Abstract
Cdk5 is a key neuronal kinase necessary for proper brain development, which has recently been implicated in modulating nociception. Conditional deletion of Cdk5 in pain-sensing neurons attenuates pain responses to heat in both the periphery and orofacial regions. Cdk5 activity is regulated by binding to the activators p35 and p39, both of which possess a cyclin box. Our previous examination of the nociceptive role of the well-characterized Cdk5 activator p35 using mice that either lack or overexpress this regulatory subunit demonstrated that Cdk5/p35 activity affects mechanical, chemical, and thermal nociception. In contrast, the nociceptive role of Cdk5’s other less-studied activator p39 is unknown. Here, we report that the knockout of p39 in mice did not affect orofacial and peripheral nociception. The lack of any algesic response to nociceptive stimuli in the p39 knockout mice contrasts with the hypoalgesic effects that result from the deletion of p35. Our data demonstrate different and nonoverlapping roles of Cdk5 activators in the regulation of orofacial as well as peripheral nociception with a crucial role for Cdk5/p35 in pain signaling.
Keywords: Cdk5, p35, p39, nociception, behavior
Introduction
Cyclin-dependent kinase 5 (Cdk5) is a unique serine/threonine kinase that shares high homology with other Cdks but has no role in normal cell cycle control.1,2 Cdk5 regulates many important neuronal functions, including neuronal migration and differentiation, synaptic plasticity, and neurotransmitter release.3–5 Because of its important role in these processes, Cdk5 is critical for brain development and survival as evident from embryonic lethality in Cdk5 knockout(−/−) mice.6
Cdk5 kinase activity depends on its binding to one of its regulatory subunits, either p35 or p39, which share about 57% amino acid homology.7 The expression of these two activators, in turn, limits Cdk5 activity primarily to postmitotic neurons. Despite their similar enzymatic activity and substrate specificity,8,9 the expression profile, stability, and degradation of these Cdk5 activators vary. Transcriptional studies revealed that developmental stage-specific as well as region-specific differences exist between the expression of p35 and p39.10 p35 expression commences during early embryonic stages and peaks in neonates, while p39 expression is delayed to peak postnatally.11–13 Furthermore, p35 levels are significantly higher in cerebral cortex and hippocampus, while p39 is expressed more in posterior regions of the brain, such as the cerebellum.10
Importantly, Cdk5/p39 complexes are less stable when compared to Cdk5/p35.9,14 Phosphorylation of both activators also determines the subcellular localization of active Cdk5 complexes, where Cdk5/p39 shows nuclear localization while Cdk5/p35 is predominantly localized to the cytoplasm.15,16 Finally, both activators differ in their proteasomal degradation in neurons, with a much slower degradation and cleavage rate for p39 compared to p35.17,18
Unlike Cdk5−/− mice, deletion of p35 does not result in embryonic lethality. Even though p35−/− mice are viable and fertile, they display subtle impaired neuronal migration and inverted neuronal layering in the cerebral cortex along with abnormal hippocampus morphology.19 In contrast, mice lacking p39 have no obvious phenotypic defects or apparent abnormalities20 but show impaired remyelination21 and exhibit defects in axonal growth and dendritic spine formation.22 Compound deletion of both Cdk5 activators results in perinatal lethality20 and a phenotype that is nearly identical to the Cdk5−/− mice,6 which suggests that both p35 and p39 are major activators of Cdk5 that contribute to in vivo activation of Cdk5 in a region-specific and developmentally regulated manner.
Even though p39 is a well-known activator of Cdk5, most studies have focused on the role of p35 in different physiological and pathological conditions, particularly since information about p39 has been limited in part due to instability of the Cdk5/p39 complex, lack of specific p39 antibodies, and no overt phenotype of p39−/− mice.
Although Cdk5 activity and p35 expression levels are highest between E18 and P14, Cdk5/p35 activity continues to maintain a role in modulating neuronal functions in adult brain as well, including nociception. For example, we and others initially determined that Cdk5 activity modulates pain signaling,23–25 as Cdk5 activity is upregulated in dorsal root ganglia and spinal cord in response to inflammation.24 In addition, intrathecal injection of the Cdk5 inhibitor roscovitine has been shown to have thermal antinociceptive effects26,27 and can also attenuate nociceptive responses to formalin.28 After establishing that Cdk5 activity influences nociception, we subsequently determined that Cdk5’s role in modulating pain sensitivity is affected by the expression levels of its activator p35. We first reported that Cdk5/p35 activity is required for the basal responses to noxious heat,24 while our more recent studies have uncovered an important role for p35-driven Cdk5 activity in orofacial mechanosensation29 and thermoregulation.30
In this study, we focused on the poorly understood role of p39 in pain perception under physiological conditions. We ascertained that p35- and p39-driven Cdk5 activity regulates peripheral and orofacial nociception differently. We have identified that loss of p39 in mouse peripheral and central nervous system is compensated by p35. In contrast to p35−/− mice, p39−/− mice exhibit similar Cdk5 activity as wild-type mice. A lack of p35, but not p39, influenced oral aversion to pungent natural compounds that are known to activate pain-sensing ion channels (TRPV1 and TRPA1) on nociceptive neurons. Furthermore, in contrast to the analgesic behavior of p35−/− mice, p39−/− mice display the same sensitivity to orofacial mechanical stimuli as wild-type littermate controls. Finally, the loss of p39 is not involved in peripheral thermo- and mechanosensation and p39−/− mice retain normoalgesia. Taken together, these results demonstrate that p39 and p35 play distinct roles in nociception and that only p35-driven Cdk5 activity is essential for modulating pain sensation.
