Abstract
Mammalian cell tissue culture has been a critical tool leading to our current understanding of cancer including many aspects of cellular transformation, growth and response to therapies. The current use of large panels of cell lines with associated phenotypic and genotypic information now allows for informatics approaches and in silico screens to rapidly test hypotheses based on simple as well as complex relationships. Current cell line panels with large amounts of associated drug sensitivity and genomics data are comprised of human cancer cell lines (i.e. NCI60 and GDSC). There is increased recognition of the contribution of canine cancer to comparative cancer research as a spontaneous large animal model with application in basic and translational studies. We have assembled a panel of canine cancer cell lines to facilitate studies in canine cancer and report here phenotypic and genotypic data associated with these cells.
Keywords: canine cancer, cell line panel, comparative oncology, doxorubicin, drug sensitivity, gene expression, microRNA
Introduction
Mammalian cell tissue culture is invaluable for studying biological processes and is a fundamental tool used in many laboratories worldwide. The practice of maintaining mammalian cells in a culture system began in the early 20th century,1 with these techniques quickly being utilized to study the growth of cancers in vitro including a breast adenocarcinoma from a dog.2 By 1950 the use of animal cell culture had become routine, and in 1951 the HeLa cell line was established, the first human cell line developed from a cancer patient.3,4 In vitro studies using cancer cell lines play a large role in cancer drug discovery and development, providing crucial data on drug effects and cancer biology in the early pre-clinical stages, many of which would be unethical to explore in patients. This information is key in the decision process for drugs moving forward into expensive and time consuming clinical trials.5
The emergence of new genomic technologies in the last decade has revolutionized cancer research and revealed to researchers that genetic heterogeneity is inherent across the whole human tumour population as well as within histological tumour types. Importantly, this heterogeneity is highly similar between primary tumours and tumour-derived cell lines according to multiple studies including breast cancer, melanoma and non-small cell lung cancer.6–8 This has led to renewed interest in creating cancer cell line panels as model systems to further explore genetic effects on cancer biology and therapeutic response.4 Te most well known human cancer cell line panel dedicated to this purpose was developed by the National Cancer Institute (NCI60 panel), consisting of 60 cell lines of various tumour types that has been used to screen over 100 000 compounds for anti-cancer activity.9 The panel has also undergone molecular profilling at the DNA, RNA, protein and chromosomal levels.10
More recently, cell line panels from the Genomics and Drug Sensitivity in Cancer project (GDSC) and the Broad Institute's Cancer Cell Line Encyclopedia (CCLE) have been established containing 1217 and 1046 cell lines, respectively. These panels have been screened against 138 and 24 cancer drugs, respectively.11,12 Gene expression, chromosomal copy number and sequencing data are available for the CCLE, whereas generated genomic data for the GDSC panel include data on gene expression, point mutations, gene amplifications and deletions, sites of microsatellite instability, and chromosomal rearrangements.11,12 Fortunately, unique genomic data from these large cell line panels can be shared for 496 cell lines that overlap CCLE and GDSC panels, and 55 cell lines of the NCI60 that are found on either the CCLE or the GDSC panels. In order to better translate discovered genetic relationships of drug response from cell lines to tumours, available genomic resources such as the NCI's Cancer Genome Atlas (TCGA) have been established, which contain exon and whole genome sequencing as well as gene expression data for thousands of tumour samples representing 33 tumour types.13 These resources are invaluable for the development of more personalized therapeutic strategies for the treatment of cancer.
Similar cancer cell line panels for canine cancer at such a scale are currently non-existent. Small collections of canine cancer cell lines exist at various institutions but the range of data is often limited. The purpose of this article is to describe the first diverse canine cancer cell line panel of its kind, comprised of 28 validated cell lines representing multiple tumour types. Herein we will report the characteristics of the Flint Animal Cancer Center (FACC) panel and the accompanying genomic profiling that have been performed as well as its potential applications for comparative and translational oncology.
Materials and methods
Cell culture
FACC cell lines were acquired from other institutions, purchased from the American Type Culture Collection (ATCC), or established from tumour samples from the FACC archive (see Table 1). During cell viability assays, all cells were maintained in RPMI 1640 culture medium containing 10% fetal bovine serum (FBS), penicillin (100 units mL−1), streptomycin (100 μg mL−1) and incubated at 37 °C in a humidified atmosphere of 5% CO2:95% air.
Table 1. Current cell lines within the FACC panel.
| Cell line name | Tumour type | Sourcea | Xenograftb | Known markers | Mutations |
|---|---|---|---|---|---|
| D17 | Osteosarcoma | ATCC | Yes | ||
| Abrams | Osteosarcoma | UWM | Yes | ||
| Moresco | Osteosarcoma | UWM | ND | ||
| Gracie | Osteosarcoma | CSU | Yes | ||
| MacKinley | Osteosarcoma | CSU | Yes | ||
| Yamane | Osteosarcoma | CSU | ND | ||
| Vogel | Osteosarcoma | CSU | ND | BRAF V595E | |
| OSA8 | Osteosarcoma | UCSF | ND | ||
| HMPOS | Osteosarcoma | Tokyo | ND | ||
| OS2.4 | Osteosarcoma | WSU | ND | ||
| 17CM98 | Melanoma | UWM | Yes | SILV, TYR, MAGE, MART-1 | |
| CML-6M | Melanoma | AU | Yes | ||
| CML-10C2 | Melanoma | AU | Yes | ||
| Jones | Melanoma | CSU | ND | SILV, TYR, MAGE, MART-1 | NRAS Q61R |
| Parks | Melanoma | CSU | ND | SILV, MAGE, MART-1 | |
| CMT12 | Mammary carcinoma | AU | ND | Cytokeratin | |
| CMT27 | Mammary carcinoma | AU | ND | Cytokeratin | |
| DEN-HSA/Fitz | Hemangiosarcoma | UWM | No | VEGFR2, αVβ3 integrin, fVIII-ra | |
| Bliley | Bladder carcinoma | CSU | ND | Cytokeratin | BRAF V595E |
| 1771 | Lymphoma | Upenn | Yes | CD45, class II MHC | |
| OSW | Lymphoma | OSU | Yes | CD45, class II MHC | |
| CLBL1 | Lymphoma | Aus | ND | CD22, CD45, class II MHC | KIT mutant |
| CLL1390 | Leukaemia | UCD | ND | CD45, CD34, CD4 | PTEN deletion |
| C2 | Mast cell | UCSF | Yes | CD5, CD117 | KIT mutant |
| DH82 | Histiocytic sarcoma | ATCC | ND | CD45, CD18 | |
| MH/Nike | Histiocytic sarcoma | CSU | ND | CD14, CD18, CD45 | |
| CTAC | Thyroid carcinoma | OSU | ND | ||
| STSA-1 | Soft-tissue sarcoma | UI | Yes |
ATCC, American Type Culture Collection; AU, Auburn University; Aus, Veterinary University of Austria; CSU, Colorado State University; ND, not determined; UCSF, University of California-San Francisco; OSU, The Ohio State University; Tokyo, University of Tokyo; UI, University of Illinois at Urbana-Champaign; Upenn, University of Pennsylvania; UWM, University of Wisconsin-Madison; WSU, Washington State University.
