Abstract
Objective
Ezetimibe improves cardiovascular outcomes when added to optimum statin treatment. It lowers LDL cholesterol and percent intestinal cholesterol absorption, but the exact cardioprotective mechanism is unknown. We tested the hypothesis that the dominant effect of ezetimibe is to increase the reverse transport of cholesterol from rapidly-mixing endogenous cholesterol pool into the stool.
Approach and Results
In a randomized, placebo-controlled, double-blind parallel trial in 24 healthy subjects with LDL cholesterol 100–200 mg/dL, we measured cholesterol metabolism before and after a 6-week treatment period with ezetimibe 10 mg/day or placebo. Plasma cholesterol was labeled by intravenous infusion of cholesterol-d7 in a lipid emulsion and dietary cholesterol with cholesterol-d5 and sitostanol-d4 solubilized in oil. Plasma and stool samples collected during a cholesterol- and phytosterol-controlled metabolic kitchen diet were analyzed by mass spectrometry. Ezetimibe reduced intestinal cholesterol absorption efficiency 30 ± 4.3% (SE, P < 0.0001) and LDL cholesterol 19.8 ± 1.9% (P = 0.0001). Body cholesterol pool size was unchanged, but fecal endogenous cholesterol excretion increased 66.6 ± 12.2% (P < 0.0001) and percent cholesterol excretion from body pools into the stool increased 74.7 ± 14.3% (P < 0.0001) while plasma cholesterol turnover rose 26.2 ± 3.6% (P = 0.0096). Fecal bile acids were unchanged.
Conclusions
Ezetimibe increased the efficiency of reverse cholesterol transport from rapidly-mixing plasma and tissue pools into the stool. Further work is needed to examine the potential relation of reverse cholesterol transport and whole body cholesterol metabolism to coronary events and the treatment of atherosclerosis.
Keywords: cholesterol absorption, excretion, controlled diet, stable isotopes, clinical trial, mass spectrometry
Introduction
Statins inhibit cholesterol biosynthesis and are highly effective in reducing plasma LDL cholesterol levels and cardiovascular disease (CVD) risk.1 In spite of these effects, CVD remains the leading cause of death in the United States and other Western countries.2 Thus, complementary strategies are needed to further reduce risk.
Inhibiting intestinal cholesterol absorption represents another approach for LDL cholesterol lowering which also affects whole body cholesterol metabolism. The drug ezetimibe reduces intestinal cholesterol absorption by targeting Niemann-Pick C1 like 1 (NPC1L1), a sterol transporter expressed in the apical membrane of enterocytes.3, 4 As a result, less cholesterol is delivered to the liver, thereby upregulating LDL receptors and reducing LDL cholesterol.5 When given to patients with primary hypercholesterolemia, ezetimibe (10 mg/day) reduces LDL cholesterol 15–20%.6, 7 Hepatic NPC1L1 is a second target of ezetimibe.8 Even though ezetimibe may increase cholesterol excretion by inhibiting intestinal NPC1L1 alone (in rodents, for example), reducing both hepatic NPC1L1 and intestinal NPC1L1 is expected to maximize endogenous cholesterol excretion in species such as humans where NPC1L1 is expressed both in enterocytes and hepatocytes. Hepatic NPC1L1 reclaims cholesterol from the bile back into the liver, inhibiting biliary cholesterol secretion.9 Ezetimibe, by inhibiting hepatic NPC1L1, stimulates biliary cholesterol secretion; simultaneous inhibition of intestinal NPC1L1 by ezetimibe facilitates excretion of this biliary cholesterol in the stool.
Reverse cholesterol transport (RCT), the process of removing excess cholesterol from peripheral tissues to the rapidly-mixing plasma and tissue pool and then directing it to the intestine for excretion,10, 11 has been an area of intense research because it is another mechanism with the potential to reduce CVD risk.12 The terminal portion of RCT involves two pathways: biliary secretion and an intestinal phase where cholesterol is both absorbed from the lumen and directly secreted into the lumen. The latter process is known as trans-intestinal cholesterol excretion (TICE).21 Cholesterol from both pathways arrives in the small intestine and is excreted into the stool. Inhibiting intestinal cholesterol absorption not only reduces plasma LDL cholesterol levels, but also significantly increases total fecal cholesterol excretion.13, 14 Because the majority of intestinal cholesterol comes from endogenous cholesterol, ezetimibe is expected to promote fecal excretion of endogenous cholesterol (FEEC) by inhibiting intestinal NPC1L1, as suggested in animal studies.15
Two published clinical studies using pioneering mass spectroscopic methodologies have examined changes in body cholesterol metabolism associated with ezetimibe treatment; both support the idea that ezetimibe increases endogenous cholesterol excretion.14,16 However, this conclusion is tempered by limitations present in one or both of those trials, including lack of a control group, lack of concurrent ezetimibe treatment during metabolic measurements, collection of stool samples without control of either dietary cholesterol or phytosterol intake, lack of measurement of intestinal cholesterol absorption efficiency or total endogenous cholesterol excretion, and a focus on analyses performed during rapid changes in cholesterol enrichment, where repeatability of calculated parameters is limited. In the present work, we tried to optimize the experimental conditions while emphasizing the critical roles of the rapidly-mixing endogenous cholesterol pool and the intestine in the action of ezetimibe. Using a well-defined intravenous cholesterol tracer and oral tracers, we carefully identified the origins of fecal cholesterol as endogenous, dietary or unlabeled cholesterol arising from newly-synthesized, non-equilibrated cholesterol.20 Measurements were made while the subjects were consuming a metabolic kitchen diet under near steady-state conditions, where results in the placebo group were reproducible. We advanced the notion that percent cholesterol excretion (PCE), the percent of the rapidly-mixing cholesterol pool excreted in the stool daily, is helpful in understanding and quantifying the effect of ezetimibe on whole body cholesterol metabolism.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement.