Methods
Mice
p39+/− mice were maintained on a C57BL/6 background. All mice were housed in standard cages in climate (22℃) and light controlled rooms (14:10 h light/dark cycle) with ad libitum food (2918 Teklad global 18% protein rodent diet, Envigo) and water, unless otherwise noted. For all experiments, age-matched wild-type littermates served as controls. All experimental procedures were approved by the Animal Care and Use Committee of the National Institute of Dental and Craniofacial Research, National Institutes of Health and adhered to the guidelines of the International Association for the Study of Pain (IASP) Committee for Research and Ethical Issues.31
Behavioral testing
Open-field test
Exploratory behavior as well as locomotor activity was evaluated using the VersaMax Animal Activity Monitoring System (AccuScan Instruments Inc, Columbus, OH). The open field arena consisted of a clear Plexiglass square chamber of 42 × 42 × 30 cm dimensions, divided into a grid of equally sized areas through infrared photocell beams. During the experiments, mice were placed individually into the center of the open field for 10-min sessions, and their behavior was monitored with an automated tracking system (VersaMax software system).
Mechanical operant behavioral assay
Mechanical sensitivity was measured using the Orofacial Stimulation Test (31300, Ugo Basile, Italy) as described previously.29 Initially, mice underwent a 10-day adaptation training (using a blank plate) after 8 h of deprivation of food and water. Mechanical sensitivity was tested using a plate with 6 + 6 wires, which induces a mechanical force on the mouse vibrissal pad. The mice were trained in the same cage and at the same time each day, and they had a day of rest in between testing. Data were recorded automatically using ORO software and consisted of the total time the mouse spent acquiring the reward (30% sucrose) over a 20-min period, with or without mechanical wires, and the number of attempts the mouse made to access the reward.
Operant lickometer test
Nociceptive responses to natural pungent compounds that activate pain-sensing neurons like capsaicin and isothiocyanates (agonists to the TRPV1 and TRPA1 receptors, respectively) were measured using a lickometer (Habitest system, Coulbourn Instruments, Whitehall, PA). Nociceptive sensation to hot taste stimuli through TRPV1 was induced by different concentrations of capsaicin and to TRPA1 by using allyl isothiocyanate (mustard oil; both Sigma-Aldrich, St Louis, MO). Before the testing, mice were deprived of water overnight and then placed into the Habitest system. A computer-operated system monitored their licking events for 1 h. Initially the mice were trained with water (n = 5 sessions), and then they were tested for consumption/aversion to different concentrations of either capsaicin or mustard oil (n = 5 sessions per concentration). All mice were tested at the same time each day and retested under the same conditions every other day.
Mechanical paw withdrawal threshold test
To determine hind paw mechanical sensitivity, the Dynamic Plantar Aesthesiometer (Ugo Basile) was used. This device consists of transparent plastic chambers over a mesh platform and movable touch stimulator unit with a probe. Paw withdrawal latency to mechanical stimulation was assessed using an automated steel rod that was pushed against the plantar surface of the paw with increasing force until the paw was withdrawn. Mice were allowed to acclimatize for 30 min prior the start of the experiment. Latency and actual force at the time of paw withdrawal were automatically detected and recorded by the unit. Measurements were taken in triplicates at least 10 min apart. Data are presented as mean time and force intensity needed for paw withdrawal.
Thermal paw withdrawal latency test
The response to a noxious thermal stimulus was determined using the Plantar Test (Ugo Basile). This device consists of a movable Infrared (IR) generator placed below the glass panel within plastic chambers. After 30 min of acclimatization, a mobile radiant heat source was placed directly under the plantar surface of the hind paw, and the time taken for hind paw withdrawal was monitored. Two different intensities of the heat source were used: low (IR30) and high (IR60). The heat stimulation was repeated three times with a 10-min interval to obtain the mean latency of paw withdrawal.
RNA extraction and q-PCR
Trigeminal ganglia, dorsal root ganglia, spinal cord, and brain were dissected out from p39−/− mice and their wild-type littermate controls. All tissues were immediately frozen and kept at −80℃. Total RNA was extracted using a RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. RNA from each sample was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, CA). Quantitative real-time polymerase chain reaction (qPCR) reactions were conducted using Assays on Demand and TaqMan Universal Master Mix (both Thermo Fisher Scientific). All samples were run in duplicate using the Real-time PCR System 7500 (Thermo Fisher Scientific). The p39, p35, Cdk5, TRPV1, and TRPA1 levels were normalized to the levels of hypoxanthine-guanine phosphoribosyl-transferase using the comparative threshold method.
Western blotting
Tissue homogenates were lysed in tissue protein extraction reagent (Thermo Fisher Scientific) containing protease (cOmplete Mini) and phosphatase (PhosSTOP) inhibitor cocktail tablets (both Roche, Indianapolis, IN). Total protein concentration was measured using a Bradford Protein Assay (Bio-Rad, Hercules, CA). Protein of 40 µg was separated on a 4% to 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Thermo Fisher Scientific), transferred to a 0.45-µm nitrocellulose membrane, and immunoblotted with antibodies directed against p39 (Abcam, Cambridge, MA), p35 (C-19), Cdk5 (C-8) (both Santa Cruz Biotechnology, Santa Cruz, CA), and tubulin (Sigma-Aldrich) overnight. Membranes were washed with phosphate-buffered saline plus 5% nonfat dry milk with 0.05% Tween 20 (Sigma-Aldrich) and then incubated for 1 h with 1:2000 dilution of anti-mouse/anti-rabbit secondary antibody conjugated to horseradish peroxidase (Santa Cruz Biotechnology). The membranes were developed with SuperSignal West Pico or Dura Chemiluminescent Substrate (Thermo Fisher Scientific).
Cdk5 assay
Kinase assays were performed as described previously.32 Cdk5 was immunoprecipitated with the polyclonal Cdk5 C8 antibody (Santa Cruz Biotechnology) for 2 h at 4℃, and immunoglobulin was isolated using protein A Sepharose beads for 2 h at 4℃. Immunoprecipitates were washed three times with lysis buffer and then once with 1× kinase buffer containing 20 mM Tris-Cl, pH 7.4, 1 mM EDTA, 1 mM ethylene glycol tetraacetic acid, 10 mM MgCl2, 10 mM sodium fluoride, and 1 mM sodium orthovanadate. The samples were then added to the reaction mixture containing kinase buffer, 50 µM of adenosine triphosphate (ATP), 20 μg of histone H1, and 0.5 Ci of [32P] ATP and incubated at 30℃ for 1 h. After the kinase reaction, 25-µl aliquots of the incubation mixture were placed on a Whatman p81 paper square, air-dried, and washed three times for 15 min each in 75 mM phosphoric acid and once in 95% ethanol. After air-drying, the squares were transferred to vials containing Bio-Safe II scintillation fluid for counting.