ND, not determined.
Cell line validation
Genomic DNA was isolated using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA, USA) from 1 to 5 –106 cells. Multiplex polymerase chain reaction (PCR) was conducted on 50–400 ng of isolated genomic DNA to confirm the species of origin of each cell line as previously described.14 Upon confirmation as a canine cell line, each line was subsequently analysed by short tandem repeat (STR) profiling using the Canine Stockmarks Genotyping Kit (Life Technologies, Carlsbad, CA USA) per the manufacturer's protocol and as previously described.15 The PCR products were analysed via capillary electrophoresis as follows: 1.5 μL of diluted PCR product (1:5 to 1:10 dilution) was mixed with 0.5 μL size standard and 10 μL of highly deionized formamide. Samples were run using POP7 polymer and the array length was 50 cm. Run conditions were identical to the default run module except injection time was increased from 15 to 24 s, and scan number was shortened from 1800 scans to 1750. Fragment lengths for each locus were recorded and for ease of analysis rounded to the nearest common allelic size whole number. This information is maintained in an Excel datasheet that can be sorted by allelic size to ease comparisons between cell lines. Where possible cell line duplicates from multiple laboratories were analysed to confirm identity.
Microarray gene expression analysis
RNA was extracted from cell lines using the RNeasy kit (Qiagen) according to manufacturer's protocol. A DNA digestion step was included using the RNase-Free DNAse Set to ensure RNA purity (Qiagen). Yield and integrity of RNA was examined via a NanoDrop 1000 spectrophotometer (Thermo Scientific, Asheville, NC, USA) and a Bioanalyzer (Agilent, Santa Clara, CA, USA). Samples were hybridized onto Affymetrix GeneChip Canine Genome 2.0 arrays, Canine Gene 1.0 ST arrays and GeneChip miRNA 4.0 arrays at the Genomics and Microarray Core at the University of Colorado Denver. Resulting CEL files were then imported into Bioconductor16 and intensity values were preprocessed with the Robust Multi-Array Average (RMA) algorithm.17
Unsupervised clustering and principal component analysis
Affymetrix Canine 2.0 Microarray Gene Expression data was processed using RMA and the log 2 transformed gene expression data was ranked based on the standard deviation of each gene across all sample sets. The top 100 most variant genes were selected for unsupervised clustering using the CIMminer website (http://discover.nci.nih.gov/cimminer/). Gene expression data were also sorted for the 522 cancer genes currently annotated in the Cosmic Cancer Gene Database (http://cancer.sanger.ac.uk/cosmic). These cancer genes were then ranked based on the standard deviation of each cancer gene across all samples to identify the most variant genes. The top 100 most variant cancer genes were used in unsupervised clustering to generate heat maps using CIMminer. Euclidian distancing and average linkage were the parameters chosen for the analysis. Principal components analysis was conducted on the whole gene expression data set and a graph of the first two components was generated using Bioconductor.
Cell viability assays
Drug sensitivity data were generated via a resazurin-based bioreductive fluorometric assay (resazurin, Sigma, St. Louis, MO) for cisplatin (CIS), carboplatin (CARBO), doxorubicin (DOX), lomustine (CCNU), paclitaxel (PTX) and vinblastine (VBL) in the FACC panel as follows: cells were plated in 96 well plates at a density of 1500–5000 cells in 100 μL per well, depending on growth rate; 24 h afer initial plating, serial doses of the drugs in 100 μL of media were added to the plates, including vehicle control wells and media-only blank wells, followed by 48 h incubation. For adherent cell lines, drug-containing media was then replaced with 200 μL fresh media, and 20 μL of resazurin solution (200 μg resazurin salt per mL in phosphate-buffered saline) was added to each well. For non-adherent cell lines, resazurin solution was added directly to the drug-containing media. Following 2–4 h of incubation with resazurin fluorescence was measured on a 96 well plate reader with emission wavelength parameters of 530 and excitation of 590. Experiments were performed at least in triplicate, and medial dose (Dm) values were calculated.
Differential expression analysis of miRNA microarray data
For comparison of resistant cells with sensitive cells for DOX, differential expression (DE) analysis was performed using the limma package in Bioconductor.18 The cut-off of significance was based on un-adjusted P-value 0.05. CIMminer was used to generate clustered images of the data from the top 100 variably expressed microRNAs (miRNAs) across all samples based on standard deviation. Unsupervised clustering for both axes was performed with the following parameters: average linkage, Euclidean distance and quantile binning with median centering of the data.
