Abstract
Cystinuria is an incompletely dominant disorder characterized by defective urinary cystine reabsorption that results in the formation of cystine-based urinary stones. Current treatment options are limited in their effectiveness at preventing stone recurrence and often poorly tolerated. We report that the nutritional supplement α-lipoic acid inhibits cystine stone formation in the Slc3a1-/- mouse model of cystinuria by increasing the solubility of urinary cystine. These findings identify a novel therapeutic strategy for the clinical treatment of cystinuria.
Kidney stones affect approximately 9% of the U.S. population, with the rate of incidence and prevalence increasing within the U.S. and globally1. While surgical techniques to remove obstructive stones have improved, few therapeutic advances have been made to prevent stone recurrence2. Cystinuria is a rare type of kidney stone disease caused by mutations in the SLC3A1 and/or SLC7A9 genes that are responsible for cystine reabsorption in the renal proximal tubule, and is characterized by aggressive and recurrent cystine stone formation. Currently available interventions aimed at preventing cystine stones in these individuals include increasing fluid intake and pharmaceutical compounds that increase urinary pH or interfere with cysteine dimerization3. However, these measures have marginal effects on stone prevention, and the medications are poorly tolerated with sometimes serious adverse side effects4,5. Furthermore, cystine stones are dense, large, and largely resistant to extracorporeal shock wave lithotripsy (SWL), often requiring multiple procedures or surgery to remove obstructive stones6. The high rate of stone recurrence and surgical burden associated with cystinuria thus places these patients at increased risk for reduced kidney function and chronic kidney damage7.
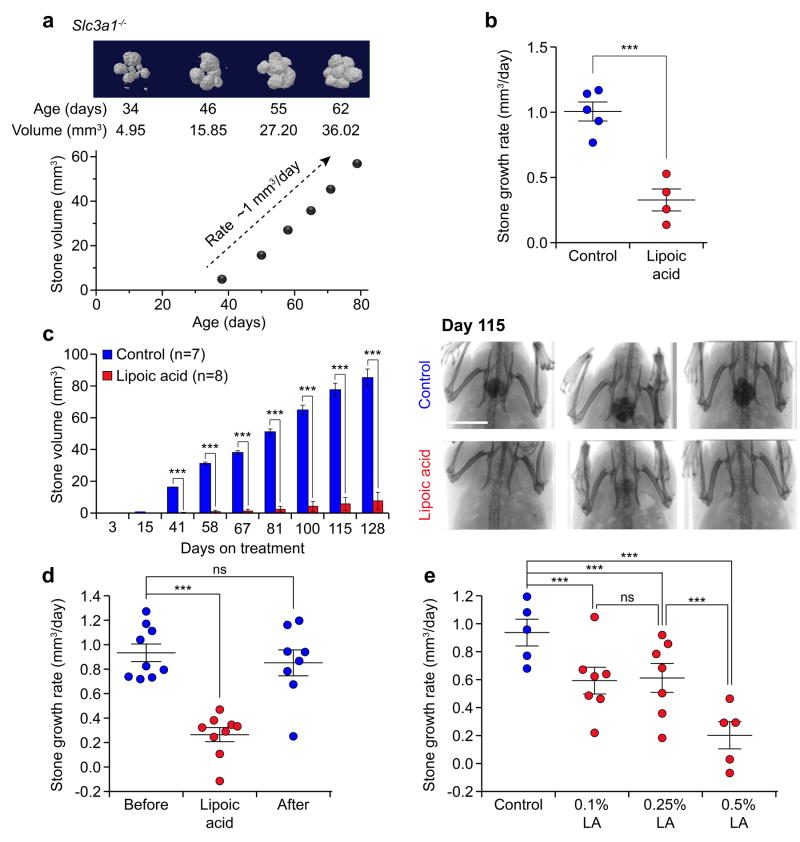
To address the clinical challenges associated with cystinuria, we utilized the Slc3a1-/- mouse model which develops cystine urolithiasis, to identify compounds that effectively inhibit stone formation8. Micro-computed tomography (μCT) analysis revealed that Slc3a1-/- mice accumulated urinary bladder stones at an average rate of 1 mm3/day (Fig. 1a). We applied this measure to evaluate compounds for their effects on cystine stone growth in vivo. Notably, treatment of mice with the drug tiopronin, a sulfhydryl drug approved for treatment of cystinuria, had no significant effect on cystine stone growth rate compared to mice on a regular diet (Supplementary Fig.1a). This result was consistent with observations that tiopronin has limited therapeutic effect9. Similarly, treatment of mice with L-cystine dimethylester (L-CDME), a cystine crystal growth inhibitor that has previously been shown to result in smaller stone size distrubtion8,10, also had no significant effect on cystine stone growth rate compared to mice on a regular diet (Supplementary Fig. 1b). To identify more effective inhibitors, we evaluated compounds for cystine stone inhibition based on two criteria: activation of the antioxidant response signaling pathway that promotes glutathione synthesis and cellular cystine uptake, and successful in vivo delivery to the renal proximal tubules and urine. Treatment with the pro-antioxidant sulforaphane11 had a modest effect on cystine stone growth (Supplementary Fig. 1c). In contrast, we found that the pro-antioxidant compound α-lipoic acid (α-LA) was a strong suppressor of stone growth as mice treated with α-LA had lower stone formation growth compared to untreated mice (Fig. 1b).
