Abstract
The objective of the present study was to quantitatively analyze the permeability of tumor entity and peritumor edema in glioma grading, using dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI). In the present retrospective study, 80 patients underwent T1-weighted DCE-MRI examination at 3.0 T and the pathological results (including astrocytoma and oligodendroglioma) were obtained between January 2012 and June 2015. All cases were surgically validated as grade I–IV gliomas. The original DCE-MRI data were analyzed using dual compartment modified Tofts model. The forward volume transfer constant (Ktrans), backflux rate (kep) and fractional volume (ve) were calculated with the region of interest selected on the highest permeability area of the tumor entity and peritumor edema. Analysis of variance with the Bonferroni correction was used to compare the values of Ktrans, kep, and ve of the tumor entity and peritumor edema in different glioma grades. The results of the present study revealed that the Ktrans, kep, and ve values in each stage were associated with the pathological grading (r=0.951, 0.804 and 0.766, respectively). There were significant differences identified between different tumor grades in Ktrans, kep, with the exception being between grades II and III in kep. In addition, there was a significant difference revealed between grade I/II and grade III/IV in ve. Receiver operator characteristics curve analysis was used to evaluate the diagnosis accuracies of permeability parameters. Ktrans was demonstrated to exhibit the highest sensitivity and specificity for evaluating the tumor grade. With the threshold values of 0.160, 0.420 and 0.935 in Ktrans on tumor, glioma grades I vs. II, II vs III and III vs. IV may be differentiated with sensitivities of 0.900, 0.950 and 0.950, and specificities of 0.950, 0.950 and 0.850, respectively. Furthermore, associations were observed between the Ktrans, kep and ve values of peritumor edema and the pathological grading in glioma (Ktrans r=0.438, P<0.001; Kep r=0.385, P<0.001; Ve r=0.397, P<0.001, respectively). Ktrans values in peritumoral edema revealed significant differences between low-grade and high-grade glioma. The sensitivity and specificity for Ktrans of peritumor edema were 0.975 and 0.950, with a threshold value of 0.007. Therefore, the DCE-MRI parameters of Ktrans of tumor entity and peritumor edema in gliomas may be used to accurately differentiate glioma grades.
Keywords: 3.0T, permeability, dynamic contrast-enhanced magnetic resonance imaging, glioma, brain
Introduction
Gliomas are the most common type of neuroepithelial tumor of the brain (1). Gliomas are classified as grade I–IV by the World Health Organization (WHO; 2007) on the basis of pathological characteristics (2). Different tumor grades require distinct treatments and exhibit varied prognoses; accurate determination of the glioma grade is important to determine the appropriate treatment strategy; high-grade gliomas are usually treated with adjuvant radiotherapy or chemotherapy after resection, whereas low-grade gliomas are not (3,4). In the majority of histological grading systems, the vascular proliferation of gliomas is a diagnostic criterion for malignant tumors (5–7). A non-invasive biomarker may enable a surgeon to classify the grade of glioma, avoiding unnecessary or inaccurate biopsies.
Magnetic resonance imaging (MRI) is a non-invasive examination technique that can enable the diagnosis and grading of gliomas. However, conventional enhancement cannot quantitatively analyze tumors. MR perfusion using T2-dynamic susceptibility contrast perfusion weighted imaging (T2-DSC-PWI) (8–11) and T1-weighted dynamic contrast-enhanced magnetic resonance imaging (T1-DCE-MRI) (12–16) methods have been widely used to assess gliomas. T2-DSC-PWI, which is based on first-pass contrast-enhanced perfusion imaging, reflects the microvascular density of a tumor; however, it ignores the permeability or blood-brain barrier (BBB) damage of tumor vasculature (17,18). A previous study demonstrated that T2-DSC-PWI may underestimate the grading of gliomas (19).
DCE-MRI focuses on tumor microvascular leakage and, using quantitative analysis of the permeability parameters, it may reflect the microvascular permeability of the glioma (20). Roberts et al (20) revealed that the microvascular permeability parameters have a markedly higher association with tumor grade compared with fractional blood volume, which was achieved by T2-DSC-PWI. Previous studies have demonstrated differences in the forward volume transfer constant (Ktrans) of DCE-MRI between the tumor entity of low-grade gliomas and that of high-grade gliomas (20–27). However, to the best of our knowledge, only a limited number of studies have investigated permeability parameter (Ktrans, kep and ve) characteristics of tumor entity and peritumoral edema of distinct glioma grades. The purpose of the present study was to quantitatively analyze the tumor entity and peritumoral edema of differing glioma grades, using permeability parameters for accurate preoperative clinical assessment of glioma. Additionally, the present study aimed to provide a non-invasive imaging method to assist in future treatment planning and in predicting the prognosis of patients with glioma.
Materials and methods
Patients
The present retrospective study was approved by the Institutional Research Board of Harbin Medical University (Harbin, China) and the requirement for informed consent was waived. Patients who underwent MRI examination prior to surgery and other treatment, with a diagnosis of glioma at The Second Affiliated Hospital of Harbin Medical University (Harbin, China) between January 2012 and June 2015 were included. MRI scans which revealed obvious motion artifacts were excluded. A total of 80 patients (48 males, 32 females; mean age, 48 years; range, 32–62 years) with pathologically validated brain glioma of grade I–IV, according to the WHO (2007) classification system for brain gliomas, were included in the present study. The gliomas included 20 cases of grade I astrocytoma, 12 cases of grade II astrocytoma, 8 cases of grade II oligodendroglioma, 20 cases of grade III astrocytoma and 20 cases of grade IV glioblastoma.
