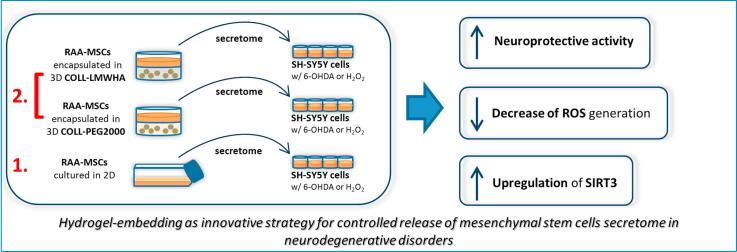
Graphical abstract
Keywords: Adipose mesenchymal stem cells, Hydrogel, 3D cell culture, Parkinson’s disease, Secretome
Abstract
Neurodegenerative diseases, as Parkinson’s disease (PD), involve irreversible neural cell damage and impairment. In PD, there is a selective degeneration of the dopaminergic neurons leading to motor symptoms. A common finding in PD neurodegeneration is the increase of reactive oxygen species (ROS), leading to oxidative stress. To date there are only interventions to relieve PD symptoms, however progress has been made in the development of therapies that target the immune system or use its components as therapeutic agents; among these, mesenchymal stem cells (MSCs), which are able to express neuroprotective factors as cytokines, chemokines and angiogenic molecules, collectively named secretome, that accumulate in MSC culture medium. However, lasting cell-free administration of secretome in vitro or in vivo is challenging. We used the conditioned media from rat adipose tissue-derived MSCs (RAA-MSCs) to check for neuroprotective activity towards pro-oxidizing agents such as hydrogen peroxide (H2O2) or the dopaminergic selective toxin 6-hydroxydopamine (6-OHDA) that is commonly used to model PD neurodegeneration. When neuroblastoma SH-SY5Y cells were pre-conditioned with 100% RAA-MSC media, then treated with H2O2 and 6-OHDA, mortality and ROS generation were reduced. We implemented the controlled release of RAA-MSC secretome from injectable biodegradable hydrogels that offer a possible in situ implant with mini-invasive techniques. The hydrogels were composed of type I bovine collagen (COLL) and low-molecular-weight hyaluronic acid (LMWHA) or COLL and polyethylene glycol (PEG). Hydrogels were suitable for RAA-MSC embedding up to 48 h and secretome from these RAA-MSCs was active and counteracted 6-OHDA toxicity, with upregulation of the antioxidant enzyme sirtuin 3 (SIRT3). These results support a biomaterials-based approach for controlled delivery of MSC-produced neuroprotective factors in a PD-relevant experimental context.
1. Introduction
Neurodegenerative diseases such Parkinson’s disease (PD) involve the progressive loss of one or more functions of the nervous system. So far this disorder is treated with symptomatic drugs, with limited results. There are several causes of neurodegeneration, such as genetic mutations, intracellular accumulation of toxic proteins, or mitochondrial dysfunction, resulting in cell death and increasing reactive oxygen species (ROS). Progress has been made in the development of therapies using immunoregulatory strategies, including recombinant proteins, immune suppression, gene therapy or cell therapy [1]. In the latter area stem cells (SCs) offer a new frontier for immunomodulation and regeneration of damaged tissue.
Depending on the stage of development and differentiation potentials, SCs are divided into embryonic or adult, including mesenchymal SCs (MSCs). MSCs are multipotent, with self-renewal capacity, and are obtained from several tissues such as bone marrow, umbilical cord, adipose tissue, or spleen. These cells are easily isolated and expandable in vitro where they carry out paracrine secretion of anti-inflammatory and neuroprotective factors [2], [3], [4]; the combination of these factors is known as secretome [5]. However, the therapeutic application of secretome in neurodegenerative disorders is challenging, mainly because the damaged tissues are not easily targeted by systemic administration and direct infusion of MSCs can arouse safety concerns, with limited therapeutic window. We have characterized the neuroprotective action of a RAA-MSC derived secretome and its controlled release from a biocompatible hydrogel, that may help overcome the limitations.
2. Materials and methods
2.1. Cell culture
2.1.1. Human neuroblastoma SH-SY5Y
Cell were cultured in polypropylene flasks (T25, Falcon) in DMEM medium (Invitrogen) supplemented with fetal bovine serum (10% v/v) (Gibco), l-glutamine 2 mM, penicillin 100 IU/mL and streptomycin 100 μg/mL (Invitrogen). Cells were maintained in an incubator at 37 °C, with 5% CO2. For treatments, cells were detached from the support with 0.05% trypsin (500 μL/25 cm2) for 5 min at 37 °C, counted through a Burker chamber and seeded at a density of 20,000 cells/well.