Figure 1.
α-Lipoic acid inhibits cystine stone formation in the Slc3a1-/- mouse model of cystinuria. (a) In vivo bladder stone growth in a representative Slc3a1-/- male mouse (n = 24), quantified by μCT analysis. (b) Stone growth rate in stone-forming Slc3a1-/- mice treated with α-lipoic acid (0.5%, w/w supplementation in the diet) (n = 4) compared to control, untreated mice (n = 5). Statistical differences in stone volume growth rate relative to the control were tested using a linear mixed regression model. (c) Stone volume in Slc3a1-/- mice reared on α-lipoic acid supplemented diet (n = 8) compared to untreated control mice (n = 7) (Left). Statistical differences in stone volume between α-lipoic acid treatment and control at each point were determined by Student’s t-tests, after adjusting for multiple comparisons (see Online Methods). (Right) Representative radiograph images (n = 361 μCT scans) of the pelvic region of control mice and α-lipoic acid-treated mice on day 115 of treatment. Results are representative of three independent experiments. Scale bar, 1 cm. (d) Stone growth rate evaluated by weekly μCT for 6 weeks of pre-treatment, α-lipoic acid treatment, and post-treatment periods in Slc3a1-/- mice (n = 9). Statistical differences in stone volume growth rate between treatment periods were tested using a linear mixed regression model with linear spline. (e) Stone growth rate in Slc3a1-/- mice upon different doses of α-lipoic acid administration (control, n = 5; 0.1% LA, n = 7; 0.25% LA, n = 7 ; 0.5% LA, n = 5). Statistical differences in stone volume growth rate were tested using a linear mixed regression model. Error bars represent mean ± s.e.m. Data are plotted for individual mice; *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
We treated 4 to 7-week old male Slc3a1-/- mice, prior to any stone formation, with oral administration of α-LA (0.5%) supplementation into the diet. While all untreated Slc3a1-/- mice developed stones within 6 weeks of the study and continued to progressively accrue stone volume, mice treated with α-LA had significantly delayed stone formation, lower overall stone volume accumulation, and formed fewer stones (Fig. 1c and Supplementary Fig. 1d-e). Further, α-LA was an effective suppressor of cystine stone growth, as Slc3a1-/- mice with existing stones demonstrated decreased stone growth rate when treated with α-LA. Withdrawal of α-LA over a period of four weeks resulted in reversion of stone growth rate to the initial rate of growth (Fig. 1d), suggesting that continuous α-LA treatment was necessary for its effect on stone inhibition.
Mouse intake of the 0.5% α-LA supplemented diet is approximately equivalent to a human dose of 2700 mg/day for a 67 kg adult12. While no human toxicity limit for α-LA supplementation has been established, we tested whether lower doses of α-LA effectively inhibited stone growth in the Slc3a1-/- mouse. 0.1% and 0.25% α-LA in the diet, approximately equivalent to human doses of 540 and 1350 mg/day respectively12, significantly attenuated cystine stone growth compared to untreated mice (Fig. 1e). However, these moderate doses were also significantly less effective than the high dose of 0.5% α-LA in the diet, indicating that the effect of α-LA on cystine stone growth was dose-dependent (Fig. 1e). Of note, clinical trials assessing α-LA daily treatment at 600 mg to 1800 mg doses have reported no major adverse reactions13.
