Abstract
Background
Normothermic machine perfusion of the liver (NMP-L) is a novel technique that preserves liver grafts under near-physiological conditions whilst maintaining their normal metabolic activity. This process requires an adequate oxygen supply, typically delivered by packed red blood cells (RBC). We present the first experience using an acellular hemoglobin-based oxygen carrier (HBOC) Hemopure in a human model of NMP-L.
Methods
Five discarded high-risk human livers were perfused with HBOC-based perfusion fluid and matched to 5 RBC-perfused livers. Perfusion parameters, oxygen extraction, metabolic activity and histological features were compared during 6 hours of NMP-L. The cytotoxicity of Hemopure was also tested on human hepatic primary cell line cultures using an in vitro model of ischemia reperfusion injury.
Results
The vascular flow parameters and the perfusate lactate clearance were similar in both groups. The HBOC-perfused livers extracted more oxygen than those perfused with RBCs (O2ER 13.75 vs 9.43 % x105 per gram of tissue, p=0.001). In vitro exposure to Hemopure did not alter intracellular levels of reactive oxygen species and there was no increase in apoptosis or necrosis observed in any of the tested cell lines. Histological findings were comparable between groups. There was no evidence of histological damage caused by Hemopure.
Conclusion
Hemopure can be used as an alternative oxygen carrier to packed red cells in NMP-L perfusion fluid.
Introduction
The rising incidence of chronic liver disease has resulted in increased demand for liver transplantation (1,2). This can be met by the progressive utilization of high-risk organs from extended criteria donors. The quality of these livers is already compromised at the time of organ recovery, and deteriorates further during static cold storage (SCS) thereby increasing the risk of early graft dysfunction and/or primary non-function (3). Machine perfusion is a novel technology that can minimize preservation-associated liver injury and several groups have already reported promising results from pilot series of patients transplanted with machine perfused grafts (4–7). Whilst the earliest clinical series used hypothermic machine perfusion without oxygenation, in order to mitigate liver damage during preservation, a model requires oxygen (8). Oxygen requirements during hypothermic or sub-normothermic machine perfusion are relatively low due to reduced liver metabolic activity and these can be met by supplying a high fraction of inspired oxygen (FiO2) dissolved in the perfusion fluid (9).
At 37°C the liver requires adequate oxygen delivery with a dedicated oxygen carrier to enable and support full liver metabolic activity (10). To date, all clinical transplant series using organs preserved by normothermic machine perfusion of the liver (NMP-L) have used red blood cells (RBC) as oxygen carriers (11–16). Whilst blood-based perfusion fluid is physiological, it has also several potential disadvantages including immune-mediated phenomena, blood-borne infectious transmission, RBC hemolysis, use of a precious resource and logistical difficulties associated with using cross-matched blood (17–20). These reasons pose problems too for the use of third-party blood in clinical practice. Recently, in a bid to overcome these issues, a team from The University of Bristol and NHS Blood and Transplant presented a feasible approach to the manufacture of red cells for clinical use from in vitro culture (21).
Acellular oxygen carriers have been developed and tested as an alternative to packed red cell transfusions (22,23). Hemopure (hemoglobin glutamer-250 [bovine]; HBOC-201, Hemoglobin Oxygen Therapeutics LLC, Cambridge, MA) is a polymerized bovine hemoglobin-based oxygen carrier (HBOC) of low immunogenicity and an oxygen carrying capacity similar to that of human hemoglobin at normothermic temperatures (23,24). Fontes et al, recently reported successful sub-normothermic machine perfusion of the liver using Hemopure in combination with a colloid in a porcine liver transplant model (25).
Here we present the first experience using an acellular HBOC-based perfusion fluid in human livers during normothermic machine perfusion.
Materials and Methods
Study design
The study was performed on 10 rejected donor livers offered to our center for research between August 2014 and July 2016. Five organs were perfused with a Hemopure-based perfusion fluid (HBOC group) and 5 with a packed red blood cell-based fluid (RBC group) and underwent 6 hours of NMP-L. The HBOC and RBC livers were matched according to type of organ donation (donor after brain death or circulatory death) and function based on the unit’s developed viability testing protocol. All 3 viable RBC livers were successfully transplanted (16).
Source of discarded human livers and liver tissues
The discarded livers included in the study were initially offered, accepted and procured (using nationally agreed surgical protocols (26)) with the intention to use them for clinical transplantation. The livers were declined by all UK transplant centers and offered for research by the National Health Service Blood and Transplant (NHSBT) coordinating office. Ethical approval for the study was granted by the London-Surrey Borders National Research Ethics Service committee as well as Loco-Regional and NHSBT Ethics Committees (reference 13/LO/1928 and 06/Q702/61). The tissue used for the cellular isolation and in vitro toxicity experiments was obtained from fully consenting adult patients undergoing hepatic explant or resection at the University Hospital Birmingham.
Normothermic machine perfusion of the liver
Normothermic machine perfusion was performed using the Liver Assist device (Organ Assist, Groningen, The Netherlands) which perfuses both hepatic arterial and portal venous systems as described previously (16). The perfusion pressure was initially set at 30mmHg in the arterial and 8mmHg in the portal circuit respectively, aiming to achieve arterial flow greater than 150mL/minute and portal flow more than 500mL/minute. The resistances differed between livers and if required the pressures were gradually increased up to 50mmHg and 12 mmHg for artery and portal vein respectively to achieve the target flows. Oxygen was supplied to maintain an approximate target arterial pO2 of 20kPa (mean hepatic artery pO2 throughout perfusion 22.23kPA). The temperature was initially set to 25°C and increased incrementally to 37°C within 30 minutes. The liver preparation was analogous to clinical transplantation. Vessels were prepared to enable cannulation with 20 French, Medos cannulae and a transparent plastic tube was inserted into the bile duct and the cystic duct was ligated. Livers were then flushed with 2L of 10% dextrose solution at room temperature to remove any excess extracellular potassium, as per our unit’s standard protocol for clinical transplantation, before being connected to the device.
Hemopure
This bovine hemoglobin product is processed to eliminate RBC constituents, bacterial endotoxins, viruses and the prions responsible for variant Creutzfeldt-Jakob disease and bovine spongiform encephalopathy. The result is a sterile glutaraldehyde-polymerized bovine hemoglobin (30-35g Hb per 250 mL) which is added to a modified Ringer’s lactate solution. Hemopure has an average molecular weight of 250 kDa and can be stored at 2 - 30°C for up to 3 years (27). The oxygen affinity of human hemoglobin is dependent on 2,3-biphosphoglycerate, which is present in red blood cells. The oxygen affinity of Hemopure however, is modulated by chloride ion concentration. As such, it’s oxygen dissociation curve is shifted to the right compared to that of corpuscular hemoglobin and hence will release oxygen more readily into the tissues. It has a P50 (oxygen pressure at which 50% of oxygen-binding sites are saturated) of 40mmHg (+/-6mmHg), compared to 27mmHg for human hemoglobin.
Perfusion fluid constitution
We used a perfusion fluid developed by our team for resuscitation of discarded livers (28). This consisted of 3 units of group-specific Rhesus-negative donor packed RBCs obtained from the local blood bank, or an equivalent volume of Hemopure. The remaining perfusion fluid constituents are detailed in Table 1 and the biochemical starting compositions of the fluids are shown in Table 2. Details of exact fluid constituents can be found in the Appendix as supplementary material.
Table 1.
