Abstract
The impact of baseline exercise capacity on clinical outcomes in patients with stable ischemic heart disease randomized to an initial strategy of optimal medical therapy (OMT) with or without percutaneous coronary intervention (PCI) in the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial has not been studied. A post hoc analysis was performed in 1,052 patients of COURAGE (PCI + OMT: n = 527, OMT: n = 525) who underwent exercise treadmill testing at baseline. Patients were categorized into 2 exercise capacity groups based on metabolic equivalents (METs) achieved during baseline exercise treadmill testing (<7 METs: n = 464, ≥7 METs: n = 588) and were followed for a median of 4.6 years. The primary composite end point of death or myocardial infarction was similar in the PCI + OMT group and the OMT group for patients with exercise capacity <7 METs (19.1% vs 16.1%, p = 0.31) and ≥7 METs (13.3% vs 10.3%, p = 0.27). After adjusting for baseline covariates, the hazard ratio (99% confidence interval) for the primary end point for the PCI + OMT group versus the OMT group was 1.42 (0.90 to 2.23, p = 0.05) and for the exercise capacity subgroups of ≥7 METs and <7 METs was 0.75 (0.46 to 1.22, p = 0.13). There was no statistically significant interaction between the original treatment arm allocation (PCI + OMT vs OMT) and baseline exercise capacity. In conclusion, there was no difference in the long-term clinical outcomes in patients with exercise capacity <7 METs compared with ≥7 METs, irrespective of whether they were assigned to initial PCI. Patients with exercise capacity <7 METs did not derive a proportionately greater clinical benefit from PCI + OMT compared with those patients who received OMT alone. Published by Elsevier Inc. (Am J Cardiol 2015;116:1509–1515)
In patients with coronary artery disease (CAD), exercise capacity, expressed as metabolic equivalents (METs; 1 MET = 3.5 ml/O2/kg/min at rest), is an inverse independent predictor of cardiovascular events, irrespective of age, sex, and race.1–8 In the Coronary Artery Surgery Study registry, in patients with 3-vessel CAD, only those who could not achieve >7 METs on a Bruce protocol exhibited better survival with coronary artery bypass surgery than with medical therapy alone, suggesting that exercise testing identified a subgroup of high-risk patients for whom surgery was the superior option.9 In the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial, there was no difference in the composite end point of death or myocardial infarction in patients with stable ischemic heart disease randomized to optimal medical therapy (OMT) alone or percutaneous coronary intervention (PCI) + OMT.10 The COURAGE trial provides an opportunity to explore the relation between baseline exercise capacity and long-term clinical outcomes. We therefore evaluated the effect of baseline exercise capacity achieved during exercise treadmill testing in patients of COURAGE trial randomized to PCI + OMT or OMT alone. We hypothesized that, compared to patients with exercise capacity ≥7 METs, those with exercise capacity <7 METs would have a higher rate of cardiac events, and hence, will derive greater benefit from an initial strategy of PCI + OMT than from OMT alone.
Methods
The rationale, design, methods, baseline characteristics, and results of the COURAGE trial (Clinical Trials no. NCT00007657) have been described previously.10–12 Enrollment required a stenosis of ≥70% in at least 1 major epicardial coronary artery on coronary angiography with objective evidence at baseline of myocardial ischemia or at least 1 coronary stenosis of ≥80% and classic angina in the absence of objective evidence of myocardial ischemia. Patients were excluded if they developed significant ST-segment depression (≥1.5 mm) or hypotension during stage 1 of a Bruce protocol, findings of which would be consistent with a “markedly positive” test for which angiography and revascularization were likely. Because this post hoc analysis sought to assess the relation between exercise capacity and outcomes, patients who were unable to exercise and who underwent pharmacologic stress myocardial perfusion imaging or dobutamine stress echocardiography or qualified for the trial with baseline ischemic ST-segment deviation on the resting electrocardiogram were excluded.
Of the 2,287 trial patients, 1,052 (46%) underwent baseline exercise treadmill testing before randomization, of whom 527 subsequently received PCI + OMT and 525 received OMT alone. Of this subgroup, 452 (43%) underwent standard exercise treadmill test with 12-lead electrocardiogram, 542 (51.5%) underwent exercise myocardial perfusion imaging, and 58 (5.5%) underwent exercise echocardiography. Patients were further categorized into 2 exercise capacity subgroups on the basis of the METs achieved during the exercise treadmill test (either <7 METs or ≥7 METs). The derivation of the study cohort is shown in Figure 1. The primary outcome measure was a composite of all-cause death or nonfatal myocardial infarction (the COURAGE trial primary endpoint) during a median follow-up of 4.6 years(range 2.5to 7 years) after randomization.
Figure 1.