Results
Subjects
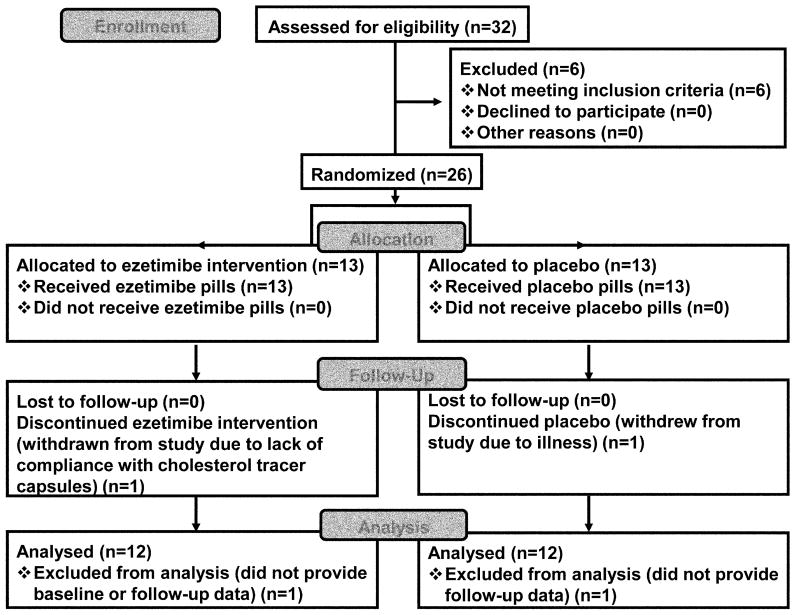
As shown in Figure 1, 26 subjects were randomized and 24 completed the protocol (12 ezetimibe, 12 placebo). Subjects included 15 females and 9 males with mean age of 55.6 ± 2.6 years. Table 1 shows the subject characteristics upon entry into the study.
Fig. 1. Consolidated Standards of Reporting Trials (CONSORT) flow diagram.
Flow of subjects throughout the trial. All completed subjects were included in the analyses.
Table 1.
Subject characteristics at screening
| N (women/men) | 24 (16/8) |
| Race: White/Black | 23/1 |
| Age (y) | 55.6 ± 2.6 |
| BMI (kg/m2) | 28.4 ± 1.0 |
| Lipids: | |
| Total cholesterol (mg/dL) | 211 ± 7 |
| LDL cholesterol (mg/dL) | 131 ± 6 |
| HDL cholesterol (mg/dL) | 56 ± 3 |
| Triglyceride (mg/dL) | 122 ± 8 |
| HbA1c (%) | 5.5 ± 0.1 |
| Glucose (mg/dL) | 111± 2 |
| Blood pressure: | |
| Systolic (mm Hg) | 127 ± 4 |
| Diastolic (mm Hg) | 78 ± 2 |
Values are means ± SE.
Cholesterol metabolism
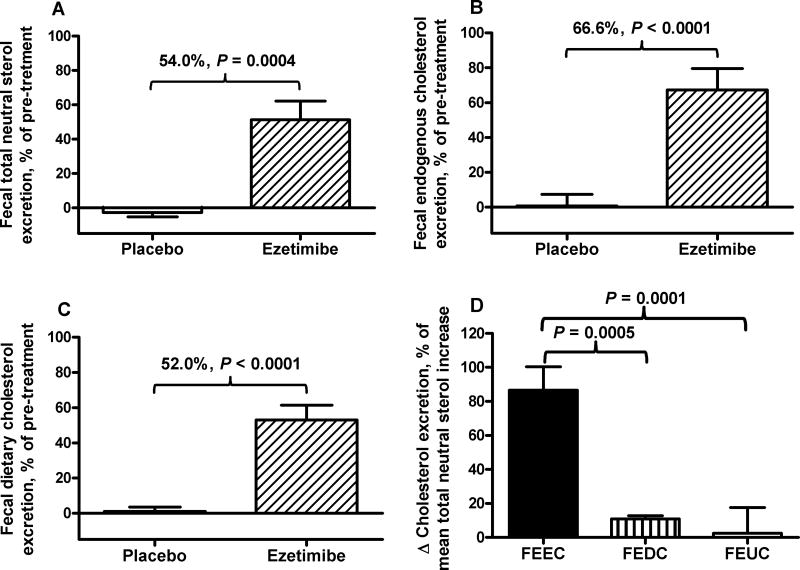
Table 2 shows the results of cholesterol metabolic measurements for the pre and post treatment periods of placebo and ezetimibe groups and the respective absolute differences in pre-post change between placebo and ezetimibe groups. Ezetimibe treatment increased fecal total neutral sterol excretion +0.37 ± 0.08 g/day relative to placebo (Table 2) and 54.0% (Fig. 2A). Additionally, ezetimibe increased FEEC (+0.31 ± 0.06 g/day, Table 2; 66.6 ± 12.2%, Fig. 2B) and dietary cholesterol (+0.038 ± 0.006 g/day, Table 2; 52.0 ± 8.5%, Fig. 2C). Endogenous cholesterol was the major contributor to the increase in fecal sterol excretion, accounting for 86.6 ± 11.9% of the increase (Fig. 2D).