MRI
Imaging was performed on 3.0 T systems (Achieva 3.0T; Philips Medical Systems B.V., Eindhoven, The Netherlands) using a quadruple birdcage head coil. All patients underwent pre-operative evaluations of the lesions by MRI and were not undergoing therapy at the time of the study. The following scanning sequences were performed: i) Conventional sequences consisting of axial and sagittal T1-weighted fast-field-echoes [repetition time (TR)/echo time (TE), 250/2.3 msec; matrix, 288×201; number of signals averaged (NSA), 1; flip angle, 75°]; axial fluid-attenuated inversion recovery [FLAIR; TR/TE/inversion time (TI), 7,000/120/2,200 msec; matrix, 232×184; NSA, 1], and T2-weighted turbo-spin-echo (TR/TE, 1,750/80 msec; matrix, 288×175; NSA, 1; flip angle, 90); ii) conventional enhancement sequence consisting of axial and sagittal T1-weighted fast-field-echo (TR/TE, 250/2.3 msec; matrix, 288×201; NSA, 1; flip angle, 75°); iii) multi-flip angle traverse T1-weighted 3D fast-field-echo (TR/TE, 14/3.2 msec; matrix, 192×144; NSA, 2; multi-flip angle, 5° and 10°); iv) DCE-MRI sequence of traverse T1-weighted 3D fast-field-echo (TR/TE, 15/3.2 msec; matrix, 192×144; NSA, 1; flip angle, 15°). The dynamic scan time/phase was 4 sec, with 120 phases and a total of 8 min. At the fifth phase, 0.1 mmol/kg body weight gadolinium (gadodiamide and Omniscan; GE Healthcare, Chicago, IL. USA) was administered intravenously with a power injector (Spectris Solaris EP, Medrad; Bayer, Newbury, UK) at a rate of 3.5 ml/sec, followed by a bolus injection of a 20 ml saline flush.
DCE-MRI analysis and region of interest (ROI) selection
The DCE-MRI data were analyzed using Omni-Kinetic software (version 1.0; GE Healthcare), with the dual-compartment pharmacokinetic model of Modified Tofts, as described previously (12,19). Filice and Crisi (28) selected the superior sagittal sinus as their venous input function as it allowed for partial volume effects to be avoided on account of artery pulse; this was adopted in the present study and used as the venous input function. The Ktrans, backflux rate (kep), and fractional volume (ve) maps were generated using Omni-Kinetic software, and the highest permeability areas of the tumor entity and peritumor edema were selected as the ROI [tumor ROI area, between 5 and 10 mm2; peritumor edema determined around the tumor, 1–2 cm away from the margin of tumor entity (the boundary of strengthen region), with an area <10 mm2). Large vessels and necrosis were avoided in ROI placement. A total of 3 ROIs were selected and the mean value was calculated.
Statistical analysis
Statistical analyses were performed using the SPSS Statistics package (version 21; IBM Corp., Armonk, NY, USA). Data are presented as the mean ± standard deviation. The Ktrans, kep and ve values of different glioma grades were analyzed using two-way analysis of variance with the Bonferroni correction. The Pearson's correlation coefficient analysis was used to analyze the association between the pathological grades and the permeability parameters. P<0.05 was considered to indicate a statistically significant difference. The receiver operating characteristic (ROC) curve was used to compare the sensitivities and specificities of different quantitative parameters, with the area under the ROC curve (AUROC) computed and the threshold values determined using the Youden Index.
Results
Correlation between permeability parameters of tumor entity, peritumor edema and the hsistopathological tumor grade
The Ktrans, kep and ve values of the tumor entity and peritumor edema of different grades are listed in Table I, all parameters increased with an increase in glioma grade. One representative case for each glioma grade is presented in Figs. 1–4. The Ktrans, kep, and ve values of acute tumor were strongly associated with the pathological grade of the gliomas, with correlation coefficients of 0.951, 0.804 and 0.766, respectively (Table II). The Ktrans, kep, and ve values of peritumor edema were moderately associated with the pathological grades of the gliomas, with correlation coefficients of 0.438, 0.385 and 0.397, respectively (Table II).
Table I.
Ktrans, kep and ve values of tumor entity and peritumor edema in different glioma grades.
| Ktrans, min−1 | kep, min−1 | ve | ||||||
|---|---|---|---|---|---|---|---|---|
| Grade | n | Tumor entity | Peritumor edema | Tumor entity | Peritumor edema | Tumor entity | Peritumor edema | |
| I | 20 | 0.063±0.053 | 0.003±0.001 | 0.314±0.126 | 0.199±0.106 | 0.251±0.205 | 0.012±0.011 | |
| II | 20 | 0.271±0.073 | 0.004±0.002 | 0.823±0.317 | 0.273±0.154 | 0.387±0.200 | 0.012±0.008 | |
| III | 20 | 0.730±0.185 | 0.018±0.016 | 0.980±0.373 | 0.321±0.193 | 0.799±0.204 | 0.049±0.047 | |
| IV | 20 | 1.153±0.140 | 0.021±0.028 | 1.424±0.269 | 0.409±0.258 | 0.817±0.121 | 0.060±0.081 | |
Ktrans, forward volume transfer constant; kep, backflux rate; ve, fractional volume.