α-LA treatment protected Slc3a1-/- kidneys from atrophy and hydronephrosis-related damage (Supplementary Fig. 2)14, likely through the prevention of obstructive stones. Slc3a1-/- mice treated with α-LA had normal body weight, and modestly greater food intake and water intake compared to mice not treated with α-LA (Supplementary Fig. 3a-c), suggesting that α-LA did not prevent stones through an anorexic mechanism15 or by promoting hydration. Additionally, urinary pH was not significantly different between mice treated with α-LA compared to untreated controls (Supplementary Fig. 3d).
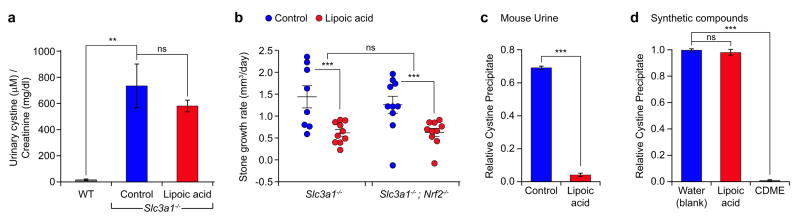
α-LA treatment did not affect urinary cystine concentration in Slc3a1-/- mice, as cystine levels in control versus α-LA treated mice were not significantly changed (Fig. 2a). This result suggests that α-LA (or its reduced form dihydrolipoic acid) did not chelate cysteine or interfere with cysteine dimerization, as is the mechanism of thiol binding drugs like tiopronin and penicillamine.
Figure 2.
α-Lipoic acid increases cystine solubility in the urine environment. (a) Urinary cystine concentration from wild-type (n = 2), control Slc3a1-/- (n = 4), and α-lipoic acid-treated Slc3a1-/- (n = 5) mice. Values are normalized to creatinine measurements. Error bars represent mean ± s.e.m. Statistical differences in urinary cystine concentration were determined by Student’s t-test. (b) Stone growth rates in Slc3a1-/- mice (control, n = 8; treated, n = 10) and Slc3a1-/-;Nrf2-/- (control, n = 10; treated, n = 10) treated with α-lipoic acid. Data are plotted for individual mice, error bars represent mean ± s.e.m, and statistical differences across mouse groups in the effects of α-lipoic acid compared to control were tested using a linear mixed regression model. (c) Relative yield of L-cystine precipitation obtained after crystallization for 3 days in the presence of urine from untreated- and α-lipoic acid-treated wild-type mice compared to blank (water). Statistical differences were determined by Student’s t-test. The error bars represent mean ± s.d. based on three measurements. (d) Relative yield of L-cystine precipitation obtained after crystallization for 3 days in the presence of α-lipoic acid (500μM) and L-cystine dimethyl ester (CDME, 500μM), a positive control10 compared to blank (water). Statistical differences were determined by student’s t-test. The error bars represent mean ± s.d. based on three measurements. *P < 0.05, **P < 0.01, ***P < 0.001. ns, not significant.
We then tested the hypothesis that α-LA inhibited cystine stone formation by promoting cystine transport and metabolism. α-LA treatment has been shown to increase Nrf2 nuclear localization and the transcription of Nrf2-mediated genes that promote glutathione biosynthesis via cystine import and cellular cysteine utilization16,17. We examined whether α-LA effects on cystine stone growth were dependent upon Nrf2 in vivo. Slc3a1-/-; Nrf2-/- double knockout mice were treated with α-LA and compared to Slc3a1-/- control littermates. Nrf2 did not contribute to cystine stone formation, as the rate of stone growth in Slc3a1-/-; Nrf2-/- mice was not significantly different compared to Slc3a1-/- mice (Fig. 2b). Additionally, the effect of α-LA on stone growth was conserved in the Slc3a1-/-; Nrf2-/- mice, indicating that α-LA regulated cystine stone formation independently of its role in activating the Nrf2-dependent antioxidant response (Fig. 2b).
Based on these results and the clinical observations that α-LA administration alters urine attributes18, we examined whether α-LA affected cystine solubility in the urine. Ex vivo cystine precipitation analysis revealed that cystine was considerably more soluble in α-LA treated urine compared to untreated urine (Fig. 2c). However, while previous studies have shown that CDME interferes with cystine crystal growth and results in crystals of distinctly different shape10, α-LA treatment did not alter the cystine hexagonal crystal habit (Supplementary Fig. 4). Further, synthetic α-LA had no detectable effect on cystine solubility, suggesting that α-LA did not directly interfere with cystine precipitation (Fig. 2d). This result is consistent with the observation that exogenous α-LA is known to undergo extensive metabolism in both mice and humans, including β-oxidation, sulfur methylation, sulfur oxidation, and glycine conjugation in mice, before being excreted into the urine19. It is likely that these α-LA-derived metabolic changes and/or metabolites in the urine are ultimately responsible for preventing cystine stone formation in Slc3a1-/- mice. Further investigation will be needed to identify the specific metabolite(s) of α-LA responsible for increasing cystine solubility, and how these α-LA-induced changes affect cystine crystallization.