Results
Cell line validation
Tumour-derived canine cancer cell lines have been established or acquired from multiple sources over the years at the FACC at Colorado State University. Currently, our panel consists of 28 cell lines representing 11 different tumour types: 10 osteosarcomas, 5 melanomas, 2 mammary carcinomas, 1 hemangiosarcoma, 1 bladder carcinoma, 3 lymphomas, 1 leukaemia, 1 mast cell tumour, 2 histiocytic sarcomas, 1 thyroid carcinoma and 1 soft tissue sarcoma. Cell line names, origins, known xenograft potential, known markers and mutation status of the ‘FACC panel’ are described in Table 1. All of the cell lines were validated previously as being canine in origin.15 To address the possibility of cross-contamination and uniqueness of the individual lines in the FACC panel, STR analysis was used for further validation. The fragment size rounded to the nearest common whole number at each allele from 10 loci is listed in Table S1, Supporting information. When possible, cell lines obtained from multiple laboratories were assessed to confirm the identity of a given cell type; however, source genomic material for these lines was not available. Based on this analysis, the genetic identity of 28 cell lines was confirmed. The Fitz and DEN-HSA hemangiosarcoma cell lines were found to be genetically identical,15 as were the MH and Nike histiocytic sarcoma cell lines. In addition, the CMT12 and CMT27 canine mammary cell lines exhibited conserved profiles at 8 of the 10 loci and 90% homology overall suggesting the potential for a common source for these cell lines.15
Molecular characterization of cell lines
In order to characterize the FACC panel on a molecular level and to facilitate future genomic studies, all 28 cell lines have undergone molecular profiling on the mRNA and miRNA expression level. The Affymetrix GeneChip Canine Genome 2.0 array contains over 43 000 probesets mapping to over 20 000 genes. In contrast to the 3′-biased probesets of the 2.0 array, the FACC panel has also recently been profiled with the Canine Gene 1.0 ST arrays, which performs a whole-transcriptome analysis with over 195 000 probesets spread out across each exon of the genes. Additionally, miRNA expression has been profiled in the panel using Affymetrix GeneChip miRNA 4.0 arrays which contain all miRNA in the miRBase Release 20 (www.affymetrix.com). To show that the two types of mRNA gene expression arrays were complementary with each other in the FACC panel, correlations were performed between both the Canine 2.0 and 1.0 ST arrays for a selection of known cancer genes in each cell line. We observed an average correlation coefficient of 0.6905 for all 28 cell lines, which was highly significant (P 1.0E-7) (Fig. S1). It is important to note that the RNA used for microarray analysis on both platforms was extracted in different labs in the FACC at different times, suggestive of strong conservation of genotypic features in these cell lines.
Histological characteristics are routinely used by pathologists for distinguishing different tumour types from each other, and gene expression patterns can be utilized in similar ways via unsupervised hierarchal cluster analysis. Using the 2.0 expression data, the first two principal components from the principal components analysis (PCA) of the gene expression data were plotted with PC1 on the x-axis and PC2 on the y-axis (Fig. 1). The distribution of the samples in this graph is in roughly three groups. Five of seven haematopoietic cell lines, including the CLBL1, CLL1390, 1771, OSW and Nike cell lines, are distributed across the bottom of the graph. A group in the middle of the top of the graph contains 8 of 10 osteosarcoma cell lines and the Bliley transitional cell carcinoma line. To the left is a group containing the other two osteosarcoma cell lines that were derived from metastatic tumours, Abrams and D17; 3 of the melanoma cell lines, the mammary carcinoma cell lines, one histiocytic sarcoma cell line DH82, the C2 mast cell line, and three soft tissue sarcomas including the two hemangiosarcoma cell lines that were genetically identical based on STR analysis.
Figure 1.
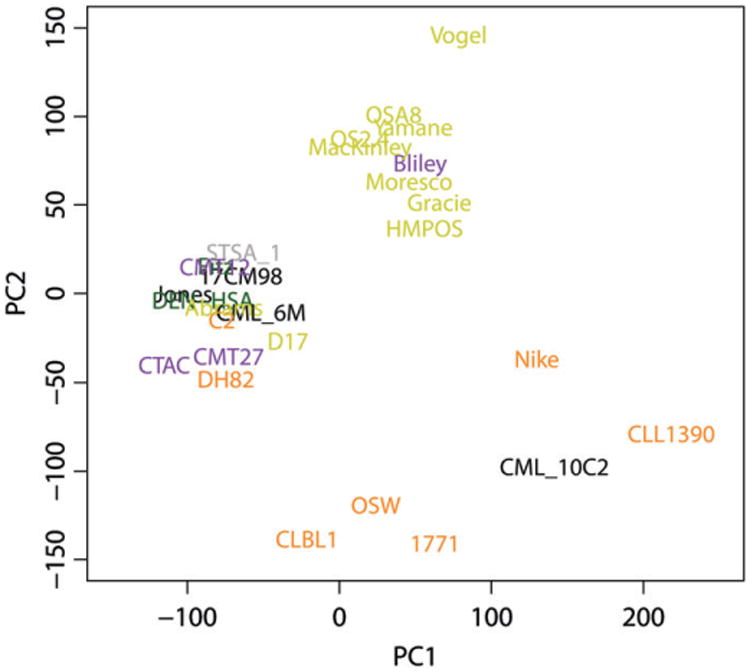
Principal component analysis of the FACC panel. RMA processed Canine 2.0 gene expression data from the FACC panel was used for principal component analysis. Tumour types are colour coded as follows: haematopoietic cancers (orange), osteosarcomas (yellow), carcinomas (purple), melanomas (black), hemangiosarcomas (green), and soft tissue sarcoma (grey).