Table 2.
Cholesterol metabolic measurements before and after treatment
| Placebo | Ezetimibe (10 mg/day) | Treatment Difference§* |
|||
|---|---|---|---|---|---|
|
|
|||||
| Pre | Post | Pre | Post | ||
| Cholesterol metabolism† | |||||
| Total fecal cholesterol (g/day) | 0.82 ± 0.07 | 0.81 ± 0.08 | 0.71 ± 0.04 | 1.07 ± 0.08ǂ | 0.37 ± 0.08ǂ |
| Endogenous origin (g/day) | 0.59 ± 0.08 | 0.58 ± 0.08 | 0.51 ± 0.05 | 0.82 ± 0.06ǂ | 0.31 ± 0.06ǂ |
| Dietary origin (g/day) | 0.090 ± 0.010 | 0.090 ± 0.010 | 0.080 ± 0.010 | 0.120 ± 0.010ǂ | 0.038 ± 0.006ǂ |
| Unlabeled (g/day) | 0.15 ± 0.06 | 0.14 ± 0.05 | 0.12 ± 0.03 | 0.13 ± 0.06 | NS |
| Percent cholesterol excretion (%/day) | 1.85 ± 0.25 | 1.86 ± 0.23 | 1.78 ± 0.17 | 2.99 ± 0.22ǂ | 1.19 ± 0.23ǂ |
| Total fecal bile acids (g/day) | 0.51 ± 0.02 | 0.63 ± 0.06 | 0.51 ± 0.02 | 0.66 ± 0.06§ | NS |
| Cholesterol rapidly-mixing pool size (g/day) | 32.0 ± 1.9 | 31.1 ± 1.5 | 29.0 ± 0.9 | 27.5 ± 1.0 | NS |
| Plasma relative cholesterol d7 enrichment | 0.28 ± 0.02 | 0.27 ± 0.01 | 0.32 ± 0.02 | 0.23 ± 0.01ǂ | −0.07 ± 0.004ǂ |
| Percent cholesterol absorption (%) | 61.7 ± 3.2 | 61.6 ± 3.3 | 61.5 ± 2.6 | 42.5 ± 2.7ǂ | −18.9 ± 3.2ǂ |
| Plasma lipids and lipoproteins | |||||
| Total cholesterol (mg/dL) | 212.3 ± 6.3 | 213.0 ± 6.7 | 222.6± 13.1 | 189.3± 9.9ǂ | −33.9 ± 8.4ǂ |
| LDL‖ cholesterol (mg/dL) | 141.2 ± 5.3 | 139.2 ± 6.0 | 144.6 ± 10.9 | 114.4 ± 8.7ǂ | −28.2 ± 6.3ǂ |
| HDL# cholesterol (mg/dL) | 48.6 ± 3.3 | 46.2 ± 3.5 | 55.2 ± 2.9 | 55.7 ± 3.2 | NS |
| Triglycerides (mg/dL) | 121.6 ± 11.5 | 141.1 ± 14.4 | 109.9 ± 8.9 | 106.3 ± 9.6 | NS |
| Plasma non-cholesterol sterols (µg/mg) | |||||
| Cholestanol / Total cholesterol | 1.20 ± 0.09 | 1.26 ± 0.08 | 1.49 ± 0.12 | 1.41 ± 0.08 | NS |
| Lathosterol / Total cholesterol | 1.18 ± 0.12 | 1.06 ± 0.13 | 1.00 ± 0.09 | 1.34 ± 0.11ǂ | 0.45 ± 0.08ǂ |
| Total phytosterols / Total cholesterol | 1.92 ± 0.34 | 1.89 ± 0.32 | 2.46 ± 0.26 | 1.42 ±0.14ǂ | −1.01 ± 0.18ǂ |
| Campesterol / Total cholesterol | 0.90 ± 0.16 | 0.87 ± 0.17 | 1.22 ± 0.14 | 0.61 ± 0.08ǂ | −0.57 ± 0.10ǂ |
| Sitosterol / Total cholesterol | 0.97 ± 0.18 | 0.98 ± 0.15 | 1.18 ± 0.11 | 0.77 ± 0.06ǂ | −0.43 ± 0.10ǂ |
| Stigmasterol / Total cholesterol | 0.045 ± 0.005 | 0.040 ± 0.004 | 0.054 ± 0.006 | 0.038 ± 0.003ǂ | NS |
All values are means ± SE, n=12 subjects per group. Significance of Pre to Post differences in each group and treatment effect are indicated by † for P < 0.05 and ‡ for P < 0.01.