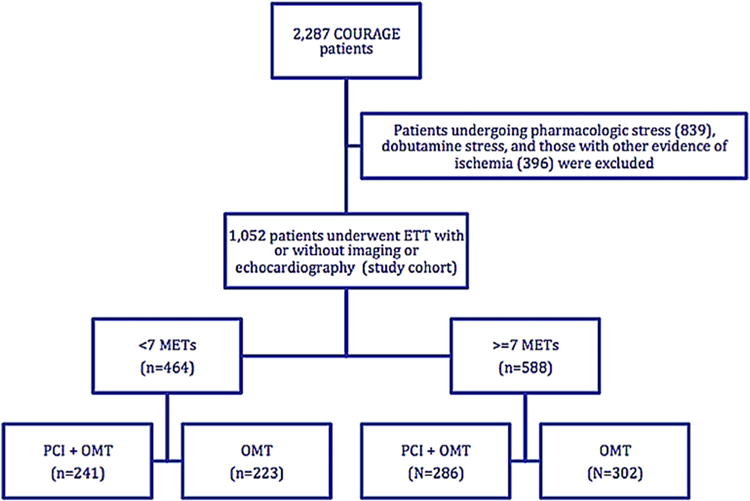
Study cohort derivation chart. ETT = exercise treadmill testing.
Exercise stress testing was performed in conformity with American College of Cardiology and American Heart Association guidelines.13 As per the trial protocol, the decision to perform treadmill exercise stress versus pharmacologic stress testing was at the discretion of the site principal investigators based on the clinical assessment of physical and functional status of patients. The use of nitrates and/or β-adrenergic blocking agents before exercise stress testing was discouraged by trial protocol.
Symptom-limited exercise treadmill testing used the standard Bruce protocol in patients who reported no limitations in their functional capacity at baseline. In patients with limited functional capacity, the modified Bruce, Naughton, or Balke protocols were used. Exercise duration, heart rate, blood pressure, functional capacity, and electrocardiographic response to exercise were recorded. Ischemic ST-segment depression was defined as >1-mm ST-segment deviation from baseline, ≥80 msec after the J-point. Exercise capacity was estimated from exercise workload achieved using a standard formula: ml/kg/min = (mph × 2.68) + (1.8 × 26.82 × mph × grade/100) ÷ 3.5.14 Exercise capacity was expressed as METs, which was calculated by dividing the estimated peak oxygen consumption (ml/kg/min) by 3.5 ml/kg/min (oxygen consumption at rest).
Continuous variables were presented as a mean ± standard deviation and compared by use of the Student t test. Dichotomous variables were presented as percentages and compared by use of the chi-square test or the Wilcoxon rank-sum test. Cumulative event-free survival curves were calculated by the Kaplan–Meier method, and the primary efficacy of PCI + OMT, compared with OMT alone, was assessed by the stratified log-rank statistic. Because of the post hoc nature of this analysis, a more rigorous estimate of the treatment effect was used, with a 99% confidence interval (CI) instead of a 95% CI surrounding the hazard ratio (HR) for the Cox proportional hazards model, with a p value for significance at <0.01. The effects in the Cox regressions were also adjusted for age, sex, body mass index, family history of CAD, angina, systolic blood pressure, and diastolic blood pressure. All statistical analyses were performed using SAS 9.3.
Results
Baseline characteristics according to METs achieved are summarized in Table 1. In comparison to patients with exercise capacity ≥7 METS, those with <7 METs were older, more often women, less likely to have a family history of CAD, and had a significantly greater resting systolic and lower diastolic blood pressure. They were also more likely to have Canadian Cardiovascular Class II or III angina and more likely to have received calcium channel blockers. In patients with either ≥7 METs or <7 METs, there were no other statistically significant differences in the baseline characteristics and angiographic data between the treatment groups (PCI + OMT vs OMT alone; Table 1).
Table 1.