Figure 1.
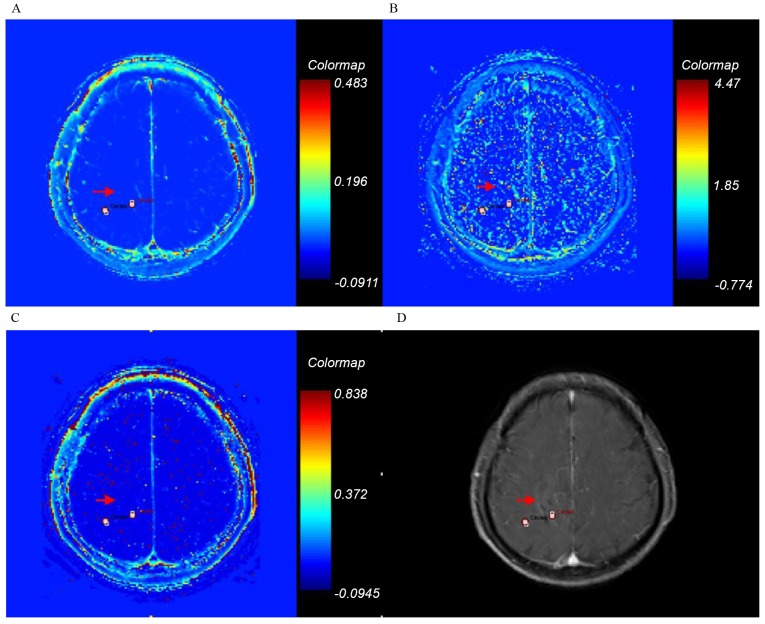
Ktrans, kep, ve and axial T1-weighted enhancement maps of grade I glioma. Glioma is on the right frontal-parietal lobe (arrow). (A) Ktrans; (B) kep; (C) ve; (D) axial T1-weighted enhancement map. Ktrans, forward volume transfer constant; kep, backflux rate; ve, fractional volume.
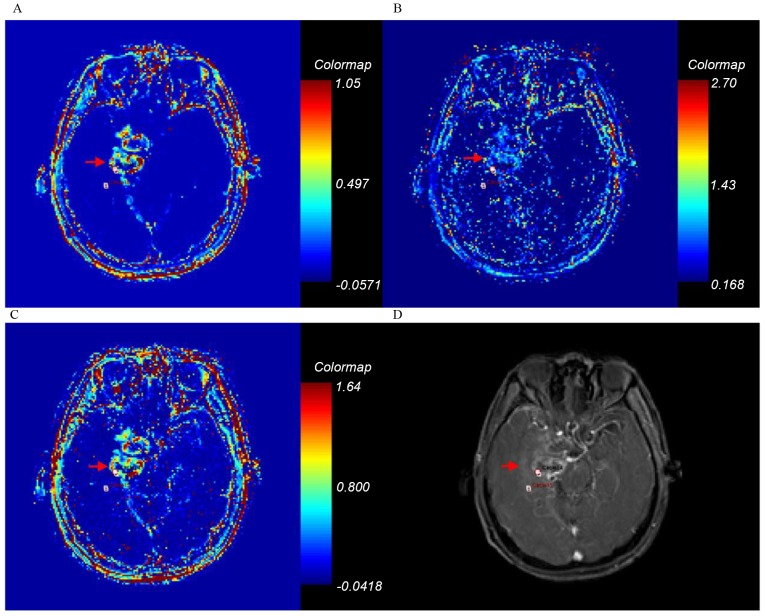
Figure 4.
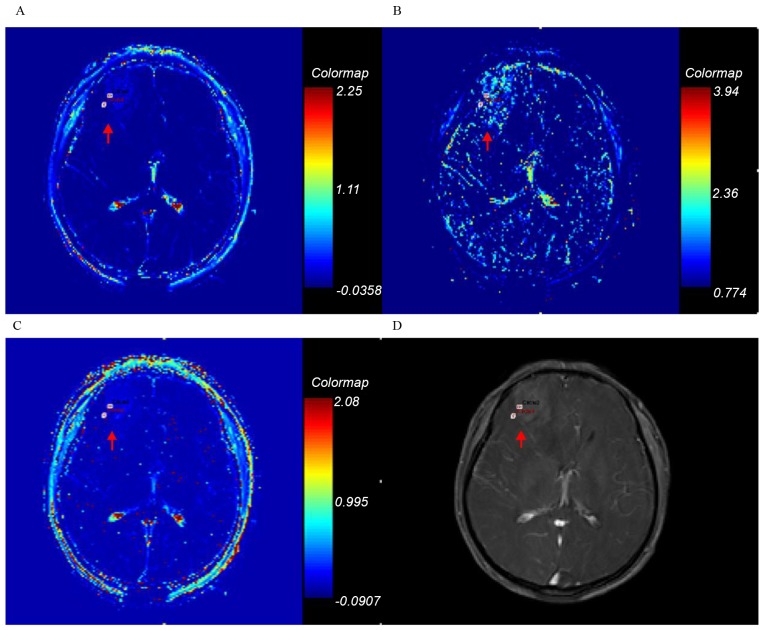
Ktrans, kep, ve and axial T1-weighted enhancement maps of grade IV glioma. Glioma is on the right parietal lobe (arrow). (A) Ktrans; (B) kep; (C) ve; (D) axial T1-weighted enhancement map. Ktrans, forward volume transfer constant; kep, backflux rate; ve, fractional volume.
