirtazapine, however, ketanserin increased the number of omitted trials even though the rats met the categorical criterion for study inclusion (see Methods; F(2,8)=8.6; p=0.003). There was no correlation between the VR schedule and number of omissions.
Figure 4.
Pretreatment with vehicle (A), ketanserin at 1.0mg/kg (B), or ketanserin at 2.5mg/kg (C) had no effect on preference for the VR lever. Ketanserin at 5.0mg/kg (D) decreased preference for the VR lever by ~25% while also increasing the number of omitted trials. In the absence of ketanserin (i.e., 24hrs post-injection), preference for the VR lever was restored. The range of the VR schedule was VR8–VR18. One-way repeated measures ANOVA with post hoc Newman-Keuls. **, p<0.01.
DISCUSSION
The goals of the present study were twofold: (i) to develop a novel rodent task of gambling-like reinforcement using ICSS as the positive reinforcer, and (ii) to determine the effects of clinically-used mixed-function serotoninergic compounds, mirtazapine and ketanserin in this task. To do so, we developed a VR task that models the unpredictable delivery of a reinforcer. During unpredictable reinforcement, both animals and humans often exhibit high response rates, even after a series of no reinforcement or losses (Madden et al., 2007). Such behavioral patterns are also seen during the random schedules of reinforcements associated with slot machines. Common animal models of impulsivity and risky decision-making involve food as the reinforcer. We demonstrate that ICSS is a viable alternative to food, and that in contrast to tasks with food reinforcers, ICSS allowed the rats to rapidly acquire the task and to be repeatedly tested without concern of satiety. Using this novel paradigm, we determined that mirtazapine and ketanserin decreased the selection of the unpredictable, large reinforcer, shifting preference to the predictable, small reinforcer. The change in preference following mirtazapine administration was not accompanied by an increase in the number of omitted trials by rats that completed the task. We previously revealed that this same dose of mirtazapine did not increase latency to lever press in a cue reactivity paradigm, alter coordinated motor function measured on a rotarod (Graves and Napier, 2011), or attenuate pramipexole-induced improvements in a test of evoked motor behavior (Holtz et al., 2016). Additionally, a pilot study (n=3) of lever-pressing vs ICSS current frequency indicated that mirtazapine did not alter the reward potency of ICSS (unpublished data). Thus, mirtazapine is likely altering the rats’ willingness to work for a positive reinforcer rather than altering motor function or decreasing the hedonic impact of ICSS. These outcomes contrast those obtained with ketanserin, for the dose of ketanserin that reduced preference for the VR also significantly increased the number of omitted trials, which was due, in part, to failure to make any choice at all in some trials. Thus, we cannot rule out an effect of motor slowing with ketanserin.
Decision-making tasks in which response costs can bias choice include altering reward delivery such that the outcome (i) requires increased physical effort in order for the reward to be obtained (effort-based decision-making), (ii) is uncertain or risky (risk/reward decision-making), or (iii) is delayed (delay discounting) (Shafiei et al., 2012). Recent studies have provided insight into the various transmitters that underlie these processes. Mirtazapine and ketanserin have high affinity for 5-HT2A/2C receptors, at which they may act as antagonists or inverse agonists (De Boer, 1995;Hoyer, 1988;Bonhaus et al., 1995;Wikstrom et al., 2002;Chanrion et al., 2008;Labasque et al., 2010). Prior to the current study, the influence of serotonergic compounds on effort-based decision-making had not been determined. Studies using delay discounting and risk/reward decision-making have revealed a role for 5-HT on these aspects of decision-making; however the particular role remains unclear. Mixed results have been reported on the effect of global reductions in 5-HT (i.e., lesions of the serotonergic system) on delay discounting (Wogar et al., 1993;Mobini et al., 2000;Winstanley et al., 2003;Winstanley et al., 2005). Ketanserin has no effect in a delay discounting task; however, SDZ SER 082, a selective 5-HT2C antagonist, increases the preference for the large reward given after a long delay (Talpos et al., 2006). In risk/reward decision-making, modeled in a rodent gambling task, depletion of dietary 5-HT increases risky decision-making (Koot et al., 2012). A recent study of healthy humans performing a card gambling task suggests that ketanserin enhances riskaversion (Macoveanu et al., 2013). As a collective, these studies indicate unique contributions of specific 5-HT receptors in various facets of decision-making. Thus, targeting multiple receptor subtypes may be beneficial.