To further assess the defining gene expression characteristics of each of these cell lines, a cluster analysis heat map was generated using the top 100 most variant genes (Fig. 2A). This unbiased cluster analysis separated the cell lines into two primary groups: the first group located at the top of the heat map contains six haematopoietic cell lines: OSW, Nike, C2, CLL1390, 1771 and CLBL1. Interestingly, the DH82 histiocytic cell line was placed in the other large group, but separated out with the Gracie osteosarcoma cell line and the CML10C2 melanoma cell line. The remainder of the cell lines were clustered into two secondary groups. The first group of 13 cell lines is dominated by 9 osteosarcoma cell lines, the STSA-1 soft tissue sarcoma cell line, and 3 melanoma cell lines. The second group, comprised of the next six cell lines as listed on the left axis of the figure, contains primarily carcinoma cell lines: CMT12, adjacent to CMT27; Bliley, and CTAC (thyroid carcinoma). In addition, the juxtaposed DEN-HSA and Fitz hemangiosarcoma cell lines are a subgroup within this branch. The genes dictating these groups are shown along the bottom of the figure. Elevated expression of cell-type specific markers such as lymphocyte cytosolic protein 1 (LCP1), cytokeratins (KRT8 and KRT18), epithelial cell adhesion molecule (EPCAM), and collagen, type I, alpha 1 (COL1A1) contribute primarily to the separation of the various cancer cell histotypes.
Figure 2.
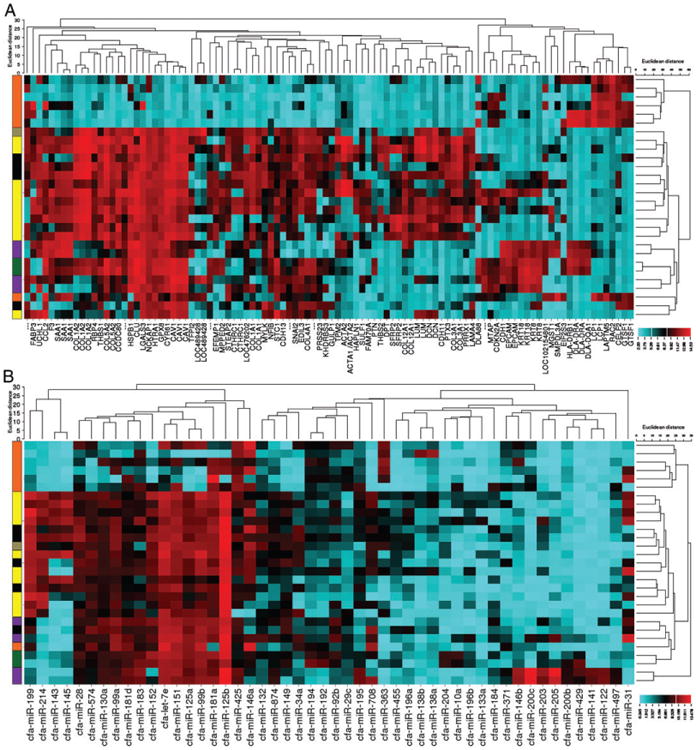
Cluster analysis using the top 100 most variant genes and microRNAs separates the FACC panel into groups with similar histotypes. A) Affymetrix Canine 2.0 gene array data and B) GeneChip miRNA 4.0 array data was ranked based on the standard deviation of each probeset across all samples and unbiased cluster analysis of the 100 most variant probesets was performed using the web-based tool CIMminer (http://discover.nci.nih.gov/cimminer/). Cells of similar developmental lineage group together: haematopoietic cancers (orange), osteosarcomas (yellow), carcinomas (purple), melanomas (black), hemangiosarcoma (green) and soft tissue sarcoma (grey).}?>
A similar unsupervised cluster analysis using expression data from the top 50 most variant canine miRNAs also resulted in cell lines clustering together based on developmental lineage (Fig. 2B). This is consistent with findings of Lu et al., who reported one of the earliest high throughput miRNA expression analysis studies using a variety of human tumour and cancer cell lines.19
In order to explore the alterations in gene expression that contribute to the unrestrained growth of these cell lines, the gene expression data were sorted for the 522 genes identified as contributing to the development and progression of various cancers in the COSMIC Cancer Gene Census. The genes were once again ranked according the standard deviation of each cancer gene across all samples to identify the most variant genes and cluster analysis of the 100 most variant cancer genes was generated (Fig. 3). As previously, the unbiased cluster analysis separated the cell lines into two primary groups: the haematopoietic cell lines at the bottom of the heat map and a large grouping containing a combination of the carcinomas and sarcomas at the top. As before, these cell lines are separated into three subgroups; the first containing a combination of the sarcoma and melanoma lines, as well as the thyroid carcinoma cell line. The next group is the mammary and transitional cell carcinomas, and the two hemangiosarcoma lines. One notable exception is the Gracie osteosarcoma cell line, which was separated from all the other sarcoma lines and grouped with the carcinoma cell lines. These separations are again largely due to the overexpression of cell-specific markers that have been shown to contribute to cancer development including COL1A1 and LCP1.
Figure 3.
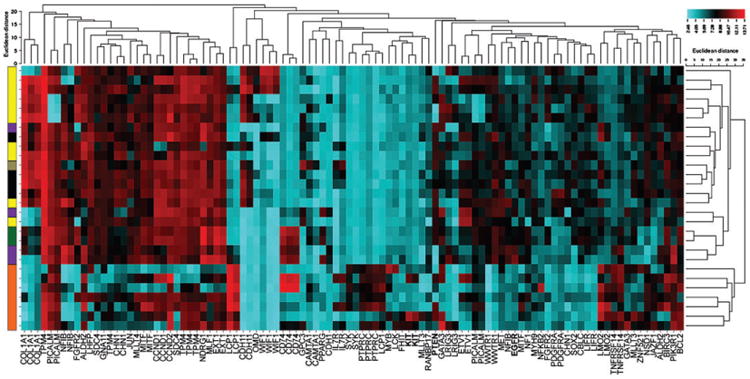
Cluster analysis using the top 100 most variant cancer genes separates the FACC panel into groups with similar histotypes and may identify critical genetic drivers. Affymetrix Canine 2.0 gene array data was sorted for the 522 cancer genes currently annotated in the Cosmic Cancer Gene Database. These cancer genes were then ranked based on the standard deviation across all samples and unbiased cluster analysis was performed using CIMminer. Tumour types are colour coded as follows: haematopoietic cancers (orange), osteosarcomas (yellow), carcinomas (purple), melanomas (black), hemangiosarcomas (green) and soft tissue sarcoma (grey).