2.1.2. Mesenchymal stem cells (MSCs)
Commercially available mesenchymal stem cells (NeuroZone, Bresso, Italy) isolated from adipose tissue of adult CD-1 rats (RAA-MSCs) were used. Cells were grown in adhesion in polypropylene flasks (T25, Falcon), in αMEM medium (Lonza) supplemented with fetal bovine serum at 10% (v/v) (Gibco), 0.5 mM L-glutamine, penicillin 100 IU/mL and streptomycin 100 μg/mL (Invitrogen). Cells were kept in an incubator at 37 °C, with 5% CO2. When required, cells were detached from the support using 0.05% trypsin (500 μL/25 cm2) for 5 min at 37 °C, centrifuged at 900 rpm for 5 min and seeded.
2.2. Conditioned medium from mesenchymal stem cells
RAA-MSCs (up to passage 6) were cultured in T25 flasks until 80% confluence. Cells were then washed with 1X D-PBS and complete fresh αMEM without FBS was added. After 24 h the secretome-enriched conditioned medium (CM) was collected, briefly centrifuged at 13,000 rpm and used immediately or frozen at −80 °C until required [6].
2.3. Oxidative stress challenge
SH-SY5Y cells were seeded in quadruplicate at a concentration of 20,000 cells/well in 96-well plates (Iwaki) and incubated overnight. The next day, the CM was added at different dilutions (10, 30, 50, 70 and 100%) and left for 24 h. The following day, the CM was removed and the pre-conditioned cells were incubated with H2O2 (50–150 μM) or 6-OHDA (50–100 μM) (Sigma) for a further 18–24 h. Then cell viability was assessed by a colorimetric assay (MTS, Promega), in which the reagent (10% v/v) is added directly to the culture medium, incubating for 3–4 h at 37 °C and recording the absorbance directly proportional to the number of viable cells at 490 nm.
2.4. Reactive oxygen species
Reactive oxygen species (ROS) were detected by 2′,7′-dichlorofluorescein diacetate (DCFDA) assay. After cell internalization, DCFDA is deacetylated by cellular esterases to a non-fluorescent compound, which is then oxidized by ROS to 2′,7′-dichlorofluorescein (DCF). This fluorescence is recorded (Infinite M200, Tecan) at wavelengths of 485 and 535 nm. DCFDA was used at the concentration of 10 μM in D-MEM without phenol red.
2.5. Mitochondrial protein
Mitochondria were isolated from SH-SY5Y cells by mechanical cell disruption followed by differential centrifugation using a dedicated kit according to the manufacturer’s instructions (Abcam). Briefly, after two centrifugations at low speed (1000g for 10 min, 4 °C) a third centrifugation (12,000g for 10 min at 4 °C) isolates the mitochondrial fraction, that can be lysed for mitochondrial protein collection.
2.6. Western blotting
Protein extract (20 μg) was separated by electrophoresis on denaturing polyacrylamide gels (SDS-PAGE) and transferred to a nitrocellulose membrane (BioRad). Nonspecific binding sites were blocked and the membrane was incubated overnight at 4 °C with the primary antibody (anti-α-tubulin 1:5000 Abcam; anti-Sirt3 1:1000 ThermoFisher Scientific; anti-SOD2 1:1000, Santa Cruz Biotechnology; anti-VDAC 1:1000 ThermoFisher Scientific; anti-Hsp70 1:200 Santa Cruz Biotechnology; anti-SIRT1 1:1000 Origen). The secondary antibody was conjugated to the enzyme horseradish peroxidase (HRP). For visualization of immunoreactive bands on the membrane a peroxide and luminol solution was applied (Millipore). After development of the photographic film, the reactive bands were quantified densitometrically using ImageJ software.
2.7. Hydrogels
We used semi-interpenetrated polymer systems (semi-IPNs) based on bovine collagen (COLL) (Sigma-Aldrich) and polyethylene glycol (MW 2000, PEG2000) or COLL and low-molecular weight hyaluronic acid (LMWHA, MW 100 kDa) (Table 1).
Table 1.
Hydrogel composition.
| Hydrogel | Type I Collagen (COLL) (mg/mL) | Polyethylenglycole (PEG) 2000 (mg/mL) | Low molecular weight hyaluronic acid (LMWHA) (mg/mL) |
|---|---|---|---|
| COLL/LMWHA | 1.2 | 0.0 | 2.5 |
| COLL/PEG2000 | 1.8 | 0.6 | 0.0 |
All matrices were prepared from a 2.4 mg/mL COLL solution, dissolved in phosphate buffered saline solution (PBS) and NaOH 0.1 M. PEG2000 (Sigma-Aldrich) 2.4 mg/mL in saline was autoclaved (121 °C, 20 min). The LMWHA (Altergon Italia) 5 mg/mL was obtained by dissolving the polymer in MilliQ water and sterilized by autoclaving (121 °C, 20 min).