Statistical analysis
All data are expressed as mean ± SEM. The statistical evaluation was performed with GraphPad Prism 7 software (GraphPad, San Diego, CA). Statistical differences between the groups were assessed using an unpaired t test. The significance level was set at p < 0.05.
Results
Cdk5 activity in both the central and peripheral nervous system of p39−/− mice is p35 dependent
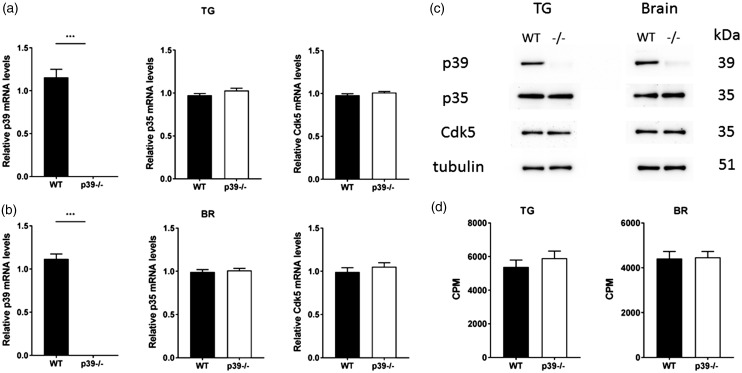
We initially determined the expression profile and activity of Cdk5/p35 in the trigeminal ganglia and brain of p39−/− mice. The qPCR analysis confirmed the targeted deletion of p39 messenger RNA (mRNA) in the knockout mice (p < 0.001). However, there were no statistically significant differences in mRNA profiles of p35 and Cdk5 in either the trigeminal ganglia (Figure 1(a)) or the brain (Figure 1(b)) of p39−/− mice as compared to the littermate controls. In addition, there were no apparent changes in the expression of the transient receptor potential channels TRPV1 or TRPA1 (Supplementary Figure 1(a) and (b)). The immunoblot analysis of p39−/− mice showed almost undetectable p39 protein levels with no obvious changes in p35 or Cdk5 protein levels in trigeminal ganglia and brain of p39−/− mice as compared to wild-type controls (Figure 1(c)). Finally, the loss of p39 did not change the activity of Cdk5 in the trigeminal ganglia and the brain as compared to the wild-type (WT) mice (Figure 1(d)), demonstrating the different regulation of Cdk5 activity in p35−/− and p39−/− mice. Interestingly, while Cdk5 activity was significantly decreased in p35−/− mice,29 it did not change in p39−/− mice, indicating that p35 can compensate for the loss of p39 in order to induce the Cdk5 activity.
Figure 1.
Levels of Cdk5 and its regulatory subunit p35, and Cdk5 kinase activity in trigeminal ganglia and brain of wild-type and p39−/− mice. Real-time PCR analysis revealed significantly reduced levels of p39 mRNA in trigeminal ganglia (a) and brain (b) of p39−/− mice. p35 and Cdk5 expression levels remained unchanged. Results obtained from six different mice are expressed as mean ± SEM and analyzed by unpaired t test (***p < 0.001). Representative Western blots showing p39, p35, and Cdk5 levels from wild-type and p39−/− mice (c). Cdk5 activity in trigeminal ganglia and brain of wild-type and p39−/− mice (d). Data obtained from 4 different mice are presented as mean ± SEM and analyzed by unpaired t test. BR: brain; CPM: counts per minute; Cdk5: cyclin-dependent kinase 5; mRNA: messenger RNA; TG: trigeminal ganglia; WT: wild type.
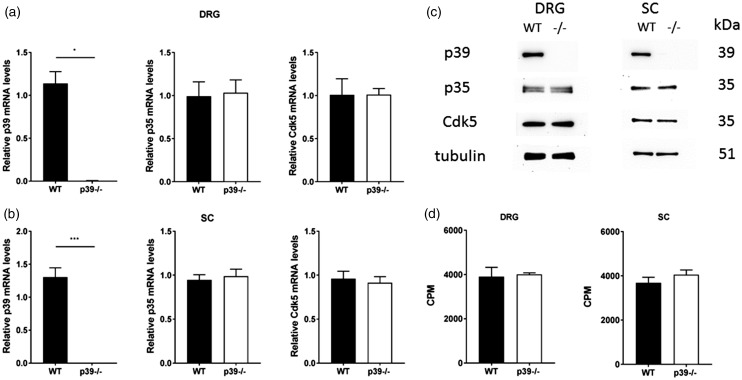
p35 compensates for the absence of p39 within the trigeminal ganglia, which relays orofacial pain, but we also wanted to determine whether p39 has any role in peripheral pain signaling pathways along the dorsal root ganglia and through the lateral spinothalamic tract. Therefore, we next performed expression and activity profile of p35 and Cdk5 in both the dorsal root ganglia and the spinal cord of p39−/− mice. Real-time PCR analysis showed significantly decreased p39 mRNA levels in the dorsal root ganglia (p < 0.05; Figure 2(a)) and the spinal cord (p < 0.001; Figure 2(b)) of p39−/− mice. Loss of p39 elicited no changes in p35/Cdk5 mRNA or protein levels versus the controls (Figure 2(a) to (c)), and no obvious changes in TRPV1 and TRPA1 mRNA levels were seen in these tissues (Supplementary Figure 1(c) and (d)). Lastly, the absence of p39 had no effect on Cdk5 activity in spinal cord and DRGs in p39−/− mice, and its activity was comparable to that in the control animals (Figure 2(d)). Overall, these findings indicate that, even with loss of p39, the presence of p35 still permits Cdk5 to be active.
Figure 2.