Table II.
Pearsons correlation between the Ktrans, kep and ve values of tumor entity and peritumor edema and the histopathological tumor grade.
| Ktrans, min−1 | kep, min−1 | ve | |||||
|---|---|---|---|---|---|---|---|
| Grade | r | P-value | r | P-value | r | P-value | |
| Tumor entity | 0.951 | <0.001a | 0.804 | <0.001a | 0.766 | <0.001a | |
| Peritumor edema | 0.438 | <0.001a | 0.385 | <0.001a | 0.397 | <0.001a | |
Ktrans, forward volume transfer constant; kep, backflux rate; ve, fractional volume.
P<0.05.
Comparison between permeability parameters of tumor entity in different glioma grades
There were significant differences determined between different glioma groups of Ktrans, as presented in Table III. kep values were different between different groups, with the exception of between grades II and III (Table III). The ve values varied between groups, with the exception being between grade I and II, and between grade III and IV (Table III). ROC curves to discriminate different glioma grades using Ktrans, kep and ve are presented respectively in Fig. 5. The threshold values of Ktrans, kep and ve were 0.160, 0.365 and 0.285 to distinguish between grades I and II, 0.420, 0.915 and 0.505 to distinguish between grades II and III, and 0.935, 1.015 and 0.615 to distinguish between grades III and IV, respectively.
Table III.
P-values, determined using analysis of variance with Bonferroni corrections, for the Ktrans, kep and ve parameters of the tumor entity and peritumor edema to differentiate between the histologically defined tumor grades.
| P-value | |||||||
|---|---|---|---|---|---|---|---|
| Ktrans, min−1 | kep, min−1 | ve | |||||
| Tumor grade | Tumor entity | Peritumor edema | Tumor entity | Peritumor edema | Tumor entity | Peritumor edema | |
| I vs. II | <0.01a | >0.05 | <0.01a | >0.05 | >0.05 | >0.05 | |
| I vs. III | <0.01a | <0.05a | <0.01a | >0.05 | <0.01a | >0.05 | |
| I vs. IV | <0.01a | <0.01a | <0.01a | <0.01a | <0.01a | <0.05a | |
| II vs. III | <0.01a | >0.05 | >0.05 | >0.05 | <0.01a | >0.05 | |
| II vs. IV | <0.01a | <0.01a | <0.01a | >0.05 | <0.01a | <0.05a | |
| III vs. IV | <0.01a | >0.05 | <0.01a | >0.05 | >0.05 | >0.05 | |
Ktrans, forward volume transfer constant; kep, backflux rate; ve, fractional volume.
P<0.05.
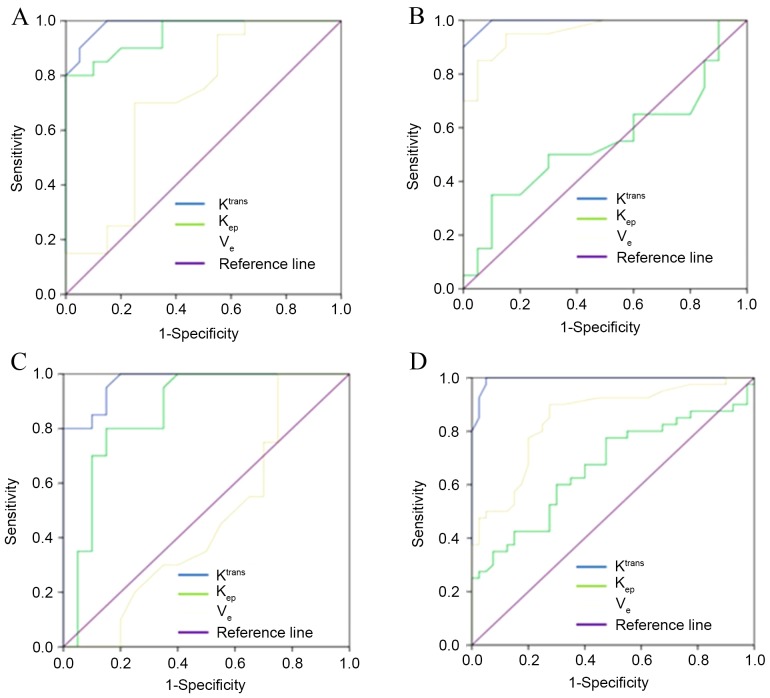
Figure 5.
