Abstract
Purpose
To determine the relationships between stereoacuity, control of exotropia, and angle of deviation in children with intermittent exotropia (IXT).
Methods
Data collected for 652 participants 3 to <11 years of age with IXT meeting eligibility criteria for enrollment into one of two multicenter, randomized clinical trials were used to evaluate relationships between stereoacuity, control, and angle of deviation at enrollment.
Results
Any level of stereoacuity and angle of deviation could be accompanied by any level of control. Worse distance exotropia control was weakly associated with poorer distance stereoacuity (R = 0.26; 99% CI, 0.17–0.36) and larger angles of deviation at distance (R = 0.27; 99% CI, 0.17–0.36). Worse near exotropia control was weakly associated with poorer near stereoacuity (R = 0.17; 99% CI, 0.07–0.27) and moderately associated with larger angles of deviation at near (R = 0.37; 99% CI, 0.28–0.45). There was no association between stereoacuity and angle of deviation at distance (R = 0.07; 99% CI, −0.03 to 0.17) or at near (R = 0.02; 99% CI, −0.08 to 0.12).
Conclusions
Although weak and moderate associations were found between stereoacuity, control, and angle of deviation, a child may exhibit any combination of stereoacuity, control, and angle of deviation. The specific roles of control, stereoacuity, and angle of deviation in the diagnosis, management, and pathogenesis of IXT are unclear, and each appears to yield somewhat independent information.
Intermittent exotropia (IXT) is the most common form of childhood-onset exotropia, with an incidence of 32.1 per 100,000 in children <19 years of age.1 IXT is characterized by an exotropia that is not constant and is mainly present at distance but may also be present at near. Many cases of IXT are treated using surgical interventions and nonsurgical interventions, such as part-time occlusion, fusional vergence exercises, and overminus lenses. Nevertheless, the relative effectiveness of such treatments has not been rigorously studied, and the natural history of IXT is unknown. The Pediatric Eye Disease Investigator Group (PEDIG) is attempting to address some of these issues through two randomized clinical trials—one comparing part-time patching to observation,2,3 continuing with a natural history component, and a second study evaluating the effectiveness of bilateral lateral rectus recession versus monocular unilateral lateral rectus recession with medial rectus resection for the treatment of IXT. The goal of the current study was to examine the relationships among baseline clinical factors in participants with IXT who were enrolled into each of these randomized clinical trials to determine whether or not there were characteristic relationships between stereoacuity, control, and angle of the deviation. We recognized that we would not be able to attribute causality to any relationship between these factors; nevertheless, if strong relationships could be shown to exist, those relationships might influence clinical assessment, where one measure might be a reasonable surrogate for another. We also assessed relationships between age and stereoacuity, control, and angle of deviation, the relationships between the presence of anisometropia and each of these characteristics, and the relationships between sex and each of these characteristics.
Subjects and Methods
The studies involved were supported through a cooperative agreement with the National Eye Institute of the National Institutes of Health and were conducted by PEDIG at 70 academic- and community-based sites. Informed consent forms compliant with the US Health Insurance Portability and Accountability Act of 1996 were approved by institutional review boards, and a parent or guardian provided written informed consent. An independent data and safety monitoring committee provided study oversight. The studies are listed on www.clinicaltrials.gov (NCT01032603 and NCT01032330, accessed 10/01/16). The full study protocols are available on the PEDIG website (www.pedig.net, accessed 10/01/16).
Data Collection
Between January 2010 and February 2014, 876 participants 1 to <11 years of age with IXT were enrolled in one of the two randomized clinical trials. The first randomized trial compared occlusion therapy with observation for the treatment of IXT among participants 1 to <11 years of age with untreated IXT (other than refractive correction).2,3 The second randomized trial will evaluate two surgical procedures for the treatment of IXT among children 3 to <11 years of age with no prior strabismus surgery or botulinum toxin injection. Data analyses were limited to 652 participants (of the total 876 enrolled in both randomized trials) who were ≥3 years of age and had measurements of stereoacuity, exotropia control, and angle of horizontal deviation (measured by prism and alternate cover test) at distance and near.
Baseline testing included measurements of near stereoacuity, distance stereoacuity, assessment of distance and near exotropia control, and assessment of ocular alignment at distance and near using the prism and alternate cover test (PACT), in that order. Testing was performed in current refractive correction, if prescribed, without prism or overminus. Cycloplegic refraction was required within 6 months prior to enrollment. At the time of enrollment, participants with >1 D of spherical equivalent (SE) anisometropia were required to have worn refractive correction for at least 1 week.
Distance stereoacuity was assessed using the Distance Randot Test (Stereo Optical Co, Chicago IL, with thresholds of 400, 200, 100, and 60 arcsec, requiring 2 of 2 presentations at each level to pass that level).4,5 Near stereoacuity was assessed using the Randot Preschool Stereotest (Stereo Optical Co, Chicago IL, with thresholds of 800, 400, 200, 100, 60, and 40 arcsec, requiring 2 of 3 presentations at each level to pass that level).6 If the child could not pass the 400 arcsec level at distance or the 800 arcsec level at near, their stereoacuity was recorded as “nil” at the corresponding distance.