COLL/PEG2000 (1.8 mg COLL/mL; PEG2000 0.6 mg/mL) was obtained by mixing 3:1 2.4 mg/mL COLL and 2.4 mg/mL PEG2000. COLL/LMWHA (COLL 1.2 mg/mL; LMWHA 2.5 mg/mL) was obtained by mixing 1:1 2.4 mg/mL COLL and 5 mg/mL LMWHA. Rheological properties and injectability were characterized beforehand [7]. For experimental purposes, we prepared 500 μL samples in 48-well plates (Costar Corning) that were incubated at 37 °C for 1 h to promote fibrillogenesis.
2.8. RAA-MSC encapsulation and conditioned medium
RAA-MSCs were resuspended in medium at a density of 2.5 × 106 cells/mL and mixed 1:10 (v/v) in PEG2000/COLL or COLL/LMWHA, 500 μL of this suspension were dispensed into 48-well plates and incubated at 37 °C for 1 h. After that, 500 μL of complete culture medium were added and replaced after overnight incubation with αMEM without FBS for secretome collection. After a further 24–48 h, the conditioned medium containing the secretome was removed and immediately frozen at −80 °C; cellular metabolic activity was evaluated by a colorimetric test (MTS, Promega).
2.9. Proliferative effect of RAA-MSC conditioned medium
To evaluate cell proliferation, we calculated cell number using a DNA content quantification assay [8]. SH-SY5Y cells were seeded at a density of 5.5 × 105 cells/cm2 in 100 μL of culture medium and grown overnight. The next day, culture medium was replaced with 100 μL/well of CM or standard culture medium (with and without FBS) as control. The next day, the medium was replaced with 100 μL/well of complete αMEM without FBS. Twenty-four hours later the medium was removed, and cells were lysed by adding sterile water (200 μL/well) and running four cycles of freezing at −80 °C and thawing at 37 °C. Then 50 μL of lysate were mixed with 50 μL of Hoechst 33258 (1 μg/μL, Thermo Fisher Scientific), dispensed in 96 well-plates (Costar Corning), and shaken for 1 min before fluorescence assessment (λexc 360 nm, λem 460 nm). DNA content was calculated from a standard curve and the number of cells in each sample was calculated by assuming that a diploid human cell contains 6.4 pg DNA [9], applying the following formula:
2.10. Statistics
The experimental data were analyzed by one-way analysis of variance (ANOVA) and Dunnett's test, two-way ANOVA and post hoc tests, or with Student’s t-test for a direct comparison of two groups. Associations with p < 0.05 were considered significant. Statistical tests were done using GraphPad Prism 6.0 software.
3. Results
3.1. SH-SY5Y cells are protected from oxidative damage by RAA-MSC conditioned medium
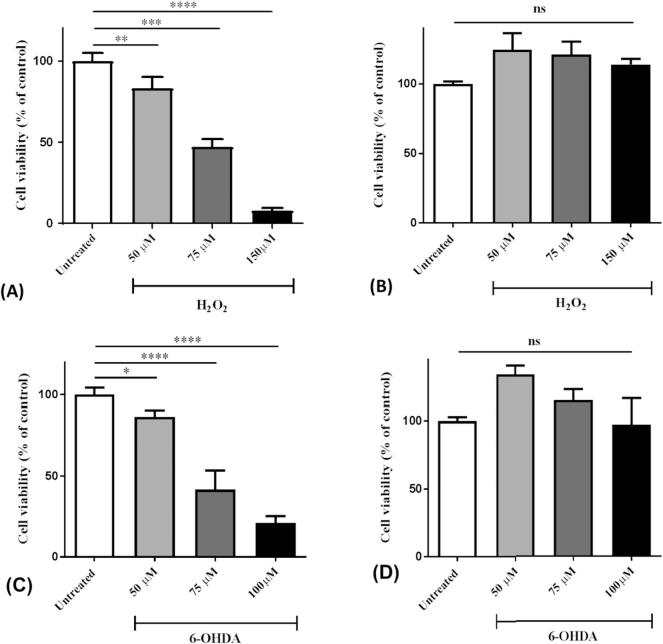
To assess whether the conditioned medium (CM) from RAA-MSCs had cytoprotective action against oxidative stress, we measured the SH-SY5Y response to increasing concentrations of H2O2 or 6-OHDA. Cells were pre-conditioned with RAA-MSC CM for 24 h and then exposed to oxidative challenge (Fig. 1). The CM exerted a protective effect against the toxicity induced by H2O2 or 6-OHDA for every concentration, with full recovery of cell viability.
Fig. 1.
Dose-response patterns of SH-SY5Y cells to hydrogen peroxide (H2O2) or 6-hydroxydopamine (6-OHDA). Cells were exposed to the oxidant stimulus without (A–C) or after (B–D) pre-conditioning with RAA-MSC conditioned media (CM) for 24 h. Cell viability was quantified by MTS assay. **p<0.01 vs. control group; ***p<0.001 vs. control group; ****p<0.0001 vs. control group; ns: not significant (one-way ANOVA and Dunnett's test).