Perfusion fluid constitution
| Oxygen carrier | |
|---|---|
| Packed red blood cells or |
3 units |
| Hemopure | 4 bags (same volume as 3 units RBC) |
| Drug | Amount (Initial fluid added to circuit) |
| Human albumin solution 5% | 1000 ml |
| Heparin | 10,000 IU1 |
| Sodium bicarbonate 8.4% | 30 ml2 |
| Calcium gluconate 10% | 10 ml |
| Vancomycin | 500 mg |
| Gentamicin | 60 mg |
| Continuous infusions | |
| Epoprostenol | 2 μg/ml, commenced at 4 ml/hour and titrated as necessary |
| Intermittent drug administration | |
| Aminoplasmal 10%3 | 50 ml bolus every 6 hours |
| Dextrose 10% | Infusion as necessary according to perfusate glucose concentration |
Note: 1bolus repeated every 3 hours; 2bolus 10-30ml administrated if perfusate pH<7.00; 3with added vitamins (Cernevit and Vitamin K)
Table 2.
Comparison of biochemical composition of RBC-based and HBOC-based perfusion fluids prior to the start of perfusion
| RBC-Based Perfusate | HBOC-Based Perfusate | p value | |
|---|---|---|---|
| pH | 7.379 (7.256-7.458) | 7.685 (7.463-7.785) | 0.008 |
| PCO2 (kPa) | 4.31 (0.86-7.78) | 0.81 (0.74-2.23) | 0.087 |
| PO2 (kPa) | 28.26 (24.60-30.36) | 56.43 (45.11-64.07) | 0.008 |
| BE (mmol/L) | -13.7 (-19.1- -1.7) | -10.7 (-12.1- -9.7) | 0.691 |
| CHCO3- (mmol/L) | 17.3 (3.7-40.4) | 8.1 (7.1-11.7) | 0.691 |
| Na+ (mmol/L) | 138.6 (116.8-157.2) | 150.9 (148.3-153.6) | 0.206 |
| K+ (mmol/L) | 8.79 (6.31-12.90) | 1.90 (1.80-1.90) | 0.008 |
| Cl- (mmol/L) | 112.4 (76.0-117.0) | 109.0 (108.4-113.4) | 0.008 |
| Ca2+ (mmol/L) | 0.637 (0.573-0.700) | 1.000 (0.900-1.000) | 0.786 |
| tHb (g/L) | 84.4 (75.5-100.2) | 57.3 (55.8-58.5) 1 | 0.008 |
| O2Hb (%) | 97.8 (94.8-98.0) | 81.1 (79.3-82.9) | 0.008 |
| COHb (%) | 1.0 (0.9-1.9) | 0.1 (0.1-0.1) | 0.008 |
| H.Hb (%) | 0.7 (0.6-3.6) | 17.6 (16.8-18.6) | 0.008 |
| MetHb (%) | 0.5 (0.4-0.6) | 1.8 (1.6-2.0) | 0.008 |
| SO2 (%) | 99.3 (96.4-99.4) | 82.0 (81.0-82.9) | 0.008 |
| Hct(c) (%) | 22.6 (16.9-30.1) | 17.2 (16.8-17.5) | 0.079 |
| Glu (mmol/L) | 8.0 (6.8-10.5) | 3.5 (3.5-5.6) | 0.008 |
| Lactate (mmol/L) | 7.7 (6.7-9.2) | 5.4 (5.3-5.8) | 0.008 |
After 6 hours the median (range) value of Hb (g/L) was 59.3 (49.6-64.1)
Assessment of liver physiology and sample collection protocol
The macroscopic appearance of the liver was assessed throughout the course of NMP-L. The perfusion and sampling protocol included recording of arterial and venous circuit flow rates (ml/min for hepatic artery, L/min for portal vein), pressure (mmHg), resistance (mmHg·min/L) and temperature (°C) at 30-minute intervals. At the same intervals, we sampled arterial and hepatic venous perfusion fluid that was immediately assessed using a Cobas b 221 point of care system (Roche Diagnostics, USA). The oxygen extraction ratio was calculated using the following equation; O2ER/g tissue = [(SaO2-SvO2)/SaO2]/liver mass (g). Bile was collected at hourly intervals and weighed at the end of the procedure. Mean or median perfusion parameter values were calculated using results recorded at 30 minute intervals. Determination of organ viability was as per our criteria for organ viability used in a pilot series of transplantation using discarded donor livers (6) (see supplementary material for more information).
Histological Assessment
Liver biopsies were taken prior to the start of NMP-L and after 6 hours of perfusion. Biopsies were fixed in formalin, embedded in paraffin and sections cut at 4μm then stained with hematoxylin and eosin (H&E) and periodic-acid Schiff (PAS). Biopsies were assessed for preexisting acute or chronic liver injury. The percentages of large and small droplet macrovesicular steatosis, coagulative necrosis, subtle zone 3 changes of detachment of hepatocyte plates from the sinusoidal endothelium and glycogen depletion was determined (29). Histological assessment was conducted by an independent experienced liver transplant pathologist without prior knowledge of the liver perfusion designated category.
Perfusate and tissue analysis
Perfusates and tissues were snap-frozen at different time points for subsequent analyses. This included analysis of tissue adenosine triphosphate (ATP) content, and analysis of the perfusate for levels of transaminases and 8-hydroxy-2’-deoxyguanosine (8-OH-dG) – an established marker of oxidative stress. The protocol for ATP extraction can be seen in the Appendix as online supplementary material. All perfusates underwent haemoglobin depletion using Hemoglobind (BioTech Support Group LLC, Monmouth Junction, NJ) as per the manufacturer’s instructions, except using a 1:8 ratio to ensure removal of all free haemoglobin. Perfusate levels of 8-OH-dG were quantified using a competitive ELISA (Abcam) as per the manufacturers protocol.
Primary human hepatocyte, human sinusoidal endothelial cell and human biliary epithelial cell isolation
The isolation of primary human hepatocytes (30), sinusoidal endothelial cells (31) and biliary endothelial cells (32) has been previously described, the detailed protocols for which are supplied In the Appendix as online supplementary material.
In vitro model of ischemia reperfusion injury
Cells were incubated in the standard media for each cell type or 50:50 mix of standard media with Hemopure (the same concentration as is present in the perfusion fluid). In experiments, human hepatocytes, HSEC and BEC were grown for 3 days in standard media, in 6-well plates coated with rat type 1 collagen, at 37°C in 5% CO2. We utilized a model of warm in vitro ischemia reperfusion injury (IRI) that we have described previously (33), the details of which are within the supplementary material.
Assessment of reactive oxygen species production, apoptosis and necrosis
Reactive oxygen species (ROS) production, apoptosis and necrosis were determined using a 3-color assay as previously described (34). For further details and precise flow cytometry protocol please refer to the supplementary material and our previous publications (35–37). All data are expressed as Median Fluorescence Intensity (MFI). Taken together these 3 markers give a comprehensive assessment of the magnitude of IRI in primary human liver cells.
Statistical analysis
Categorical data is presented as numbers and percentage and were compared with Fischer’s exact test. Continuous variables are expressed as mean and standard deviation or median with range (where appropriate) and were compared using t tests or 2-tailed Mann-Whitney U test. A p value of <0.05 was deemed significant and was rounded to 3 decimal places for the presentation of results. All statistical analyses were performed using Prism 6 for Mac software (Graphpad Software Inc, La Jolla, CA, USA).
Results
Donor characteristics
Most livers (8 out of 10) were from donors after circulatory death (DCD). The median (range) donor age was 48 (25 - 70) years, the donor body mass index 26 (21 – 45) kg/m2 and the liver weight 1998 (1555-2486) grams. The median static cold storage time was 450 (380 – 754) minutes. The mean donor risk index for the RBC and HBOC groups were 2.21 and 2.36 respectively (38). The most common reason for the organ being declined for transplantation was prolonged donor warm ischemic time in combination with suboptimal macroscopic liver appearance. The detailed characteristics are provided in Table 3, and examples of these livers in Figure 1.
Table 3.
