Abstract
Background
Previous studies have suggested that acute necrotising gingivitis precedes noma disease and that noma clusters in some villages in certain regions of low- and middle-income countries. We sought to assess the prevalence of gingivitis with bleeding in young children from villages with or without a history of noma and to analyse epidemiological differences related to sociodemographic characteristics, nutritional status and oral hygiene practices.
Methods
We conducted a cross-sectional study in 440 children aged between 2 and 6 years from four villages in the Zinder region of southeast Niger in Africa. In two villages, cases of noma have repeatedly been detected; in the other two, noma has never been identified. We randomly selected 110 participants from each village.
Results
The prevalence of acute necrotising gingivitis was significantly higher in the noma villages compared with the non-noma villages (6.8% vs 0.9%; p=0.001). We found differences between the four villages regarding socioeconomic factors, stunting, undernourishment and oral hygiene practices. The type of oral hygiene procedures influenced the amount of dental plaque and gingival inflammation. Children using sand, coal or other abrasive products instead of a toothbrush had a significantly increased likelihood to be diagnosed with acute necrotising gingivitis (p=0.041).
Conclusions
Our data suggest that efforts to prevent noma should focus on populations with a high prevalence of acute necrotising gingivitis and include nutritional support and attempts to introduce safe and efficient oral hygiene practices to improve gingival health.
Keywords: acute necrotizing gingivitis, noma, epidemiology, Niger, risk factors, cross-sectional study
Key questions.
What is already known about this topic?
Noma is a facial gangrenous disease affecting children living in the poorest areas of low- and middle-income countries, especially in Africa.
The mortality during the acute phase of the disease is high and the evolution is extremely rapid, causing the loss of soft and bony facial tissues in a few days only.
Recent studies comparing the microbiota associated with active lesions of noma and acute necrotising gingivitis (ANG) suggest that ANG might be a condition immediately preceding noma.
What are the new findings?
The present findings corroborate the known risk factors identified for noma disease and reinforce the notion that its eradication needs concerted efforts to alleviate poverty, improve nutrition and search for early signs of the disease.
Recommendations for policy
Our results suggest the need to promote safe and efficient oral hygiene practices, particularly in populations with a high prevalence of ANG and a history of noma.
Introduction
Noma is a facial gangrenous disease affecting children living in the poorest areas of low- and middle-income countries, especially in west Africa.1 2 The mortality during the acute phase of the disease is high and the evolution is extremely rapid, causing the loss of soft and bony facial tissues in a few days only. Survivors suffer from disfigurement and functional impairment affecting speech, breathing, chewing or even vision.3 Often, the aesthetic damage becomes a source of stigma leading to isolation from society and the family.4 Recent studies comparing the microbiota associated with active lesions of noma and acute necrotising gingivitis (ANG) suggest that ANG might be a condition immediately preceding noma.5 6
The principal characteristics of ANG are painful, bleeding gums and ulceration at the gingival margin, especially in the interdental region (figure 1A, B). ANG may be limited to one or few interdental papillae, or may be generalised.7 In Europe and North America, ANG is associated with tobacco or substance abuse, stress and immunodeficiency and affects mainly young adults.8 9 Studies undertaken in Nigeria and Senegal showed that the prevalence of ANG in Africa is much higher (between 11% and 73% depending on the region) and affects also young children.10–13 Since ANG is relatively simple to diagnose clinically, it may be useful to focus efforts to prevent noma disease among patients with ANG or among populations with a high prevalence of ANG. Indeed, our previous research on noma,14 and our collaboration with ‘Sentinelles’, a Swiss non-governmental humanitarian organisation active in Niger, have indicated that some villages are regularly affected by noma, while others are not.
Figure 1.
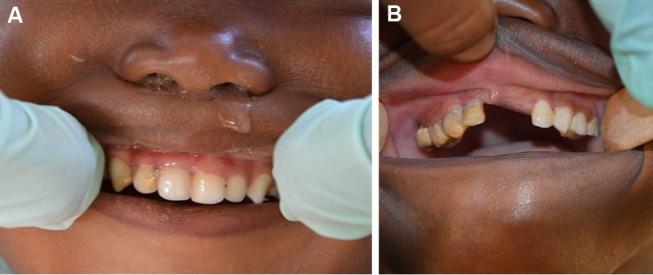
(A) Mild to moderate acute necrotising gingivitis (ANG) in a 3-year-old girl from the village of Droum. In the region of the upper-right incisors and the canine, the gingiva is oedematous and the interdental papillae have been lost due to ulceration. Soft and hard bacterial deposits are visible. (B) Advanced ANG in a 4-year-old girl from the village of Guidimouni. There are marked signs of ulceration and necrosis of the gingiva, with greyish pseudo-membranes and disappearance of the gingival papillae. The crowns of the teeth are covered with large amounts of soft and hard bacterial deposits.
This study aimed to assess the prevalence of gingivitis with bleeding, especially ANG, in young children from villages with or without cases of noma in the same geographic region in west Africa. In addition, we analysed differences related to sociodemographic factors, anthropometric indices for nutritional status and oral hygiene practices.