Cholesterol metabolism: Total fecal cholesterol was measured as the sum of intact cholesterol and its bacterial metabolites, coprostanol and coprostanone. It is comprised of material of endogenous origin labeled with cholesterol-d7, dietary origin measured with cholesterol-d5, or unlabeled cholesterol. Percent cholesterol excretion is the percent of the rapidly-mixing cholesterol pool per day excreted in the feces. Plasma relative cholesterol d7 enrichment for pre-treatment is calculated as (plasma cholesterol d7 enrichment on day 15 - plasma cholesterol d7 enrichment on day 1) / (plasma cholesterol d7 enrichment on day 2 - plasma cholesterol d7 enrichment on day 1); for post-treatment it is calculated as (plasma cholesterol d7 enrichment on day 57-plasma cholesterol d7 enrichment on day 43) / (plasma cholesterol d7 enrichment on day 44 - plasma cholesterol d7 enrichment on day 43).
LDL: low-density lipoprotein;
HDL: high-density lipoprotein.
Treatment difference is the absolute difference in pre-post change between placebo and ezetimibe groups,
P < 0.01,
P < 0.05. NS, not significant.
Fig. 2. Effects of ezetimibe on fecal excretion of total (A), endogenous (B), and dietary cholesterol (C), and relative contributions to fecal excretion of total neutral sterols by ezetimibe (D).
Fecal excretion of total and endogenous cholesterol was determined in subjects receiving 10 mg/day ezetimibe (n=12) or placebo (n=12) for 6 weeks, as described in Methods. Fecal excretion of unabsorbed dietary cholesterol and unlabeled cholesterol were calculated as described in Methods. Results of A, B, and C are expressed as percent change relative to pre-treatment in each group. Treatment effects and the P values are shown above the bars of placebo and ezetimibe. Results of D are increases of fecal excretion of endogenous cholesterol (FEEC), of dietary cholesterol excretion (FEDC), and of unlabeled cholesterol (FEUC), as percent of the mean increase in fecal excretion of total neutral sterols by ezetimibe.
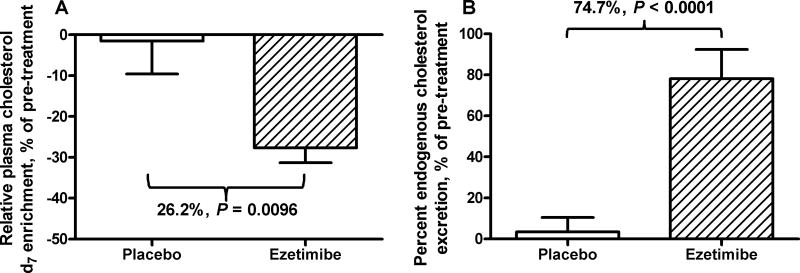
Ezetimibe did not alter excretion of unlabeled cholesterol, which represents newly synthesized cholesterol that has not equilibrated with tracer, or excretion of bile acids (Table 2). Furthermore, the rapidly-mixing pool of body cholesterol was not affected by ezetimibe (Table 2). However, ezetimibe reduced relative cholesterol-d7 enrichment by 0.07 ± 0.004 (Table 2) or 26.2 ± 3.6% (Fig. 3A), indicating an increased turnover of body cholesterol by ezetimibe. In addition, ezetimibe treatment increased PCE by +1.19 ± 0.23 percentage points (Table 2) or 74.7 ± 14.3% (P < 0.0001) (Fig. 3B). The treatment effect on intestinal cholesterol absorption was −18.9 ± 3.2 percentage points (Table 2) or −30.0 ± 4.3% with ezetimibe treatment relative to the pre-treatment period.
Fig. 3. Effect of ezetimibe on plasma relative cholesterol d7 enrichment and percent endogenous cholesterol excretion.
Plasma relative cholesterol d7 enrichment (A) is the cholesterol d7 enrichment ratio during each metabolic measurement period. For post treatment, it is calculated as the ratio of net plasma cholesterol d7 enrichment on day 57 (difference between day 57 and day 43) to that on day 44 (difference between day 44 and day 43). For pre-treatment, it is expressed as the cholesterol d7 enrichment ratio of plasma cholesterol d7 enrichment on day 15 to that on day 2. Percent cholesterol excretion (B) is expressed as percent fecal endogenous cholesterol excretion of the rapidly-mixing cholesterol pool. Treatment effects and the P values are shown above the bars of placebo (n=12) and ezetimibe (10 mg/day for 6 weeks, n=12).
Plasma lipids and lipoproteins
Ezetimibe treatment significantly reduced plasma LDL cholesterol levels by 28.2 ± 6.3 mg/dL (Table 2) or 19.8 ± 1.9% (P = 0.0001) relative to the pre-treatment and total cholesterol by 33.9 ± 8.4 mg/dL (Table 2) or 15 ± 0.02% (P = 0.0003). Plasma triglyceride and HDL cholesterol concentrations were not affected.