Donor demographic, liver characteristics and machine perfusion data
| RBC 1 | RBC 2 | RBC 3 | RBC 4 | RBC 5 | HBOC 1 | HBOC 2 | HBOC 3 | HBOC 4 | HBOC 5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Donor information | ||||||||||
| Donor type | DBD | DCD | DCD | DCD | DCD | DCD | DCD | DBD | DCD | DCD |
| Age | 46 | 49 | 60 | 46 | 30 | 70 | 35 | 50 | 25 | 60 |
| Sex | male | female | female | male | male | male | male | male | male | male |
| BMI (kg/m2) | 23 | 45 | 36 | 28 | 25 | 29 | 21 | 25 | 26 | 21 |
| Blood Group | O+ | O+ | A+ | O+ | A+ | A+ | A+ | O+ | O+ | A+ |
| Cause of death | ICH | HBI | HBI | HBI | HBI | ICH | ICH | ICH | Trauma | Trauma |
| Reason for rejection | Length of ITU stay | WIT and donor history | Steatosis | WIT and appearance | 100 minutes agonal period | Steatosis | Poor in situ perfusion | Donor history of malignancy | Segment VII laceration and patchy perfusion | Donor malignancy (renal) |
| Liver Characteristics | ||||||||||
| Liver weight | 1961 | 1943 | 1712 | 2486 | 1997 | 2208 | 2218 | 2380 | 1998 | 1555 |
| Cold ischemic time | 380 | 406 | 491 | 453 | 445 | 400 | 453 | 754 | 612 | 446 |
| Donor WIT | - | 36 | 32 | 31 | 12 | 24 | 20 | - | 22 | 21 |
| Donor risk index | 1.41 | 2.86 | 2.77 | 2.25 | 1.76 | 3.20 | 2.47 | 1.58 | 2.20 | 2.26 |
| Machine perfusion parameters | ||||||||||
| Lactate (mmol/L) | ||||||||||
| Highest | >20.0 | 5.5 | 13.3 | 12.4 | 13.3 | 9.6 | >20.0 | 10.3 | 10.4 | 9.0 |
| Lowest | 4.4 | 1.4 | 9.0 | 1.2 | 0.8 | 3.8 | 8.8 | 0.3 | 1.2 | 0.6 |
| Last | >20.0 | 1.4 | 9.0 | 1.2 | 0.8 | 5.2 | >20.0 | 0.3 | 1.6 | 1.4 |
| Total Bile production (g) | 2.6 | 0 | 0 | 11.3 | 27 | 0 | 0 | 17.6 | 26.2 | 24 |
| Mean Arterial flow (mL/min) | 277 | 476 | 582 | 654 | 558 | 535 | 256 | 761 | 529 | 616 |
| Mean Portal vein flow (L/min) | 1188 | 926 | 1112 | 1015 | 963 | 865 | 1237 | 1505 | 1286 | 1021 |
| Mean Arterial flow per gram liver tissue (mL/min/g) | 0.14 | 0.24 | 0.34 | 0.26 | 0.28 | 0.24 | 0.12 | 0.32 | 0.26 | 0.40 |
| Mean O2 ER per gram liver tissue (x105) | 14.57 | 9.98 | 8.47 | 3.86 | 12.90 | 11.64 | 19.80 | 8.49 | 16.31 | 12.02 |
Abbreviations:
DBD, donation after brain death; DCD, donation after circulatory death; ER, Extraction ratio; HBI, hypoxic brain injury; ICH, intracranial hemorrhage; ITU, intensive treatment unit; WIT, warm ischemic time
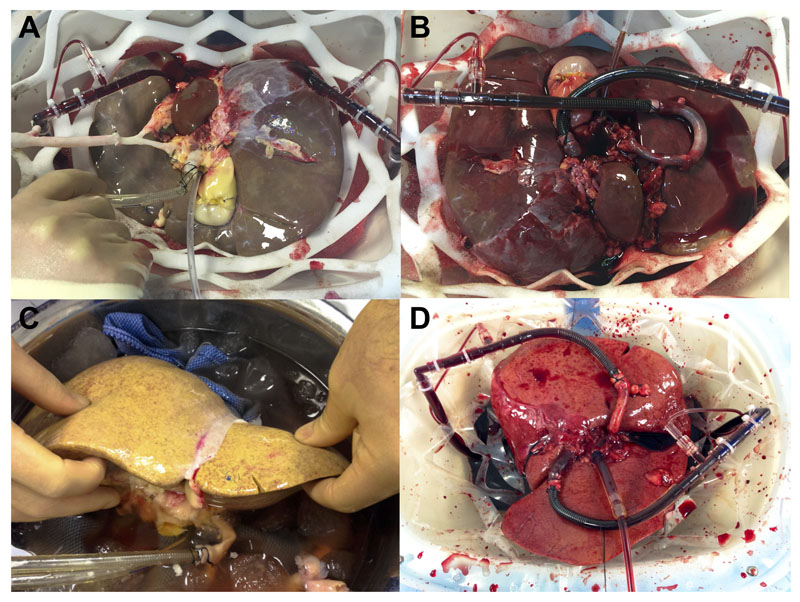
Figure 1. Macroscopic liver appearance.
Hemopure perfused liver #5 before (A) and 1 minute after (B) commencing the perfusion. This liver was poorly perfused in situ and on the back table during the retrieval process, however performed very well and a homogenous perfusion was achieved almost immediately, helped by the low viscosity of the fluid. Hemopure perfused liver #1 before (C) and 5 minutes after (D) commencing the perfusion. Despite the severely steatotic nature of the graft, a homogenous perfusion was still achieved shortly after almost 7 hours of cold storage.
Machine perfusion parameters
The HBOC group livers established global perfusion rapidly and the liver surface appeared homogenous within the first 5 minutes. This observation was reflected in the lower initial hepatic arterial resistance and pressure required to achieve the target flow rates within the initial 30 minutes of perfusion (resistance 0.26mmHg·min/L (range 0.20-0.32) in HBOC group versus 0.39mmHg·min/L (0.22-0.56), p=0.667; Figure 2). By the time the target perfusate temperature of 37°C was reached and stabilized, the appearance of the RBC livers improved and both groups demonstrated similar perfusion parameters throughout the remaining perfusion course. The detail is shown in Figure 2 and Tables 3 and 4.
Figure 2. Perfusion parameters.
Hepatic artery flow rates (A) and portal vein flow rates (B) in Hemopure and RBC perfused livers. Hepatic artery pressure (C) and resistance (D) showed slight differences in the pressure settings used, however the resistances over the course of the perfusion were similar. The resistance in cold livers were observably lower (within first 30 minutes as liver warmed) in the Hemopure group, likely due to the low viscosity of the fluid. There were no differences in lactate metabolism (E), 8-OH-2-dG production (G) or ATP replenishment (H). O2ER (F) was increased in livers perfused with Hemopure.
Table 4.
Perfusion parameters of both perfused groups with associated p values
| RBC | HBOC | p value | |
|---|---|---|---|
| HA Pressure (mmHg) | 53.0 (36.5-56.0) | 56.6 (41.8-58.2) | 0.002 |
| HA Resistance T0 | 0.39 (0.22-0.56) | 0.26 (0.20-0.32) | 0.667 |
| HA Resistance T0-6.0 | 0.12 (0.07-0.56) | 0.11 (0.10-0.32) | 0.471 |
| HA Flow/gram (mL/min/g) | 0.26 (0.10-0.36) | 0.30 (0.09-0.32) | 0.828 |
| PV Flow/gram (mL/min/g) | 0.59 (0.29-0.65) | 0.63 (0.23-0.67) | 0.385 |
| Haemoglobin (g/dL) | 73.80 (70.54-85.80) | 56.24 (52.80-61.40) | <0.001 |
| O2ER/gram x 105 | 9.43 (3.45-13.67) | 13.75 (5.53-17.40) | 0.001 |
| Lactate level | |||
| Highest | 13.3 (5.4-20.0) | 10.1 (8.6-19.3) | 0.389 |
| Lowest | 1.4 (0.8-9.0) | 1.2 (0.3-9.1) | 0.524 |
| Last | 1.5 (0.8-20.0) | 1.6 (0.3-11.6) | 0.889 |
| Tissue ATP content (nmol/g protein) | 0.836 | ||
| T0 (preperfusion) | 3.9 (2.9) | 5.2 (6.0) | |
| T6 (postperfusion) | 18.9 (22.5) | 23.1 (32.6) | |
Abbreviations:
ATP, adenosine triphosphate; HA, Hepatic Artery; HBOC, Hemopure perfusion group; O2ER, Oxygen extraction ratio; PV, Portal Vein; RBC, Red blood cell perfusion group
Note:
T0 designates the median resistance immediately after perfusion was commenced; T0-6.0 designates the median resistance over the course of the perfusion.