Methods
Study design and participants
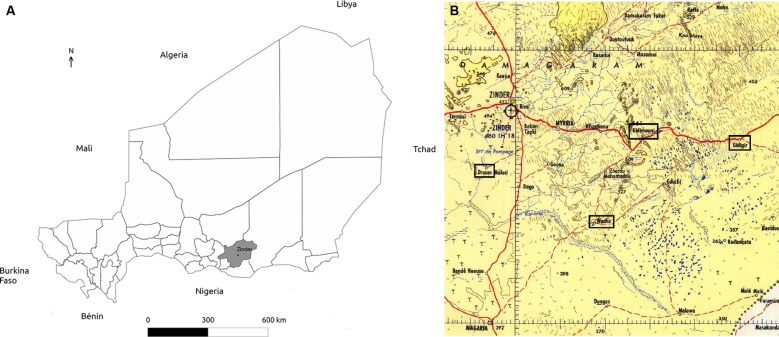
We conducted a cross-sectional study between 14 March 2014 and 4 April 2014 in four villages in the Zinder region of southeast Niger (figure 2A). In Droum and Guidimouni, cases of noma have repeatedly been identified, while noma has never been detected in Wacha and Guidiguir. The four villages are similar with regards to environment (agricultural setting, similar climate and cultural habits), access to healthcare (vaccination programmes) and distance from the region’s main city (Zinder; figure 2B). In each village, we randomly selected 110 children aged between 2 and 6 years. The recruitment of the 440 participants was performed in two steps. First, local coworkers informed the inhabitants of each village about the planned survey and invited families with children aged between 2 and 6 years to attend a meeting. On this occasion, the local staff explained the study in the native language using the written information that had been prepared in French. Children were randomly selected by two-stage random cluster sampling.15 Informed consent was obtained from the parent or guardian accompanying the child by signature or a fingerprint. Selected children were offered a bracelet that was locked on their wrist until the next appointment.
Figure 2.
(A) Geographical localisation of the study population, Zinder region (in grey), Niger, Africa. (B) Geographical localisation of the four villages (framed in black) included in the study: Droum, Wacha, Guidimouni and Guidiguir.
Data collection
A study dentist (MAM) and a study nurse collected the clinical data. The study dentist was trained and validated by a study investigator (NC) in Niamey, Niger, 1 month ahead of the trial. The study nurse recorded weight, height, arm circumference, presence of fever and signs of malnutrition, halitosis or gingival pain.14 The study dentist then interviewed the child’s parents or guardians using a structured questionnaire to obtain information about the living conditions, pregnancy, the breastfeeding period, nutritional habits and oral hygiene of the child.14 All information was accepted as given without verification.
The intraoral exam started by counting the number of decayed, missing or filled teeth and by noting the presence or absence of any gingival ulceration. The presence of plaque and gingival inflammation were assessed at three sites (mesial-buccal, mid-buccal and distal-buccal) of every erupted tooth using the plaque index (PI) and gingival index (GI), respectively.16 17 A PI and GI score between 0 and 3 was attributed for each of the three surfaces as follows: PI score 0: no plaque; 1: plaque only visible on probing; 2: moderate soft deposits visible by naked eye; 3: abundant soft matter. GI score 0: absence of inflammation; 1: mild inflammation with slight redness, slight oedema and no bleeding on probing; 2: moderate inflammation, redness, oedema and bleeding on probing; 3: severe inflammation, marked redness, oedema and tendency to spontaneous bleeding. We estimated a mean value for the PI and GI per child by calculating the arithmetic mean of the scores from all evaluated sites per individual. In addition, we calculated the individual number of sites with a PI score >1 (accumulation of soft matter visible with the naked eye) and a GI score >1 (moderate or severe inflammation of the gingiva with bleeding on examination). Using algorithms proposed by WHO and the United States Centers for Disease Control and Prevention,18 we calculated the weight-for-height (WHZ), the height-for-age (HAZ) and the weight-for-age (WAZ) z-scores as anthropometric estimates for wasting (WHZ), stunting (HAZ) and undernourishment (WAZ). The scores were compared with the standard values of the United States National Center for Health Statistics.
For the assessment of oral hygiene procedures, respondents were not required to choose answers from a predetermined list. Initial screening showed the following answers given frequently: use of a toothbrush, rubbing the teeth with a finger and water, rubbing with water and soap, rubbing with water and sand, rubbing with water and coal and use of combinations of these or other abrasive products. For the analysis, we compared toothbrushing or rubbing with plain water to all other procedures involving potentially abrasive substances.
Dependent variables
The presence of ANG,19 defined as a painful gingivitis with ulceration of the gingival margin (figure 1A) and/or necrosis of the interdental papilla (figure 1B) and/or spontaneous bleeding (ie, one or several sites with a GI score 3), was the primary outcome. We explored factors differentiating children from noma and non-noma villages in a secondary analysis.
Statistical analysis
Based on the literature,10 11 we hypothesised that approximately 20% of children in the four villages would be diagnosed with ANG. We needed to include a total of 440 children (110 per village) to obtain the power to describe the prevalence of ANG with a precision of ±3.75%. We described continuous variables by their mean (±SD) and median, and categorical variables by their frequency and relative percentage per category. The prevalence of ANG was presented with its 95% CI in the two noma villages using the exact binomial approximation. We compared continuous variables between the four villages using analysis of variance (ANOVA) or the Kruskal-Wallis test when variables were not normally distributed (Kurtosis and Skewness tests). Categorical variables were compared by χ2 or by Fisher’s exact test when expected numbers were <5. In a second step, we grouped together all children living in the two noma villages and all children living in the two non-noma villages.