ROC curve was used to compare the sensitivities and specificities of different quantitative parameters in gliomas. ROC discriminated the tumor entity between (A) grades I and II (AUC, 0.986 for Ktrans; 0.951 for kep; and 0.709 for ve); (B) between grades II and III (AUC, 0.995 for Ktrans; 0.548 for kep; and 0.959 for ve); and (C) between grades III and IV (AUC, 0.971 for Ktrans; 0.861 for kep; and 0.465 for ve). (D) ROC discriminated the peritumor edema between low and high-grade (AUC, 0.994 for Ktrans; 0.668 for kep; and 0.848 for ve). ROC, receiver operating characteristic; AUC, area under the curve; Ktrans, forward volume transfer constant; kep, backflux rate; ve, fractional volume.
The AUROC sensitivity and specificity threshold values for Ktrans of tumor entity are listed in Table IV. With threshold values of 0.160 and 0.420 and 0.935, the AUROC using Ktrans to differentiate between glioma grade I and II, grade II and III, grade III and IV were 0.986, 0.995 and 0.971, respectively.
Table IV.
AUC, threshold, sensitivity and specificity of Ktrans values of tumor entity to differentiate different tumor grades.
| Ktrans, min−1 | AUC | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|
| I vs. II | 0.986 | 0.160 | 0.900 | 0.950 |
| II vs. III | 0.995 | 0.420 | 0.950 | 0.950 |
| III vs. IV | 0.971 | 0.935 | 0.950 | 0.850 |
AUC, area under the curve; Ktrans, forward volume transfer constant.
Comparison between permeability parameters of peritumor edema in different glioma grades
The Ktrans, kep and ve values of peritumor edema in different grades are presented in Table I. Ktrans values of peritumor edema revealed significant differences between grades I and grade III or IV, and between grades II and grade III or IV. There were no differences in Ktrans of peritumor edema between grade I and II, or between grade III and IV (Table III). The AUROC sensitivity and specificity thresholds for Ktrans of peritumor edema in ROC analysis are listed in Table V. The Ktrans and ve values of peritumor edema were associated with the pathological grade of the gliomas, as presented in Table II. Using ROC curve analysis, a threshold Ktrans value of 0.007 was used to differentiate low and high-grade glioma, with a sensitivity of 0.975 and a specificity of 0.950 (Fig. 5; Table V).
Table V.
AUC, threshold, sensitivity and specificity of Ktrans values of peritumor edema to differentiate low-grade from high-grade gliomas.
| I/II vs. III/IV | AUC | Threshold | Sensitivity | Specificity |
|---|---|---|---|---|
| Ktrans, min−1 | 0.994 | 0.007 | 0.975 | 0.950 |
AUC, area under the curve; Ktrans, forward volume transfer constant.
Discussion
The present study quantitatively analyzed the permeability parameters of different glioma grades, and provided ranges for accurate pre-operative clinical assessment of tumor grades. Ktrans, kep and ve values in tumor entity and peritumor edema revealed significant differences between low and high glioma grades. The microvascular permeability parameter Ktrans has a higher diagnostic performance, compared with kep and ve, with significant differences identified between tumor entities in different glioma grades (Table III). kep values were not different between grades II and III and ve values were not different between grades I and II, or between grades III and IV. In addition, the results indicated that the Ktrans values were positively associated with the tumor grade (Table II).
The results of the present study indicated that permeability parametric changes were associated with the biological characteristics of the lesion. Compared with low-grade gliomas, the cells of high-grade gliomas exhibited a high degree of vigorous growth and tumor angiogenesis increased. The BBB of tumor angiogenesis is incomplete or immature, the vessels affected by the tumor lack integrity and the distance between endothelial cells increases thus causing vascular permeability to be increased, leading to increases in Ktrans and kep. For increased tumor grades, the damage to the BBB increases, with increased vascular permeability and significantly increased Ktrans.
kep is a rate constant which reflects the rate of the contrast agents between the vasculature and extravascular extracellular space (EES). The kep parameter may reflect the destruction of the BBB by the tumor; however, it may reflect the pressure of the tissue around the tumor and, thus, indirectly reflect the degree of malignancy of the tumor. Compared with low-grade gliomas, high-grade gliomas invaded an increased amount of peritumor edema and the tissue pressure around the tumor was increased, which resulted in increased kep. kep directly reflects the degree of damage to the vascular BBB, and indirectly reflects the degree of peritumoral edema and the interstitial pressure around the tumor. The kep values of certain tumor grades are statistically different, with the exception being between grade II and III (Table III). The values of the ve increased with the increasing glioma grades. However, there was no statistically significant difference identified between grade I and II, and between grades III and IV.
In the peritumor edema, Ktrans values demonstrated a significant difference between low- and high-grade gliomas, demonstrating that there was increased damaged to the BBB damage in high-grade, compared with low-grade glioma. This difference indicated that the microvascular permeability of peritumor edema increased with the increasing pathological grades of the gliomas. Whereas in conventional MR enhancement, peritumor edema cannot be quantitatively analyzed and did not provide an accurate guidance for glioma classification.