Liver viability and oxygen consumption
HBOC perfusion fluid provided sufficient oxygen delivery for livers to perform metabolic functions that indicate their viability (Figure 2). Active liver metabolism was also confirmed by the progressive storage of glycogen in hepatocytes (Figure 3). There was progressive regeneration of ATP stores over the course of the perfusion and there were no differences between the RBC and HBOC groups (Table 4 and Figure 2 panel H). There was an increase in perfusate levels of 8-hydroxy-2’-deoxyguanosine (8-OH-dG) – an established marker of oxidative stress, although this appeared to plateau after the first 2 hours of perfusion and again, there were no differences between the RBC and HBOC perfused groups (Figure 2 panel G). There was a significantly higher oxygen extraction observed in the HBOC group compared to the RBC group and this difference was apparent throughout the course of the perfusion (Figure 2 panel F).
Figure 3. Histological findings.
Figure 3a: H&E sections of Hemopure-perfused livers. A: H&E stained section of part of a large portal tract following 6 hours of perfusion showing normal bile ducts (BD), artery (HA) and portal vein (PV). There is some portal edema present (black arrow) (objective x10). B: H&E stained section showing an intra-parenchymal portal tract with normal bile duct, artery and vein (objective x20). C: H&E stained section of extrahepatic bile duct following 6 hours of perfusion demonstrating normal architecture of the epithelium within the deep peri-biliary plexus (objective x20). D: H&E stained section prior to perfusion showing small droplet steatosis (black arrows) with empty sinusoids (objective x20). E: H&E stained section following 6 hours of perfusion showing a similar degree of small droplet steatosis of hepatocytes (black arrows). The Hemopure fluid fills the sinusoids and central vein and stains pink (objective x20). F: H&E stained section following 6 hours of perfusion and flushing with 2L 10% dextrose showing the Hemopure has been flushed out of the vasculature. The hepatocytes and sinusoids appear normal (objective x20).
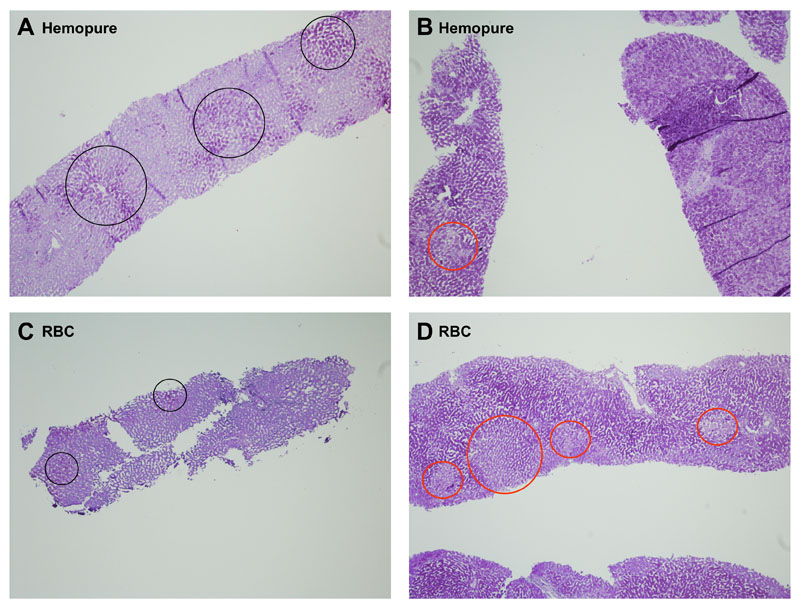
Figure 3b: PAS sections of Hemopure and RBC-perfused livers. A and C: PAS stained section of Hemopure-perfused and RBC-perfused livers respectively, showing marked glycogen depletion prior to perfusion with black circles showing scanty glycogen stores (objective x4). B and D: PAS stained section of Hemopure-perfused and RBC-perfused livers respectively, showing increased glycogen within hepatocytes following 6 hours of perfusion with red circles showing scanty areas which lack glycogen. (objective x4).
Histological assessment
The viable livers in the Hemopure group had a similar histological appearance to those perfused with packed RBC (not shown) with the majority of hepatocytes showing normal morphology with an intact hepatocyte plate/sinusoidal lining (Figure 3a.A-D). Following perfusion with Hemopure the vasculature appeared to contain a pink-staining solution (3a.E) which was not present following RBC-based perfusions and which appeared to be flushed out effectively with 2L 10% dextrose at the end of the perfusion process (Figure 3a.F). Extrahepatic bile ducts perfused with Hemopure maintained normal morphology (Figure 3a.C) with a largely intact surface epithelium, viable epithelial lining of the deep peribiliary glands and no loss of stromal nuclei, arterial medial nuclei or evidence of thrombosis. Within both groups, the livers deemed viable (based on perfusion characteristics) demonstrated an increase in glycogen storage (Table 5, Figure 3b) or maintained high glycogen stores during perfusion, whilst those which were deemed non-viable failed to restore glycogen reserves (Table 5). PAS stain was unaffected by the presence of Hemopure. Importantly, there was no histological evidence of damage caused by Hemopure infusion and livers that were viable according to our criteria, had similar histological features in both RBC and HBOC-infused groups. Although we do not use the scoring system at our center, when we compared the 2 groups histologically using our own system or Suzuki’s criteria for IRI (39), there were no observable differences.
Table 5.
Histological features on liver biopsies
| RBC 1 | RBC 2 | RBC 3 | RBC 4 | RBC 5 | HBOC 1 | HBOC 2 | HBOC 3 | HBOC 4 | HBOC 5 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Designated viability | Nonviable | Viable | Nonviable | Viable | Viable | Nonviable | Nonviable | Viable | Viable | Viable | |
| Large droplet steatosis1 (%) | 0 | <1 | 15 | <5 | 0 | 80 | 0 | 0 | <1 | 0 | |
| Small droplet steatosis2 (%) | 0 | 10 | 40 | 20 | 5 | 70 | <1 | 1 | 80 | 0 | |
| Glycogen depletion3 (pre-NMP-L/post-NMP-L) |
- | 95/10 | 90/80 | 90/10 | 80/15 | 90/85 | 60/65 | 10/10-15 | 80/50 | 60/15 | |
| Detached hepatocytes4 (%) (pre-NMP-L/post-NMP-L) |
- | 1 / 2 | 20 / 15 | - | 0 / 1 | 0/5 | 0/50 | 0/0 | 0/0 | 0/0 | |
| Coagulative Necrosis5 (%) (pre-NMP-L/post-NMP-L) |
- | 0 / 5 | 0 / 5 | 0 / 30 | 0 / 0 | 0/1 | 0/2 | 0/0 | 0/0 | 0/0 | |
| Other finding | Hepatitis with severe cholestasis | Patchy congestion | Steatohepatitis | Congested, did not flush well | |||||||
Abbreviations:
HBOC, Hemopure group; NMP-L, normothermic machine perfusion – Liver ; RBC, Red blood cell group
Note: Values designated with “–“ are missing; Steatosis determined in the pre-NMP-L biopsy
Large droplet macrovesicular steatosis is defined as a single large fat droplet within the hepatocyte cytoplasm displacing the nucleus. Values are % of hepatocytes containing fat
Small droplet macrovesicular steatosis is defined as fat droplets, usually multiple within the cytoplasm of the hepatocyte which do not displace the nucleus. Values are % of hepatocytes containing fat
Glycogen depletion is graded as % of hepatocytes which do not contain glycogen.