Baseline characteristics and angiographic data categorized based on METs achieved
| Variable | Exercise Work Load (N = 1,052)
|
P value between MET categories | ||||||
|---|---|---|---|---|---|---|---|---|
| <7 METs (N=464)
|
P value | ≥7 METs (N=588)
|
P value | |||||
| PCI+OMT (N=241) |
OMT (N=223) |
PCI+OMT (N=286) |
OMT (N=302) |
|||||
| Age (years) mean ± SD | 64 ± 9 | 65 ± 9 | 0.30 | 59 ± 9 | 58 ± 9 | 0.23 | <0.001* | |
| Men | 187 (78%) | 173 (78%) | 1.00 | 258 (90%) | 281 (93%) | 0.21 | <0.001* | |
| White | 209 (87%) | 196 (88%) | 0.78 | 262 (92%) | 256 (86%) | 0.03 | 0.67 | |
| BMI mean ± SD (kg/m2) | 30 ± 5 | 29 ± 5 | 0.13 | 29 ± 4 | 29 ± 5 | 0.75 | 0.11 | |
| Never smoked | 48 (20%) | 52 (23%) | 0.37 | 54 (19%) | 74 (25%) | 0.10 | 0.93 | |
| Hypertension | 152 (63%) | 136 (62%) | 0.74 | 164 (59%) | 172 (58%) | 0.84 | 0.14 | |
| Diabetes mellitus | 72 (30%) | 60 (27%) | 0.50 | 66 (23%) | 85 (29%) | 0.15 | 0.34 | |
| Family history of CAD | 106 (48%) | 88 (44%) | 0.36 | 153 (58%) | 157 (56%) | 0.65 | <0.001* | |
| Myocardial infarction | 68 (29%) | 76 (35%) | 0.17 | 97 (34%) | 96 (32%) | 0.64 | 0.54 | |
| CABG | 17 (7%) | 21 (9%) | 0.35 | 27 (9%) | 19 (6%) | 0.16 | 0.83 | |
| PCI | 35 (15%) | 19 (9%) | 0.04 | 40 (14%) | 44 (15%) | 0.85 | 0.21 | |
| Angina pectoris | 217 (90%) | 200 (90%) | 0.79 | 241 (85%) | 256 (85%) | 0.94 | 0.01* | |
| CCS Angina Class | 0 | 23 (10%) | 23 (10%) | 0.46 | 44(15%) | 46 (15%) | 0.12 | <0.001* |
| 1 | 57 (24%) | 58 (26%) | 89 (31%) | 104 (34%) | ||||
| 2 | 94 (39%) | 95(43%) | 106(37%) | 123 (41%) | ||||
| 3 | 66 (28%) | 47 (21%) | 46 (16%) | 29 (10%) | ||||
| PAD | 17 (7%) | 10 (5%) | 0.25 | 9 (3%) | 13 (4%) | 0.46 | 0.11 | |
| Heart failure | 6 (3%) | 6 (3%) | 0.88 | 14 (5%) | 7 (2%) | 0.09 | 0.36 | |
| Stroke/TIA | 15 (6%) | 15 (7%) | 0.84 | 13 (5%) | 20 (7%) | 0.28 | 0.55 | |
| Renal disease | 5 (2%) | 0 | 0.03 | 6 (2%) | 7 (2%) | 0.86 | 0.16 | |
| Heart rate (bpm) mean ± SD | 64 ± 10 | 66 ± 13 | 0.08 | 64 ±11 | 65 ± 11 | 0.39 | 0.64 | |
| SBP (mm Hg) mean ± SD | 135 ± 21 | 134 ± 18 | 0.71 | 131 ± 18 | 130 ± 18 | 0.50 | 0.002* | |
| DBP (mm Hg) mean ± SD | 64 ± 11 | 73 ± 11 | 0.83 | 76 ± 11 | 76 ± 11 | 0.99 | <0.001* | |
| Number of arteries narrowed | 1 | 80 (33%) | 65 (29%) | 0.28 | 105 (37%) | 99 (33) | 0.61 | 0.49 |
| 2 | 100 (41%) | 87 (39%) | 105 (37%) | 118 (39%) | ||||
| 3 | 61 (25%) | 71 (32%) | 76 (27%) | 85 (28%) | ||||
| Ejection Fraction | 63 ± 11% | 63 ± 10% | 0.55 | 63 ± 11% | 62 ± 9% | 0.54 | 0.28 | |
| Medications (n) | 206 | 183 | 233 | 247 | ||||
| Aspirin | 185 (90%) | 167 (91%) | 0.63 | 202 (87%) | 216 (87%) | 0.81 | 0.12 | |
| ACEi/ARB | 101 (49%) | 89 (49%) | 0.98 | 115 (49%) | 123 (50%) | 0.85 | 0.89 | |
| Beta-blocker | 155 (75%) | 129 (71%) | 0.33 | 165(70%) | 176 (71%) | 0.80 | 0.44 | |
| Calcium channel blocker | 72 (35%) | 60 (33%) | 0.68 | 59 (25%) | 61 (25%) | 0.92 | 0.003* | |
ACEi = angiotensin converting enzyme inhibitor; ARB = angiotensin receptor blocker; BMI = body mass index; CAD = coronary artery disease; CABG = coronary artery bypass grafting; CCS = Canadian Cardiovascular Class; DBP = diastolic blood pressure; METs = metabolic equivalents; OMT = optimal medical therapy; PAD = peripheral arterial disease; PCI = percutaneous coronary intervention; SBP = systolic blood pressure; SD = standard deviation; TIA = transient ischemic attack.
p <0.01 significant.