We assessed the linear correlation by Pearson's correlation coefficients. First between the mean PI and the mean GI, then between the number of sites with a score >1 for PI and the number of sites with a score >1 for GI. Differences of clinical and anamnestic data of children from villages with or without noma were analysed by ANOVA for continuous variables and χ2 test for categorical variables. Differences in the PI or GI with regards to the type of oral hygiene (toothbrushing or rubbing with plain water vs procedures involving potentially abrasive substances) were analysed by Student’s t-test (PI and GI) or the Mann-Whitney non-parametric test (number of sites with PI >1 and number of sites with GI >1).
We assessed factors associated with ANG using unconditional logistic regression at univariable level. We applied the rule of seven events per variable to select the final multivariable model assessing the likelihood of ANG.20 We tested the same variables that were associated with noma disease in our previous study.14 All variables associated with a p<0.05 with the dependent variable (ANG) were kept in the final model. We then assessed factors associated with the type of village (noma vs non-noma) at univariable level. We included all variables associated with an increased likelihood to live in a noma village in univariable analysis at a significance level of p<0.25 in a multivariable model using a forward stepwise procedure. All tests were two-tailed and p values <0.05 were regarded as significant. We used Stata (intercooled V.14.0) for all analyses.
Results
Participants’ sociodemographic and clinical characteristics are presented in table 1. The 440 children participating in this study had a mean age of 3.5 years (±SD 1.2 years: median 3). We identified differences between the four villages regarding age, weight, arm circumference, HAZ or WAZ scores, number of persons or children at home, number of living children per mother, sibling order and mother’s age and duration of breast feeding.
Table 1.
Sociodemographic and clinical characteristics of children globally and by village
| Total (n=440) | Droum (n=110) | Guidimouni (n=110) | Guidiguir (n=110) | Wacha (n=110) | p Value | |
| Mean age, months (±SD, median) | 42.6 (±13.9, 36) | 40.1 (±12.0, 36) | 45.8 (±14.2, 48) | 41.3 (±14.4, 36) | 43.33 (±14.5, 36) | 0.01 |
| Male gender (vs female), n (%) | 210 (47.7) | 48 (43.6) | 61 (55.5) | 50 (45.5) | 51 (46.4) | 0.30 |
| Mean weight, kg (±SD, median) | 12.0 (±2.8, 12) | 11.3 (±2.6, 11) | 12.4 (±3.0, 12) | 12.2 (±2.6, 12) | 11.9 (±2.9, 11) | 0.02 |
| Mean height, cm (±SD, median) | 91.1 (±10.1, 91) | 88.9 (±9.3, 88) | 92.6 (±10.4, 93.5) | 91.3 (±10.7, 89.5) | 91.6 (±9.7, 91) | 0.41 |
| Mean arm circumference, cm (±SD, median) | 14.4 (±3.6, 14.2) | 14.3 (±1.0, 14.3) | 14.3 (±6.6, 13.7) | 14.4 (±1.1, 14.5) | 14.7 (±2.4, 14.6) | <0.001 |
| Wasting (vs no wasting), n (%) | 5 (1.1) | 0 | 5 (4.5) | 0 | 0 | – |
| Fever (vs no fever), n (%) | 53 (12.2) | 10 (9.1) | 13† (11.9) | 14 (12.7) | 16 (15.1) | 0.60 |
| Halitosis (vs no halitosis), n (%) | 1 (0.23) | 1 (0.9) | 0 | 0 | 0 | 0.99 |
| Gingival pain (vs no pain), n (%) | 1 (0.23) | 0 | 1 (0.9) | 0 | 0 | 0.99 |
| Mean height-for-age z-score (±SD, median) | −2.0 (±1.7,–2.0) | −2.2 (±1.4, –2.0) | 2.2 (±1.6, –2.2) | 1.6 (±2.1, –1.7) | 2.0 (±1.3, –1.9) | 0.045 |
| Categories of height-for-age z-score, n (%) | 0.85 | |||||
| No stunting | 202 (50.3) | 51 (47.2) | 46 (47.4) | 54 (54.0) | 51 (52.6) | |
| Moderate stunting | 95 (23.6) | 29 (26.9) | 21 (21.7) | 23 (23.0) | 22 (22.7) | |
| Severe stunting | 105 (26.1) | 28 (25.9) | 30 (30.9) | 23 (23.0) | 24 (24.7) | |
| Mean weight-for-age z-score (±SD, median) | −2.0 (±1.3, –2.0) | −2.2 (±1.3, –1.9) | −2.2 (±1.3, –2.4) | −1.6 (±1.2, –1.6) | −2.1 (±1.3, –2.2) | 0.01 |
| Mean weight-for-height z-score (±SD, median) | −1.3 (±1.7, –1.2) | −1.4(±1.8, –1.2) | −1.3 (±1.4, –1.4) | −0.98 (±1.6, –1.2) | −1.4 (±1.9, –1.4) | 0.243 |
| Categories of weight-for-height z-score, n (%) | 0.069 | |||||
| No wasting | 281 (69.9) | 75 (69.4) | 69 (71.1) | 74 (74.0) | 63 (65.0) | |
| Moderate wasting | 64 (15.9) | 14 (13.0) | 16 (16.5) | 20 (20.0) | 14 (14.4) | |
| Severe wasting | 57 (14.2) | 19 (17.6) | 12 (12.4) | 6 (6.0) | 20 (20.6) | |
| Mean body mass index z-score (±SD, median) | −1.1 (±1.8, –1.0) | −1.2 (±1.9, –1.0) | −1.0 (±1.5, –1.1) | −0.8 (±1.8, –0.9) | −1.2 (±1.9, –1.1) | 0.407 |
| Mean number of persons at home (±SD, median) | 9.0 (±6.6, 7) | 10.0 (±6.5, 9) | 11.0 (±9.9, 8) | 6.5 (±3.3, 6) | 8.3 (±3.3, 8) | <0.001 |