We report here a new therapeutic application of α-LA treatment that protects against cystine stone formation in a mouse model of cystinuria. While α-LA has previously been implicated for its beneficial effects in promoting stress-activated signaling mechanisms, our data identifies a novel function of α-LA on that relies upon increasing urinary cystine solubility, likely via the excretion of downstream α-LA metabolites into the urine. This increase in cystine solubility is achieved without inducing changes to urinary pH, which predisposes to other types of stone formation (e.g. calcium phosphate)20. That α-LA is a widely available nutritional supplement with few adverse side effects13 makes it potentially advantageous over currently available medications and a particularly attractive candidate for assessment in individuals with cystinuria. These findings highlight a new avenue for treating and preventing cystine stone recurrence, and have implications for understanding treatment approaches for other types of urinary stone disease.
Online Methods
Animals
Homozygous Slc3a1-/- male mice (on a mixed C57Bl/6 and 129/SvJ background) were bred and maintained as previously described21. Specifically, Slc3a1-/- mice were generated by homologous recombination in embryonic stem (ES) cells. A 24 kb DNA fragment was isolated by screening a 129/SvJ genomic library. A targeting vector was constructed by replacing 5.7 kb of the wild-type sequence that included exon 1 with a 1.7 kb neomycin cassette under the control of a PGK1 promotor. The vector also included a 2.9 kb 5’ homology arm, a 5.6 kb 3’ homology arm, and a thymidine kinase sequence upstream of the 5’ homology arm. Southern blotting with 5’ and 3’ screening probes located external to the homology arms were used to verify the difference in fragment size between the wild-type (24 kb) and knockout (20 kb) alleles. The targeting vector was linearized with the restriction enzyme AflII and then electroporated into 129/SvJ ES cells. Recombinant clones were identified using the 5’ and 3’ probes and the presence of the PGK-neo cassette in the knockout allele was confirmed using a neo probe. For each experiment, Slc3a1-/- littermate mice were randomly assigned to treatment or control groups. Wild-type (129X1/SvJ) and Nrf2-/- mice were purchased from Jackson laboratories, Nrf2-/- were bred to Slc3a1-/- mice to generate Slc3a1-/-; Nrf2-/- and Slc3a1-/-; Nrf2+/+ littermate controls.
α-lipoic acid was administered via 0.5%, 0.25%, or 0.1% (w/w) supplementation in the diet (Envigo); control mice were administered a non-supplemented equivalent diet. Tiopronin, N-(2-mercaptopropionyl)glycine, was administered via daily oral gavage at 3.7 mg/day, the mouse dose equivalent of the tiopronin 1000 mg/day dose prescribed to patients, calculated according to the body surface area normalization method12. L-CDME was administered via daily oral gavage at 2 mg/day, as was previously done8. Control mice for these experiments were administered water only as a vehicle control. Sulforaphane was administered via intraperitoneal injection three times per week at 25 mg/kg in PBS. Control mice were administered PBS only as a vehicle control.
24-hour food and water intake was measured using group-housed mice (3 to 4 mice per cage) in metabolic cages (Promethion). For urinary pH determination, urine from Slc3a1-/- mice was collected from voluntary expulsion and measured using a micro combination pH electrode (Lazar).
All experiments detailed here involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committees (IACUC) of the Buck Institute and Rutgers University.
Micro-computed tomography (μCT)
Stone growth rate was calculated from weekly measurements taken during a period of 6 weeks. As Slc3a1-/- mice are susceptible to mortality, measurements from time points immediately prior to any deaths were excluded from analysis14,22. Mice were anesthetized and scanned weekly using Skyscan 1176 microcomputed tomography (μCT) scanner (Bruker Corp, Billerica, MA). The Skyscan reconstruction program NRecon was used for image reconstruction, and bladder stone volume was quantified using the Bruker CT-Analyzer (CTAn, Version 1.14) program. 3-D image models were created using CTAn and Bruker CT-Volume (CTVol, Version 2.2).