Table 2 provides the baseline exercise treadmill test characteristics of the study cohort. In patients with either <7 METs or ≥7 METs, there was no significant difference in the mean exercise duration and ischemic ST-segment depression between the PCI + OMT group and the OMT group. In patients who achieved ≥7 METs, the mean METs achieved was statistically greater in the patients randomized to PCI + OMT compared with OMT alone.
Table 2.
Baseline exercise stress test characteristics categorized based on METs achieved
| Variable | Exercise Work Load (N=1,052)
|
||||||
|---|---|---|---|---|---|---|---|
| <7 METs (N=464)
|
≥7 METs (N=588)
|
||||||
| PCI+OMT (N=241) |
OMT (N=223) |
P value | PCI+OMT (N=286) |
OMT (N=302) |
P value | ||
| Exercise Protocol | Bruce | 145 (60%) | 138 (62%) | 285 (99.7%) | 298 (99%) | ||
| Modified Bruce | 61(25%) | 55 (25%) | 0 | 4 (1%) | |||
| Naughton | 9 (4%) | 9 (4%) | 0 | 0 | |||
| Balke/Other | 26 (11%) | 21 (9%) | 1 (0.3%) | 0 | |||
| Exercise Duration | <5 minutes | 123 (51%) | 110 (49%) | 0 | 0 | ||
| 5–7 minutes | 77 (32%) | 77 (35%) | 71 (25%) | 97 (32%) | |||
| 7–9 minutes | 21 (9%) | 19 (9%) | 137 (48%) | 138 (46%) | |||
| >9 minutes | 20 (8%) | 17 (8%) | 78 (27%) | 67 (22%) | |||
| Exercise Duration (mean ± SD) | 5.3 ± 2.3 | 5.3 ± 2.2 | 0.99 | 8.4 ± 2.2 | 8.0 ± 1.7 | 0.02 | |
| METs achieved | <3 | 80 (33%) | 69 (31%) | 0 | 0 | ||
| 4–6 | 161 (67%) | 154 (69%) | 0 | 0 | |||
| 7–9 | 0 | 0 | 180 (63%) | 213 (71%) | |||
| >9 | 0 | 0 | 106 (37%) | 89 (29%) | |||
| METs achieved (mean ± SD) | 4.1 ± 1.3 | 4.2 ± 1.3 | 0.59 | 8.4 ± 2.1 | 8.0 ± 1.6 | 0.009* | |
| ST segment depressions (mm) | <1 | 67 (28%) | 63 (29%) | 0.80 | 95 (33%) | 101 (34%) | 0.80 |
| 1–2 | 126 (53%) | 120 (54%) | 132 (46%) | 142 (48%) | |||
| >2 | 47 (20%) | 38 (17%) | 59 (21%) | 55 (18%) | |||
METs = metabolic equivalents; mins = minutes; OMT = optimal medical therapy; PCI = percutaneous coronary intervention; SD = standard deviation.
p <0.01 significant.
During follow-up, a total of 58 patients died, and 108 patients sustained a nonfatal myocardial infarction. The event rates for the exercise capacity subgroups by randomized treatment assignment are provided in Table 3. In patients with exercise capacity <7 METs, there was no difference in the primary end point between patients assigned to PCI + OMT versus OMT alone (19.1% vs 16.1%, p = 0.31). In patients with exercise capacity ≥7 METs, those assigned to PCI + OMT, despite achieving a higher mean MET level, had numerically more primary end points than those with OMT alone (13.3% vs 10.3%, p = 0.27). Overall, 67 patients in the OMT group crossed over to the PCI group in the first year after randomization, 27 (12%) in the <7 METs group, and 40 (13%) in the ≥7 METs group. There was no significant difference in the Kaplan–Meier cumulative event-free survival curves in patients with METs ≥7 and METs <7 by randomized treatment assignment (Figures 2 and 3).
Table 3.
Event rates categorized by METs achieved
| Outcomes | <7 METs (N=464)
|
≥7 METs (N=588)
|
||
|---|---|---|---|---|
| PCI+OMT (N=241) |
OMT (N=223) |
PCI+OMT (N=286) |
OMT (N=302) |
|
| *Death/MI (151 events) | 46(19%) | 36(16%) | 38(13%) | 31(10%) |
| Death (58 events) | 18(7%) | 13(6%) | 11(4%) | 16(5%) |
| MI (108 events) | 32(13%) | 28(13%) | 29(10%) | 19(6%) |
METs = metabolic equivalents; MI = myocardial infarction; OMT = optimal medical therapy; PCI = percutaneous coronary intervention.
Some patients had MI before their subsequent death. For the primary outcome analysis of Death/MI, only the first event was included (time to first event). Hence, the overall Death/MI events are lesser than Death plus MI alone.
Figure 2.