| Mean number of children at home (±SD, median) | 5.6 (±3.8, 5) | 6.6(±4.3, 6) | 6.1 (±4.7, 5) | 4.2 (2.8, 4) | 5.5 (2.4, 5) | <0.001 |
| Mean number of past pregnancies of the mother (±SD, median) | 5.6 (±2.9, 5) | 6.1 (±3.2, 6) | 6.3 (±2.7, 6) | 4.4 (±2.4, 4) | 5.7 (±2.7, 5) | <0.001 |
| Mean number of living children per mother (±SD, median) | 4.7±2.2, 4) | 4.9(±2.6, 4) | 5.2 (±2.0, 5) | 4.0 (±2.1, 4) | 4.6 (±2.1, 4) | <0.001 |
| Sibling chronological order, n (%) | <0.001 | |||||
| First position | 57 (13.0) | 14 (12.7) | 8 (7.3) | 20 (18.4) | 15 (13.6) | |
| Second position | 49 (11.2) | 11 (10.0) | 5 (4.6) | 18 (16.5) | 15 (13.6) | |
| Third position | 73 (16.6) | 21 (19.1) | 19 (17.3) | 25 (22.9) | 8 (7.3) | |
| Fourth to fifth position | 111 (25.3) | 18 (16.4) | 34 (30.9) | 23 (21.1) | 36 (32.7) | |
| Greater than fifth position | 149 (33.9) | 46 (41.8) | 44 (40.0) | 23 (21.1) | 36 (32.7) | |
| Mean duration since last pregnancy, years (±SD, median) | 2.2 (±1.0, 2) | 2.2 (±0.9, 2) | 2.2 (±0.8, 2) | 2.2 (±1.4, 2) | 2.2 (±1.1, 2) | 0.99 |
| Mean mother age, years (±SD, median) | 29.5 (±7.7, 30) | 29.7 (±8.0, 28) | 30.1(±7.0, 30) | 27.8 (±8.0, 25) | 30.5 (±7.4, 30) | 0.04 |
| Mean breast feeding duration, months (±SD, median) | 4.0(±2.6, 6) | 2.1 (±2.5, 1) | 4.9 (±2.2, 6) | 3.3 (±2.8, 4) | 5.6 (±1.3, 6) | <0.001 |
The intraoral exam revealed that none of the examined children had fillings and none of the teeth were missing due to extraction. Results from the dental examination are shown in table 2. The four villages differed with regards to PI, GI, number of cases with ANG, history of gingival bleeding or gingival lesions. The mean PI and the mean GI were highly and positively correlated (r=0.737), as were the individual numbers of sites with a score >1 of PI and GI (r=0.726). We found differences between the four villages regarding oral hygiene procedures. These differences remained significant when we compared the type of products used by the children in noma and non-noma villages (p<0.001). The mean values for PI, GI, the number of cases with ANG, history of gingival bleeding or gingival lesions depended on the type of oral hygiene procedures. Mean values were the lowest in children using methods based on friction of the teeth (PI 1.02±0.31; GI 1.02±0.21; number of sites with PI >1 50.18±10.92; number of sites with GI >1 30.67±7.05). They were significantly higher (except for the number of sites with PI >1 or GI >1) in children using any kind of abrasive method (PI 1.09±0.37 (p=0.035); GI 1.07±0.26 (p=0.04); number of sites with PI >1 70.53±13.79 (p=0.171); number of sites with GI >1 50.84±10.48 (p=0.103)).
Table 2.
Dental examination globally and by village
| Total (n=440) | Droum (n=110) | Guidimouni (n=110) | Guidiguir (n=110) | Wacha (n=110) | p Value* | |
| Mean number of teeth examined (±SD, median) | 17.6 (±1.5, 18) | 17.7 (±1.2, 18) | 17.6 (±1.4, 18) | 17.6 (±1.5, 18) | 17.4 (±1.8, 18) | 0.840 |
| Mean number of decayed teeth (±SD, median) | 0.5 (±1.4, 0) | 0.7 (±1.6, 0) | 0.5 (±1.5, 0) | 0.4 (±1.5, 0) | 0.3 (±0.9, 0) | 0.208 |
| Number of decayed teeth, n (%) | 0.097 | |||||
| None | 369 (83.9) | 83 (75.5) | 93 (84.6) | 99 (90.0) | 94 (85.5) | |
| 1–2 | 39 (8.9) | 16 (14.5) | 7 (6.4) | 4 (3.6) | 12 (10.9) | |
| 3–4 | 18 (4.1) | 7 (6.4) | 6 (5.4) | 3 (2.7) | 2 (1.8) | |
| ≥5 | 14 (3.2) | 4 (3.6) | 4 (3.6) | 4 (3.6) | 2 (1.8) | |
| Mean PI on all teeth (±SD, median) | 1.1 (±0.4, 1) | 1.1 (±0.4, 1) | 1.1 (±0.3, 1) | 1.0 (±0.3, 1) | 1.1 (±0.3, 1) | 0.0483 |
| Mean number of sites with PI>1 (±SD, median) | 6.5 (±12.7, 0) | 10.2 (±16.2, 0.5) | 5.9 (±10.9, 0) | 4.1 (±9.8, 0) | 5.9 (±12.2, 0) | 0.0079 |
| Mean GI on all teeth (±SD, median) | 1.1 (±0.2, 1) | 1.1 (±0.3, 1.1) | 1.0 (±0.2, 1) | 1.0 (±0.2, 1) | 1.0 (±0.3, 1) | <0.001 |
| Mean number of sites with GI>1 (±SD, median) | 4.9 (±9.2, 0) | 7.9 (±11.8, 4) | 3.7 (±7.8, 0) | 3.9 (±7.7, 0) | 4.1 (±8.3, 0) | <0.001 |
| Number of children with ANG | 17 (3.9) | 10 (9.1) | 5 (4.69) | 0 (0) | 2 (1.8) | 0.002 |
| Number of children with spontaneous bleeding | 9 (2.1) | 5 (4.6) | 3 (2.7) | 0 (0) | 1 (0.9) | 0.087 |
| Products used for oral hygiene in the child, n (%) | <0.001 | |||||
| Toothbrush | 97 (22.1) | 30 (27.3) | 13 (11.8) | 9 (8.2) | 45 (40.9) | |
| Water | 94 (21.4) | 3 (2.7) | 31 (28.2) | 56 (50.9) | 4 (3.6) | |
| Water and soap | 165 (37.5) | 64 (58.2) | 19 (17.3) | 45 (40.9) | 37 (33.6) | |
| Water and sand | 20 (4.6) | 5 (4.6) | 12 (10.9) | 0 (0) | 3 (2.7) | |
| Water and coal | 19 (4.3) | 1 (0.9) | 14 (12.7) | 0 (0) | 4 (3.6) | |