Kidney histology
Kidney tissues were fixed in formalin and embedded in paraffin. 7 μM sections were then stained with Hematoxylin and Eosin.
Cystine determination
Urine from Slc3a1-/- mice collected from voluntary expulsion, and internal standard DL-cystine (3,3,3’,3’-D4, Cambridge Isotope Laboratories) was added immediately to the samples. Urine samples were then sored at -80°C prior to cystine estimation. Urinary cystine was measured using the EZ-FAAST® kit from Phenomenex, Inc. (Torrance, CA) coupled with LC-MS/MS, following vendor-provided directions. Creatinine levels were determined using an assay kit according to manufacturer’s instructions (Cayman Chemical).
Cystine precipitation assay
100 mL of 4 mM supersaturated L-cystine solution was prepared by heating a cystine suspension in water, under reflux at 100°C for 20 min with continuous stirring to completely dissolve L-cystine, as previously described10. 400 μL of this supersaturated L-cystine solution was added to borosilicate glass tubes (3 technical replicates per condition per experiment) containing 100 μL of water (blank), synthetic compounds (at least 3 independent experiments performed), or mouse urine (at least 2 independent experiments performed) and vortexed briefly to homogenize the contents. The solutions were subsequently incubated at 4°C for 3 days to allow cystine precipitation. At the end of the incubation period, the tubes were centrifuged at 4000 rpm for 20 min and the supernatant discarded. The remaining cystine precipitate was dissolved in 3.6 mL of 25 μM d4-cystine (internal standard) solution and 2 μL injected for LC-MS/MS analysis. Mouse urine samples were obtained from 24 h urine collections from 129X1/SvJ male mice treated with or without 0.5% α-lipoic acid (Tecniplast).
Liquid chromatography tandem mass spectrometry (LC-MS/MS)
Liquid chromatography (LC) was performed using a Shimadzu UFLC prominence system fitted with following modules: CBM-20A (Communication bus module), DGU-A3 (degasser), two LC-20AD (liquid chromatograph, binary pump), SIL-20AC HT (auto sampler) and connected to a Phenomenex Luna® NH2 column (2 × 150 mm, 3 μm, 100 Å). Mass spectrometry (MS) was performed using a 4000 QTRAP® LC-MS/MS mass spectrometer from AB SCIEX fitted with a Turbo V™ ion source. AB SCiex’s Analyst® v1.6.1 was used for all forms of data acquisition, development of LC method, and optimization of analyte-specific MRM (multiple reaction monitoring) transitions. AB SCiex’s Peakview® v2.1 and Skyline® v3.523 was used for LC-MS/MS data analysis.
Optimization of cystine- and d4-cystine-specific multiple reaction monitoring (MRM) transitions, such as determination of suitable precursor and product ions and optimal MS parameters for each transition (Q1, precursor → Q3, product) were achieved by isocratic flow injection of the 10 μM solution for each compounds. The most intense (Q1→ Q3) transition was used as quantifier, whereas the next best transition was used as qualifier for each compound (see table below). For LC separation, a solvent gradient of 95% of 20 mM ammonium acetate/20 mM ammonium hydroxide (pH~9.5) in water and 5% acetonitrile (aqueous) - acetonitrile (organic) was used with 0.4 mL/min flow rate, starting with an acetonitrile content of 50% for 1.5 min, which was decreased to 2% over 3.5 min and held at 2% for 1.5 min. The LC column was subsequently reconstituted to its initial condition (organic content of 50%) over the next 1 min and re-equilibrated for 4 min. For cystine estimation (retention time = 4.9 min), the mass spectrometer was operated in positive ion mode. Source conditions were as follows: curtain gas (CUR) 20, nebulizer gas (GS1) 60, auxiliary gas (GS2) 50, ionspray voltage (IS) 4500 V, and source temperature (TEM) 450°C. Quantification of cystine was based on integration of corresponding LC-MS/MS-specific quantifier peaks (peak areas) and the corresponding quantifier peak area for d4-cystine.
Table.