In addition, examination of the generated heat map does provide potential evidence of genetic drivers of cancer development. For example, elevated expression of KIT is observed in the C2 and 1771 cell lines. Previous studies from our lab and others have shown that the C2 cell line carries an activating mutation in the KIT gene.20 Decreased expression of PTEN is observed in the CMT12 and CMT27 mammary cell lines, the HMPOS, Abrams, and OSA8 osteosarcoma cell lines, and the CLL1390 and DH82 cell lines. A smaller decrease in expression is also observed in the OSW cell line. Previous studies using fluorescence in situ hybridization analysis of the CLL1390 cell line indicated complete loss of the PTEN gene.21 Similarly, deletions of the region in chromosome 26 containing the PTEN gene have been observed in 40.7% of canine histiocytic sarcomas and 30% of canine osteosarcomas.22,23 Interestingly, the canine mammary carcinomas both appear to over-express epidermal growth factor receptor (EGFR). This finding supports a study which reported the malignant phenotype of CMT12 and CMT27 cells to be stimulated by EGF, making it a potential model for studying anti-EGFR therapies.24 The Gracie osteosarcoma cell line is clustered separately from the other osteosarcoma cell lines. Examination of the Gracie cell line gene expression profile reveals the elevated expression of ETV1, notable for its fusion to the EWS gene in human Ewing's sarcoma and TMPRSS2 in prostate cancers.25 This cell line also exhibits reduced expression of several markers elevated in the majority of the osteosarcoma cell lines, including COL1A1, FGFR2, and CDH11.
Pharmacologic screening
Currently, there are three cancer drugs approved by the US Food and Drug Administration in canine oncology. Toceranib phosphate and the conditionally approved masitinib are indicated for the treatment of canine mast cell tumours.26,27 Paccal Vet-CA1 is conditionally approved to treat squamous cell carcinoma and mammary carcinoma.28 Many drugs commonly used to treat dogs with cancer are considered ‘off-label’. One of the purposes of this panel is for drug screening in order to identify beneficial pairings of human-approved or novel therapeutics with a given tumour type in canine cancer. Cytotoxic chemotherapy is commonly used in the treatment of canine cancers, thus we have begun to screen the FACC panel with these drugs. In Fig. 4, the drug sensitivity data in the FACC panel are compared with the human NCI60 cancer panel for six antineoplastic drugs: CIS, PTX, CCNU, VBL, CARBO and DOX. Statistical testing revealed that with the exception of CIS, the means of the drug sensitivity ranges between human and canine panels were significantly different from each other. Additionally, the variances in the data were also significantly different for CARBO and PTX. Overall, however, the patterns of drug sensitivity and variances observed for each agent in both human and canine panels shared general trends, and the mean Log GI50 values for each agent showed a significant cross-species correlation (r = 0.88, P = 0.0194, Pearson). These data suggest that human and canine cancer cell lines respond in similar ranges to cytotoxic agents.
Figure 4.
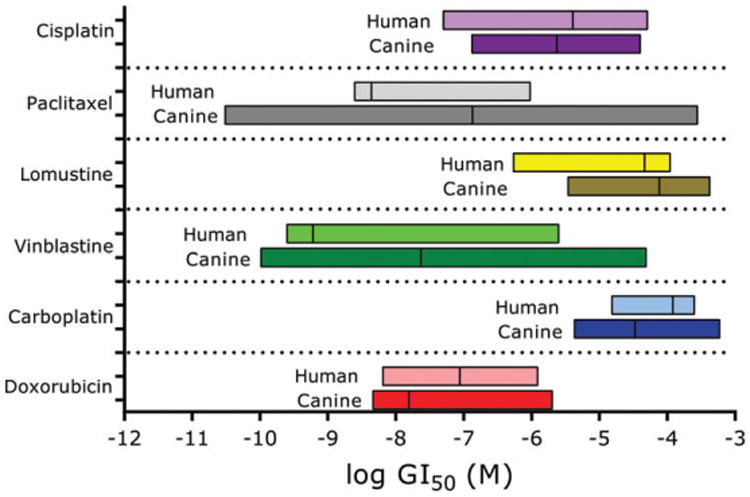
Human and canine cancer cells are similarly sensitive to chemotherapy. GI50 ranges of the human NCI60 panel to six chemotherapeutics were compared with the ranges generated in the canine FACC panel via resazurin assays.
miRNA signature for DOX sensitivity is highly conserved between human and dogs
It is well known that there is high conservation of miRNAs in mammals. To demonstrate the similarity between miRNA expression data generated for human and canine miRNAs in canine OS cells we performed DE analyses for DOX resistant cells compared with sensitive cells; 25 of 30 (83.3%) differentially expressed canine miRNAs were identical to human miRNAs identified on the same miRNA microarray chip with similar fold changes, all in the same direction (Table 2). Given the high conservation seen in this study, the larger set of human miRNAs identified in the analysis compared with the canine set may lead to an expansion of miRNA knowledge in canine cancer, where annotation is not as advanced as is seen in human research.