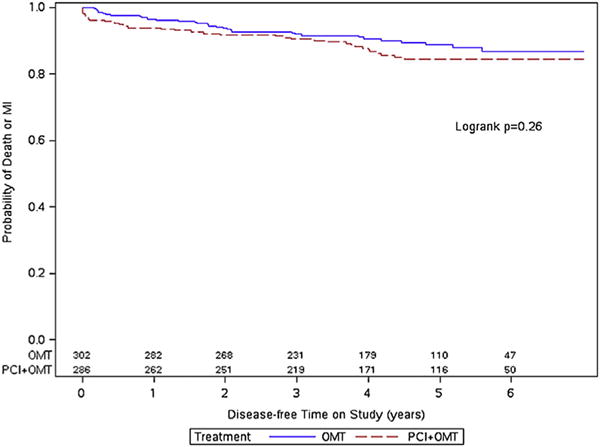
Kaplan–Meier curves for the primary end point in patients with exercise capacity ≥7 METs.
Figure 3.
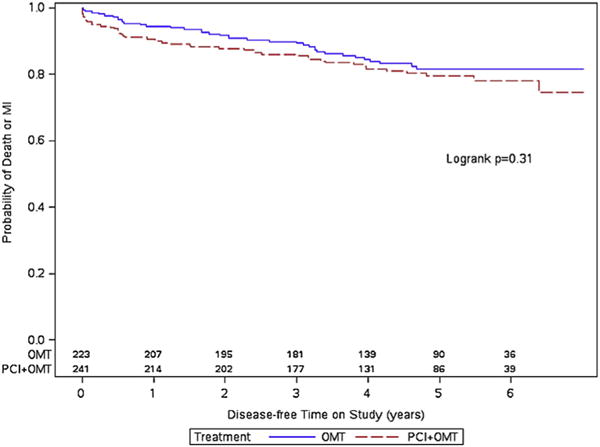
Kaplan–Meier curves for the primary end point in patients with exercise capacity <7 METs.
Table 4 provides the results of multivariate Cox models for death, myocardial infarction, and death or myocardial infarction for the overall cohort stratified by exercise capacity and treatment assignment. After adjustment for the previously mentioned covariates, HR (99% CI) for the primary outcome of death or myocardial infarction for the PCI + OMT versus the OMT group was 1.42 (0.90 to 2.23, p = 0.05) and for exercise capacity subgroups of ≥7 METs and <7 METs was 0.75 (0.46 to 1.22, p = 0.13). The adjusted results for models with death and myocardial infarction as the outcomes were not different for the PCI + OMT group versus the OMT group and exercise capacity subgroups. The interaction between treatment assignment and exercise capacity achieved was not significant in any of the statistical analyses. When METs were used as a continuous variable, the adjusted HR (99% CI) for the primary outcome for the PCI + OMT versus the OMT group was 1.44 (0.92 to 2.27, p = 0.04). Similarly, the adjusted HR for METs as a continuous variable was 0.91 (0.83 to 1.01, p = 0.02). The interaction between treatment assignment and METs as a continuous variable was not significant.
Table 4.
Cox proportional hazard models for various clinical end points stratified by treatment assignment and exercise capacity
| Outcomes | Variables | Unadjusted Model
|
Adjusted Models*
|
||
|---|---|---|---|---|---|
| HR (99% CI) | P value | HR (99% CI) | P value | ||
| Death/MI (151 events) | PCI + OMT vs. OMT | 1.28 (0.84,1.95) | 0.13 | 1.42 (0.90,2.23) | 0.05 |
| <7 METs vs. ≥7 METs | 0.66 (0.43,1.00) | 0.01 | 0.75 (0.46,1.22) | 0.13 | |
| Death (58 events) | PCI + OMT vs. OMT | 0.93 (0.55,1.58) | 0.78 | 1.11 (0.53,2.34) | 0.72 |
| <7 METs vs. ≥7 METs | 0.70 (0.42,1.19) | 0.19 | 0.92 (0.41,2.05) | 0.78 | |
| MI (108 events) | PCI + OMT vs. OMT | 1.38 (0.93,2.05) | 0.11 | 1.51 (0.88,2.57) | 0.05 |
| <7 METs vs. ≥7 METs | 0.61 (0.42,0.90) | 0.01 | 0.73 (0.41,1.29) | 0.16 | |
Data on METs as a continuous variable are not shown in the table.
METs = metabolic equivalents; MI = myocardial infarction; OMT = optimal medical therapy; PCI = percutaneous coronary intervention.
Adjusted for age, gender, body mass index, family history of coronary artery disease, angina, systolic blood pressure and diastolic blood pressure.
To further examine the impact of even more reduced levels of baseline exercise capacity on outcomes, we performed a separate analysis using 5 METs as an alternative cut-point to better define those patients with the lowest exercise capacity. Of the 1,052 patients, only 170 (16%) patients achieved <5 METs, of whom 91 patients were randomized to PCI + OMT and 79 patients to OMT alone. The primary composite end point occurred in 16 patients who received PCI + OMT compared with 14 patients assigned to OMT alone (18% vs 16%, HR 1.06, 99% CI 0.41 to 2.71, p = 0.88).