| Other products/combinations | 45 (10.2) | 7 (6.4) | 21 (19.1) | 0 (0) | 17 (15.5) | |
| Past gingival pain, n (%) | 16 (3.6) | 7 (6.4) | 6 (5.5) | 3 (2.7) | 0 (0) | 0.051 |
| Past gingival bleeding, n (%) | 15 (3.4) | 7 (6.4) | 6 (5.5) | 2 (1.8) | 0 (0) | 0.02 |
| Past gingival lesion, n (%) | 7 (1.6) | 6 (5.5) | 1 (0.9) | 0 (0) | 0 (0) | 0.003 |
| Type of past gingival lesion, n (%) | – | |||||
| Ulceration | 1 (11.1) | 0 (0) | 1 (100.0) | 0 (0) | 0 (0) | |
| Gingivitis | 8 (88.9) | 6 (100.0) | 0 (0) | 0 (0) | 0 (0) |
*Kruskal-Wallis test,
ANG, acute necrotising gingivitis; GI, gingival index; PI, plaque index.
The clinical examinations revealed a total of 17 cases of ANG of which 15 were identified in the noma villages (table 2). With 3.9% (95% CI 2.3 to 6.1) in Droum and 6.8% (95% CI 3.9 to 11.0) in Guidimouni, the prevalence of ANG was significantly higher in the noma villages compared with the non-noma villages (p=0.001). None of the children examined had a history of noma. The likelihood of ANG was significantly associated with only two variables: the type of village and the method of tooth cleaning (table 3). Due to the small number of cases, we only kept these two variables in the multivariable model. Children living in noma villages had a higher likelihood of ANG compared with those living in non-noma villages (OR 6.90; 95% CI 1.55 to 30.77 (p=0.011)) and there was a trend for an association between ANG and the type of products used for oral hygiene (p=0.100). The likelihood of ANG was significantly increased 2.92-fold (95% CI 0.81 to 10.44) in children using procedures involving abrasive products compared with the use of water with or without a toothbrush.
Table 3.
Factors associated with the likelihood of acute necrotising gingivitis (univariable analyses)
| OR (95% CI) | p Value | |
| Noma villages (reference non-noma villages) | 7.98 (1.80 to 35.31) | 0.006 |
| Age >3 years (reference<3 years) | 2.19 (0.49 to 9.76) | 0.303 |
| Arm circumference (cm) | 1.18 (0.77 to 1.82) | 0.444 |
| Height-for-age z-score | 0.77 (0.55 to 1.09) | 0.144 |
| Weight-for-height z-score | 0.95 (0.69 to 1.31) | 0.757 |
| Weight-for-age z-score | 0.76 (0.50 to 1.15) | 0.193 |
| Number of persons at home≥10 (reference<10) | 2.48 (0.94 to 6.57) | 0.068 |
| Number of past pregnancies of the mother | 1.05 (0.95 to 1.15) | 0.330 |
| Number of past deliveries of the mother | 1.14 (0.97 to 1.33) | 0.106 |
| Sibling chronological order>fifth position (reference first to fourth position) | 1.38 (0.51 to 3.70) | 0.522 |
| Exclusive breastfeeding duration in months | 0.90 (0.76 to 1.08) | 0.262 |
| Products used for tooth cleaning (reference toothbrush and water) | ||
| Water and soap/water and sand/water and coal/other products/combinations | 3.73 (1.06 to 13.18) | 0.041 |
| Past gingival pain | 0.15 (0.04 to 0.58) | 0.006 |
| Past gingival bleeding | 0.14 (0.03 to 0.54) | 0.004 |
| Mean number of diseased sites for plaque index | 1.07 (1.04 to 1.09) | <0.001 |
| Mean number of diseased sites for gingival index | 1.11 (1.07 to 1.14) | <0.001 |
Table 4 shows factors associated with the likelihood of living in a noma versus a non-noma village. We found differences in some anthropometric measurements between the two types of villages. The arm circumference, HAZ and WAZ were significantly lower in children from noma villages compared with non-noma villages. We also found differences for several sociological variables between the types of villages. The number of persons living at home, the number of past pregnancies of the mother, being born after the fifth position in the chronological sibling order and a short duration or absence of exclusive breast feeding all significantly increased the likelihood of belonging to a noma village. The type of products used for oral hygiene was also significantly associated with noma villages. Unexpectedly, a history of past gingival pain or bleeding decreased the likelihood of belonging to a noma village. In the multivariable model, the likelihood of belonging to a noma village was significantly increased for children above the fifth position in the sibling order, for children not exclusively breast fed during their childhood or breast fed during 1–3 months compared with 4–6 months, for those using water and sand, water and coal or other aggressive products for oral hygiene compared with the use of a toothbrush or water alone. The likelihood of belonging to a noma village was lowest in the children with the greatest arm circumference or HAZ (table 4).