The permeability parameters determined in peritumor edema may indicate the breakdown of the BBB, which suggests that tumor cells may infiltrate in peritumor edema. Bruger et al (29) validated the boundary of gliomas using pathology. The boundary of gliomas is neither an area of T1WI enhancement nor a region of T2WI high signal. Winkler et al (30) identified malignant tumor cells invasion followed myelinated fiber tracts and perivascular space to the region of peritumor edema. Tumor cells invasion is more evident in the highly malignant tumor than lowly malignant tumor. The present study revealed that the increase in Ktrans, kep and ve values in a region of peritumor edema, with and without enhancement, may suggest the possibility of tumor invasion. The results of the present study suggested that tumor cell invasion may be of increased severity in the peritumor edema region of high-grade gliomas compared with low-grade gliomas. Quantitative parameters achieved by DCE-MRI may define the boundaries of the tumor more accurately compared with conventional MR enhancement and guide surgical treatment, and reduce the recurrence rate following surgery.
DCE-MRI is on the basis of pharmacokinetic modeling using a two-compartment model (12,19), with the quantitative parameters reflecting the leakage of the contrast agent, and consequently the degree of damage to the vascular BBB of the glioma. Roberts et al (20) demonstrated that microvascular permeability exhibited an association with the grade of a tumor.
A previous study revealed that small molecule contrast agents may be used to quantify microvascular permeability and identified that the degree of malignant tumor increased with high vascular permeability (21). High-grade gliomas exhibit high vascular permeability due to the destruction of the BBB of the vessels and tumor angiogenesis (20,31–33). Compared with other permeability parameters (kep and ve), Ktrans may be more accurate in the evaluation of glioma. Previous studies evaluated tumor grades by calculating the microvascular permeability of the gliomas; the relative permeability values of malignant gliomas were significantly increased, compared with those of benign gliomas (20–27). However, a limited number of studies performed a quantitative analysis of the Ktrans values in the peritumor edema area. Microvascular permeability parameters of peritumor edema were analyzed by Jensen et al (34), but grading measurement or ROC curve analysis using peritumor edema was not performed. In the present study, the Ktrans values of peritumor edema of high and low gliomas were identified to be significantly different, and the corresponding threshold value was obtained.
The present study identified specific thresholds, and identified that tumor entity and peritumor edema revealed significant differences between different glioma grades, which may enable an accurate tumor boundary to be determined. The present study had a number of limitations. A selection bias of ROI may be present in the present study, because the measuring area and surgical resection area are not the same. Therefore, the grade of glioma may have been underestimated or overestimated.
DCE-MRI is a valuable technique that may distinguish different glioma grades using the quantitative permeability parameter of Ktrans, which may assist with the choice of clinical treatment for gliomas, including surgery, radiotherapy and chemotherapy. Furthermore, the peritumor edema provides information on the invasion degree of tumor cells and may serve a function in clinical treatment and prognosis evaluation.
Figure 2.
Ktrans, kep, ve and axial T1-weighted enhancement maps of grade II glioma. Glioma is on the right frontal lobe (arrow). (A) Ktrans; (B) kep; (C) ve; (D) axial T1-weighted enhancement map. Ktrans, forward volume transfer constant; kep, backflux rate; ve, fractional volume.
Figure 3.
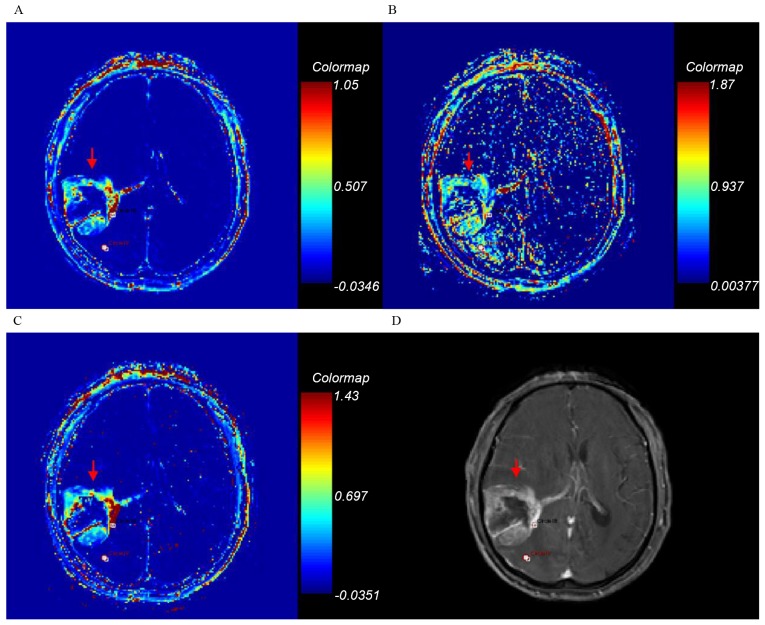
Ktrans, kep, ve and axial T1-weighted enhancement maps of grade III glioma. Glioma is on the right depth of temporal lobe (arrow). (A) Ktrans; (B) kep; (C) ve; (D) axial T1-weighted enhancement map. Ktrans, forward volume transfer constant; kep, backflux rate; ve, fractional volume.
Acknowledgements
The present study was supported by the Health and Family Planning Commission Foundation of Heilongjiang province (grant no. 2014-438).