Expression and activity profile of p39, p35, and Cdk5 in dorsal root ganglia and spinal cord of p39−/− mice. qPCR analysis revealed significantly decreased levels of p39 mRNA in the DRG (a) and spinal cord (b) of p39−/− mice but no changes in p35 or Cdk5 mRNA levels. All the data were normalized to the expression level seen in control animals. Results obtained from 6 to 10 animals are expressed as mean ± SEM and analyzed by an unpaired t test (*p < 0.05, ***p < 0.001). Representative Western blots showing p39, p35, and Cdk5 protein levels in DRG and spinal cord of control and p39−/− animals (c). Cdk5 activity in DRG (n = 6) and spinal cord (n = 5) of WT and p39−/− mice (d). Data are presented as mean ± SEM. Cdk5: cyclin-dependent kinase 5; CPM: counts per minute; DRG: dorsal root ganglion; mRNA: messenger RNA; SC: spinal cord; WT: wild type.
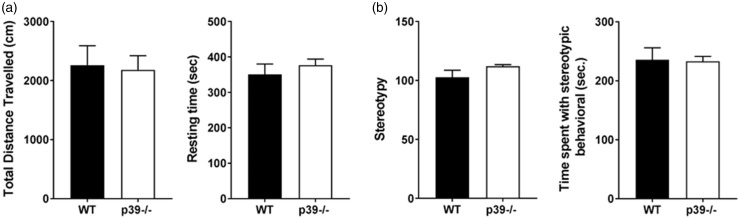
Normal exploratory activity and anxiety in p39−/− mice
Prior to investigating the role of p39 in the orofacial pain operant assays, we first ascertained whether different expression of this gene might affect locomotor and exploratory behavior using an open-field test. The total distance travelled and resting time, a measure of exploration, were essentially the same in both groups (Figure 3(a)), indicating that depletion of p39 does not cause any exploratory changes. Since the center of a nonfamiliar arena could be anxiogenic, anxiety was studied by analyzing the time and distance the mice spent in the center of the arena. There were no changes in the time that mice spent in the middle of the cage as well as the center distance travelled between p39−/− mice and their littermate controls. In conclusion, the loss of p39 did not cause any significant changes in stereotypical behavior (Figure 3(b)) including anxiety or exploratory behavior, both of which could, in turn, affect the pain testing.
Figure 3.
Normal activity and anxiety levels in p39−/− mice. Total distance travelled and resting time (a), stereotypy and time control and p39−/− mice spent displaying stereotypic behavior (b). Values represent the mean from four mice during 10-min interval sessions. Data are presented as mean ± SEM and analyzed by unpaired t test. WT: wild type.
Oral aversion to capsaicin is p39 independent
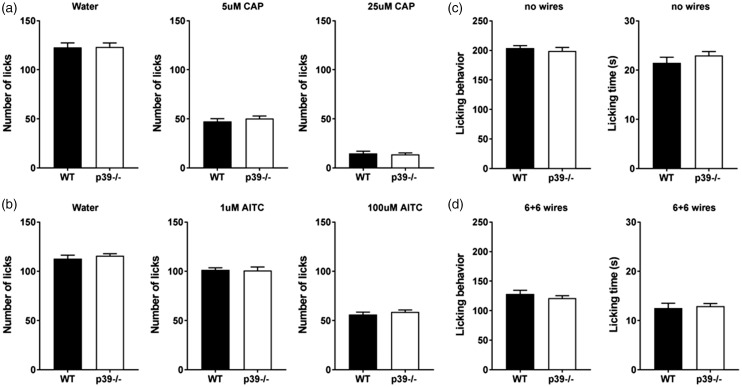
It is well established that a reward–conflict paradigm can be employed to measure pain in the orofacial area. To determine whether the Cdk5 activator p39 affects cheminociceptive behavior, we examined the sensitivity of p39−/− mice to capsaicin, a specific activator of TRPV1. Oral administration of capsaicin causes an unpleasant burning sensation. We have previously reported that p35-driven Cdk5 activity serves as an important factor for the development of oral aversion to capsaicin. Studies on genetically engineered mice revealed that mice overexpressing p35 (with hyperactivated Cdk5) were more sensitive to capsaicin consumption, whereas p35−/− (with low Cdk5 activity) showed decreased aversion to capsaicin consumption. Moreover, conditional deletion of Cdk5 in nociceptive neurons showed similar results as complete p35−/−.30
After establishing this relationship, we next asked how the loss of p39 may affect cheminociception to a TRPV1 agonist. Using a lickometer, we assayed the sensitivity of p39−/− mice as well as their littermate controls to different concentrations of capsaicin (5 and 25 µM). Targeted deletion of p39 did not affect licking behavior during the training sessions (water; Figure 4(a)). Interestingly, unlike the analgesic phenotype in p35−/− mice, loss of p39 did not result in any hypoalgesic behavior, as the p39−/− showed the same licking response as the wild-type controls. Specifically, we did not notice any changes in aversion to either low or high concentrations of capsaicin as indicated by similar consumption of capsaicin as compared to control mice (Figure 4(a)). These data indicate that p39 does not modulate orofacial cheminociception induced by capsaicin, where p35 apparently compensates for the loss of p39 to induce Cdk5 activity to levels equivalent to those in wild-type mice.
Figure 4.
Behavioral responses of wild-type and p39−/− mice during orofacial operant assays. Responses of wild type and p39−/− mice to TRPV1 and TRPA1 agonists. Water-deprived mice were tested using a lickometer. Initially, mean licking responses were measured using water. After five training sessions, water was replaced with 5 or 25 μM capsaicin, and aversion to the agonist was measured during 1 h (a). Data are presented as mean ± SEM from four mice during five differenct measurements. Effect of activation of the TRPA1 receptor. Mean licking responses of wild-type p39−/− mice during lickometer testing using water and two different concentrations of the TRPA1 agonist mustard oil-1 and 100 μM (b). During mechanical testing, mice were able to access a 30% sucrose reward by inserting their snout through the drinking window. Licking behaviour and the time spent licking the reward were measured with no wires (c) or using a plate of 6 + 6 wires (d). Data are presented as mean ± SEM from four mice measured five times in case of the baseline and three times after the induction of nociception. AITC: allyl isothiocyanate; CAP: capsaicin; WT: wild type.