While most people who gamble do so for entertainment and without harmful consequences, a significant portion (~2.5%) exhibit some form of gambling disorder (Potenza, 2013). In conjunction with 5-HT-mediated impulse control, several neurotransmitter systems are implicated in various aspects of gambling behaviors, including dopamine- and opioid-mediated reward, norepinephrine-mediated arousal, and glutamate-mediated compulsion (for review, see (Potenza, 2013)). Mixed-function compounds like mirtazapine and ketanserin have not yet been evaluated in human problem gamblers. The majority of monoaminergic pharmacotherapies tested in clinical trials for gambling disorders have been selective serotonin reuptake inhibitors (SSRIs) that target the 5-HT transporter, e.g., fluvoxamine, sertraline, escitalopram and paroxetine. The outcomes of these studies are inconsistent and the incidence of a placebo effect often is high (Hollander et al., 2000;Blanco et al., 2002;Kim et al., 2002;Grant et al., 2003;Saiz-Ruiz et al., 2005;Grant and Potenza, 2006). As the mechanism of action of SSRIs is to increase global 5-HT and do not differentiate 5-HT receptors, the potential benefits of targeting the 5-HT system to treat gambling may have been missed. In support of this possibility, a small, open-label study of nefazodone, an antidepressant that acts primarily as an antagonist at 5-HT2 receptors, showed improvement in gambling behaviors (Pallanti et al., 2002). Preclinical and clinical studies suggest drug addiction and impulsivity share common underlying neurotransmitter systems, including 5-HT (for reviews, see (Kirby et al., 2011;Bullock and Potenza, 2012;Cunningham and Anastasio, 2014)). Studies in laboratory rats indicate that mixed-function drugs whose target includes 5-HT2 receptors can attenuate the behavioral and neurobiological effects of several abused drugs, including methamphetamine (McDaid et al., 2007;Herrold et al., 2009;Bhatia et al., 2011;Graves and Napier, 2011;Voigt and Napier, 2011), cocaine (Burmeister et al., 2004), morphine (Graves et al., 2012a) and nicotine (Levin et al., 2008). Mirtazapine also shows benefits for treating drug addiction in humans (for review, see (Graves et al., 2012b)). This is in agreement with the current study, wherein mirtazapine decreased preference for the VR lever without altering the number of omitted trials. As mirtazapine is already deemed a safe and well-tolerated therapy for humans, they could be deployed in the clinical setting relatively rapidly.
CONCLUSION
We described here a novel decision-making task for rats that is independent of drug or food reinforcers that provides a platform in which to screen potential compounds for their utility to influence aspects of cost/benefit decision-making. Reductions in choice preference for larger, costlier rewards may be beneficial when considering maladaptive decision-making features often observed in behavioral addictions. We revealed that mirtazapine and ketanserin can reduce preference for a large reward on unpredictable, gambling-like schedules of reinforcement. These results illustrate the utility of this novel ICSS-mediated task and indicate the merit of additional research into the potential utility of mirtazapine and other 5-HT2A/2C ligands as therapies for gambling-like disorders.
HIGHLIGHTS.
A novel model of cost/benefit decision-making is described.
Mirtazapine decreased preference for high effort/large reinforcers.
Dose-related effects of ketanserin in this task are revealed.
This model may help identify ligands as therapies for gambling-like disorders.
Acknowledgments
This work was supported by the National Center for Responsible Gaming and USPHSG #NS074014.
ABBREVIATIONS
- 5-HT
serotonin
- FR
fixed-ratio
- ICSS
intracranial self-stimulation
- VR
variable-ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
ALP was involved in the conception and organization of the research project, its design and execution, statistical analyses, and the writing of the manuscript.