Relevant LC-MS/MS parameters.
| Analyte | MRM Precurso r Q1 (m/z) | MRM Product Q3 (m/z) | Nature of MRM Transition Q1 → Q3 (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| Cystine | 241.0 | 152.0 | Quantifier | 32 | 5.8 | 19.8 | 10.9 |
| 74.0 | Qualifier | 32 | 5.8 | 41.1 | 5.6 | ||
| d4-Cystine | 245.1 | 154.0 | Quantifier | 42 | 6.5 | 18.7 | 9.3 |
| 74.0 | Qualifier | 42 | 6.5 | 39.0 | 5.4 |
Note: DP = Declusturing potential, EP = Entrance potential, CE = Collision energy, and CXP = Collision exit potential.
Statistical analyses
Littermate mice were randomly assigned to treatment or control groups for each experiment. Investigators were not blinded to group allocation. Most experiments included a control-only period before randomization to ensure mice would survive long enough for study. Those that did not survive to randomization were removed from the study (n = 7 mice). Means and standard errors were calculated in each treatment and control group. Two-sided Student’s t-tests were used to compare differences in mean stone volume at single time points, urinary cystine levels, and relative cystine precipitate. Experiments involving multiple t-tests were adjusted for multiple comparisons using the Benjamini-Hochberg-Yekutieli procedure, which controls for false discovery rate among correlated tests24. Linear mixed regression models were used to assess the associations between treatment groups and stone growth over time. Sample size calculations were conducted assuming linear mixed regression models with six time points during each treatment period and stone growth of 1 mm3 per day in the control group. Assuming stone growth of 0.6 mm3 per day in the treatment group, at least n = 4 mice were required in each group to have at least 80% power to detect differences across treatment and control groups. Assuming stone growth of 0.7 mm3 per day, n = 6 mice in each group were needed.
Given the approximately linear increase in stone volume, time was used as a continuous variable in linear mixed regression models. An interaction term between time and treatment group was included to test for differences in mean stone growth across treatment groups. For experiments with more than two treatment groups, interaction terms were first jointly tested across all treatment groups. If the overall test indicated a significant interaction, differences between each pair of treatment groups were tested. Random intercepts for each mouse were included in regression models to account for the correlated, repeated stone volume measures within mice. For experiments in which mice were switched from one diet to another, linear splines were included in models with knots at the times of switch to allow for different stone growth rates while on each diet. For experiments in which mice were randomized to α-lipoic acid treatments or control after a control-only period, mean stone growth rates were adjusted for baseline stone growth, defined by the mean stone growth rate in each mouse prior to randomization. A three-way interaction between mouse group, treatment group, and time was used in the regression model to test whether Nrf2-/- modified the effect of α-lipoic acid compared to treatment. Two-sided tests were used in all models.
Stone volumes over time were plotted prior to modeling to ensure volume increased approximately linearly in each experiment and the continuous form of time in models was appropriate. Random intercepts and residuals estimated from models were plotted using histograms and quantile-quantile plots to check for normality. There was no evidence of any violations of these model assumptions. A Poisson regression model was used to compare the counts of stones between the control and α-lipoic acid groups. Model assumptions, including overdispersion and zero-inflation, were checked and there was no evidence of assumption violations. All statistical analyses were conducted using R 3.2.3 software (R Foundation for Statistical Computing, Vienna, Austria).
Supplementary Material
Acknowledgments
We thank R. Murphy and S. Melov for their advice regarding μCT, T. Te Koi for histology assistance, M. Chamoli and N. Mathew for help with imaging, and D. Chrzan for helpful discussions. This work was supported by grants from the American Federation of Aging Research (to P.K.), Larry L. Hillblom Foundation (to P.K.), Boston Scientific Foundation (to M.S.) and the NIH (R01 AG038688 & R01 AG045835 to P.K.; R21 DK091727 to P.K. and M.S.; P20 DK100863 & R21 DE025961 to M.S.; K12 DK083021 & R21 DK109433 to T.C.).
Footnotes
Author Contributions
T.Z., A.K., M.S., and P.K. conceived the experiments. T.Z. and N.B. developed methodology and designed experiments. T.Z., N.B., and J.Z. analyzed and interpreted data. T.Z., J.N.B., S.Y., J.P., M.Y., and S.D. performed in vivo experiments. N.B., T.Z., D.H., and A.R. performed ex vivo and in vitro experiments. M.N.O. and T.Z. performed food and water intake measurements. J.T. and A.S. designed and generated the Slc3a1-/- mouse. T.Z., N.B., and J.Z. wrote the manuscript, D.K., A.S., R.R.G., T.C., A.K., M.S., and P.K. revised the manuscript.
Competing Financial Interests Statement:
The authors declare no competing financial interests.