Table 4.
Factors associated with the likelihood of living in a noma village versus non-noma village (univariable and multivariable analyses)
| Univariable analyses | Multivariable model | |||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age, months | 1.00 | 0.99 to 1.02 | 0.703 | – | – | – |
| Arm circumference (cm) | 0.69 | 0.57 to 0.82 | <0.001 | 0.73 | 0.60 to 0.89 | 0.002 |
| Height-for-age z-score | 0.86 | 0.76 to 0.97 | 0.014 | 0.85 | 0.74 to 0.98 | 0.022 |
| Weight-for-height z-score | 0.96 | 0.85 to 1.07 | 0.461 | – | – | – |
| Weight-for-age z-score | 0.83 | 0.71 to 0.97 | 0.022 | – | – | – |
| Number of persons at home | 1.13 | 1.07 to 1.19 | <0.001 | – | – | – |
| Number of past pregnancies of the mother | 1.16 | 1.08 to 1.24 | <0.001 | – | – | – |
| Sibling chronological order>fifth position (reference first to fourth position) | 1.83 | 1.22 to 2.73 | 0.003 | 1.90 | 1.20 to 3.01 | 0.006 |
| Categories of exclusive breast feeding (reference 4–6 months) | 2.15 | <0.001 | <0.001 | |||
| No exclusive breast feeding | 2.15 | 1.35 to 3.43 | 0.001 | 2.52 | 1.50 to 4.23 | <0.001 |
| 1–3 months | 2.85 | 1.52 to 5.35 | 0.001 | 2.76 | 1.41 to 5.42 | 0.003 |
| Products used for tooth cleaning (reference toothbrush or water) | <0.001 | 0.002 | ||||
| Water and soap/water and sand/water and coal/other products/combinations | 2.00 | 1.36 to 2.93 | 2.00 | 1.28 to 3.11 | ||
| Past gingival pain | 0.22 | (0.06 to 0.78) | 0.019 | – | – | – |
| Past gingival bleeding | 0.15 | (0.03 to 0.66) | 0.012 | – | – | – |
Discussion
The main finding of this investigation among young children in the Zinder region of southeast Niger was the significant association between the residency of a child in a village with or without reported cases of noma and the prevalence of ANG. The 3% overall prevalence of ANG in this survey was smaller than the 20% expected at study implementation. This may be due to the activities of the humanitarian organisation ‘Sentinelles’ that has continuously conducted public health programmes in the Zinder region over three decades with a focus on prevention and the early diagnosis of noma. The inhabitants of the four selected villages benefited from these campaigns to a similar degree. Although considerably lower than expected, the prevalence of ANG described in the current study is still high compared with reported prevalence rates≤0.5% in industrialised countries.21 22 Due to the small number of cases of ANG, we were underpowered to assess multiple risk factors. Nevertheless, we proposed a multivariable model including only two variables, that is, the children’s origin and oral hygiene practices, and observed a trend for an association between ANG and the type of products used for oral hygiene. Children using water and sand or water and coal instead of a toothbrush had a significantly increased likelihood to be diagnosed with ANG. In addition, the type of oral hygiene procedures influenced both the amount of dental plaque and gingival inflammation.
The causal relationship between dental plaque and gingivitis has been demonstrated convincingly by experiments where healthy participants refrain from tooth cleaning for several days to allow undisturbed growth of bacterial deposits on teeth.23 In these experiments, the accumulation of dental plaque is reproducibly accompanied by the development of gingivitis, whereas the removal of the bacterial deposits restores gingival health. Furthermore, it is known that inappropriate tooth cleaning methods can cause gingival abrasion, which could lead to secondary ANG.23 Thus, it is conceivable that by cleaning children’s teeth with sand or coal instead of a toothbrush or finger friction with water, the risk to develop ANG may increase due to inefficient plaque removal and recurrent gingival injuries. We also showed that the use of abrasive methods for oral hygiene was significantly more prevalent in noma compared with non-noma villages, thus suggesting that these practices could be a trigger for further consequences of ANG. However, we could not conclude clearly on the causal relationship due to the observational nature of the study design.