SET was involved in the conception of the research project, its design and execution, and the writing, review and critique of the manuscript.
TCN was involved in the conception and organization of the research project, the design, review and critique of the statistical analyses, the writing, and the review and critique of the manuscript. All authors approved the final version of the manuscript
COMPETING INTERESTS
The authors have no financial conflict of interest with respect to the content of this manuscript.
References
- Baarendse PJ, Winstanley CA, Vanderschuren LJ. Simultaneous blockade of dopamine and noradrenaline reuptake promotes disadvantageous decision making in a rat gambling task. Psychopharmacology (Berl) 2013;225:719–731. doi: 10.1007/s00213-012-2857-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bhatia KS, Szabo ST, Fowler JC, Wetsel WC, Lee TH. Reversal of long-term methamphetamine sensitization by combination of pergolide with ondansetron or ketanserin, but not mirtazapine. Behav Brain Res. 2011;223:227–232. doi: 10.1016/j.bbr.2011.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco C, Petkova E, Ibanez A, Saiz-Ruiz J. A pilot placebo-controlled study of fluvoxamine for pathological gambling. Ann Clin Psychiatry. 2002;14:9–15. doi: 10.1023/a:1015215809770. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Bach C, DeSouza A, Salazar FH, Matsuoka BD, Zuppan P, Chan HW, Eglen RM. The pharmacology and distribution of human 5-hydroxytryptamine2B (5-HT2B) receptor gene products: comparison with 5-HT2A and 5-HT2C receptors. Br J Pharmacol. 1995;115:622–628. doi: 10.1111/j.1476-5381.1995.tb14977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M, Kalbe E, Labudda K, Fujiwara E, Kessler J, Markowitsch HJ. Decision-making impairments in patients with pathological gambling. Psychiatry Res. 2005;133:91–99. doi: 10.1016/j.psychres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bullock SA, Potenza MN. Pathological gambling: neuropsychopharmacology and treatment. Current Psychopharmacology. 2012;1:67–85. doi: 10.2174/2211556011201010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology. 2004;29:660–668. doi: 10.1038/sj.npp.1300346. [DOI] [PubMed] [Google Scholar]
- Chanrion B, Mannoury la CC, Gavarini S, Seimandi M, Vincent L, Pujol JF, Bockaert J, Marin P, Millan MJ. Inverse agonist and neutral antagonist actions of antidepressants at recombinant and native 5-hydroxytryptamine2C receptors: differential modulation of cell surface expression and signal transduction. Mol Pharmacol. 2008;73:748–757. doi: 10.1124/mol.107.041574. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC. Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology. 2014;76(Pt B):460–478. doi: 10.1016/j.neuropharm.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- De Boer T. The effects of mirtazapine on central noradrenergic and serotonergic neurotransmission. Int Clin Psychopharmacol. 1995;10(Suppl 4):19–23. doi: 10.1097/00004850-199512004-00004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Fawcett J, Barkin RL. A meta-analysis of eight randomized, double-blind, controlled clinical trials of mirtazapine for the treatment of patients with major depression and symptoms of anxiety. J Clin Psychiatry. 1998a;59:123–127. [PubMed] [Google Scholar]