As dilutions of CM may be cytoprotective [10], we then examined the antioxidant effect of serial dilutions of RAA-MSC CM against 100 μM 6-OHDA (Fig. 2A). Cell viability was recovered only with undiluted CM. The result was similar for H2O2 (data not shown). To verify the specificity of the protective effect of the RAA-MSC, as negative control we used conditioned media from dermal fibroblasts (HuDe), which share mesenchymal phenotypes with MSCs, but lack the differentiation and colony-forming potential [11]. We used undiluted HuDe conditioned medium, but there was no recovery of cell viability (Fig. 2B).
Fig. 2.
Concentration-dependent effect and specificity of RAA-MSC conditioned media (CM) against oxidative stress. (A). Dose-response to 6-hydroxydopamine (6-OHDA) 100 μM of decreasing dilutions of RAA-MSCs CM. SH-SY5Y were pre-conditioned for 24 h before challenge with the toxin for a further 24 h. Cell viability was quantified by MTS assay. (B) The CM from dermal fibroblasts was unable to prevent the oxidative damage triggered by 6-OHDA. ****p < 0.0001 vs. control group; ns: not significant (one-way ANOVA and Dunnett's test). (C) Dose-response pattern to H2O2 and ROS generation. SH-SY5Y cells were pre-conditioned for 24 h with RAA-MSC CM 100% and treated with DCFDA 10 μM to measure intracellular ROS levels. The fluorescence was quantified using a fluorescence reader. *p < 0.05 vs. control group; ns: not significant; two-way ANOVA, Tukey's post hoc test.
After proving that the CM from RAA-MSC had protective action against oxidative stress, we measured ROS by DCFDA assay in the same experimental setting (Fig. 2C). There was a slight reduction in the ROS level in SH-SY5Y pre-conditioned with CM compared to control, supportive of an antioxidant response.
3.2. Neuroprotective effect of conditioned medium collected from hydrogel-embedded RAA-MSCs
Once it was clear that RAA-MSC CM promoted an antioxidant response in SH-SY5Y cells, we implemented secretome release from hydrogel-embedded RAA-MSCs. First, we assessed the cytocompatibility of hydrogel-embedded RAA-MSC CM (Fig. 3A). There were no deleterious effects due to the hydrogel matrices. Then we measured the metabolic activity of SH-SY5Y cells treated for 24 h with the CM exposed for 24 or 48 h to RAA-MSCs encapsulated in COLL/PEG2000 or COLL/LMWHA. As a reference, we used the CM from RAA-MSCs grown in standard conditions (Flask) without the hydrogel matrices (Fig. 3B and C). When SH-SY5Y cells were exposed to CM enriched for 24 h (Fig. 3B), the medium containing secretome from RAA-MSCs encapsulated in PEG2000/COLL allowed recovery of metabolic activity, ranging from 50 to 62% of the reference condition (SH-SH5Y cells exposed to 6-OHDA only). Recovery in cell viability was comparable also in CM exposed for 24 h to RAA-MSCs included in COLL/LMWHA gel.
Fig. 3.
Cytocompatibility and antioxidant effect of conditioned media (CM) from hydrogel-embedded RAA-MSCs. (A) Metabolic activity of SH-SY5Y cells after 24 h incubation with CM from RAA-MSCs cells included in hydrogels. Statistical analysis was done considering αMEM without FBS as control. One-way ANOVA followed by Tukey's multiple comparisons test (mean ± SD, n = 5). *p = 0.0226 and 0.025, and ns: not significant. (B) Metabolic activity of SH-SY5Y cells exposed to hydrogel-embedded RAA-MSC CM, collected after 24 h conditioning followed by 24 h challenge with 6-OHDA. Results are mean ± SD (n = 12). Statistical analysis was done using two-way ANOVA followed by Tukey's multiple comparisons test: *p = 0.012; ****p < 0.0001. (C) Metabolic activity of SH-SY5Y cells exposed to hydrogel-embedded RAA-MSC CM collected after 48 h of conditioning. Cells were then challenged for 24 h with 6-OHDA. The results are mean ± SD (n = 12). Statistical analysis was done using two-way ANOVA followed by Tukey's multiple comparisons test: ****p < 0.0001. Flask: CM from RAA-MSCs grown in standard conditions without the hydrogel matrices.
We repeated the experiment using the CM containing secretome produced by RAA-MSCs encapsulated in the hydrogels for 48 h (Fig. 3C). The CM from COLL/PEG2000 embedded cells had a protective effect, leading to an increase of cell viability ranging from 60 to 75% of 6-OHDA alone, similarly to the CM from COLL/LMWHA.