Detached hepatocytes are the % of hepatocytes which have lost cohesion from each other and from the sinusoidal lining
Necrosis is depicted as the percent of total hepatocytes in the biopsy which show classical ischemic-type coagulative necrosis.
In vitro cytotoxicity testing of Hemopure
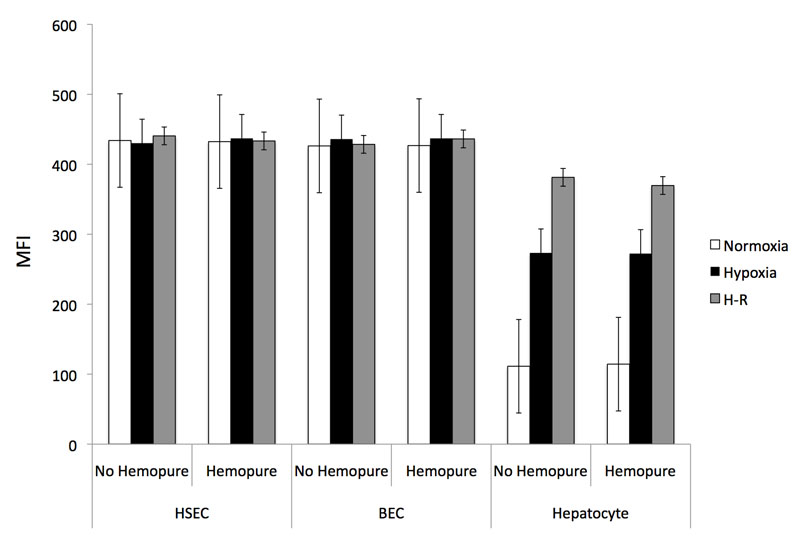
HSEC and BEC did not increase intracellular ROS production during in vitro IRI when cultured in standard media (Figure 4a). Human hepatocytes demonstrated increased ROS accumulation when exposed to hypoxia that was accentuated during H-R as we have previously demonstrated (35). When human hepatocytes, HSEC or BEC were cultured in Hemopure-containing media, there was no significant increase in intracellular ROS production during normoxia, hypoxia or H-R. These results demonstrate that Hemopure does not increase ROS accumulation in isolated primary liver cells during in vitro IRI.
Figure 4. Toxicity testing - The Effects of Hemopure on ROS Production and Apoptosis in Human Hepatocytes, Human HSEC and Human BEC.
Figure 4a: Isolated human hepatocytes, HSEC and BEC were exposed to the in vitro model of IRI in the presence and absence of Hemopure and the effect upon intracellular ROS accumulation was assessed using 2',7'-dichlorofluorescin. Data are expressed as MFI and calculated as described in the Methods and Materials section (n=3-6).
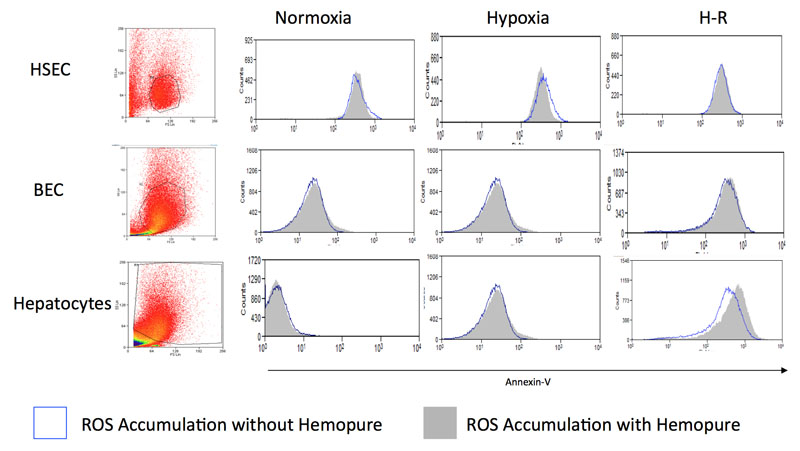
Figure 4b: The bottom panel shows representative flow cytometry plots demonstrating the effects of Hemopure on apoptosis in human hepatocytes, HSEC and BEC during hypoxia. Similar plots were obtained during normoxia and H-R (data not shown).
Our previous work has shown that increases in intracellular ROS increase cell death in parenchymal liver cells primarily via apoptosis but also necrosis (35). As Figure 4b demonstrates, when human hepatocytes, HSEC or BEC were cultured in Hemopure during normoxia, hypoxia or H-R there was no increase in apoptosis relative to cells cultured in standard media. There was no increase in necrosis in human hepatocytes, HSEC or BEC during in vitro IRI when cultured with Hemopure (data not shown). Hemopure therefore shows no increase in cytotoxicity in primary human liver cells during IRI.
Discussion
Organ machine perfusion is becoming an increasingly attractive preservation method since experimental studies have demonstrated it mitigate IRI and potentially improve allograft function (8). In particular, data from early clinical transplant series using normothermic machine perfused grafts show promising results and provide a potential means of overcoming the critical shortage of donor organs (11,12,16,40,41). Regardless of perfusion temperature, it is generally accepted that oxygenation of the perfusate is advantageous (4). Here we show for the first time that Hemopure, an acellular oxygen carrier, has the potential to replace packed red cells as the oxygen carrier of choice in a human NMP-L model. This study was primarily designed to assess the feasibility of Hemopure to replace RBC in a model of viability testing using NMP-L. This is not a model of true reperfusion given that we do not use whole blood and there is no immune cell population (other than resident immune cells) in the perfusate, however, including a subsequent reperfusion step to simulate a clinical transplantation would not have added any additional information to the chosen study endpoint.
In the present experiments, we observed increased oxygen consumption in the HBOC liver group. We believe this to be a result of the physiological and rheological properties of Hemopure. As previously described, the oxygen dissociation curve of Hemopure lies to the right of corpuscular hemoglobin (with a p50 of 40mmHg) and therefore gives up oxygen to tissues more readily (41,42). This difference was more pronounced at the initial phase of the perfusion, prior to the liver core and the perfusate temperature reaching 37°C, during which Hb-O2 affinity would normally be increased, giving oxygen to tissues less freely. Across all temperatures therefore, Hemopure will give up more oxygen to tissues than corpuscular hemoglobin. Additional properties such as a molecular diameter approximately 1/1000th the diameter of a red-blood cell and the fact Hemopure is a less viscous fluid, result in a more homogenous perfusion (42) and facilitate the diffusive transport of oxygen in the microcirculation improving tissue oxygenation. Low-viscosity preservation fluids may protect against the development of posttransplant biliary complications however this aspect of NMP-L requires further research (43–45).
The apparent advantage of a lower O2 affinity did not translate to a reduction in intracellular ROS when using Hemopure in the IRI model in vitro and the reasons for this remain the focus of ongoing research in our laboratory. Crucially, Hemopure did not induce cell death in primary human liver cells during IRI. One of the acknowledged protective mechanisms of NMP-L is attenuation of IRI because the organ replenishes energy stores within an environment free from recipient immune-mediated injury, thereby minimizing ROS accumulation at true reperfusion – a central trigger of allograft necroapoptosis observed following transplantation (33). Porcine HBOC’s have been shown to exhibit antioxidant activity in vitro and significantly inhibit hydrogen peroxide-mediated endothelial cell damage and apoptosis (46). They have also been shown to have a protective effect on focal cerebral IRI in an animal model (47).