Discussion
The principal finding of this post hoc analysis is that there was no difference in the primary outcome of death or myocardial infarction in patients with impaired exercise capacity (<7 METs) compared with intact exercise capacity (≥7 METs), irrespective of the initial treatment strategy of PCI + OMT versus OMT alone during a 2.5- to 7-year follow-up. Patients with exercise capacity <7 METs did not derive a proportionately greater clinical benefit from PCI + OMT compared with those patients who received OMT alone.
Multiple previous studies have reported exercise capacity to be a strong independent predictor of survival in patients with CAD, with better outcomes in patients achieving a higher aerobic workload.1–8 These studies used various MET cut-points to classify patients at increased risk for cardiac events.3 In an attempt to identify the age-specific exercise capacity threshold for mortality risk assessment, a recent study by Kokkinos et al15 found that in men within the age group of 60 to 69 years, the peak MET level of 6 to 7 was not associated with an increase in mortality risk (HR 1.0). In the present study, mean age of the entire cohort was 61.4 years at baseline and 85% were men, and therefore, the use of a MET cut point of <7 versus ≥7 was chosen based on this report.
In the Coronary Artery Surgery Study registry, patients who reached ≤stage 2 on the Bruce protocol (≤7 METs) had significantly better survival with coronary artery bypass grafting than medical therapy alone.9 However, in the present study, there was no evidence to suggest an incremental clinical benefit from adding revascularization with PCI to OMT in patients with lower exercise capacity, when defined as less than 5 or 7 METs. These differences between the previous observational studies and our study could possibly be explained by either the mode of revascularization used (coronary artery bypass grafting vs PCI) or the evolution of medical therapy over the past 2 decades.
Stringent guideline-directed management strategies were used in the design and execution of the COURAGE trial compared with the observational cohort studies from an earlier era, when there were fewer options for disease-modifying pharmacotherapy and less was known about secondary prevention. OMT in COURAGE trial consisted of aggressive lifestyle modifications including smoking cessation, adoption of a healthy diet, weight loss, and regular aerobic exercise consisting of 30 to 45 minutes of moderate intensity activity, 5 times per week, with intensive medical therapy. The medical therapy used in the Coronary Artery Surgery Study registry9 (43% received β blockers and 60% received long-acting nitrates, whereas none received angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, statins, or aspirin therapy) and in other observational trials either antedated the contemporary use of disease-modifying pharmacotherapies1–3,5 or was not as aggressive4,6,7 compared to the COURAGE trial (on an average approximately 95% received aspirin, 93% received statins, 86% received β blockers, and 69% received angiotensin-converting enzyme inhibitors/angiotensin receptor blockers).10
Several previous studies have demonstrated that multiple risk factor interventions with aggressive lifestyle modifications including regular exercise, dietary interventions, and intensive lipid-lowering therapy with statins not only slows the progression of CAD but also may result in regression of CAD.16–21 Regular physical exercise has been shown to significantly improve exercise capacity and reduce myocardial ischemia by improving endothelium-mediated vasodilation in patients with stable CAD.17,22,23 Thus, the more aggressive use of OMT and intensive therapeutic lifestyle modifications may have offset the adverse effects of lower exercise capacity as observed in the current post hoc analysis.
These findings suggest that in stable ischemic heart disease, patients with decreased baseline exercise capacity, an initial strategy of multifaceted, aggressive lifestyle modifications, regular aerobic exercise to improve exercise capacity, and intensive medical therapy may decrease future cardiovascular events.
Several limitations of this post hoc analysis warrant comment. First, the present study comprised a modest sample size of patients who underwent exercise treadmill testing. Second, follow-up exercise treadmill testing was not available for all patients to assess the interval change in the exercise capacity in response to assigned treatment. Third, exercise capacity was estimated using a formula based on the speed and grade of the treadmill that estimates peak aerobic capacity by workload. The formula does not account for the amount of time doing a specific workload, and we did not measure ventilatory gas exchange, which is considered to be a more accurate predictor of outcomes.24,25 However, METs achieved on exercise treadmill testing are a more common and practical measure of exercise capacity in clinical practice and have been associated with clinical outcomes as well. Fourth, we used a cut-point of 7 METs to examine differences in outcomes in the treatment groups. To assess whether a further decrease in exercise capacity would affect outcomes, we did a separate analysis using a cut-point of 5 METs. There was no difference in clinical outcomes between groups of patients who achieved <5 and ≥5 METs, although the small sample size may have resulted in a type II error. Fifth, 67 patients in this analysis crossed over from OMT to PCI + OMT group, and thus, the results do not address the issue of whether PCI would be superior to OMT alone in patients with lower exercise capacity in the absence of crossovers. Finally, patients in this trial were excluded if they had baseline severe left ventricular systolic dysfunction (ejection fraction <30%) or had high-risk features such as significant ST depressions (≥1.5 mm) or hypotension during stage 1 of a standard Bruce protocol. Hence, it is unclear from this post hoc analysis whether such high-risk patients with impaired exercise capacity would derive clinical benefit from PCI in addition to OMT.