Table 2. Differentially expressed canine and human miRNAs between doxorubicin sensitive and resistant canine cancer cell lines.
| miR name | logFC | P value | miR name | logFC | P value |
|---|---|---|---|---|---|
| Canine miRNAs | |||||
| cfa-let-7f | 1.24 | 0.0025 | cfa-miR-19a | 3.55 | 0.0179 |
| cfa-let-7g | 1.24 | 0.0015 | cfa-miR-19b | 2 | 0.0244 |
| cfa-miR-103 | 0.81 | 0.0436 | cfa-miR-20a | 1.54 | 0.0182 |
| cfa-miR-106a | 1.49 | 0.0181 | cfa-miR-25 | 1.16 | 0.033 |
| cfa-miR-106b | 1.41 | 0.022 | cfa-miR-26b | 2.83 | 0.0022 |
| cfa-miR-10a | 4.05 | 0.0021 | cfa-miR-30b | 1.5 | 0.0028 |
| cfa-miR-128 | 2.03 | 0.0227 | cfa-miR-30c | 1.37 | 0.0019 |
| cfa-miR-148b | 2.3 | 0.0281 | cfa-miR-30e | 2.13 | 0.0094 |
| cfa-miR-15b | 1.19 | 0.0463 | cfa-miR-342 | 1.93 | 0.0422 |
| cfa-miR-17 | 2.9 | 0.0081 | cfa-miR-421 | 2.48 | 0.0363 |
| cfa-miR-1841 | 0.97 | 0.05 | cfa-miR-450b | 0.9 | 0.0273 |
| cfa-miR-1842 | 2.82 | 0.0154 | cfa-miR-503 | 1.37 | 0.0383 |
| cfa-miR-186 | 2.11 | 0.0036 | cfa-miR-92b | 3.14 | 0.0152 |
| cfa-miR-192 | 3.92 | 0.0017 | cfa-miR-93 | 0.73 | 0.034 |
| cfa-miR-194 | 3.96 | 0.0015 | cfa-miR-98 | 2.26 | 0.0013 |
| Human miRNAs | |||||
| hsa-let-7f-5p | 1.24 | 0.0012 | hsa-miR-1303 | −0.46 | 0.0111 |
| hsa-let-7g-3p | 0.27 | 0.0437 | hsa-miR-1306-5p | 1.6 | 0.0103 |
| hsa-let-7g-5p | 1.24 | 0.0006 | hsa-miR-1307-5p | 1.06 | 0.0086 |
| hsa-let-7i-3p | 0.75 | 0.0323 | hsa-miR-136-3p | −0.3 | 0.0274 |
| hsa-let-7i-5p | 1.55 | 0.0211 | hsa-miR-147a | 0.35 | 0.0496 |
| hsa-miR-101-3p | 1.06 | 0.0436 | hsa-miR-148b-3p | 2.3 | 0.0273 |
| hsa-miR-101-5p | 0.57 | 0.0338 | hsa-miR-152-5p | 0.37 | 0.0282 |
| hsa-miR-103a-2-5p | 1.8 | 0.0225 | hsa-miR-106a-5p | 1.47 | 0.013 |
| hsa-miR-103a-3p | 0.81 | 0.0313 | hsa-miR-15a-3p | 0.79 | 0.0354 |
| hsa-miR-211-5p | −0.3 | 0.0419 | hsa-miR-16-2-3p | 1.08 | 0.0253 |
| hsa-miR-106b-3p | 0.78 | 0.0179 | hsa-miR-17-3p | 2.9 | 0.0078 |
| hsa-miR-29b-2-5p | 2.32 | 0.0288 | hsa-miR-17-5p | 1.48 | 0.0122 |
| hsa-miR-301a-3p | 2.82 | 0.0231 | hsa-miR-186-5p | 2.11 | 0.0031 |
| hsa-miR-30b-5p | 1.5 | 0.0019 | hsa-miR-18a-3p | 2.66 | 0.0217 |
| hsa-miR-30c-5p | 1.47 | 0.0014 | hsa-miR-18a-5p | 2.32 | 0.0117 |
| hsa-miR-30d-5p | 1.07 | 0.0034 | hsa-miR-18b-5p | 2.9 | 0.0215 |
| hsa-miR-30e-3p | 2.13 | 0.0085 | hsa-miR-1908-5p | −0.53 | 0.0353 |
| hsa-miR-30e-5p | 2.86 | 0.0076 | hsa-miR-1915-3p | −0.66 | 0.0479 |
| hsa-miR-328-5p | −0.57 | 0.009 | hsa-miR-192-5p | 3.92 | 0.0017 |
| hsa-miR-339-5p | 0.86 | 0.0414 | hsa-miR-194-5p | 3.96 | 0.0014 |
| hsa-miR-33a-3p | 0.38 | 0.0439 | hsa-miR-19a-3p | 3.55 | 0.0179 |
| hsa-miR-342-3p | 1.93 | 0.0408 | hsa-miR-19b-3p | 2.04 | 0.0199 |
| hsa-miR-342-5p | 1.27 | 0.029 | hsa-miR-202-5p | 0.79 | 0.0361 |
| hsa-miR-378d | 1.53 | 0.0498 | hsa-miR-20a-5p | 1.54 | 0.0158 |
| hsa-miR-422a | 1.44 | 0.0498 | hsa-miR-20b-5p | 2 | 0.0323 |
| hsa-miR-494-3p | 2.27 | 0.0176 | hsa-miR-503-5p | 1.85 | 0.0322 |
| hsa-miR-496 | 0.34 | 0.0123 | hsa-miR-25-3p | 1.16 | 0.0277 |
| hsa-miR-502-5p | 2.04 | 0.0193 | hsa-miR-26b-5p | 2.7 | 0.0022 |
| hsa-miR-505-3p | 2 | 0.0091 | hsa-miR-509-3-5p | 0.36 | 0.0371 |
| hsa-miR-505-5p | 1 | 0.0348 | hsa-miR-509-3p | −0.27 | 0.0364 |
| hsa-miR-106b-5p | 1.41 | 0.0189 | hsa-miR-512-3p | 0.8 | 0.0481 |
| hsa-miR-107 | 0.8 | 0.033 | hsa-miR-523-3p | −0.26 | 0.0254 |
| hsa-miR-10a-5p | 4.88 | 0.0018 | hsa-miR-551b-3p | −0.29 | 0.0441 |
| hsa-miR-10b-3p | 2.18 | 0.0398 | hsa-miR-619-5p | 0.98 | 0.0287 |
| hsa-miR-1180-3p | −0.64 | 0.0376 | hsa-miR-638 | −0.68 | 0.0248 |
| hsa-miR-1207-5p | −0.62 | 0.0085 | hsa-miR-758-5p | −0.41 | 0.0305 |
| hsa-miR-1225-3p | −0.42 | 0.0491 | hsa-miR-769-5p | 1.43 | 0.0365 |
| hsa-miR-1227-5p | −0.69 | 0.0324 | hsa-miR-888-5p | -0.24 | 0.0362 |
| hsa-miR-1228-3p | 0.9 | 0.0073 | hsa-miR-891a-3p | 0.42 | 0.0164 |
| hsa-miR-1228-5p | −0.86 | 0.0282 | hsa-miR-891b | 0.33 | 0.045 |
| hsa-miR-1233-5p | −0.44 | 0.032 | hsa-miR-92b-3p | 3.14 | 0.015 |
| hsa-miR-1246 | 2.55 | 0.0476 | hsa-miR-93-3p | 1.26 | 0.0269 |
| hsa-miR-1272 | 1 | 0.0065 | hsa-miR-93-5p | 0.73 | 0.0199 |
| hsa-miR-128-3p | 2.03 | 0.0215 | hsa-miR-940 | 0.92 | 0.0472 |
| hsa-miR-1285-5p | −0.23 | 0.0477 | hsa-miR-98-5p | 2.26 | 0.0011 |
| hsa-miR-1287-3p | 0.49 | 0.0127 | hsa-miR-99b-3p | 0.44 | 0.0315 |
logFC, log fold change.