There is mounting evidence that circulating resident leukocytes, or those few that may be present in blood products, can activate proinflammatory signaling pathways that accentuate organ damage (48). While a leukocyte filter decreases the amount of circulating leukocytes, cells trapped in the filter can still potentially trigger proinflammatory cascades that activate ROS, damage-associated molecular pattern molecules (DAMPs) and cytokine production, impacting upon organ quality (48–50). DAMPs filters are starting to be trialed in some perfusion research settings. Third party blood can also sensitize the organ recipient and although its significance in preventing liver damage will require further research, replacing RBC with HBOC has would avoid these phenomena (51).
Some models do not require the use of third party blood products. At present, for normothermic perfusion of the heart, the organ recovery team obtains whole donor blood at the time of organ procurement (11). However, this approach is not feasible for all potentially recovered donor organs as the blood volume to prime the perfusion devices would be more than the circulatory volume of the donor. The logistics of retrieving donor blood is also unfavorable as it causes severe hypotension and leads to a delay in cross clamping the aorta.
Whilst third party blood provides good results for NMP-L, there are several reasons why finding alternatives may be an important development for the clinical adoption of machine perfusion in the future. The obvious reason is to avoid any unnecessary blood usage – a scarce resource and vital for major surgical procedures or other therapeutic interventions. Complying with ethical and legislative regulations, acquiring approval for third party blood to be used in NMP-L research is a lengthy process. Using an acellular oxygen carrier would avoid this and overcome other challenges that are associated with the use of blood products such as traceability. Additionally, HBOC’s do not require cross-matching and have a long shelf-life at room temperature. In our experience this prevented delays and minimized the cold ischemic time which is a key factor when attempting to utilize extended criteria donor livers.
Whereas our research team have focused on viability testing and maximizing organ utilization in a model of NMP-L, others have pursued subnormothermic (25) or hypothermic in a bid to improve the long-term results in organs from DCD donors (4,9). The optimal temperature for liver perfusion remains a matter of continuing discussion and an area of intensive research (52–54). Hemopure can deliver oxygen within the wide range of the conventionally used machine perfusion temperatures (10°C to 37°C), currently being trialed in clinical and research settings (25,53).
NMP-L can also be used to improve logistics through significantly extending the organ preservation time (14). Hemolysis caused by sheer stress from the device pump and circuit tubing decreases the perfusate oxygen carrying capacity over time and is currently 1 of the main limiting factors for further extension of organ preservation times (55). The hemolysed RBC debris, whilst eventually cleared by the liver, may adversely disrupt the hepatic microcirculation, particularly in the peribiliary vascular plexus during the NMP-L procedure. A purified acellular fluid theoretically avoids these limitations. The half-life of Hemopure is 16 hours and recurring anemia in the clinical setting is usually noted 24 hours after administration. We have not observed any degradation of the product however its stability beyond 6 hours of perfusion is yet to be tested (56). One of the constraints for wider and faster implementation of the NMP technology into the organ preservation pathway is its high cost. Hemopure costs more per unit than RBCs however this cost could be offset by the numerous advantages its use offers (57).
There has been a longstanding interest in developing an efficient and safe alternative to donor blood. Several products have been tested mainly in the preclinical setting with promising results although they have not been adopted into routine clinical practice (22,58). Despite negative reports from a meta-analysis examining the use of 5 different HBOC products, Hemopure has demonstrated clinical efficacy in trials investigating its use in general, urological, orthopedic, vascular and cardiac surgery, though it demonstrated some side effects, most commonly hypertension and bradycardia (23,59–62). Liver machine perfusion with Hemopure ex situ avoids the potential complexities of systemic in vivo interactions and their potential side effects. Histological assessment also showed that flushing the liver at the end of NMP-L effectively removes Hemopure from the liver, so only a very small volume (if any) would reach the recipient circulation.
The main limitation of our study is being unable to assess the effect of true reperfusion during transplantation as the livers were not transplanted. We also chose not to simulate the reperfusion effect by NMP-L with whole blood containing immune cell populations. This model however, provided reassurance that Hemopure does not cause any apparent histological damage and it is able to deliver enough oxygen to fully support human liver metabolism at normothermic condition. Such confirmation was necessary prior to evaluating Hemopure in a clinical transplant setting.
In conclusion, this study suggests that Hemopure-based perfusion fluid is a feasible alternative to the blood-based solution currently used for NMP-L. Hemopure may be logistically, rheologically and immunologically superior to packed red cells when used in a normothermic perfusion model. Our findings warrant further HBOC-based machine perfusion fluid testing in a pilot clinical trial.
Supplementary Material
Acknowledgments
This study was funded by QEHB Charities (Liver Foundation) at University Hospitals Birmingham NHS Foundation Trust. The Hemopure fluid was kindly provided free of charge by Zaf Zafirelis from HbO2 Therapeutics. The Liver Assist device used for this project was provided by the Organ Assist company. Mr Bhogal is funded by the Academy of Medical Sciences. We gratefully acknowledge the generous project support provided by all the team members of the Liver Unit at Queen Elizabeth Hospital Birmingham. We thank Dr Gary Reynolds of the Centre for Liver Research for tissue and histological processing, and Ms Bridget Gunson for her assistance in obtaining the regulatory approval for the study. Finally, we would like to thank our organ donors, their families and the NHSBT network for allowing us to perform this work.
This paper presents independent research supported by the NIHR Birmingham Liver Biomedical Research Unit and the views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
Funding
The project was funded by QEHB Charity (Liver Foundation). The Liver Assist device used was on loan from Organ Assist Company. Hemopure was provided free of charge by Hemoglobin Oxygen Therapeutics LLC. Neither of the 2 companies had any role in the study design, data collection, analysis, interpretation or manuscript preparation. The authors are employees of the University Hospital Birmingham or University of Birmingham and none of them received any payment or have any conflict of interest related to this manuscript.
Abbreviations
- ATP
adenosine triphosphate
- BEC
biliary epithelial cells
- DAMPs
damage-associated molecular pattern molecules
- DBD
donation after brain-stem death
- DCD
donation after circulatory death
- ER
extraction ratio
- FiO2
fraction of inspired oxygen
- HBI
hypoxic brain injury
- HBOC
hemoglobin-based oxygen carrier
- H&E
hematoxylin and eosin
- H-R
hypoxia reoxygenation
- HSEC
human sinusoidal endothelial cells
- ICH
intracranial hemorrhage
- IRI
ischemia reperfusion injury
- ITU
intensive therapy unit
- MFI
medium fluorescence intensity
- NHSBT
National Health Service Blood and Transplant
- NMP-L
normothermic machine perfusion of the liver
- PAS
periodic-acid Schiff
- RBC
red blood cells
- ROS
reactive oxygen species
- SCS
static cold storage
- WIT
warm ischemic time
- 8-OH-dG
8-hydroxy-2-deoxyguanosine
Footnotes
Authors' Contribution
HM and SCA initiated the study and were responsible for the management of the research project. RWL, BTFS, ASm and HM performed the machine perfusions; RHB performed the in vitro toxicity testing; RWL and RHB collected the samples and data; DAHN and SGH analyzed the histology samples; DFM and HM provided the surgical expertise and prepared livers for the perfusions; RWL, RHB and HM performed the statistical analysis; RWL, LW and YB performed lab-based experiments; HM, DAHN, RWL, RHB, and ASc were responsible for the data interpretation, manuscript drafting and submission; all co-authors actively contributed to manuscript preparation and approved the final manuscript.