3.3. Conditioned medium from hydrogel-embedded RAA-MSCs counteracts oxidative damage even after correction for its proliferative effect
The findings illustrated in Fig. 3 indicate that the secretome from RAA-MSCs embedded in hydrogels has a complete protective effect on SH-SY5Y cells exposed to 6-OHDA. However, others have reported a proliferative capacity of MSCs secretome on neuron-like cells [12]. Consequently, the protection may depend partly on an underlying increase in the number of cells compared to the control unexposed to CM. To test this, we measured DNA content as a marker of cell proliferation (Fig. 4). Samples treated with CM had a significantly higher DNA content than the reference (αMEM medium without FBS). Otherwise, SH-SY5Y cells treated with CM gave values not dissimilar from samples grown in αMEM with FBS (p > 0.05). We then replicated the experiment depicted in Fig. 3, correcting for the increased number of cells by weighting cell viability for the corresponding DNA concentration. This corrected analysis is reported in Fig. 4B. We were able to replicate a reduced but still significant effect of CM with secretome from RAA-MSCs encapsulated in COLL/PEG2000 or COLL/LMWHA hydrogels. There was a 26% recovery, compared with the reference in the case of CM produced from COLL/PEG2000 and 24% for COLL/LMWHA.
Fig. 4.
Contribution to neuroprotection of the proliferative effect of conditioned medium (CM) of hydrogel-embedded RAA-MSCs (A). Concentration of DNA in the SH-SY5Y cells treated with CM quantified by Hoechst. One-way ANOVA followed by Tukey's multiple comparisons test (mean ± SD, n = 6). **p = 0.0011. (B) Metabolic activity of SH-SY5Y cells exposed to CM from hydrogel embedded RAA-MSCs collected after 48 h of conditioning. Cells were the exposed for 24 h to oxidative stress triggered by 6-OHDA 75 μM. The results are weighted on the relative cell proliferation compared to the control without FBS (mean ± SD, n = 5). One-way ANOVA followed by Tukey 's multiple comparisons test: ****p < 0.0001; **p = 0.0024 and 0.0023.
3.4. Expression of antioxidant proteins after exposure to conditioned medium
To seek the molecular mediators of the antioxidant response in SH-SY5Y cells exposed to hydrogel-embedded RAA-MSCs CM, we examined the expression of key proteins linked to oxidative stress, such as Hsp70, SOD2 and sirtuins-1 (Sirt1) and 3 (Sirt3) (Fig. 5) [13], [14], [15]. Quantitative analysis of Hsp70 and SOD2 did not show any difference between control SH-SY5Y cells and secretome pre-conditioning (Fig. 5A and B). Sirt-1 was equally unchanged (Fig. 5C), while Sirt3 was overexpressed in CM-exposed cells (Fig. 5D).
Fig. 5.
Western blotting to assess expression of antioxidant proteins. (A) Analysis of the expression of Hsp70. SH-SY5Y cells were pre-conditioned with RAA-CM 100% for 24 h. The bar graph shows the densitometric analysis of the bands relative to the expression levels of α-Tubulin; ns: not significant. (B) Expression of mitochondrial SOD2. SH-SY5Y cells were pre-conditioned as above. The densitometric quantification reported was normalized to the mitochondrial protein VDAC; ns: not significant. (C) Expression of Sirt1. SH-SY5Y cells were pre-conditioned as described. Densitometric analysis relative to α-Tubulin was ns: not significant. (D) Expression of mitochondrial Sirt3. SH-SY5Y cells were pre-conditioned with RAA-CM 100% for 24 h, then mitochondrial proteins were extracted and analyzed. The bar graphs show the densitometric quantification, relative to the expression levels of VDAC; **p<0.01, unpaired Student’s t-test. Each experiment was independently replicated twice.
4. Discussion
MSCs regulate neuroinflammation and regeneration through action on microglia and secretion of specific bioactive factors, including growth factors, chemokines, cytokines and hormones [3], [16]. There are numerous applications of the medium conditioned by MSCs. In vivo, it increases neurogenic activity, reduces cognitive impairment and oxidative stress in mouse models of Alzheimer's disease [17]; in vitro it boosts the resistance to oxidative stress in cells of patients suffering from Friedreich ataxia [18], has antioxidant effects in skin aging processes [19] and in the protection of neurons exposed to glutamate excitotoxicity [10]. We assessed the antioxidant capacity of the CM exposed to the metabolic activity of RAA-MSCs in an in vitro model relevant for neurodegeneration. CM contrasted oxidative stress caused by the dopaminergic-selective toxin 6-OHDA [20]. CM reduced cell death in a concentration-dependent manner, with optimal protective effect when undiluted. This is partly in line with the literature, as others have described a CM dilution of 50% as optimal [10], while 100% appears even to be deleterious [21]. This probably depends on different sources of the cells (rat or human adipose tissue) and on the different cell type used (neuroblastoma or primary culture of rat neurons). Nevertheless, the cytoprotective action is specific to this CM, as CM from human fibroblasts (HuDe) did not lead to any recovery in cell viability.