Loss of p39 did not affect aversion to isothiocyanates
To further validate whether the loss of p39 affects cheminociception, we again conducted behavioral testing using the lickometer against another transient receptor potential channel agonist, the isothiocyanate mustard oil, which is known to activate TRPA1, a pain-sensing ion channel that is highly colocalized with TRPV1 in nociceptors. We, therefore, examined the licking behavior of wild-type and p39−/− mice toward water dosed with different concentrations of mustard oil. As with capsaicin, p39−/− did not show any changes in aversion to mustard oil as compared to wild-type controls (Figure 4(b)), demonstrating that loss of p39 does not alter the aversion to the TRPA1 agonist mustard oil, indicating that p35 plays a compensatory role in Cdk5 activity in terms of cheminociception.
Orofacial mechanosensation is not affected by the loss of p39
Although sensitivity to noxious heat and cold can be relayed through TRPV1 lineage neurons, mechanical pain sensitivity is transmitted via another subpopulation of somatosensory neurons.33 We therefore tested whether this particular set of mechanosensors was also modulated by changes in Cdk5 activity and, if so, what are the contributions of each Cdk5 activator? We previously reported that mice overexpressing or lacking p35 showed altered responses to noxious mechanical stimulation in the trigeminal area. Mice with increased Cdk5 activity (driven by p35) displayed aversive behavior to mechanical stimulation, whereas mice deficient in Cdk5 activity displayed mechanical hypoalgesia.29 In the present study, we examined whether p39-mediated Cdk5 activity affects mechanical pain perception in the orofacial area. Mechanosensation was measured using a mouse orofacial stimulation test where mice must push their snout through a series of abrasive wires to drink a solution of 30% sucrose. Mice were initially trained to access the reward through an innocuous module with no wires. During the baseline measurements, p39−/− and WT C57 mice did not show any aversive behavior, as there were no differences in licking behavior or the time spent in licking the reward (Figure 4(c)). Unlike the p35−/− mice, which showed increased licking time (less aversion to the wires) as compared to the controls, the p39−/− mice exhibited the same aversion to mechanical stimuli as the wild-type controls (Figure 4(d)), indicating that p39 deficiency has no analgesic properties.
Responses to peripheral mechanical stimulation are p35 dependent
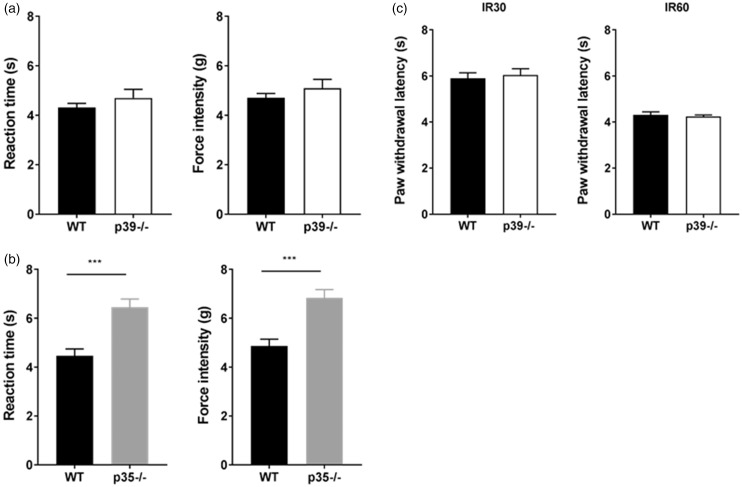
Mechanical hyperalgesia and allodynia are commonly reported symptoms of acute and chronic pain, so, in the next series of experiments, we examined the behavioral responses of p39−/− and p35−/− mice to peripheral mechanical stimulation. To quantify this behavior, we employed Dynamic Plantar Aesthesiometer to measure time and force needed for the paw withdrawal. p39−/− mice experienced normal mechanosensation and the time/force needed for paw withdrawal was similar to the wild-type controls (Figure 5(a)). On the contrary, p35−/− mice developed mechanical hypoalgesia indicating an important role of p35 in the regulation of mechanosensation by Cdk5 (Figure 5(b)). Collectively, these results indicate that only p35-mediated Cdk5 activity is involved in the establishment of sensitivity to mechanical stimuli, and they are in agreement with our previous report.29
Figure 5.
Behavioral responses of control, p35−/− and p39−/− mice to mechanical and thermal stimulation at the periphery. Reaction time and force intensity needed for paw withdrawal was unaffected in the control as well as the p39−/− mice (a). Results are expressed as mean ± SEM of time and force required to induce paw withdrawal in eight animals per group. p35−/− mice exhibited a delayed basal response to mechanical stimulation and experienced hypoalgesia compared to control animals (t test, p < 0.001) (b). Data are presented as a mean ± SEM (n = 8). Basal thermal responses were unaffected in p39−/− mice. Mean paw withdrawal latencies during low (IR30) and high (IR 60) heat stimulation (c). Data are presented as a mean ± SEM (n = 8). WT: wild type.
Thermal normoalgesia in p39−/− mice
To test whether p39 plays a role in peripheral thermosensation, we extended behavioral testing and measured responses to peripheral thermal stimulation in p39−/− mice. As per our earlier report, p35−/− mice (with significantly decreased Cdk5 activity) displayed peripheral hypoalgesia to thermal stimuli. Our current work demonstrates that p39−/− mice did not show any behavioral changes to thermal stimulation and displayed the same paw withdrawal latencies as wild-type controls. Normoalgesic responses in p39−/− were IR intensity independent (Figure 5(c)). Taken together, these results indicate that p35, but not p39, is required to regulate peripheral thermosensation, and p35-driven Cdk5 activity exclusively contributes to the transduction of this type of nociception.