Disclosure
The authors declare no conflicts of interest
References
- 1.Williams R, Aspinall R, Bellis M, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384(9958):1953–1997. doi: 10.1016/S0140-6736(14)61838-9. [DOI] [PubMed] [Google Scholar]
- 2.Williams R, Ashton K, Aspinall R, et al. Implementation of the Lancet Standing Commission on Liver Disease in the UK. Lancet. 2015;386(10008):2098–2111. doi: 10.1016/S0140-6736(15)00680-7. [DOI] [PubMed] [Google Scholar]
- 3.Olthoff KM, Kulik L, Samstein B, et al. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16(8):943–949. doi: 10.1002/lt.22091. [DOI] [PubMed] [Google Scholar]
- 4.Dutkowski P, Schlegel A, de Oliveira M, Mullhaupt B, Neff F, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60(4):765–772. doi: 10.1016/j.jhep.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16(6):1779–1787. doi: 10.1111/ajt.13708. [DOI] [PubMed] [Google Scholar]
- 6.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16(11):3235–3245. doi: 10.1111/ajt.13875. [DOI] [PubMed] [Google Scholar]
- 7.Laing RW, Mergental H, Mirza DF. Normothermic ex-situ liver preservation: the new gold standard. Curr Opin Organ Transplant. doi: 10.1097/MOT.0000000000000414. [published online March 22 2017] [DOI] [PubMed] [Google Scholar]
- 8.Schlegel A, Rougemont O, Graf R, Clavien PA, Dutkowski P. Protective mechanisms of end-ischemic cold machine perfusion in DCD liver grafts. J Hepatol. 2013;58(2):278–286. doi: 10.1016/j.jhep.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Dutkowski P, Polak WG, Muiesan P, et al. First comparison of hypothermic oxygenated perfusion versus static cold storage of human donation after cardiac death liver transplants: an international-matched case analysis. Ann Surg. 2015;262(5):764–770. doi: 10.1097/SLA.0000000000001473. [DOI] [PubMed] [Google Scholar]
- 10.Liu Q, Nassar A, Farias K, et al. Comparing normothermic machine perfusion preservation with different perfusates on porcine livers from donors after circulatory death. Am J Transplant. 2016;16(3):794–807. doi: 10.1111/ajt.13546. [DOI] [PubMed] [Google Scholar]
- 11.Dhital KK, Iyer A, Connellan M, et al. Adult heart transplantation with distant procurement and ex-vivo preservation of donor hearts after circulatory death: a case series. Lancet. 2015;385(9987):2585–2591. doi: 10.1016/S0140-6736(15)60038-1. [DOI] [PubMed] [Google Scholar]
- 12.Boucek MM, Mashburn C, Dunn SM, et al. Pediatric heart transplantation after declaration of cardiocirculatory death. N Engl J Med. 2008;359(7):709–714. doi: 10.1056/NEJMoa0800660. [DOI] [PubMed] [Google Scholar]
- 13.Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet. 2015;385(9987):2577–2584. doi: 10.1016/S0140-6736(15)60261-6. [DOI] [PubMed] [Google Scholar]
- 14.Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (first-in-man) clinical trial. Am J Transplant. 2016;16(6):1779–1787. doi: 10.1111/ajt.13708. [DOI] [PubMed] [Google Scholar]
- 15.Watson CJ, Kosmoliaptsis V, Randle LV, et al. Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant. 2016;16(1):353–357. doi: 10.1111/ajt.13448. [DOI] [PubMed] [Google Scholar]
- 16.Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex-situ evaluation. Am J Transplant. 2016;16(11):3235–3245. doi: 10.1111/ajt.13875. [DOI] [PubMed] [Google Scholar]
- 17.Schreiber GB, Busch MP, Kleinman SH, Korelitz JJ. The risk of transfusion-transmitted viral infections. The Retrovirus Epidemiology Donor Study. N Engl J Med. 1996;334(26):1685–1690. doi: 10.1056/NEJM199606273342601. [DOI] [PubMed] [Google Scholar]
- 18.Senay S, Toraman F, Gunaydin S, Kilercik M, Karabulut H, Alhan C. The impact of allogenic red cell transfusion and coated bypass circuit on the inflammatory response during cardiopulmonary bypass: a randomized study. Interact Cardiovasc Thorac Surg. 2009;8(1):93–99. doi: 10.1510/icvts.2008.183608. [DOI] [PubMed] [Google Scholar]
- 19.NHS Blood and Transplant. Hepatitis E transmission in blood components: new recommendations for immunocompromised patients. http://hospital.blood.co.uk/media/27888/nhsbt-hep-e-presentation-october-2015.pdf. Published online October 2015.
- 20.Moritz ED, Winton CS, Tonnetti L, et al. Screening for Babesia microti in the U.S. Blood Supply. N Engl J Med. 2016;375(23):2236–2245. doi: 10.1056/NEJMoa1600897. [DOI] [PubMed] [Google Scholar]
- 21.Trakarnsanga K, Griffiths RE, Wilson MC, et al. An immortalized adult human erythroid line facilitates sustainable and scalable generation of functional red cells. Nat Commun. 2017;8:14750. doi: 10.1038/ncomms14750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozzarelli A, Ronda L, Faggiano S, Bettati S, Bruno S. Haemoglobin-based oxygen carriers: research and reality towards an alternative to blood transfusions. Blood Transfus. 2010;8(Suppl 3):s59–68. doi: 10.2450/2010.010S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy JH, Goodnough LT, Greilich PE, et al. Polymerized bovine hemoglobin solution as a replacement for allogeneic red blood cell transfusion after cardiac surgery: results of a randomized, double-blind trial. J Thorac Cardiovasc Surg. 2002;124(1):35–42. doi: 10.1067/mtc.2002.121505. [DOI] [PubMed] [Google Scholar]
- 24.Sprung J, Kindscher JD, Wahr JA, et al. The use of bovine hemoglobin glutamer-250 (Hemopure) in surgical patients: results of a multicenter, randomized, single-blinded trial. Anesth Analg. 2002;94(4):799–808. doi: 10.1097/00000539-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Fontes P, Lopez R, van der Plaats A, et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant. 2015;15(2):381–394. doi: 10.1111/ajt.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NHS Blood and Transplant. Organ donation and transplantation: national organ retrieval service retrieval standards. http://www.odt.nhs.uk/pdf/nors_retrieval_standards.pdf. Published October 3 2016.