As previously stated, one limitation of possible MSC-based therapies, particularly in the field of chronic neurodegeneration, is the need for repeated treatment with the secretome, generally preferred to direct infusion of MSCs, although the latter is a possible option [22], [23], [24]. To help solve this, local injection of hydrogel-embedded MSCs may be a strategy combining long-lasting secretome production with increased control over the cell fate. This has already been explored in several fields, for instance bone or cartilage regeneration [25], [26], but also acute neurodegeneration as in traumatic brain injury [27], but there is no information in the field of chronic neurodegenerative disorders such as Parkinson’s disease. We have developed biocompatible hydrogels that may be valuable in this area. They have proved able to host RAA-MSCs in a 3D environment and at the same time do not alter the protective effect of the MSCs CM. The hydrogels showed no degradation in vitro up to 48 h, which is positive in terms of lasting control of the encapsulated cells. The secretome produced by gel-embedded cells sustained recovery of viability at all concentrations of the 6-OHDA tested, with no obvious inferiority in comparison to the CM collected from RAA-MSCs cultured in standard conditions.
An important added value of culturing MSCs in 3D may be a qualitative improvement of their secretome. Huang et al. suggested that mimicking the extracellular 3D structure led to a more physiologic behavior of MSCs, which in turn affects the phenotype [28]. In addition, Suri et al. reported that Schwann cells encapsulated in a 3D matrix based on hyaluronic acid retained their viability and were capable of releasing larger amounts of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) [29]. This requires further analysis, for instance by measuring the 3D secretome content of BDNF, NGF or antioxidant proteins such as Hsp70.
Finally, we can also suggest some molecular pathways that may contribute to the neuroprotective effect. First, we measured the reported proliferative effect of MSC CM [12]. Quantification of the proliferative effect of secretome from hydrogel-embedded RAA-MSCs confirmed that there is a partial increase in cell number when exposed to secretome both in standard and 3D conditions. However, the neuroprotective effect is not entirely due to proliferation, though this must be taken into account to avoid over-estimating the effect in our models.
We were able to link the CM protective effect to a reduction of oxidative stress in terms of ROS generation. As for the molecular players underlying this, Dey et al. showed that the MSC CM acts on PI3K/Akt signaling by increasing the phosphorylation levels of Akt and reducing the levels of phospho-p38 and phospho-JNK, while raising the basal levels of superoxide dismutase 1 (SOD1) and 2 (SOD2) by about 75% [18]. Since in our experiments SOD2 levels appeared similar in SH-SY5Y pre-conditioned and control cells, we examined the expression of other proteins active in oxidative stress, such as heat shock protein 70 (Hsp70) and two members of the sirtuin family, sirtuin 1 (Sirt1) and sirtuin 3 (Sirt3) [13], [14], [15]. The levels of expression of Hsp70 and Sirt1 appeared unchanged after CM exposure, while an expression of Sirt3 increased. Sirt3 is considered the key mitochondrial deacetylase [30] and this enables it to regulate subunits of the mitochondrial complex of the electron transport chain (ETC), directly linked to ROS generation. Sirt3 also deacetylates and positively regulates ROS detoxifying enzymes such as SOD2 or Idh2 [31]. In this respect, even if the increased Sirt3 expression was not paralleled by SOD2 upregulation, this does not exclude a role for SOD2 in the antioxidant response, as its level of acetylation may be lower at an equal level of expression. Further analysis of the level of acetylation of SOD2 is therefore needed to support this possible molecular link.
5. Conclusions
Our findings demonstrate the feasibility of a biomaterials-based approach coupling MSC neuroprotective action to injectable hydrogels, that may increase the controlled release of RAA-MSCs CM in models relevant for Parkinson’s disease toxicity mechanisms, first in vitro and then in vivo.
Acknowledgements
This study received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 646990 - NICHOID) awarded to MTR. The results reflect only the authors’ views and the Agency is not responsible for any use that may be made of the information.