Plasma non-cholesterol sterols
Ezetimibe significantly reduced plasma concentrations of total phytosterols and the two major individual phytosterols: campesterol and sitosterol (Table 2). Ezetimibe increased plasma lathosterol to total cholesterol ratio, but did not affect plasma 5α-cholestanol to total cholesterol ratio (Table 2).
Discussion
The major finding of our study is that ezetimibe increased FEEC and endogenous cholesterol turnover without affecting the size of the rapidly-mixing body cholesterol pool. As expected, ezetimibe reduced plasma total cholesterol (15.0%) and LDL cholesterol (19.8%) without affecting the concentrations of plasma HDL cholesterol or triglycerides. Ezetimibe also reduced intestinal cholesterol absorption (30.0%). The effect of ezetimibe on whole body cholesterol metabolism was much larger than its effect on LDL cholesterol and intestinal cholesterol absorption. Ezetimibe increased fecal excretion of total neutral sterols by 54.0%, which is consistent with a 64.0% increase from our previous study of ezetimibe.13 Both the amount and the efficiency of endogenous cholesterol excretion from the rapidly-mixing cholesterol plasma and tissue pool into the stool increased. FEEC rose 66.6% and PCE rose by 74.7%.
Fecal neutral sterols arise from endogenous cholesterol in the rapidly-mixing body pool, unabsorbed dietary cholesterol, and unlabeled newly-synthesized cholesterol17, all of which might potentially be altered by ezetimibe and contribute to the rise in total fecal sterol output observed. In the present study, the use of oral and intravenous cholesterol tracers revealed that the increase in FEEC accounted for 86.6% of the increase in fecal excretion of total neutral sterols due to ezetimibe. In contrast, excretion of unabsorbed dietary cholesterol and unlabeled cholesterol accounted for only 11.0% and 2.4%, respectively. The exclusion of unlabeled newly-synthesized cholesterol as a small component of fecal cholesterol with ezetimibe treatment demonstrates that the effect of ezetimibe is not due simply to a futile cycle characterized by increased local intestinal synthesis of cholesterol. Moreover, the data show that RCT from liver, plasma and intestine into the stool rises.
Endogenous cholesterol is likely secreted into the intestine both in bile and directly through TICE.18 The intestinal sterol transporter NPC1L1, located in the apical membrane of enterocytes, mediates both dietary and endogenous cholesterol uptake into the enterocyte. Because the proximal small intestine is actively involved in TICE,19 the endogenous cholesterol available for reuptake into the enterocyte comprises not only biliary cholesterol, but also cholesterol secreted through TICE. By inhibiting NPC1L1, ezetimibe reduces the intestinal reabsorption of dietary, biliary, and TICE-derived cholesterol, leading to losses in the stool. Ezetimibe may have stimulated both biliary RCT and non-biliary RCT (i.e. TICE) in the present study. The action of ezetimibe to increase fecal excretion of labeled cholesterol from macrophages (macrophage-specific RCT, mRCT) by inhibiting intestinal NPC1L1 was demonstrated in mice,15, 20 a species in which NPC1L1 is not measurably expressed in the liver. Whether this happens in humans cannot be determined from our data. More recently it has been demonstrated in vitro that ezetimibe may promote cholesterol efflux from the brush-border membrane of enterocytes into the lumen, which may help the drug both reduce intestinal cholesterol absorption and enhance cellular cholesterol flux back to the intestinal lumen.21
In humans, NPC1L1 also is expressed in the liver, where NPC1L1 inhibits biliary cholesterol secretion. Ezetimibe targets hepatic NPC1L1, stimulating biliary cholesterol secretion.8 The additional potential biliary cholesterol resulting from the inhibition of hepatic NPC1L1 arrives in the small intestine, where it is expected to be excreted in the stool because intestinal NPC1L1 is inhibited simultaneously. In the current study, the increase in FEEC by ezetimibe may reflect increased biliary cholesterol secretion (due to inhibition of hepatic NPC1L1) facilitated by reduced reabsorption of biliary cholesterol (due to inhibition of intestinal NPC1L1). The unique dual-target mechanism of ezetimibe may maximize reverse cholesterol transport by the combined inhibition of both hepatic NPC1L1 and intestinal NPC1L1 and thus provide better athero-protection in species with NPC1L1 expressed in both the liver and intestine, such as humans. The importance of inhibiting intestinal cholesterol absorption is supported by two elegant studies with LDL receptor knockout mice that overexpressed hepatic ABCG5 and ABCG8 and had increased biliary cholesterol secretion.22 However, no effects were observed on intestinal cholesterol absorption, fecal sterol excretion, or aortic atherosclerosis with functional intestinal NPC1L1.22 In the same transgenic mouse model, ezetimibe inhibited intestinal cholesterol absorption, leading to an increase in fecal sterol excretion and a reduction in proximal aortic atherosclerosis.23 These results suggest that the terminal portion of the RCT pathway focused on liver and intestine may be important in determining CVD risk.