Discussion
Multiple molecular changes in the peripheral and central nervous system contribute to the transduction of noxious stimuli. Nociception triggers a variety of behavioral and emotional responses that can intensify the reaction to pain. We and others have determined that changes in Cdk5 activity affect sensitivity to painful stimuli.24 Diverse aspects of neurogenesis are influenced by Cdk5, and numerous studies have revealed its important and multifunctional roles in physiological and pathological neuronal processes, including pain perception. In terms of nociception, administration of the Cdk5 inhibitor roscovitine promotes analgesia in various pain models. Roscovitine specifically interferes with Cdk5 activity by inhibiting ATP binding, which is required for its kinase activity.34 Intrathecal injections of roscovitine attenuated formalin-induced pain28 and suppressed heat hyperalgesia upon peripheral inflammation.25–27,35 Recent reports have also revealed an antinociceptive effect of roscovitine for neuropathic pain as well.36,37 Moreover, roscovitine can prevent remifentanil-induced postoperative thermal and mechanical hyperalgesia.38 Finally, downregulation of NMDA receptors by roscovitine was indicated in a model of cancer pain, where roscovitine significantly reduced mechanical allodynia and thermal hyperalgesia via inhibition of NR2B receptor.39 Although overall inhibition of Cdk5 by roscovitine shows an analgesic potential in different pain models, these studies have not examined which regulatory subunit, p35 or p39, contributes most to the pain perception. Here, we have investigated which Cdk5 regulatory subunit is important for normal pain sensation, assayed their importance in mechano, chemo, and thermosensation, and showed that p35 exclusively controls Cdk5-mediated regulation of pain sensitivity to various noxious stimuli.
The critical roles of Cdk5 in controlling normal neuronal development and function have been well established, but emerging evidence now implicates a role for Cdk5 in nociception. However, the mechanisms that activate Cdk5 during pain sensitization still remain elusive. Although Cdk5 must associate with either p35 or p39 for its kinase activity, most studies on Cdk5 have not focused on differentiating between p35-driven and p39-driven activity. We believe that our study is the first report distinguishing the specific roles of Cdk5/p35 versus Cdk5/p39 activity in terms of pain sensation. Previously, we demonstrated that peripheral and orofacial nociception are both regulated by Cdk5/p35 activity. Our current study shows that the lack of p39 does not lead to changes in pain perception, and that only the lack of p35 causes reduced Cdk5 activity in nociceptors to produce an analgesic effect, thereby demonstrating that p35 plays the essential role in the regulation of Cdk5 activity during nociception.
The importance of both the p35 and p39 subunits in regulating Cdk5 activity is seen in mice that lack both activators,20 where deletion of both p35 and p39 is needed to replicate the perinatal lethal phenotype of the Cdk5−/− mice.6 Mice lacking p35 alone, however, show lamination defects in the cerebral cortex but are still viable without any gross behavioral defects. Cdk5 activity mediated through p39 must therefore compensate for the loss of p35 to prevent embryonic lethality. The brain developmental defects in the p35−/− mice may result from its early expression during embryonic stages as compared to p39, which is expressed later (mainly postnatally) to induce Cdk5 activity. In contrast to the p35−/− mice, p39−/− mice do not display any obvious developmental phenotype, and most p39 functions are compensated by p35. Nonetheless, both embryonic Cdk5/p35 and postnatal Cdk5/p39 activity is needed for proper brain development, and this precise control of Cdk5 is crucial for physiological brain functions.
More recent evidences indicate nonoverlapping or even opposing roles for these two Cdk5 activators in multiple aspects of neurogenesis. As we already described, both activators display distinct expression profiles, stability, and degradation profiles. In addition, p35 and p39 also regulate diverse stages of oligodendrocyte development and myelination.40 Loss of p35 perturbs oligodendrocyte progenitor cell differentiation, process outgrowth, and myelination, whereas loss of p39 only delays maturation to myelin basic protein-positive oligodendrocytes. The expression of p39, but not p35, is upregulated during remyelinization and is essential for the repair of myelin lesions.21 In addition, both regulatory subunits also have different abilities to phosphorylate tau, but only Cdk5/p39 is involved in the in vivo phosphorylation of tau at Ser-202 and Thr-205 during brain development.12 Discrete-specific roles for both activators have been demonstrated in nonneuronal cells as well. Calcium-dependent insulin secretion from beta cells by phosphorylation of Munc 18-1 is promoted by Cdk5/p39, but not by Cdk5/p35.41 Finally, unlike the proepileptic phenotype in p35−/− mice, loss of p39 ameliorates responses to kainic acid-induced seizures.22 However, while the knockout mice for each regulatory subunit have revealed some distinct functions between p35 and p39 in terms of regulating Cdk5 activity, the exact role for either activator in pain signaling remains to be determined.
Our previous in vivo studies have demonstrated that genetically engineered mice with either increased or decreased Cdk5 activity have altered thermal and mechanical nociception.29,30 We established that Cdk5/p35 activity, in particular, is important in orofacial mechanosensation. Mice overexpressing or lacking p35 showed altered phenotypes in response to noxious mechanical stimulation of the orofacial region. Whereas mice with increased levels of p35 and Cdk5 activity display mechanical hyperalgesia, mice deficient in p35 and Cdk5 activity show mechanical hypoalgesia.29
Along with mechanonociception, Cdk5 activity also affects responses to noxious heat.30 A part of these effects is mediated by the modulation of TRPV1-mediated calcium influx. The TRPV1 receptor, which is an ion channel expressed in the peripheral and central nervous system, is activated by noxious heat, lipids, acidity, and specific agonists such as capsaicin. We have previously reported that Cdk5 modulates nociceptive signaling through the phosphorylation of TRPV1 at threonine-407.23,30 Moreover, p35−/− mice exhibited nonaversive behavior when licking capsaicin. In contrast, mice with increased p35-driven Cdk5 activity showed significantly increased aversion to capsaicin consumption.30
In our present study, we have characterized p39−/− mice and identified that in these mice, lack of p39 is functionally compensated by the expression of p35. Using an in vitro kinase assay, we confirmed that there is no difference in the level of Cdk5 activity in p39−/− mice as compared to the wild-type mice. Using different operant orofacial assays, we have demonstrated that there is no change in orofacial mechanosensation in p39−/− mice. In agreement with these findings, the responses of p39−/− mice to oral administration of capsaicin or mustard oil were similar to those of wild-type mice. Operant behavioral testing of pain in the orofacial area is of interest since this region is one of the most densely innervated areas of the body. The study of orofacial pain readily lends itself to operant behavioral testing, where a conflict–reward paradigm can be established. Operant testing also allows for the measurement of pain hypersensitivity, which is seen with chronic pain, while minimizing investigator interference and bias.