References
- 1.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inoue T, Ogasawara K, Beppu T, Ogawa A, Kabasawa H. Diffusion tensor imaging for preoperative evaluation of tumor grade in gliomas. Clin Neurol Neurosurg. 2005;107:174–180. doi: 10.1016/j.clineuro.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Zonari P, Baraldi P, Crisi G. Multimodal MRI in the characterization of glial neoplasms: The combined role of singl-evoxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging. Neuroradiology. 2007;49:795–803. doi: 10.1007/s00234-007-0253-x. [DOI] [PubMed] [Google Scholar]
- 5.Takekawa Y, Sawada T. Vascular endothelial growth factor and neovascularization in astrocytic tumors. Pathol Int. 1998;48:109–114. doi: 10.1111/j.1440-1827.1998.tb03879.x. [DOI] [PubMed] [Google Scholar]
- 6.Abdulrauf SI, Edvardsen K, Ho KL, Yang XY, Rock JP, Rosenblum ML. Vascular endothelial growth factor expression and vascular density as prognostic markers of survival in patients with low-grade astrocytoma. J Neurosur. 1998;88:513–520. doi: 10.3171/jns.1998.88.3.0513. [DOI] [PubMed] [Google Scholar]
- 7.Brem S, Cotran R, Folkman J. Tumor angiogenesis: A quantitative method for histologic grading. J Natl Cancer Inst. 1972;48:347–356. [PubMed] [Google Scholar]
- 8.Maeda M, Itoh S, Kimura H, Iwasaki T, Hayashi N, Yamamoto K, Ishii Y, Kubota T. Tumor vascularity in the brain: Evaluation with dynamic susceptibility-contrast MR imaging. Radiology. 1993;189:233–238. doi: 10.1148/radiology.189.1.8372199. [DOI] [PubMed] [Google Scholar]
- 9.Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, Harsh GR, Cosgrove GR, Halpern EF, Hochberg FH, et al. Cerebral blood volume maps of gliomas: Comparison with tumor grade and histologic findings. Radiology. 1994;191:41–51. doi: 10.1148/radiology.191.1.8134596. [DOI] [PubMed] [Google Scholar]
- 10.Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, Shigematsu Y, Liang L, Ge Y, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol. 1998;171:1479–1486. doi: 10.2214/ajr.171.6.9843274. [DOI] [PubMed] [Google Scholar]
- 11.Knopp EA, Cha S, Johnson G, Mazumdar A, Golfinos JG, Zagzag D, Miller DC, Kelly PJ, Kricheff II. Glial neoplasms: Dynamic contrast-enhanced T2*-weighted MR imaging. Radiology. 1999;211:791–798. doi: 10.1148/radiology.211.3.r99jn46791. [DOI] [PubMed] [Google Scholar]
- 12.Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(SICI)1522-2586(199909)10:3<223::AID-JMRI2>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 13.Zhang N, Zhang L, Qiu B, Meng L, Wang X, Hou BL. Correlation of volume transfer coefficient Ktrans with histopathologic grades of gliomas. J Magn Reson Imaging. 2012;36:355–363. doi: 10.1002/jmri.23675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jia Z, Geng D, Xie T, Zhang J, Liu Y. Quantitative analysis of neovascular permeability in glioma by dynamic contrast-enhanced MR imaging. J Clin Neurosci. 2012;19:820–823. doi: 10.1016/j.jocn.2011.08.030. [DOI] [PubMed] [Google Scholar]
- 15.Dujardin MI, Sourbron SP, Chaskis C, Verellen D, Stadnik T, de Mey J, Luypaert R. Quantification of cerebral tumour blood flow and permeability with T1-weighted dynamic contrast enhanced MRI: A feasibility study. J Neuroradiol. 2012;39:227–235. doi: 10.1016/j.neurad.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Sourbron SP, Buckley DL. Tracer kinetic modelling in MRI: Estimating perfusion and capillary permeability. Phys Med Biol. 2012;57:R1–33. doi: 10.1088/0031-9155/57/2/R1. [DOI] [PubMed] [Google Scholar]
- 17.Johnson G, Wetzel SG, Cha S, Babb J, Tofts PS. Measuring blood volume and vascular transfer constant from dynamic, T(2)*-weighted contrast-enhanced MRI. Magn Reson Med. 2004;51:961–968. doi: 10.1002/mrm.20049. [DOI] [PubMed] [Google Scholar]
- 18.Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14:249–265. doi: 10.1002/mrm.1910140211. [DOI] [PubMed] [Google Scholar]
- 19.Murase K. Efficient method for calculating kinetic parameters using T1-weighted dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Med. 2004;51:858–862. doi: 10.1002/mrm.20022. [DOI] [PubMed] [Google Scholar]
- 20.Roberts HC, Roberts TP, Brasch RC, Dillon WP. Quantitative measurement of microvascular permeability in human brain tumors achieved using dynamic contrast-enhanced MR imaging: Correlation with histologic grade. AJNR Am J Neuroradiol. 2000;21:891–899. [PMC free article] [PubMed] [Google Scholar]