References
- 1.Villoslada P., Moreno B., Melero I., Pablos J.L., Martino G., Uccelli A., Montalban X., Avila J., Rivest S., Acarin L., Appel S., Khoury S.J., McGeer P., Ferrer I., Delgado M., Obeso J., Schwartz M. Immunotherapy for neurological diseases. Clin. Immunol. 2008 Sep;128(3):294–305. doi: 10.1016/j.clim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Yang H., Yang H., Xie Z., Wei L., Bi J. Systemic transplantation of human umbilical cord derived mesenchymal stem cells-educated T regulatory cells improved the impaired cognition in AβPPswe/PS1dE9 transgenic mice. PLoS One. 2013 Jul 25;8(7):e69129. doi: 10.1371/journal.pone.0069129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.C. Tran, M.S. Damaser, Stem cells as drug delivery methods: application of stem cell secretome for regeneration. Adv. Drug Deliv. Rev. 82–83C (2015) 1–11, doi:10.1016/j.addr.2014.10.007. [DOI] [PMC free article] [PubMed]
- 4.Ager R.R., Davis J.L., Agazaryan A., Benavente F., Poon W.W., LaFerla F.M., Blurton-Jones M. Human neural stem cells improve cognition and promote synaptic growth in two complementary transgenic models of Alzheimer's disease and neuronal loss. Hippocampus. 2015 Jul;25(7):813–826. doi: 10.1002/hipo.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pires A.O., Mendes-Pinheiro B., Teixeira F.G., Anjo S.I., Ribeiro-Samy S., Gomes E.D., Serra S.C., Silva N.A., Manadas B., Sousa N., Salgado A.J. Unveiling the differences of secretome of human bone marrow mesenchymal stem cells, adipose tissue-derived stem cells, and human umbilical cord perivascular cells: a proteomic analysis. Stem Cells Dev. 2016;25(14):1073–1083. doi: 10.1089/scd.2016.0048. [DOI] [PubMed] [Google Scholar]
- 6.Gnecchi M., Melo L.G. Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol. Biol. 2009;482:281–294. doi: 10.1007/978-1-59745-060-7_18. [DOI] [PubMed] [Google Scholar]
- 7.Tunesi M., Batelli S., Rodilossi S., Russo T., Grimaldi A., Forloni G., Ambrosio L., Cigada A., Gloria A., Albani D., Giordano C. Development and analysis of semi-interpenetrating polymer networks for brain injection in neurodegenerative disorders. Int. J. Artif. Organs. 2013 Nov;36(11):762–774. doi: 10.5301/ijao.5000282. [DOI] [PubMed] [Google Scholar]
- 8.Tunesi M., Fusco F., Fiordaliso F., Corbelli A., Biella G., Raimondi M.T. Optimization of a 3D dynamic culturing system for in vitro modeling of frontotemporal neurodegeneration-relevant pathologic features. Front. Aging Neurosci. 2016 Jun;22(8):146. doi: 10.3389/fnagi.2016.00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dolezel J., Bartos J., Voglmayr H., Greilhuber J. Nuclear DNA content and genome size of trout and human. Cytometry A. 2003;51(2):127–128. doi: 10.1002/cyto.a.10013. [DOI] [PubMed] [Google Scholar]
- 10.Hao P., Liang Z., Piao H., Ji X., Wang Y., Liu Y., Liu J. Conditioned medium of human adipose-derived mesenchymal stem cells mediates protection in neurons following glutamate excitotoxicity by regulating energy metabolism and GAP-43 expression. Metab. Brain Dis. 2014;29(1):193–205. doi: 10.1007/s11011-014-9490-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alt E., Yan Y., Gehmert S., Song Y.H., Altman A., Gehmert S., Vykoukal D., Bai X. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103(4):197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- 12.Lattanzi W., Geloso M.C., Saulnier N., Giannetti S., Puglisi M.A., Corvino V., Gasbarrini A., Michetti F. Neurotrophic features of human adipose tissue-derived stromal cells: in vitro and in vivo studies. J. Biomed. Biotechnol. 2011;2011:468705. doi: 10.1155/2011/468705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prasad S.N., Bharath Muralidhara M.M. Neurorestorative effects of eugenol, a spice bioactive: evidence in cell model and its efficacy as an intervention molecule to abrogate brain oxidative dysfunctions in the streptozotocin diabetic rat. Neurochem. Int. 2016;95:24–36. doi: 10.1016/j.neuint.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara Y., Takemoto T., Itoh K., Ishida A., Yamazaki T. Dual role of superoxide dismutase 2 induced in activated microglia: oxidative stress tolerance and convergence of inflammatory responses. J. Biol. Chem. 2015;290(37):22805–22817. doi: 10.1074/jbc.M115.659151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haigis M.C., Guarente L.P. Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev. 2006;20(21):2913–2921. doi: 10.1101/gad.1467506. [DOI] [PubMed] [Google Scholar]
- 16.Salgado A.J., Reis R.L., Sousa N.J., Gimble J.M. Adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr. Stem Cell Res. Ther. 2010;5(2):103–110. doi: 10.2174/157488810791268564. [DOI] [PubMed] [Google Scholar]