Orofacial pain is transmitted by primary afferents through the trigeminal ganglia into the brain. However, due to the rich innervation of craniofacial structures, orofacial pain entities are often very complex, debilitating to the patient, and difficult to diagnose and treat in part due to the limited understanding of the pathophysiology of pain that arises from structures innervated by trigeminal ganglia. Although clinical interest is high, the proportion of experimental studies performed on animal models is low due to the lack of suitable animal models to measure pain in the orofacial area. There are relatively few behavioral models in laboratory animals dedicated to the study of nociception in the trigeminal region compared to the large variety of tests that measure peripheral nociception. Hence, in our study, we focused primarily on using the currently available orofacial operant assays that have advantages over basic reflex testing, but there are a limited number of existing behavioral assays around to test pain in the orofacial area.
After concluding that p39 has no role in orofacial pain sensitivity, we next decided to perform conventional pain testing that are primarily restricted to measuring peripheral responses. Using paw withdrawal testing, we were also able to verify that peripheral mechano- and thermosensation is not significantly altered between controls and p39−/− mice, indicating compensatory role of p35. These results establish that only Cdk5/p35 activity modulates orofacial as well as peripheral pain signaling, and that inhibition of Cdk5 activity controlled by p35 could serve as a novel therapeutic strategy for the development of new class of analgesics.
Conclusion
The Cdk5 activator p39 has no substantial role in nociception despite the fact that it is expressed in both types of sensory ganglia. Reward–conflict operant testing revealed that, unlike the p35−/− mice, the targeted deletion of p39 did not affect orofacial mechanical and chemical nociception, which shows the important contribution of p35 toward compensating for the lack of p39. Reflex testing showed that p39−/− mice retain normal peripheral sensation to noxious heat and mechanical stimulation. All together, these results indicate that orofacial nociception is mediated exclusively by p35-mediated Cdk5 activity, suggesting that p35 could be a new potential therapeutic target for alleviating mechanical and thermal pain.
Authors’ Contributions
MP, HCP, and ABK designed the experiments. MP, BH, MH, TO, JR, BKB, NDA, and ER were responsible for the execution, data collection, and evaluations. MP and ABK wrote the manuscript. All authors read, edited, and approved the final manuscript.
Declaring of Conflict of Interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported in part by the NIDCR Gene Transfer Core (DE000744-03) and the Intramural Research Programs of NIDCR and NINDS, NIH.
Supplementary Material
Supplementary material is available for this article online.
References
- 1.Malumbres M. Cyclin-dependent kinases. Genome Biol 2014; 15: 122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su SC, Tsai LH. Cyclin-dependent kinases in brain development and disease. Annu Rev Cell Dev Biol 2011; 27: 465–491. [DOI] [PubMed] [Google Scholar]
- 3.Dhariwala FA, Rajadhyaksha MS. An unusual member of the Cdk family: Cdk5. Cell Mol Neurobiol 2008; 28: 351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhavan R, Tsai LH. A decade of CDK5. Nat Rev Mol Cell Biol 2001; 2: 749–759. [DOI] [PubMed] [Google Scholar]
- 5.Lalioti V, Pulido D, Sandoval IV. Cdk5, the multifunctional surveyor. Cell Cycle 2010; 9: 284–311. [DOI] [PubMed] [Google Scholar]
- 6.Ohshima T, Ward JM, Huh CG, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci USA 1996; 93: 11173–11178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang DM, Yeung J, Lee KY, et al. An isoform of the neuronal cyclin-dependent kinase-5 (Cdk5) activator. J Biol Chem 1995; 270: 26897–26903. [DOI] [PubMed] [Google Scholar]
- 8.Humbert S, Dhavan R, Tsai LH. p39 activates cdk5 in neurons, and is associated with the actin cytoskeleton. J Cell Sci 2000; 113: 975–983. [DOI] [PubMed] [Google Scholar]
- 9.Yamada M, Saito T, Sato Y, et al. Cdk5-p39 is a labile complex with the similar substrate specificity to Cdk5-p35. J Neurochem 2007; 102: 1477–1487. [DOI] [PubMed] [Google Scholar]
- 10.Wu DC, Yu YP, Lee NTK, et al. The expression of Cdk5, p35, p39, and Cdk5 kinase activity in developing, adult, and aged rat brains. Neurochem Res 2000; 25: 923–929. [DOI] [PubMed] [Google Scholar]
- 11.Cai XH, Tomizawa K, Tang DM, et al. Changes in the expression of novel Cdk5 activator messenger RNA (p39(nck5ai) mRNA) during rat brain development. Neurosci Res 1997; 28: 355–360. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi S, Saito T, Hisanaga S, et al. Tau phosphorylation by cyclin-dependent kinase 5/p39 during brain development reduces its affinity for microtubules. J Biol Chem 2003; 278: 10506–10515. [DOI] [PubMed] [Google Scholar]
- 13.Tomizawa K, Matsui H, Matsushita M, et al. Localization and developmental changes in the neuron-specific cyclin-dependent kinase 5 activator (p35(nck5a)) in the rat brain. Neuroscience 1996; 74: 519–529. [DOI] [PubMed] [Google Scholar]
- 14.Saito T, Yano M, Kawai Y, et al. Structural basis for the different stability and activity between the Cdk5 complexes with p35 and p39 activators. J Biol Chem 2013; 288: 32433–32439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asada A, Saito T, Hisanaga S. Phosphorylation of p35 and p39 by Cdk5 determines the subcellular location of the holokinase in a phosphorylation-site-specific manner. J Cell Sci 2012; 125: 3421–3429. [DOI] [PubMed] [Google Scholar]