- Fawcett J, Barkin RL. Review of the results from clinical studies on the efficacy, safety and tolerability of mirtazapine for the treatment of patients with major depression. J Affect Disord. 1998b;51:267–285. doi: 10.1016/s0165-0327(98)00224-9. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tampakeras M, Sinyard J, Higgins GA. Opposing effects of 5-HT(2A) and 5-HT(2C) receptor antagonists in the rat and mouse on premature responding in the five-choice serial reaction time test. Psychopharmacology (Berl) 2007;195:223–234. doi: 10.1007/s00213-007-0891-z. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Jentsch JD. Pharmacological enhancement of memory and executive functioning in laboratory animals. Neuropsychopharmacology. 2011;36:227–250. doi: 10.1038/npp.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham SJ, Langley RW, Balboa VA, Bradshaw CM, Szabadi E. Effects of ketanserin and haloperidol on prepulse inhibition of the acoustic startle (eyeblink) response and the N1/P2 auditory evoked response in man. J Psychopharmacol. 2002;16:15–22. doi: 10.1177/026988110201600101. [DOI] [PubMed] [Google Scholar]
- Grant JE, Kim SW, Potenza MN, Blanco C, Ibanez A, Stevens L, Hektner JM, Zaninelli R. Paroxetine treatment of pathological gambling: a multi-centre randomized controlled trial. Int Clin Psychopharmacol. 2003;18:243–249. doi: 10.1097/00004850-200307000-00007. [DOI] [PubMed] [Google Scholar]
- Grant JE, Potenza MN. Escitalopram treatment of pathological gambling with co-occurring anxiety: an open-label pilot study with double-blind discontinuation. Int Clin Psychopharmacol. 2006;21:203–209. doi: 10.1097/00004850-200607000-00002. [DOI] [PubMed] [Google Scholar]
- Graves SM, Napier TC. Mirtazapine alters cue-associated methamphetamine seeking in rats. Biol Psychiatry. 2011;69:275–281. doi: 10.1016/j.biopsych.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SM, Persons AL, Riddle JL, Napier TC. The atypical antidepressant mirtazapine attenuates expression of morphine-induced place preference and motor sensitization. Brain Res. 2012a;1472:45–53. doi: 10.1016/j.brainres.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SM, Rafeyan R, Watts J, Napier TC. Mirtazapine, and mirtazapine-like compounds as possible pharmacotherapy for substance abuse disorders: evidence from the bench and the bedside. Pharmacol Ther. 2012b;136:343–353. doi: 10.1016/j.pharmthera.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrold AA, Shen F, Graham MP, Harper LK, Specio SE, Tedford CE, Napier TC. Mirtazapine treatment after conditioning with methamphetamine alters subsequent expression of place preference. Drug Alcohol Depend. 2009;99:231–239. doi: 10.1016/j.drugalcdep.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Hollander E, DeCaria CM, Finkell JN, Begaz T, Wong CM, Cartwright C. A randomized double-blind fluvoxamine/placebo crossover trial in pathologic gambling. Biol Psychiatry. 2000;47:813–817. doi: 10.1016/s0006-3223(00)00241-9. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Tedford SE, Persons AL, Grasso SA, Napier TC. Pharmacologically distinct pramipexole-mediated akinesia vs. risk-taking in a rat model of Parkinson's disease. Prog Neuropsychopharmacol Biol Psychiatry. 2016;70:77–84. doi: 10.1016/j.pnpbp.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D. Functional correlates of serotonin 5-HT1 recognition sites. 1988:59–71. doi: 10.3109/10799898809048978. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Bahk WM. Sleep-related eating disorder associated with mirtazapine. J Clin Psychopharmacol. 2014;34:752–753. doi: 10.1097/JCP.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Madden GJ, Brewer AT, Pinkston JW, Fowler SC. Effects of acute pramipexole on preference for gambling-like schedules of reinforcement in rats. Psychopharmacology (Berl) 2011;231:11–18. doi: 10.1007/s00213-010-2006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PS, Madden GJ, Stein JS. Effects of acute pramipexole on male rats' preference for gambling-like rewards II. Exp Clin Psychopharmacol. 2012;20:167–172. doi: 10.1037/a0027117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Grant JE, Adson DE, Shin YC, Zaninelli R. A double-blind placebo-controlled study of the efficacy and safety of paroxetine in the treatment of pathological gambling. J Clin Psychiatry. 2002;63:501–507. doi: 10.4088/jcp.v63n0606. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koot S, Zoratto F, Cassano T, Colangeli R, Laviola G, Van den Bos R, Adriani W. Compromised decision-making and increased gambling proneness following dietary serotonin depletion in rats. Neuropharmacology. 2012;62:1640–1650. doi: 10.1016/j.neuropharm.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Labasque M, Meffre J, Carrat G, Becamel C, Bockaert J, Marin P. Constitutive activity of serotonin2C receptors at G protein-independent signaling: modulation by RNA editing and antidepressants. Mol Pharmacol. 2010;78:818–826. doi: 10.1124/mol.110.066035. [DOI] [PubMed] [Google Scholar]
- Levin ED, Slade S, Johnson M, Petro A, Horton K, Williams P, Rezvani AH, Rose JE. Ketanserin, a 5-HT2 receptor antagonist, decreases nicotine self-administration in rats. Eur J Pharmacol. 2008;600:93–97. doi: 10.1016/j.ejphar.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liechti ME, Saur MR, Gamma A, Hell D, Vollenweider FX. Psychological and physiological effects of MDMA ("Ecstasy") after pretreatment with the 5-HT(2) antagonist ketanserin in healthy humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- Macoveanu J, Rowe JB, Hornboll B, Elliott R, Paulson OB, Knudsen GM, Siebner HR. Serotonin 2A receptors contribute to the regulation of risk-averse decisions. Neuroimage. 2013;83:35–44. doi: 10.1016/j.neuroimage.2013.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Ewan EE, Lagorio CH. Toward an animal model of gambling: delay discounting and the allure of unpredictable outcomes. J Gambl Stud. 2007;23:63–83. doi: 10.1007/s10899-006-9041-5. [DOI] [PubMed] [Google Scholar]
- McDaid J, Tedford CE, Mackie AR, Dallimore JE, Mickiewicz AL, Shen F, Angle JM, Napier TC. Nullifying drug-induced sensitization: behavioral and electrophysiological evaluations of dopaminergic and serotonergic ligands in methamphetamine-sensitized rats. Drug Alcohol Depend. 2007;86:55–66. doi: 10.1016/j.drugalcdep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Effects of central 5-hydroxytryptamine depletion on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology (Berl) 2000;152:390–397. doi: 10.1007/s002130000542. [DOI] [PubMed] [Google Scholar]
- Muller CP, Homberg JR. The role of serotonin in drug use and addiction. Behav Brain Res. 2015;277:146–192. doi: 10.1016/j.bbr.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Nordin C, Eklundh T. Altered CSF 5-HIAA Disposition in Pathologic Male Gamblers. CNS Spectr. 1999;4:25–33. doi: 10.1017/s1092852900006799. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Moorman DE, Young JW, Setlow B, Floresco SB. Neural mechanisms regulating different forms of risk-related decision-making: Insights from animal models. Neurosci Biobehav Rev. 2015;58:147–167. doi: 10.1016/j.neubiorev.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanti S, Baldini RN, Sood E, Hollander E. Nefazodone treatment of pathological gambling: a prospective open-label controlled trial. J Clin Psychiatry. 2002;63:1034–1039. doi: 10.4088/jcp.v63n1114. [DOI] [PubMed] [Google Scholar]
- Pallanti S, Bernardi S, Quercioli L, DeCaria C, Hollander E. Serotonin dysfunction in pathological gamblers: increased prolactin response to oral m-CPP versus placebo. CNS Spectr. 2006;11:956–964. doi: 10.1017/s1092852900015145. [DOI] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, Robbins TW. Double dissociation of serotonergic and dopaminergic mechanisms on attentional performance using a rodent five-choice reaction time task. Psychopharmacology (Berl) 2003;165:136–145. doi: 10.1007/s00213-002-1227-7. [DOI] [PubMed] [Google Scholar]