It is not clear from our current study whether ezetimibe mainly increased biliary secretion or TICE. In mice, ezetimibe increased mRCT by inhibiting intestinal NPC1L1, which required efficient biliary cholesterol secretion.24 Similarly, in hamsters, ezetimibe has been demonstrated to increase mRCT mainly through increased biliary cholesterol secretion and independent of TICE.25 In contrast, ezetimibe enhanced endogenous cholesterol excretion in humans predominantly by increasing TICE, mediated by intestinal ABCG5/ABCG8.26
It has been demonstrated that ezetimibe increases cholesterol synthesis in humans.5, 14 In the present study, ezetimibe increased total fecal sterols as well as plasma lathosterol/total cholesterol ratio, a biomarker for cholesterol synthesis.27 This result suggests that increased plasma lathosterol may result from relative cholesterol deficiency and does not necessarily indicate a primary disorder of cholesterol overproduction. Likewise, the observed reduction in phytosterol/cholesterol levels likely was due to reduced absorption rather than a change in dietary phytosterol intake.
Even though mRCT represents only a small fraction of whole-body cholesterol flux, there is interest in targeting mRCT in the prevention or regression of atherosclerosis. Several studies have demonstrated such an effect mediated by ezetimibe in mice15, 20, 24 and in hamsters.25 In the present study, cholesterol fluxes from macrophages or peripheral tissues into plasma were not assessed directly. However, ezetimibe in the present study may have increased cholesterol efflux from extra-hepatic tissues, including macrophages. The immediate source of endogenous cholesterol excretion is from the rapidly-mixing cholesterol pool. The size of this rapid pool was unchanged in the present study, suggesting that cholesterol efflux from peripheral tissues or cholesterol biosynthesis were responsible for the increase in cholesterol excretion. Ezetimibe significantly increased the turnover of cholesterol in body pools and plasma. In mice, ezetimibe reduced plasma LDL cholesterol mainly by reducing hepatic lipoprotein secretion and, to a lesser extent, by increasing lipoprotein clearance mediated by the upregulated LDL receptors.28 In insulin-resistant obese humans, a weight-loss diet alone and a weight-loss diet plus ezetimibe both reduced VLDL-apoB100 secretion, with no significant added effect of ezetimibe.29 Taken together, it is possible that ezetimibe increased cholesterol efflux from extra-hepatic tissues into the plasma. Ezetimibe did not affect plasma HDL cholesterol levels, as reported previously,14, 16 which suggests that ezetimibe may increase endogenous cholesterol excretion independently of HDL.
Our study is limited in that de novo cholesterol biosynthesis was not measured directly and RCT from the periphery to the rapidly-mixing cholesterol pool was not assessed. Nevertheless, the work focuses on the terminal components of the RCT pathway that appear to be of critical importance based on animal studies and quantitatively clarifies the role of ezetimibe in regulating fecal sterol output. The use of near-equilibrium conditions, multiple isotope tracers, and a controlled diet creates an experimental system in which most parameters can be replicated with a coefficient of variation of 10% or less (in the placebo group), allowing greater statistical power.
Ezetimibe treatment increased the overall efficiency of the reverse cholesterol transport pathway. In addition to inhibiting intestinal cholesterol absorption and reducing LDL cholesterol, ezetimibe increased FEEC without affecting the size of the rapidly-mixing cholesterol pool, leading to increased percent endogenous cholesterol excretion. The ability of ezetimibe to increase reverse cholesterol transport was quantitatively much larger than the effect on LDL cholesterol. Additional clinical investigation is needed to determine whether ezetimibe-enhanced RCT adds clinical benefit to LDL reduction.
Supplementary Material
Highlights.
Ezetimibe (10 mg/day for 6 weeks) significantly increased fecal excretion of total neutral sterols 54.0 ± 11.0% (P = 0.0004), endogenous cholesterol 66.6 ± 12.2% (P < 0.0001), and unabsorbed dietary cholesterol 52.0 ± 8.5% (P < 0.0001).
Ezetimibe did not alter fecal excretion of bile acids.
Endogenous cholesterol excretion accounted for 86.6 ±11.9% of the increase in fecal neutral sterols in response to ezetimibe.
Ezetimibe did not alter the size of the rapidly mixing cholesterol pool, but increased its turnover, as indicated by a 26.2 ± 3.6% decrease in plasma cholesterol d7 enrichment (P = 0.0096).
Ezetimibe increased the efficiency of whole-body cholesterol excretion (i.e., greater cholesterol flux out of the rapid cholesterol pool).
Acknowledgments
We thank the Alvin J. Siteman, Cancer Center at Washington University School of Medicine for use of the Biologic Therapy Core Facility. We are grateful for the skilled assistance from the Center for Clinical Studies, the Clinical Research Unit nursing staff and the metabolic kitchen staff at Washington University Institute of Clinical and Translational Sciences. We appreciate the dedication of the study participants.