Footnotes
Disclosures
The authors have no conflicts of interest to disclose.
References
- 1.McNeer JF, Margolis JR, Lee KL, Kisslo JA, Peter RH, Kong Y, Behar VS, Wallace AG, McCants CB, Rosati RA. The role of the exercise test in the evaluation of patients for ischemic heart disease. Circulation. 1978;57:64–70. doi: 10.1161/01.cir.57.1.64. [DOI] [PubMed] [Google Scholar]
- 2.Podrid PJ, Graboys TB, Lown B. Prognosis of medically treated patients with coronary-artery disease with profound ST-segment depression during exercise testing. N Engl J Med. 1981;305:1111–1116. doi: 10.1056/NEJM198111053051903. [DOI] [PubMed] [Google Scholar]
- 3.Morris CK, Ueshima K, Kawaguchi T, Hideg A, Froelicher VF. The prognostic value of exercise capacity: a review of the literature. Am Heart J. 1991;122:1423–1431. doi: 10.1016/0002-8703(91)90586-7. [DOI] [PubMed] [Google Scholar]
- 4.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 5.Weiner DA, Ryan TJ, McCabe CH, Chaitman BR, Sheffield LT, Ferguson JC, Fisher LD, Tristani F. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. J Am Coll Cardiol. 1984;3:772–779. doi: 10.1016/s0735-1097(84)80254-5. [DOI] [PubMed] [Google Scholar]
- 6.Hung RK, Al-Mallah MH, McEvoy JW, Whelton SP, Blumenthal RS, Nasir K, Schairer JR, Brawner C, Alam M, Keteyian SJ, Blaha MJ. Prognostic value of exercise capacity in patients with coronary artery disease: the FIT (Henry Ford ExercIse Testing) project. Mayo Clin Proc. 2014;89:1644–1654. doi: 10.1016/j.mayocp.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Padala SK, Ghatak A, Padala S, Katten DM, Polk DM, Heller GV. Cardiovascular risk stratification in diabetic patients following stress single-photon emission-computed tomography myocardial perfusion imaging: the impact of achieved exercise level. J Nucl Cardiol. 2014;21:1132–1143. doi: 10.1007/s12350-014-9986-1. [DOI] [PubMed] [Google Scholar]
- 8.Thompson CA, Jabbour S, Goldberg RJ, McClean RY, Bilchik BZ, Blatt CM, Ravid S, Graboys TB. Exercise performance-based outcomes of medically treated patients with coronary artery disease and profound ST segment depression. J Am Coll Cardiol. 2000;36:2140–2145. doi: 10.1016/s0735-1097(00)01004-4. [DOI] [PubMed] [Google Scholar]
- 9.Weiner DA, Ryan TJ, McCabe CH, Chaitman BR, Sheffield LT, Fisher LD, Tristani F. Value of exercise testing in determining the risk classification and the response to coronary artery bypass grafting in three-vessel coronary artery disease: a report from the Coronary Artery Surgery Study (CASS) registry. Am J Cardiol. 1987;60:262–266. doi: 10.1016/0002-9149(87)90224-4. [DOI] [PubMed] [Google Scholar]
- 10.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, COURAGE Trial Research Group Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 11.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk W, Knudtson M, Dada M, Casperson P, Harris CL, Spertus JA, Shaw L, Chaitman BR, Mancini GB, Berman DS, Weintraub WS. Design and rationale of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial Veterans Affairs Cooperative Studies Program no. 424. Am Heart J. 2006;151:1173–1179. doi: 10.1016/j.ahj.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Boden WE, O’Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk W, Knudtson M, Dada M, Casperson P, Harris CL, Spertus JA, Shaw L, Chaitman BR, Mancini GB, Berman DS, Gau G, Weintraub WS. The evolving pattern of symptomatic coronary artery disease in the United States and Canada: baseline characteristics of the Clinical Outcomes Utilizing Revascularization and Aggressive DruG Evaluation (COURAGE) trial. Am J Cardiol. 2007;99:208–212. doi: 10.1016/j.amjcard.2006.07.082. [DOI] [PubMed] [Google Scholar]
- 13.Gibbons RJ, Balady GJ, Bricker JT, Chaitman BR, Fletcher GF, Froelicher VF, Mark DB, McCallister BD, Mooss AN, O’Reilly MG, Winters WL, Jr, Gibbons RJ, Antman EM, Alpert JS, Faxon DP, Fuster V, Gregoratos G, Hiratzka LF, Jacobs AK, Russell RO, Smith SC., Jr ACC/AHA 2002 guideline update for exercise testing: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Circulation. 2002;106:1883–1892. doi: 10.1161/01.cir.0000034670.06526.15. [DOI] [PubMed] [Google Scholar]
- 14.Treadmill Stress Test Protocols | Social Security Advisory Service. Available at: http://www.ssas.com/disability-medical-tests/cardiovascular/treadmill-stress-testing/protocols/. Accessed on March 2, 2014.