- 17.Yan Y., Ma T., Gong K., Ao Q., Zhang X., Gong Y. Adipose-derived mesenchymal stem cell transplantation promotes adult neurogenesis in the brains of Alzheimer’s disease mice. Neural Regen. Res. 2014;9(8):798–805. doi: 10.4103/1673-5374.131596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dey R., Kemp K., Gray E., Rice C., Scolding N., Wilkins A. Human mesenchymal stem cells increase anti-oxidant defences in cells derived from patients with Friedreich's ataxia. Cerebellum. 2012;11(4):861–871. doi: 10.1007/s12311-012-0406-2. [DOI] [PubMed] [Google Scholar]
- 19.Kim W.S., Park B.S., Sung J.H. Protective role of adipose-derived stem cells and their soluble factors in photoaging. Arch. Dermatol. Res. 2009;301(5):329–336. doi: 10.1007/s00403-009-0951-9. Jun. [DOI] [PubMed] [Google Scholar]
- 20.Tobón-Velasco J.C., Vázquez-Victorio G., Macías-Silva M., Cuevas E., Ali S.F., Maldonado P.D., González-Trujano M.E., Cuadrado A., Pedraza-Chaverrí J., Santamaría A. S-allyl cysteine protects against 6-hydroxydopamine-induced neurotoxicity in the rat striatum: involvement of Nrf2 transcription factor activation and modulation of signaling kinase cascades. Free Radic. Biol. Med. 2012;53(5):1024–1040. doi: 10.1016/j.freeradbiomed.2012.06.040. Sep 1. [DOI] [PubMed] [Google Scholar]
- 21.Isele N.B., Lee H.S., Landshamer S., Straube A., Padovan C.S., Plesnila N., Culmsee C. Bone marrow stromal cells mediate protection through stimulation of PI3-K/Akt and MAPK signaling in neurons. Neurochem. Int. 2007 Jan;50(1):243–250. doi: 10.1016/j.neuint.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Yousefi F., Ebtekar M., Soudi S., Soleimani M., Hashemi S.M. In vivo immunomodulatory effects of adipose-derived mesenchymal stem cells conditioned medium in experimental autoimmune encephalomyelitis. Immunol. Lett. 2016 Apr;172:94–105. doi: 10.1016/j.imlet.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 23.Cui Y., Ma S., Zhang C., Cao W., Liu M., Li D., Lv P., Xing Q., Qu R., Yao N., Yang B., Guan F. Human umbilical cord mesenchymal stem cells transplantation improves cognitive function in Alzheimer's disease mice by decreasing oxidative stress and promoting hippocampal neurogenesis. Behav. Brain Res. 2017 Mar;1(320):291–301. doi: 10.1016/j.bbr.2016.12.021. [DOI] [PubMed] [Google Scholar]
- 24.Uccelli A., Milanese M., Principato M.C., Morando S., Bonifacino T., Vergani L., Giunti D., Voci A., Carminati E., Giribaldi F., Caponnetto C., Bonanno G. Intravenous mesenchymal stem cells improve survival and motor function in experimental amyotrophic lateral sclerosis. Mol. Med. 2012;18(18):794–804. doi: 10.2119/molmed.2011.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park Y.B., Ha C.W., Kim J.A., Rhim J.H., Park Y.G., Chung J.Y., Lee H.J. Effect of transplanting various concentrations of a composite of human umbilical cord blood-derived mesenchymal stem cells and hyaluronic acid hydrogel on articular cartilage repair in a rabbit model. PLoS One. 2016;11(11):e0165446. doi: 10.1371/journal.pone.0165446. Nov 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wise J.K., Alford A.I., Goldstein S.A., Stegemann J.P. Synergistic enhancement of ectopic bone formation by supplementation of freshly isolated marrow cells with purified MSC in collagen-chitosan hydrogel microbeads. Connect Tissue Res. 2016 Nov;57(6):516–525. doi: 10.3109/03008207.2015.1072519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi W., Huang C.J., Xu X.D., Jin G.H., Huang R.Q., Huang J.F., Chen Y.N., Ju S.Q., Wang Y., Shi Y.W., Qin J.B., Zhang Y.Q., Liu Q.Q., Wang X.B., Zhang X.H., Chen J. Transplantation of RADA16-BDNF peptide scaffold with human umbilical cord mesenchymal stem cells forced with CXCR4 and activated astrocytes for repair of traumatic brain injury. Acta Biomater. 2016 Nov;45:247–261. doi: 10.1016/j.actbio.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Huang G., Wang L., Wang S., Han Y., Wu J., Zhang Q., Xu F., Lu T.J. Engineering three-dimensional cell mechanical microenvironment with hydrogels. Biofabrication. 2012 Dec;4(4):042001. doi: 10.1088/1758-5082/4/4/042001. [DOI] [PubMed] [Google Scholar]
- 29.Suri S., Schmidt C.E. Cell-laden hydrogel constructs of hyaluronic acid, collagen, and laminin for neural tissue engineering. Tissue Eng Part A. 2010;16(5):1703–1716. doi: 10.1089/ten.tea.2009.0381. [DOI] [PubMed] [Google Scholar]
- 30.Lombard D.B., Alt F.W., Cheng H.L., Bunkenborg J., Streeper R.S., Mostoslavsky R., Kim J., Yancopoulos G., Valenzuela D., Murphy A., Yang Y., Chen Y., Hirschey M.D., Bronson R.T., Haigis M., Guarente L.P., Farese R.V., Jr, Weissman S., Verdin E., Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol. Cell. Biol. 2007;27(24):8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Someya S., Yu W., Hallows W.C., Xu J., Vann J.M., Leeuwenburgh C., Tanokura M., Denu J.M., Prolla T.A. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143(5):802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]