- 16.Asada A, Yamamoto N, Gohda M, et al. Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cycline-dependent kinase 5 complexes. J Neurochem 2008; 106: 1325–1336. [DOI] [PubMed] [Google Scholar]
- 17.Minegishi S, Asada A, Miyauchi S, et al. Membrane association facilitates degradation and cleavage of the cyclin-dependent kinase 5 activators p35 and p39. Biochemistry 2010; 49: 5482–5493. [DOI] [PubMed] [Google Scholar]
- 18.Patzke H, Tsai LH. Calpain-mediated cleavage of the cyclin-dependent kinase-5 activator p39 to p29. J Biol Chem 2002; 277: 8054–8060. [DOI] [PubMed] [Google Scholar]
- 19.Chae T, Kwon YT, Bronson R, et al. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron 1997; 18: 29–42. [DOI] [PubMed] [Google Scholar]
- 20.Ko J, Humbert S, Bronson RT, et al. p35 and p39 are essential for cyclin-dependent kinase 5 function during neurodevelopment. J Neurosci 2001; 21: 6758–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bankston AN, Li WQ, Zhang H, et al. p39, the primary activator for cyclin-dependent kinase 5 (Cdk5) in oligodendroglia, is essential for oligodendroglia differentiation and myelin repair. J Biol Chem 2013; 288: 18047–18057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Allen ME, Rui Y, et al. p39 is responsible for increasing Cdk5 activity during postnatal neuron differentiation and governs neuronal network formation and epileptic responses. J Neurosci 2016; 36: 11283–11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pareek TK, Keller J, Kesavapany S, et al. Cyclin-dependent signaling through transient receptor kinase 5 modulates nociceptive direct phosphorylation of potential vanilloid 1. Proc Natl Acad Sci USA 2007; 104: 660–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pareek TK, Keller J, Kesavapany S, et al. Cyclin-dependent kinase 5 activity regulates pain signaling. Proc Natl Acad Sci USA 2006; 103: 791–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang YR, He Y, Zhang Y, et al. Activation of cyclin-dependent kinase 5 (Cdk5) in primary sensory and dorsal horn neurons by peripheral inflammation contributes to heat hyperalgesia. Pain 2007; 127: 109–120. [DOI] [PubMed] [Google Scholar]
- 26.Zhang HH, Zhang XQ, Wang WY, et al. Increased synaptophysin is involved in inflammation-induced heat hyperalgesia mediated by cyclin-dependent kinase 5 in rats. PLoS One 2012; 7: e46666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang HH, Zhang XQ, Xue QS, et al. The BDNF/TrkB signaling pathway is involved in heat hyperalgesia mediated by Cdk5 in rats. PLoS One 2014; 9: e85536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CH, Chou WY, Hung KS, et al. Intrathecal administration of roscovitine inhibits Cdk5 activity and attenuates formalin-induced nociceptive response in rats. Acta Pharmacol Sin 2005; 26: 46–50. [DOI] [PubMed] [Google Scholar]
- 29.Prochazkova M, Terse A, Amin ND, et al. Activation of cyclin-dependent kinase 5 mediates orofacial mechanical hyperalgesia. Mol Pain 2013; 9: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jendryke T, Prochazkova M, Hall BE, et al. TRPV1 function is modulated by Cdk5-mediated phosphorylation: insights into the molecular mechanism of nociception. Sci Rep 2016; 6: 22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 32.Binukumar BK, Shukla V, Amin ND, et al. Peptide TFP5/TP5 derived from Cdk5 activator P35 provides neuroprotection in the MPTP model of Parkinson’s disease. Mol Biol Cell 2015; 26: 4478–4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra SK, Tisel SM, Orestes P, et al. TRPV1-lineage neurons are required for thermal sensation. EMBO J 2011; 30: 582–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Azevedo WF, Gaspar RT, Canduri F, et al. Molecular model of cyclin-dependent kinase 5 complexed with roscovitine. Biochem Biophys Res Comun 2002; 297: 1154–1158. [DOI] [PubMed] [Google Scholar]
- 35.Zhang X, Zhang H, Shao H, et al. ERK MAP kinase activation in spinal cord regulates phosphorylation of Cdk5 at serine 159 and contributes to peripheral inflammation induced pain/hypersensitivity. PLoS One 2014; 9: e87788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li L, Zhang C, Zi X, et al. Epigenetic modulation of Cdk5 contributes to memory deficiency induced by amyloid fibrils. Exp Brain Res 2015; 233: 165–173. [DOI] [PubMed] [Google Scholar]
- 37.Yang L, Gu X, Zhang W, et al. Cdk5 inhibitor roscovitine alleviates neuropathic pain in the dorsal root ganglia by downregulating N-methyl-D-aspartate receptor subunit 2A. Neurol Sci 2014; 35: 1365–1371. [DOI] [PubMed] [Google Scholar]
- 38.Liu X, Liu Y, Zhang J, et al. Intrathecal administration of roscovitine prevents remifentanil-induced postoperative hyperalgesia and decreases the phosphorylation of N-methyl-D-aspartate receptor and metabotropic glutamate receptor 5 in spinal cord. Brain Res Bull 2014; 106: 9–16. [DOI] [PubMed] [Google Scholar]
- 39.Zhang R, Liu Y, Zhang J, et al. Intrathecal administration of roscovitine attenuates cancer pain and inhibits the expression of NMDA receptor 2B subunit mRNA. Pharmacol Biochem Behav 2012; 102: 139–145. [DOI] [PubMed] [Google Scholar]
- 40.Luo FC, Zhang J, Burke K, et al. The activators of cyclin-dependent kinase 5 p35 and p39 are essential for oligodendrocyte maturation, process formation, and myelination. J Neurosci 2016; 36: 3024–3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lilja L, Johansson JU, Gromada J, et al. Cyclin-dependent kinase 5 associated with p39 promotes Munc18-1 phosphorylation and Ca(2+)-dependent exocytosis. J Biol Chem 2004; 279: 29534–29541. [DOI] [PubMed] [Google Scholar]