- 15.Kokkinos P, Faselis C, Myers J, Sui X, Zhang J, Blair SN. Age-specific exercise capacity threshold for mortality risk assessment in male veterans. Circulation. 2014;130:653–658. doi: 10.1161/CIRCULATIONAHA.114.009666. [DOI] [PubMed] [Google Scholar]
- 16.Gielen S, Laughlin MH, O’Conner C, Duncker DJ. Exercise training in patients with heart disease: review of beneficial effects and clinical recommendations. Prog Cardiovasc Dis. 2015;57:347–355. doi: 10.1016/j.pcad.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Schuler G, Hambrecht R, Schlierf G, Niebauer J, Hauer K, Neumann J, Hoberg E, Drinkmann A, Bacher F, Grunze M, Kübler W. Regular physical exercise and low-fat diet. Effects on progression of coronary artery disease. Circulation. 1992;86:1–11. doi: 10.1161/01.cir.86.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Haskell WL, Alderman EL, Fair JM, Maron DJ, Mackey SF, Superko HR, Williams PT, Johnstone IM, Champagne MA, Krauss RM, Far-quhar JW. Effects of intensive multiple risk factor reduction on coronary atherosclerosis and clinical cardiac events in men and women with coronary artery disease. The Stanford Coronary Risk Intervention Project (SCRIP) Circulation. 1994;89:975–990. doi: 10.1161/01.cir.89.3.975. [DOI] [PubMed] [Google Scholar]
- 19.Niebauer J, Hambrecht R, Marburger C, Hauer K, Velich T, von Hodenberg E, Schlierf G, Kübler W, Schuler G. Impact of intensive physical exercise and low-fat diet on collateral vessel formation in stable angina pectoris and angiographically confirmed coronary artery disease. Am J Cardiol. 1995;76:771–775. doi: 10.1016/s0002-9149(99)80224-0. [DOI] [PubMed] [Google Scholar]
- 20.Gould KL, Ornish D, Scherwitz L, Brown S, Edens RP, Hess MJ, Mullani N, Bolomey L, Dobbs F, Armstrong WT, Merritt T, Ports T, Sparler S, Billings J. Changes in myocardial perfusion abnormalities by positron emission tomography after long-term, intense risk factor modification. JAMA. 1995;274:894–901. doi: 10.1001/jama.1995.03530110056036. [DOI] [PubMed] [Google Scholar]
- 21.Niebauer J, Hambrecht R, Velich T, Hauer K, Marburger C, Kälberer B, Weiss C, von Hodenberg E, Schlierf G, Schuler G, Zimmermann R, Kübler W. Attenuated progression of coronary artery disease after 6 years of multifactorial risk intervention: role of physical exercise. Circulation. 1997;96:2534–2541. doi: 10.1161/01.cir.96.8.2534. [DOI] [PubMed] [Google Scholar]
- 22.Hambrecht R, Wolf A, Gielen S, Linke A, Hofer J, Erbs S, Schoene N, Schuler G. Effect of exercise on coronary endothelial function in patients with coronary artery disease. N Engl J Med. 2000;342:454–460. doi: 10.1056/NEJM200002173420702. [DOI] [PubMed] [Google Scholar]
- 23.Schuler G, Adams V, Goto Y. Role of exercise in the prevention of cardiovascular disease: results, mechanisms, and new perspectives. Eur Heart J. 2013;34:1790–1799. doi: 10.1093/eurheartj/eht111. [DOI] [PubMed] [Google Scholar]
- 24.Stelken AM, Younis LT, Jennison SH, Miller DD, Miller LW, Shaw LJ, Kargl D, Chaitman BR. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J Am Coll Cardiol. 1996;27:345–352. doi: 10.1016/0735-1097(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 25.Myers J, Gullestad L, Vagelos R, Do D, Bellin D, Ross H, Fowler MB. Clinical, hemodynamic, and cardiopulmonary exercise test determinants of survival in patients referred for evaluation of heart failure. Ann Intern Med. 1998;129:286–293. doi: 10.7326/0003-4819-129-4-199808150-00004. [DOI] [PubMed] [Google Scholar]