Sources of Funding
This work was supported by NIH grant R01 HL108160, the Washington University Mass Spectrometry Resource NIH / NIGMS grant P41GM103422, the Washington University Diabetes Research Center NIDDK grant P30 DK020579, and the Washington University Nutrition Obesity Research Center NIH grant P30 DK056341. Research reported in this publication also was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR000448 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Nonstandard Abbreviations and Acronyms
- RCT
Reverse cholesterol transport
- mRCT
Macrophage-specific reverse cholesterol transport
- TICE
Trans-intestinal cholesterol excretion
- FEEC
Fecal excretion of endogenous cholesterol
- PCE
Percent cholesterol excretion
Footnotes
ClinicalTrials.gov Identifier: URL: http://www.clinicaltrials.gov. Unique identifier: NCT01603758
Disclosures
Ezetimibe and placebo pills were provided by Merck (Kenilworth, NJ, USA).
References
- 1.Mills EJ, Rachlis B, Wu P, Devereaux PJ, Arora P, Perri D. Primary prevention of cardiovascular mortality and events with statin treatments: A network meta-analysis involving more than 65,000 patients. J Am Coll Cardiol. 2008;52:1769–1781. doi: 10.1016/j.jacc.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Mozaffarian D, Roger VL, et al. Executive summary: Heart disease and stroke statistics--2013 update: A report from the american heart association. Circulation. 2013;127:143–152. doi: 10.1161/CIR.0b013e318282ab8f. [DOI] [PubMed] [Google Scholar]
- 3.Altmann SW, Davis HR, Jr, Zhu LJ, Yao X, Hoos LM, Tetzloff G, Iyer SP, Maguire M, Golovko A, Zeng M, Wang L, Murgolo N, Graziano MP. Niemann-pick c1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 4.Davis HR, Jr, Zhu LJ, Hoos LM, Tetzloff G, Maguire M, Liu J, Yao X, Iyer SP, Lam MH, Lund EG, Detmers PA, Graziano MP, Altmann SW. Niemann-pick c1 like 1 (npc1l1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592. doi: 10.1074/jbc.M405817200. [DOI] [PubMed] [Google Scholar]
- 5.Sudhop T, Reber M, Tribble D, Sapre A, Taggart W, Gibbons P, Musliner T, von Bergmann K, Lutjohann D. Changes in cholesterol absorption and cholesterol synthesis caused by ezetimibe and/or simvastatin in men. J Lipid Res. 2009;50:2117–2123. doi: 10.1194/jlr.P900004-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knopp RH, Dujovne CA, Le Beaut A, Lipka LJ, Suresh R, Veltri EP. Evaluation of the efficacy, safety, and tolerability of ezetimibe in primary hypercholesterolaemia: A pooled analysis from two controlled phase iii clinical studies. Int J Clin Pract. 2003;57:363–368. [PubMed] [Google Scholar]
- 7.Bays HE, Moore PB, Drehobl MA, Rosenblatt S, Toth PD, Dujovne CA, Knopp RH, Lipka LJ, Lebeaut AP, Yang B, Mellars LE, Cuffie-Jackson C, Veltri EP. Effectiveness and tolerability of ezetimibe in patients with primary hypercholesterolemia: Pooled analysis of two phase ii studies. Clin Ther. 2001;23:1209–1230. doi: 10.1016/s0149-2918(01)80102-8. [DOI] [PubMed] [Google Scholar]
- 8.Temel RE, Tang W, Ma Y, Rudel LL, Willingham MC, Ioannou YA, Davies JP, Nilsson LM, Yu L. Hepatic niemann-pick c1-like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J Clin Invest. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pramfalk C, Jiang ZY, Parini P. Hepatic niemann-pick c1-like 1. Curr Opin Lipidol. 2011;22:225–230. doi: 10.1097/MOL.0b013e3283468c28. [DOI] [PubMed] [Google Scholar]
- 10.Rader DJ, Alexander ET, Weibel GL, Billheimer J, Rothblat GH. The role of reverse cholesterol transport in animals and humans and relationship to atherosclerosis. J Lipid Res. 2009;50(Suppl):S189–194. doi: 10.1194/jlr.R800088-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenson RS, Brewer HB, Jr, Davidson WS, Fayad ZA, Fuster V, Goldstein J, Hellerstein M, Jiang XC, Phillips MC, Rader DJ, Remaley AT, Rothblat GH, Tall AR, Yvan-Charvet L. Cholesterol efflux and atheroprotection: Advancing the concept of reverse cholesterol transport. Circulation. 2012;125:1905–1919. doi: 10.1161/CIRCULATIONAHA.111.066589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohashi R, Mu H, Wang X, Yao Q, Chen C. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. Qjm. 2005;98:845–856. doi: 10.1093/qjmed/hci136. [DOI] [PubMed] [Google Scholar]