- 27.Anbari KK, Garino JP, Mackenzie CF. Hemoglobin substitutes. Eur Spine J. 2004;13(Suppl 1):S76–82. doi: 10.1007/s00586-004-0737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perera T, Mergental H, Stephenson B, et al. First human liver transplantation using a marginal allograft resuscitated by normothermic machine perfusion. Liver Transpl. 2016;22(1):120–124. doi: 10.1002/lt.24369. [DOI] [PubMed] [Google Scholar]
- 29.Silva MA, Mirza DF, Murphy N, et al. Intrahepatic complement activation, sinusoidal endothelial injury, and lactic acidosis are associated with initial poor function of the liver after transplantation. Transplantation. 2008;85(5):718–725. doi: 10.1097/TP.0b013e3181663366. [DOI] [PubMed] [Google Scholar]
- 30.Bhogal RH, Hodson J, Bartlett DC, et al. Isolation of primary human hepatocytes from normal and diseased liver tissue: a one hundred liver experience. PLoS One. 2011;6(3):e18222. doi: 10.1371/journal.pone.0018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lalor PF, Edwards S, McNab G, Salmi M, Jalkanen S, Adams DH. Vascular adhesion protein-1 mediates adhesion and transmigration of lymphocytes on human hepatic endothelial cells. J Immunol. 2002;169(2):983–992. doi: 10.4049/jimmunol.169.2.983. [DOI] [PubMed] [Google Scholar]
- 32.Joplin R. Isolation and culture of biliary epithelial cells. Gut. 1994;35(7):875–878. doi: 10.1136/gut.35.7.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 2010;16(11):1303–1313. doi: 10.1002/lt.22157. [DOI] [PubMed] [Google Scholar]
- 34.Bhogal RH, Weston CJ, Curbishley SM, Adams DH, Afford SC. Autophagy: a cyto-protective mechanism which prevents primary human hepatocyte apoptosis during oxidative stress. Autophagy. 2012;8(4):545–558. doi: 10.4161/auto.19012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhogal RH, Curbishley SM, Weston CJ, Adams DH, Afford SC. Reactive oxygen species mediate human hepatocyte injury during hypoxia/reoxygenation. Liver Transpl. 2010;16(11):1303–1313. doi: 10.1002/lt.22157. [DOI] [PubMed] [Google Scholar]
- 36.Bhogal RH, Weston CJ, Curbishley SM, Adams DH, Afford SC. Activation of CD40 with platelet derived CD154 promotes reactive oxygen species dependent death of human hepatocytes during hypoxia and reoxygenation. PLoS One. 2012;7(1):e30867. doi: 10.1371/journal.pone.0030867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhogal RH, Weston CJ, Curbishley SM, Bhatt AN, Adams DH, Afford SC. Variable responses of small and large human hepatocytes to hypoxia and hypoxia/reoxygenation (H-R) FEBS Lett. 2011;585(6):935–941. doi: 10.1016/j.febslet.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6(4):783–790. doi: 10.1111/j.1600-6143.2006.01242.x. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55(6):1265–1272. doi: 10.1097/00007890-199306000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Warnecke G, Moradiellos J, Tudorache I, et al. Normothermic perfusion of donor lungs for preservation and assessment with the Organ Care System Lung before bilateral transplantation: a pilot study of 12 patients. Lancet. 2012;380(9856):1851–1858. doi: 10.1016/S0140-6736(12)61344-0. [DOI] [PubMed] [Google Scholar]
- 41.Hosgood SA, Saeb-Parsy K, Hamed MO, Nicholson ML. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 2016;16(11):3282–3285. doi: 10.1111/ajt.13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mackenzie CF, Moon-Massat PF, Shander A, Javidroozi M, Greenburg AG. When blood is not an option: factors affecting survival after the use of a hemoglobin-based oxygen carrier in 54 patients with life-threatening anemia. Anesth Analg. 2010;110(3):685–693. doi: 10.1213/ANE.0b013e3181cd473b. [DOI] [PubMed] [Google Scholar]
- 43.Pirenne J, Van Gelder F, Coosemans W, et al. Type of donor aortic preservation solution and not cold ischemia time is a major determinant of biliary strictures after liver transplantation. Liver Transpl. 2001;7(6):540–545. doi: 10.1053/jlts.2001.24641. [DOI] [PubMed] [Google Scholar]
- 44.Erhard J, Lange R, Scherer R, et al. Comparison of histidine-tryptophan-ketoglutarate (HTK) solution versus University of Wisconsin (UW) solution for organ preservation in human liver transplantation. A prospective, randomized study. Transpl Int. 1994;7(3):177–181. doi: 10.1007/BF00327084. [DOI] [PubMed] [Google Scholar]
- 45.Moench C, Moench K, Lohse AW, Thies J, Otto G. Prevention of ischemic-type biliary lesions by arterial back-table pressure perfusion. Liver Transpl. 2003;9(3):285–289. doi: 10.1053/jlts.2003.50015. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W, Yan K, Dai P, Tian J, Zhu H, Chen C. A novel hemoglobin-based oxygen carrier, polymerized porcine hemoglobin, inhibits H(2)O(2)-induced cytotoxicity of endothelial cells. Artificial Organs. 2012;36(2):151–160. doi: 10.1111/j.1525-1594.2011.01305.x. [DOI] [PubMed] [Google Scholar]
- 47.Xie Z, Liu L, Zhu W, et al. The protective effect of polymerized porcine hemoglobin (pPolyHb) on transient focal cerebral ischemia/reperfusion injury. Artif Cells Nanomed Biotechnol. 2015;43(3):180–185. doi: 10.3109/21691401.2015.1037886. [DOI] [PubMed] [Google Scholar]
- 48.Noda K, H S, Zaldonis D, D'Cunha J, Luketich JD, Shigemura Circulating cytokines vs. leukocytes: a therapeutic target during ex vivo lung perfusion. J Heart Lung Transplant. 2016;35(4S):S181–182. [Google Scholar]
- 49.van Golen RF, van Gulik TM, Heger M. Mechanistic overview of reactive species-induced degradation of the endothelial glycocalyx during hepatic ischemia/reperfusion injury. Free Radic Biol Med. 2012;52(8):1382–1402. doi: 10.1016/j.freeradbiomed.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 50.van Golen RF, van Gulik TM, Heger M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev. 2012;23(3):69–84. doi: 10.1016/j.cytogfr.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Rabinovici R. The status of hemoglobin-based red cell substitutes. Isr Med Assoc J. 2001;3(9):691–697. [PubMed] [Google Scholar]
- 52.Hoyer DP, Mathe Z, Gallinat A, et al. Controlled oxygenated rewarming of cold stored livers prior to transplantation: first clinical application of a new concept. Transplantation. 2016;100(1):147–152. doi: 10.1097/TP.0000000000000915. [DOI] [PubMed] [Google Scholar]
- 53.Karangwa SA, Dutkowski P, Fontes P, et al. Machine perfusion of donor livers for transplantation: a proposal for standardized nomenclature and reporting guidelines. Am J Transplant. doi: 10.1111/ajt.13843. [published online April 29 2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Graham JA, Guarrera JV. “Resuscitation” of marginal liver allografts for transplantation with machine perfusion technology. J Hepatol. 2014;61(2):418–431. doi: 10.1016/j.jhep.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 55.Sakota D, Sakamoto R, Sobajima H, et al. Mechanical damage of red blood cells by rotary blood pumps: selective destruction of aged red blood cells and subhemolytic trauma. Artif Organs. 2008;32(10):785–791. doi: 10.1111/j.1525-1594.2008.00631.x. [DOI] [PubMed] [Google Scholar]
- 56.McNeil JD, Propper B, Walker J, et al. A bovine hemoglobin-based oxygen carrier as pump prime for cardiopulmonary bypass: reduced systemic lactic acidosis and improved cerebral oxygen metabolism during low flow in a porcine model. J Thorac Cardiovasc Surg. 2011;142(2):411–417. doi: 10.1016/j.jtcvs.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 57.Shander A, Hofmann A, Ozawa S, Theusinger OM, Gombotz H, Spahn DR. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–765. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 58.Jahr JS, Walker V, Manoochehri K. Blood substitutes as pharmacotherapies in clinical practice. Curr Opin Anaesthesiol. 2007;20(4):325–330. doi: 10.1097/ACO.0b013e328172225a. [DOI] [PubMed] [Google Scholar]
- 59.LaMuraglia GM, O'Hara PJ, Baker WH, et al. The reduction of the allogenic transfusion requirement in aortic surgery with a hemoglobin-based solution. J Vasc Surg. 2000;31(2):299–308. doi: 10.1016/s0741-5214(00)90161-7. [DOI] [PubMed] [Google Scholar]
- 60.Jahr JS, Liu H, Albert OK, et al. Does HBOC-201 (Hemopure) affect platelet function in orthopedic surgery: a single-site analysis from a multicenter study. Am J Ther. 2010;17(2):140–147. doi: 10.1097/MJT.0b013e3181a2b08d. [DOI] [PubMed] [Google Scholar]
- 61.Ashenden MJ, Schumacher YO, Sharpe K, Varlet-Marie E, Audran M. Effects of Hemopure on maximal oxygen uptake and endurance performance in healthy humans. Int J Sports Med. 2007;28(5):381–385. doi: 10.1055/s-2006-924365. [DOI] [PubMed] [Google Scholar]
- 62.Van Hemelrijck J, Levien LJ, Veeckman L, Pitman A, Zafirelis Z, Standl T. A safety and efficacy evaluation of hemoglobin-based oxygen carrier HBOC-201 in a randomized, multicenter red blood cell controlled trial in noncardiac surgery patients. Anesth Analg. 2014;119(4):766–776. doi: 10.1213/ANE.0000000000000305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.