References
- 1.Scales CD, Jr, Smith AC, Hanley JM, Saigal CS. Urologic Diseases in America, P. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160–165. doi: 10.1016/j.eururo.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan MS, Pearle MS. Medical management of renal stones. BMJ. 2016;352:i52. doi: 10.1136/bmj.i52. [DOI] [PubMed] [Google Scholar]
- 3.Mattoo A, Goldfarb DS. Cystinuria. Semin Nephrol. 2008;28:181–191. doi: 10.1016/j.semnephrol.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Zheng Z, et al. Tiopronin-induced membranous nephropathy: a case report. Ren Fail. 2014;36:1455–1460. doi: 10.3109/0886022X.2014.926754. [DOI] [PubMed] [Google Scholar]
- 5.Ishak R, Abbas O. Penicillamine revisited: historic overview and review of the clinical uses and cutaneous adverse effects. Am J Clin Dermatol. 2013;14:223–233. doi: 10.1007/s40257-013-0022-z. [DOI] [PubMed] [Google Scholar]
- 6.Varda BK, et al. Imaging and surgical utilization for pediatric cystinuria patients: A single-institution cohort study. Journal of pediatric urology. 2016;12106:e101–107. doi: 10.1016/j.jpurol.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Rule AD, Krambeck AE, Lieske JC. Chronic kidney disease in kidney stone formers. Clin J Am Soc Nephrol. 2011;6:2069–2075. doi: 10.2215/CJN.10651110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahota A, et al. Novel cystine ester mimics for the treatment of cystinuria-induced urolithiasis in a knockout mouse model. Urology. 2014;84:1249 e1249–1215. doi: 10.1016/j.urology.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker G. Caring for Australians with Renal, I. The CARI guidelines. Kidney stones: cystine stones. Nephrology (Carlton) 2007;12(Suppl 1):S4–10. doi: 10.1111/j.1440-1797.2006.00722.x. [DOI] [PubMed] [Google Scholar]
- 10.Rimer JD, et al. Crystal growth inhibitors for the prevention of L-cystine kidney stones through molecular design. Science. 2010;330:337–341. doi: 10.1126/science.1191968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoon HY, et al. Sulforaphane protects kidneys against ischemia-reperfusion injury through induction of the Nrf2-dependent phase 2 enzyme. Biochem Pharmacol. 2008;75:2214–2223. doi: 10.1016/j.bcp.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler D, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes care. 2006;29:2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 14.Livrozet M, et al. An animal model of type A cystinuria due to spontaneous mutation in 129S2/SvPasCrl mice. PLoS One. 2014;9:e102700. doi: 10.1371/journal.pone.0102700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim MS, et al. Anti-obesity effects of alpha-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nat Med. 2004;10:727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 16.Suh JH, et al. Decline in transcriptional activity of Nrf2 causes age-related loss of glutathione synthesis, which is reversible with lipoic acid. Proc Natl Acad Sci U S A. 2004;101:3381–3386. doi: 10.1073/pnas.0400282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han D, et al. Lipoic acid increases de novo synthesis of cellular glutathione by improving cystine utilization. Biofactors. 1997;6:321–338. doi: 10.1002/biof.5520060303. [DOI] [PubMed] [Google Scholar]
- 18.Teichert J, et al. Pharmacokinetics of alpha-lipoic acid in subjects with severe kidney damage and end-stage renal disease. J Clin Pharmacol. 2005;45:313–328. doi: 10.1177/0091270004270792. [DOI] [PubMed] [Google Scholar]
- 19.Schupke H, et al. New metabolic pathways of alpha-lipoic acid. Drug metabolism and disposition: the biological fate of chemicals. 2001;29:855–862. [PubMed] [Google Scholar]
- 20.Wagner CA, Mohebbi N. Urinary pH and stone formation. J Nephrol. 2010;23(Suppl 16):S165–169. [PubMed] [Google Scholar]
- 21.Ercolani M, et al. Bladder outlet obstruction in male cystinuria mice. Int Urol Nephrol. 2010;42:57–63. doi: 10.1007/s11255-009-9597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feliubadalo L, et al. Non-type I cystinuria caused by mutations in SLC7A9, encoding a subunit (bo,+AT) of rBAT. Nat Genet. 1999;23:52–57. doi: 10.1038/12652. [DOI] [PubMed] [Google Scholar]
- 23.MacLean B, et al. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–1188. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.