- 13.Lin X, Racette SB, Lefevre M, Ma L, Spearie CA, Steger-May K, Ostlund RE., Jr Combined effects of ezetimibe and phytosterols on cholesterol metabolism: A randomized, controlled feeding study in humans. Circulation. 2011;124:596–601. doi: 10.1161/CIRCULATIONAHA.110.006692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davidson MH, Voogt J, Luchoomun J, et al. Inhibition of intestinal cholesterol absorption with ezetimibe increases components of reverse cholesterol transport in humans. Atherosclerosis. 2013;230:322–329. doi: 10.1016/j.atherosclerosis.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Sehayek E, Hazen SL. Cholesterol absorption from the intestine is a major determinant of reverse cholesterol transport from peripheral tissue macrophages. Arterioscler Thromb Vasc Biol. 2008;28:1296–1297. doi: 10.1161/ATVBAHA.108.165803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakulj L, van Dijk TH, Freark de Boer J, Kootte RS, Schonewille M, Paalvast Y, Boer T, Bloks VW, Boverhof R, Nieuwdorp M, Beuers UH, Stroes ES, Groen AK. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 2016 doi: 10.1016/j.cmet.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Ferezou J, Coste T, Chevallier F. Origins of neutral sterols in human feces studied by stable isotope labeling (d and 13c). Existence of an external secretion of cholesterol. Digestion. 1981;21:232–243. doi: 10.1159/000198568. [DOI] [PubMed] [Google Scholar]
- 18.Temel RE, Brown JM. A new model of reverse cholesterol transport: Enticeing strategies to stimulate intestinal cholesterol excretion. Trends Pharmacol Sci. 2015;36:440–451. doi: 10.1016/j.tips.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Velde AE, Brufau G, Groen AK. Transintestinal cholesterol efflux. Curr Opin Lipidol. 2010;21:167–171. doi: 10.1097/MOL.0b013e3283395e45. [DOI] [PubMed] [Google Scholar]
- 20.Briand F, Naik SU, Fuki I, Millar JS, Macphee C, Walker M, Billheimer J, Rothblat G, Rader DJ. Both the peroxisome proliferator-activated receptor delta agonist, gw0742, and ezetimibe promote reverse cholesterol transport in mice by reducing intestinal reabsorption of hdl-derived cholesterol. Clin Transl Sci. 2009;2:127–133. doi: 10.1111/j.1752-8062.2009.00098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakano T, Inoue I, Takenaka Y, Ono H, Katayama S, Awata T, Murakoshi T. Ezetimibe promotes brush border membrane-to-lumen cholesterol efflux in the small intestine. PLoS One. 2016;11:e0152207. doi: 10.1371/journal.pone.0152207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu JE, Basso F, Shamburek RD, Amar MJ, Vaisman B, Szakacs G, Joyce C, Tansey T, Freeman L, Paigen BJ, Thomas F, Brewer HB, Jr, Santamarina-Fojo S. Hepatic abcg5 and abcg8 overexpression increases hepatobiliary sterol transport but does not alter aortic atherosclerosis in transgenic mice. J Biol Chem. 2004;279:22913–22925. doi: 10.1074/jbc.M402838200. [DOI] [PubMed] [Google Scholar]
- 23.Basso F, Freeman LA, Ko C, Joyce C, Amar MJ, Shamburek RD, Tansey T, Thomas F, Wu J, Paigen B, Remaley AT, Santamarina-Fojo S, Brewer HB., Jr Hepatic abcg5/g8 overexpression reduces apob-lipoproteins and atherosclerosis when cholesterol absorption is inhibited. J Lipid Res. 2007;48:114–126. doi: 10.1194/jlr.M600353-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Xie P, Jia L, Ma Y, Ou J, Miao H, Wang N, Guo F, Yazdanyar A, Jiang XC, Yu L. Ezetimibe inhibits hepatic niemann-pick c1-like 1 to facilitate macrophage reverse cholesterol transport in mice. Arterioscler Thromb Vasc Biol. 2013;33:920–925. doi: 10.1161/ATVBAHA.112.301187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Uto-Kondo H, Ayaori M, Sotherden GM, Nakaya K, Sasaki M, Yogo M, Komatsu T, Takiguchi S, Yakushiji E, Ogura M, Nishida T, Endo Y, Ikewaki K. Ezetimibe enhances macrophage reverse cholesterol transport in hamsters: Contribution of hepato-biliary pathway. Biochim Biophys Acta. 2014;1841:1247–1255. doi: 10.1016/j.bbalip.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Jakulj L, van Dijk TH, de Boer JF, Kootte RS, Schonewille M, Paalvast Y, Boer T, Bloks VW, Boverhof R, Nieuwdorp M, Beuers UH, Stroes ES, Groen AK. Transintestinal cholesterol transport is active in mice and humans and controls ezetimibe-induced fecal neutral sterol excretion. Cell Metab. 2016;24:783–794. doi: 10.1016/j.cmet.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Miettinen TA, Tilvis RS, Kesäniemi YA. Serum cholestanol and plant sterol levels in relation to cholesterol metabolism in middle-aged men. Metabolism. 1989;38:136–140. doi: 10.1016/0026-0495(89)90252-7. [DOI] [PubMed] [Google Scholar]
- 28.Repa JJ, Turley SD, Quan G, Dietschy JM. Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption. J Lipid Res. 2005;46:779–789. doi: 10.1194/jlr.M400475-JLR200. [DOI] [PubMed] [Google Scholar]
- 29.Chan DC, Watts GF, Gan SK, Ooi EM, Barrett PH. Effect of ezetimibe on hepatic fat, inflammatory markers, and apolipoprotein b-100 kinetics in insulin-resistant obese subjects on a weight loss diet. Diabetes Care. 2010;33:1134–1139. doi: 10.2337/dc09-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.