Bold names are common microRNAs found differentially expressed for both canine and human.
Discussion
Since the early 1900s, tissue culture cell lines from canine tumours have been established. A recent Pub Med search using the terms ‘canine cancer cell line’ retrieved almost 1200 articles. Despite the long history and the substantial quantity of research being performed with canine cancer cells, in vitro panels have not yet been developed to the scale that has occurred in human cancer. As a step toward that goal, we have introduced and described a validated panel of 28 canine cancer cell lines collected from multiple sources or established at the FACC at Colorado State University. Individual cell lines in the panel have already been used in several studies. A Pub Med search using the name of each cell line combined with the canine tumour type as keywords resulted in 92 articles involving one or more of 20 of 28 cell lines in the FACC panel. In these studies, the canine cancer cell lines were used to investigate the effects of genes or drugs or both on different cancer processes.
In this new genomics era for cancer research, combining genotypic, phenotypic and pharmacologic data to reveal novel relationships has been essential for the many recent discoveries that have culminated in improved clinical outcomes. Our canine panel has undergone both mRNA and miRNA expression profiling, and has been used for screening of several established and novel anti-cancer agents. With these new tools, the possibilities for comparative and translational applications with human cancer research are becoming readily apparent. Dogs with cancer can potentially benefit from new discoveries made in human oncology, and conversely, human research can benefit through the integration of canine cancer models for pre-clinical validation studies.
Currently, the size of the FACC panel is relatively small when compared with similar human panels such as the NCI60, the GDSC or the CCLE. There are challenges inherent to that fact. Although there are 11 different tumour types within the panel, 6 of these types are represented by a single cell line, making it extremely difficult to form experimental conclusions with a large degree of confidence. Indeed, the panel should be considered a ‘jumping of’ point toward further advanced experimentation in a similar way as initial microarray analyses are typically viewed as ‘hypothesis-generating’ experiments. It would be advantageous to increase the size of the panel, although establishing new cell lines from tumour samples is difficult regardless of species because of low take rate, the danger of contamination and outgrowth of competing fibroblasts, as well as the obstacle of overcoming anoikis-related cell death after loss of contact with their extracellular matrix.29 A possible solution to this problem for the future would be for a call for a greater collaborative effort across several institutions. The sharing of canine cell line resources has helped the FACC panel grow to what it is today, and the fastest way for future growth would undoubtedly be from an increase of collaborations with fellow researchers that have additional established cell lines to contribute. The formation of the FACC panel is a useful step in the right direction towards improving informatics approaches to canine cancer, but it is only one step of many.
Another future direction is to expand upon available gene expression profiling to include next generation genomic data such as whole exome and genome sequencing, and array comparative genome hybridization (array-CGH) data. Sequencing would be an invaluable tool in identifying potential mutations and/or deletions in oncogenes and tumour suppressors across the entire genome. Array-CGH would allow investigation of alterations in copy number of genes and their role in canine cancer progression. The integration of multiple types of genomic data will facilitate identification of the most significant drivers in canine cancer.
In conclusion, we have introduced a new valuable resource for canine cancer studies in the FACC panel. With its potential for testing various cancer processes and pharmacological screening connected with genomic data, we hope it will serve to facilitate studies that can further shorten the gap between human and canine oncology, leading to novel discoveries and better designed treatments for dogs with cancer.
Supplementary Material
Acknowledgments
The study was supported by Morris Animal Foundation D13CA-058, CSU Cancer Supercluster, and Colorado State University College of Veterinary Medicine and Biomedical Sciences Foundation Funds.
Footnotes
Conflict of interest: None of the authors have any conflicts of interest to disclose that pertain to studies performed for this manuscript.