Our survey revealed significant and clinically relevant differences in noma and non-noma villages regarding general health conditions, household structure and nutritional habits in early childhood. Stunting and undernourishment assessed by anthropometric measures were more prevalent in children from noma villages compared with non-noma villages, indicating differences with regards to poverty among these communities. The significant differences in the numbers of persons living together, numbers of the mother’s past pregnancies and the absence or short duration of exclusive breast feeding further strengthen this hypothesis. Our group previously conducted a case–control study of all children diagnosed with acute noma between 1 August 2001 and 31 October 2006 in the same region of southeast Niger.14 Severe wasting and stunting and a high number of past pregnancies of the mother were significant risk factors for noma. In the present study, associations between these risk factors and ANG were not significant because of insufficient power. However, the presence of risk factors for noma identified in the previous study increased the likelihood of living in a noma village compared with a non-noma village.
The current study has unique strengths. First, participants were randomly selected from four villages of the Zinder region in southeast Niger with two villages presenting a history of noma and two without. Thus, the study sample is representative of the target population. Another critical strength is the use of objective measurements to define ANG and the assessment based on a standardised clinical examination by a dental professional trained and validated by the study investigators. Thus, estimates of ANG prevalence can be considered as reliable.
Our study has some limitations. First, we cannot exclude the existence of information bias, in particular, recall bias affecting responses on pregnancy, the breastfeeding period or nutritional habits. Therefore, we did not test these variables as risk factors for ANG or for the village origin. Second, due to the observational design, we cannot infer causation from correlations between risk factors and outcomes. Nevertheless, the epidemiological associations found between ANG or the village origin and oral hygiene procedures constitute important insights for further investigation. The more frequent use of abrasive products in noma villages could be due to a false interpretation of recommendations given by non-governmental organisations to improve oral hygiene in an effort to prevent noma. It could also be explained by the existence of traditional oral hygiene practices that should be modified to prevent ANG and consequently noma.
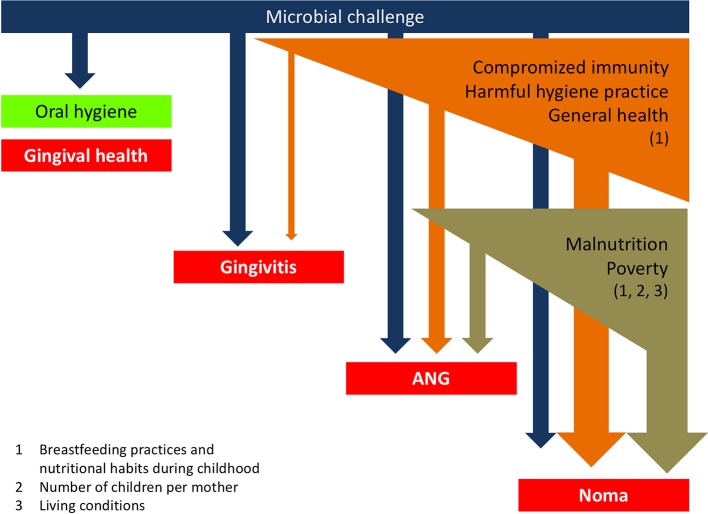
A simple framework organising the links between the different factors associated with ANG and, consequently, noma can be proposed (figure 3). The present findings corroborate the known risk factors identified for noma and reinforce the notion that its eradication needs concerted efforts to alleviate poverty, improve nutrition and to search for early signs of the disease. In addition, our results suggest a need for promoting safe and efficient oral hygiene practices, especially in populations with a high prevalence of ANG and where a history of noma has been previously described. Future studies should elucidate the reasons for using abrasive products for oral hygiene with the aim to develop intervention strategies to improve oral hygiene in children and adults, particularly in the noma villages.
Figure 3.
Schematic representation of the links between different factors associated with acute necrotising gingivitis (ANG) and noma.
Acknowledgments
Members of the Geneva Study Group on Noma (GESNOMA) are D Baratti-Mayer, J-E Bornand, A Gayet-Ageron, A Gervaix, A Mombelli, D Montandon, B Pittet-Cuénod, D Pittet, M Rusconi and J Schrenzel. The authors thank the GESNOMA healthcare workers in Zinder, Niger, the healthcare workers of ‘Sentinelles’ in Zinder, Niger and Lausanne, Switzerland. They also thank Rosemary Sudan for editorial assistance. The continued generous financial support and encouragement of Ms Gertrude Hirzel (Gertrude Hirzel Foundation) is greatly appreciated. The authors also thank Jean-Marc Theler, Unit of Population Epidemiology, University of Geneva Hospitals, for the creation of figure 2A (Quantum GIS (http://www.qguis.org/ OSGeo (open source geospatial location); coordinates obtained by openstreetmap and itouchmap).
Footnotes
Contributors: DB-M, AG-A, DP and AM had responsibility for the overall study design, management, conduct, analysis and writing of the paper. MAM and NC carried out the clinical part of the study. DB-M, AG-A, DP and AM were involved in the data analysis, interpretation, synthesis and writing. All authors reviewed and approved the final version.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: Ethics committee of the Ministry of Health in Niger and the institutional ethics committee of the University of Geneva Hospitals, Geneva, Switzerland.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional unpublished data are available.