- 21.Daldrup H, Shames DM, Wendland M, Okuhata Y, Link TM, Rosenau W, Lu Y, Brasch RC. Correlation of dynamic contrast-enhanced MR imaging with histologic tumor grade: Comparison of macromolecular and small-molecular contrast media. AJR Am J Roentgenol. 1998;171:941–949. doi: 10.2214/ajr.171.4.9762973. [DOI] [PubMed] [Google Scholar]
- 22.Provenzale JM, Wang GR, Brenner T, Petrella JR, Sorensen AG. Comparison of permeability in high-grade and low-grade brain tumors using dynamic susceptibility contrast MR imaging. AJR Am J Roentgenol. 2002;178:711–716. doi: 10.2214/ajr.178.3.1780711. [DOI] [PubMed] [Google Scholar]
- 23.Roberts HC, Roberts TP, Ley S, Dillon WP, Brasch RC. Quantitative estimation of microvascular permeability in human brain tumors: Correlation of dynamic Gd-DTPA-enhanced MR imaging with histopathologic grading. Acad Radiol. 2002;9(Suppl 1):S151–S155. doi: 10.1016/S1076-6332(03)80425-7. [DOI] [PubMed] [Google Scholar]
- 24.Falk A, Fahlström M, Rostrup E, Berntsson S, Zetterling M, Morell A, Larsson HB, Smits A, Larsson EM. Discrimination between glioma grades II and III in suspected low-grade gliomas using dynamic contrast-enhanced and dynamic susceptibility contrast perfusion MR imaging: A histogram analysis approach. Neuroradiology. 2014;56:1031–1038. doi: 10.1007/s00234-014-1426-z. [DOI] [PubMed] [Google Scholar]
- 25.Roy B, Awasthi R, Bindal A, Sahoo P, Kumar R, Behari S, Ojha BK, Husain N, Pandey CM, Rathore RK, Gupta RK. Comparative evaluation of 3-dimensional pseudocontinuous arterial spin labeling with dynamic contrast-enhanced perfusion magnetic resonance imaging in grading of human glioma. J Comput Assist Tomogr. 2013;37:321–326. doi: 10.1097/RCT.0b013e318282d7e2. [DOI] [PubMed] [Google Scholar]
- 26.Choi HS, Kim AH, Ahn SS, Shin NY, Kim J, Lee SK. Glioma grading capability: Comparisons among parameters from dynamic contrast-enhanced MRI and ADC value on DWI. Korean J Radiol. 2013;14:487–492. doi: 10.3348/kjr.2013.14.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung SC, Yeom JA, Kim JH, Ryoo I, Kim SC, Shin H, Lee AL, Yun TJ, Park CK, Sohn CH, et al. Glioma: Application of histogram analysis of pharmacokinetic parameters from T1-weighted dynamic contrast-enhanced MR imaging to tumor grading. AJNR Am J Neuroradiol. 2014;35:1103–1110. doi: 10.3174/ajnr.A3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filice S, Crisi G. Dynamic contrast-enhanced perfusion MRI of high grade brain gliomas obtained with arterial or venous waveform input function. J Neuroimaging. 2016;26:124–129. doi: 10.1111/jon.12254. [DOI] [PubMed] [Google Scholar]
- 29.Bruger PC, Vogel FS, Green SB, Strike TA. Glioblastoma multiforme and anaplastie astrocytoma: Pathologic criteria and prognostic implications. Cancer. 1985;565:1106–1111. doi: 10.1002/1097-0142(19850901)56:5<1106::AID-CNCR2820560525>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Winkler F, Kienast Y, Fuhrmann M, Von Baumgarten L, Burgold S, Mitteregger G, Kretzschmar H, Herms J. Imaging glioma cell invasion in vivo reveals mechanisms of dissemination and peritumoral angiogenesis. Glia. 2009;57:1306–1315. doi: 10.1002/glia.20850. [DOI] [PubMed] [Google Scholar]
- 31.van Dijke CF, Brasch RC, Roberts TP, Weidner N, Mathur A, Shames DM, Mann JS, Demsar F, Lang P, Schwickert HC. Mammary carcinoma model: Correlation of macromolecular contrast-enhanced MR imaging characterizations of tumor microvasculature and histologic capillary density. Radiology. 1996;198:813–818. doi: 10.1148/radiology.198.3.8628876. [DOI] [PubMed] [Google Scholar]
- 32.Schwickert HC, Stiskal M, Roberts TP, van Dijke CF, Mann J, Mühler A, Shames DM, Demsar F, Disston A, Brasch RC. Contrast-enhanced MR imaging assessment of tumor capillary permeability: Effect of irradiation on delivery of chemotherapy. Radiology. 1996;198:893–898. doi: 10.1148/radiology.198.3.8628889. [DOI] [PubMed] [Google Scholar]
- 33.Taylor JS, Tofts PS, Port R, Evelhoch JL, Knopp M, Reddick WE, Runge VM, Mayr N. MR imaging of tumor microcirculation: Promise for the new millennium. J Magn Reson Imaging. 1999;10:903–907. doi: 10.1002/(SICI)1522-2586(199912)10:6<903::AID-JMRI1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 34.Jensen RL, Mumert ML, Gillespie DL, Kinney AY, Schabel MC, Salzman KL. Preoperative dynamic contrast-enhanced MRI correlates with molecular markers of hypoxia and vascularity in specific areas of intratumoral microenvironment and is predictive of patient outcome. Neuro Oncol. 2014;16:280–291. doi: 10.1093/neuonc/nou206.47. [DOI] [PMC free article] [PubMed] [Google Scholar]