Supporting Information: Additional Supporting Information may be found in the online version of this article:
References
- 1.Harrison RG. The outgrowth of the nerve fiber as a mode of protoplasmic movement. The Journal of Experimental Zoology. 1910;9:787–846. doi: 10.1002/jez.1401420103. [DOI] [PubMed] [Google Scholar]
- 2.Carrel A, Burrows MT. Cultivation in vitro of malignant tumors. The Journal of Experimental Medicine. 1911;13:571–575. doi: 10.1084/jem.13.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scherer WF, Syverton JT, Gey GO. Studies on the propagation in vitro of poliomyelitis viruses. IV Viral multiplication in a stable strain of human malignant epithelial cells (strain HeLa) derived from an epidermoid carcinoma of the cervix. The Journal of Experimental Medicine. 1953;97:695–710. doi: 10.1084/jem.97.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma SV, Haber DA, Settleman J. Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nature Reviews Cancer. 2010;10:241–253. doi: 10.1038/nrc2820. [DOI] [PubMed] [Google Scholar]
- 5.Weinstein JN. Drug discovery: cell lines battle cancer. Nature. 2012;483:544–545. doi: 10.1038/483544a. [DOI] [PubMed] [Google Scholar]
- 6.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin WM, Baker AC, Beroukhim R, Winckler W, Feng W, Marmion JM, et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Research. 2008;68:664–673. doi: 10.1158/0008-5472.CAN-07-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sos ML, Michel K, Zander T, Weiss J, Frommolt P, Peifer M, et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. The Journal of Clinical Investigation. 2009;119:1727–1740. doi: 10.1172/JCI37127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nature Reviews Cancer. 2006;6:813–823. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 10.Weinstein JN, Myers TG, O'Connor PM, Friend SH, Fornace AJ, Jr, Kohn KW, et al. An information-intensive approach to the molecular pharmacology of cancer. Science. 1997;275:343–349. doi: 10.1126/science.275.5298.343. [DOI] [PubMed] [Google Scholar]
- 11.Yang W, Soares J, Greninger P, Edelman EJ, Lightfoot H, Forbes S, et al. Genomics of Drug Sensitivity in Cancer (GDSC): a resource for therapeutic biomarker discovery in cancer cells. Nucleic Acids Research. 2013;41:D955–D961. doi: 10.1093/nar/gks1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper JK, Sykes G, King S, Cottrill K, Ivanova NV, Hanner R, et al. Species identification in cell culture: a two-pronged molecular approach. In Vitro Cellular & Developmental Biology Animal. 2007;43:344–351. doi: 10.1007/s11626-007-9060-2. [DOI] [PubMed] [Google Scholar]
- 15.O'Donoghue LE, Rivest JP, Duval DL. Polymerase chain reaction-based species verification and microsatellite analysis for canine cell line validation. Journal of Veterinary Diagnostic Investigation. 2011;23:780–785. doi: 10.1177/1040638711408064. [DOI] [PubMed] [Google Scholar]
- 16.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, et al. Bioconductor: open software development for computational biology and bioinformatics. Genome Biology. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 18.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 20.Halsey CH, Gustafson DL, Rose BJ, Wolf-Ringwall A, Burnett RC, Duval DL, et al. Development of an in vitro model of acquired resistance to toceranib phosphate (Palladia(R)) in canine mast cell tumor. BMC Veterinary Research. 2014;10:105. doi: 10.1186/1746-6148-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seiser EL, Thomas R, Richards KL, Kelley MK, Moore P, Suter SE, et al. Reading between the lines: molecular characterization of five widely used canine lymphoid tumour cell lines. Veterinary and Comparative Oncology. 2013;11:30–50. doi: 10.1111/j.1476-5829.2011.00299.x. [DOI] [PubMed] [Google Scholar]
- 22.Angstadt AY, Motsinger-Reif A, Thomas R, Kisseberth WC, Couto GC, Duval DL, et al. Characterization of canine osteosarcoma by array comparative genomic hybridization and RT-qPCR: signatures of genomic imbalance in canine osteosarcoma parallel the human counterpart. Genes, Chromosomes & Cancer. 2011;50:859–874. doi: 10.1002/gcc.20908. [DOI] [PubMed] [Google Scholar]
- 23.Hedan B, Thomas R, Motsinger-Reif A, Abadie J, Andre C, Cullen J, et al. Molecular cytogenetic characterization of canine histiocytic sarcoma: a spontaneous model for human histiocytic cancer identifies deletion of tumor suppressor genes and highlights influence of genetic background on tumor behavior. BMC Cancer. 2011;11:201. doi: 10.1186/1471-2407-11-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy KC, Qurollo BA, Rose BJ, Thamm DH. Epidermal growth factor enhances the malignant phenotype in canine mammary carcinoma cell lines. Veterinary and Comparative Oncology. 2011;9:196–206. doi: 10.1111/j.1476-5829.2010.00248.x. [DOI] [PubMed] [Google Scholar]
- 25.Oh S, Shin S, Janknecht R. ETV1, 4 and 5: an oncogenic subfamily of ETS transcription factors. Biochimica et Biophysica Acta. 1826;2012:1–12. doi: 10.1016/j.bbcan.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn KA, Ogilvie G, Rusk T, Devauchelle P, Leblanc A, Legendre A, et al. Masitinib is safe and effective for the treatment of canine mast cell tumors. Journal of Veterinary Internal Medicine. 2008;22:1301–1309. doi: 10.1111/j.1939-1676.2008.0190.x. [DOI] [PubMed] [Google Scholar]
- 27.London CA, Malpas PB, Wood-Follis SL, Boucher JF, Rusk AW, Rosenberg MP, et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clinical Cancer Research. 2009;15:3856–3865. doi: 10.1158/1078-0432.CCR-08-1860. [DOI] [PubMed] [Google Scholar]
- 28.Food US, Administration Drug. FDA Provides Important Information for Veterinarians about PACCAL VAT-CA1 for Treating Cancer in Dogs. http://www.fda.gov/AnimalVeterinary/ResourcesforYou/ucm402476.htm. Last updated: 01/13/2015.
- 29.Cheung PF, Yip CW, Ng LW, Lo KW, Wong N, Choy KW, et al. Establishment and characterization of a novel primary hepatocellular carcinoma cell line with metastatic ability in vivo. Cancer Cell International. 2014;14:103. doi: 10.1186/s12935-014-0103-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