References
- 1. Barmes DE, Enwonwu CO, Leclercq MH, et al. . The need for action against oro-facial gangrene (noma). Trop Med Int Health 1997;2:1111–4. 10.1046/j.1365-3156.1997.d01-220.x [DOI] [PubMed] [Google Scholar]
- 2. Bourgeois DM, Diallo B, Frieh C, et al. . Epidemiology of the incidence of oro-facial noma: a study of cases in Dakar, Senegal, 1981-1993. Am J Trop Med Hyg 1999;61:909–13. 10.4269/ajtmh.1999.61.909 [DOI] [PubMed] [Google Scholar]
- 3. Baratti-Mayer D, Pittet B, Montandon D, et al. . Noma: an "infectious" disease of unknown aetiology. Lancet Infect Dis 2003;3:419–31. 10.1016/S1473-3099(03)00670-4 [DOI] [PubMed] [Google Scholar]
- 4. Leila Srour M, Marck KW, Baratti-Mayer D. Noma: neglected, forgotten and a human rights issue. Int Health 2015;7:149–50. 10.1093/inthealth/ihv001 [DOI] [PubMed] [Google Scholar]
- 5. Bolivar I, Whiteson K, Stadelmann B, et al. . Bacterial diversity in oral samples of children in Niger with acute noma, acute necrotizing gingivitis, and healthy controls. PLoS Negl Trop Dis 2012;6:e1556 10.1371/journal.pntd.0001556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huyghe A, François P, Mombelli A, et al. . Microarray analysis of Microbiota of gingival lesions in noma patients. PLoS Negl Trop Dis 2013;7:e2453 10.1371/journal.pntd.0002453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giddon DB, Zackin SJ, Goldhaber P. Acute necrotizing ulcerative gingivitis in college students. J Am Dent Assoc 1964;68:381–6. 10.14219/jada.archive.1964.0076 [DOI] [PubMed] [Google Scholar]
- 8. Loesche WJ, Syed SA, Laughon BE, et al. . The bacteriology of acute necrotizing ulcerative gingivitis. J Periodontol 1982;53:223–30. 10.1902/jop.1982.53.4.223 [DOI] [PubMed] [Google Scholar]
- 9. Melnick SL, Roseman JM, Engel D, et al. . Epidemiology of acute necrotizing ulcerative gingivitis. Epidemiol Rev 1988;10:191–211. 10.1093/oxfordjournals.epirev.a036022 [DOI] [PubMed] [Google Scholar]
- 10. Idigbe EO, Enwonwu CO, Falkler WA, et al. . Living conditions of children at risk for noma: nigerian experience. Oral Dis 1999;5:156–62. 10.1111/j.1601-0825.1999.tb00082.x [DOI] [PubMed] [Google Scholar]
- 11. Ndiaye FC, Bourgeois D, Leclercq MH, et al. . Noma: public health problem in Senegal and epidemilogical surveillance. Oral Dis 1999;5:163–6. 10.1111/j.1601-0825.1999.tb00083.x [DOI] [PubMed] [Google Scholar]
- 12. Sheiham A. The epidemiology of chronic periodontal disease in western nigerian schoolchildren. J Periodontal Res 1968;3:257–67. 10.1111/j.1600-0765.1968.tb01928.x [DOI] [PubMed] [Google Scholar]
- 13. Diouf M, Cisse D, Faye A, et al. . Prevalence of necrotizing ulcerative gingivitis and associated factors in koranic boarding schools in Senegal. Community Dent Health 2012;29:184–7. [PubMed] [Google Scholar]
- 14. Baratti-Mayer D, Gayet-Ageron A, Hugonnet S, et al. . Risk factors for noma disease: a 6-year, prospective, matched case-control study in Niger. Lancet Glob Health 2013;1:e87–96. 10.1016/S2214-109X(13)70015-9 [DOI] [PubMed] [Google Scholar]
- 15. Saifuddin A. Methods in sample surveys. John Hopkins Bloomberg School of Public Health 2009. http://ocw.jhsph.edu/courses/statmethodsforsamplesurveys/pdfs/lecture5.pdf. [Google Scholar]
- 16. Loe H, Silness J. Periodontal disease in pregnancy. I. prevalence and severity. Acta Odontol Scand 1963;21:533–51. 10.3109/00016356309011240 [DOI] [PubMed] [Google Scholar]
- 17. Silness J, Loe H. Periodontal disease in pregnancy. ii. correlation between oral hygiene and periodontal condtion. Acta Odontol Scand 1964;22:121–35. 10.3109/00016356408993968 [DOI] [PubMed] [Google Scholar]
- 18. WHO Working Group. Use and interpretation of anthropometric indicators of nutritional status. Bull World Health Organ 1986;64:929–41. [PMC free article] [PubMed] [Google Scholar]
- 19. Rowland RW. Necrotizing ulcerative gingivitis. Ann Periodontol 1999;4:65–73. 10.1902/annals.1999.4.1.65 [DOI] [PubMed] [Google Scholar]
- 20. Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and cox regression. Am J Epidemiol 2007;165:710–8. 10.1093/aje/kwk052 [DOI] [PubMed] [Google Scholar]
- 21. Barnes GP, Bowles WF, Carter HG. Acute necrotizing ulcerative gingivitis: a survey of 218 cases. J Periodontol 1973;44:35–42. 10.1902/jop.1973.44.1.35 [DOI] [PubMed] [Google Scholar]
- 22. Horning GM, Hatch CL, Lutskus J. The prevalence of periodontitis in a military treatment population. J Am Dent Assoc 1990;121:616–22. 10.14219/jada.archive.1990.0221 [DOI] [PubMed] [Google Scholar]
- 23. Breitenmoser J, Mörmann W, Mühlemann HR. Damaging effects of toothbrush bristle end form on gingiva. J Periodontol 1979;50:212–6. 10.1902/jop.1979.50.4.212 [DOI] [PubMed] [Google Scholar]