- Potenza MN. Neurobiology of gambling behaviors. Curr Opin Neurobiol. 2013;23:660–667. doi: 10.1016/j.conb.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza MN, Walderhaug E, Henry S, Gallezot JD, Planeta-Wilson B, Ropchan J, Neumeister A. Serotonin 1B receptor imaging in pathological gambling. World J Biol Psychiatry. 2013;14:139–145. doi: 10.3109/15622975.2011.598559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt WE, Clissold KA, Lin P, Cain AE, Ciesinski AF, Hopkins TR, Ilesanmi AO, Kelly EA, Pierce-Messick Z, Powell DS, Rosner IA. A systematic investigation of the differential roles for ventral tegmentum serotonin 1- and 2-type receptors on food intake in the rat. Brain Res. 2016 doi: 10.1016/j.brainres.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risselada AJ, Mulder H, Heerdink ER, Grube AM, Wilmink FW, Egberts TC. The association between serotonin 2C receptor polymorphisms and weight gain and eating behavior in patients using mirtazapine: a prospective follow-up study. J Clin Psychopharmacol. 2010;30:207–209. doi: 10.1097/JCP.0b013e3181d489d7. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol. 2001;11:250–257. doi: 10.1016/s0959-4388(00)00204-x. [DOI] [PubMed] [Google Scholar]
- Rokosik SL, Napier TC. Intracranial self-stimulation as a positive reinforcer to study impulsivity in a probability discounting paradigm. J Neurosci Methods. 2011;198:260–269. doi: 10.1016/j.jneumeth.2011.04.025. [DOI] [PubMed] [Google Scholar]
- Rokosik SL, Napier TC. Pramipexole-induced increased probabilistic discounting: comparison between a rodent model of Parkinson's disease and controls. Neuropsychopharmacology. 2012;37:1397–1408. doi: 10.1038/npp.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiz-Ruiz J, Blanco C, Ibanez A, Masramon X, Gomez MM, Madrigal M, Diez T. Sertraline treatment of pathological gambling: a pilot study. J Clin Psychiatry. 2005;66:28–33. doi: 10.4088/jcp.v66n0104. [DOI] [PubMed] [Google Scholar]
- Shafiei N, Gray M, Viau V, Floresco SB. Acute stress induces selective alterations in cost/benefit decision-making. Neuropsychopharmacology. 2012;37:2194–2209. doi: 10.1038/npp.2012.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpos JC, Wilkinson LS, Robbins TW. A comparison of multiple 5-HT receptors in two tasks measuring impulsivity. J Psychopharmacol. 2006;20:47–58. doi: 10.1177/0269881105056639. [DOI] [PubMed] [Google Scholar]
- Tedford SE, Persons AL, Napier TC. Dopaminergic lesions of the dorsolateral striatum in rats increase delay discounting in an impulsive choice task. PLoS ONE. 2015;10:e0122063. doi: 10.1371/journal.pone.0122063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Mickiewicz AL, Napier TC. Repeated mirtazapine nullifies the maintenance of previously established methamphetamine-induced conditioned place preference in rats. Behav Brain Res. 2011;225:91–96. doi: 10.1016/j.bbr.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Napier TC. Context-dependent effects of a single administration of mirtazapine on the expression of methamphetamine-induced conditioned place preference. Front Behav Neurosci. 2011;5:92. doi: 10.3389/fnbeh.2011.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikstrom HV, Mensonides-Harsema MM, Cremers TI, Moltzen EK, Arnt J. Synthesis and pharmacological testing of 1,2,3,4,10,14b-hexahydro-6-methoxy-2-methyldibenzo[c,f]pyrazino[1,2-a]azepin and its enantiomers in comparison with the two antidepressants mianserin and mirtazapine. J Med Chem. 2002;45:3280–3285. doi: 10.1021/jm010566d. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Global 5-HT depletion attenuates the ability of amphetamine to decrease impulsive choice on a delay-discounting task in rats. Psychopharmacology (Berl) 2003;170:320–331. doi: 10.1007/s00213-003-1546-3. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wogar MA, Bradshaw CM, Szabadi E. Effect of lesions of the ascending 5-hydroxytryptaminergic pathways on choice between delayed reinforcers. Psychopharmacology (Berl) 1993;111:239–243. doi: 10.1007/BF02245530. [DOI] [PubMed] [Google Scholar]