Control of the exodeviation was assessed at distance (6 m) and at near (1/3 m) using the Office Control Score for IXT,7 which ranges from 0 (phoria, best control) to 5 (constant exotropia, worst control). Control levels 3 to 5 were assigned based on the proportion of time that a manifest exotropia was present during a 30-second observation period before any dissociation. If no exotropia was observed during this period, control levels 0 to 2 were assigned based on the longest time it took for fusion to be reestablished following three consecutive 10-second periods of dissociation.
Definitions of Anisometropia and Bifoveal Fusion
To assess if anisometropia was associated with other factors and to account for the potential confounding effect of anisometropia on stereoacuity, participants were classified as anisometropic if they met one of two conditions: (1) absolute difference between the eyes in SE was ≥0.50 D, or (2) absolute difference between the eyes in cylindrical values was ≥1.50 D.
To further explore relationships involving stereoacuity, stereoacuity was categorized as either bifoveal or monofixational based on age-normal values6,8 (Table 1). This classification was thought to be more informative than using the raw stereoacuity threshold not accounting for age. Bifoveal was defined as 60 arcsec or better.9 Monofixational was defined as subnormal for age.6,8,9 Participants whose stereoacuity was worse than 60 arcsec but within normal for age were classified as “uncertain” (n = 199 at distance, n = 166 at near) and were not included in analyses of bifoveal versus monofixational.
Table 1.
| Age Group (Years) | Bifoveal Fixation (Normal) |
Uncertain | Sensory Monofixation (Abnormal) |
|---|---|---|---|
| Distance Stereoacuity | |||
| 3 | 60 | 100 to 400 | Nil |
| 4, 5 | 60 | 100 to 200 | 400 to Nil |
| 6 to <13 | 60 | 100 | 200 to Nil |
| Near Stereoacuity | |||
| 3 | 40 to 60 | 100 to 400 | 800 to Nil |
| 4, 5 | 40 to 60 | 100 to 200 | 400 to Nil |
| 6 | 40 to 60 | 100 | 200 to Nil |
| 7 to <13 | 40 to 60 | 100 to Nil | |
Statistical Analysis
Pearson correlation coefficients (R) and 99% confidence intervals were calculated to evaluate the relationships between stereoacuity, exotropia control score, angle of deviation, and age; 99% CIs were used rather than 95% CIs to account for multiple comparisons. Correlation strengths were interpreted using Dancey’s categorization10: R ≤ 0.10, no association; 0.10 < R ≤ 0.30, a weak association; 0.30 < R ≤ 0.60, a moderate association; and R > 0.60, a strong association.
Analysis of covariance models were used to evaluate the effect of anisometropia (yes versus no) on stereoacuity. Analysis of variance (ANOVA) models were used to evaluate the effect of sex as well as anisometropia on exotropia control score and angle of deviation. ANOVA models were also used to evaluate whether there was a difference in exotropia control score and in angle of deviation between bifoveal and monofixational participants. Logistic regression models were used to evaluate the effect of exotropia control score, angle of deviation, and anisometropia on the odds of being classified as monofixational versus bifoveal.
Analyses evaluating monofixational (vs bifoveal fusion) or involving stereoacuity, control score, or angle of deviation were evaluated at both distance and near, between parameters measured at the same distance.
Regarding statistical adjustment for potentially confounding variables, all analyses were adjusted for treatment prior to enrollment (yes versus no). Prior treatment was defined as treatment for amblyopia or strabismus, including refractive correction. Correlations involving stereoacuity and analyses for which stereoacuity was the dependent variable were adjusted for both age and anisometropia in addition to prior treatment. Analyses evaluating the odds of being classified as monofixational were adjusted for anisometropia in addition to prior treatment.
When analyzed as a continuous outcome, stereoacuity was converted from seconds of arc to logarithm of seconds of arc (in parentheses) as follows: 40 (1.60), 60 (1.78), 100 (2.00), 200 (2.30), 400 (2.60). 800 (2.90). Participants with no detectable stereoacuity (nil) were assigned a value of 1600 (3.20).
Testing multiple hypotheses increases the chance of falsely concluding an association exists. To control for this inflation of the type 1 error rate, statistical significance was defined as a relationship that was significant at the 0.01 level (P ≤ 0.01). All analyses were performed using SAS version 9.4 (SAS Institute Inc, Cary, NC).
Results
The average age of the 652 participants was 6.1 ± 2 years (standard deviation; range, 3–10.9 years); 60% were female, 61% were white, and 39% received nonsurgical treatment for amblyopia or strabismus prior to enrollment. The mean exotropia control score was 2.8 ± 1.4 at distance and 1.4 ± 1.2 at near (range for both distance and near, 0.0–5.0). Mean angle of deviation was 25Δ ± 7Δ (range, 10 to 50) at distance and 20Δ ± 10Δ (range, −4 to 50) at near. Median stereoacuity was 100 arcsec (range, 60 to nil) at distance and 60 arcsec (range, 40 to nil) at near. Additional baseline characteristics are provided in Tables 2 and 3.
Table 2.
Distribution of Baseline Characteristics
| N | % | |
|---|---|---|
| Total Participants | 652 | 100% |
| Sex | ||
| Female | 388 | 60% |
| Male | 264 | 40% |
| Age (years) | ||
| 3 to <5 | 241 | 37% |
| 5 to <7 | 216 | 33% |
| 7 to <9 | 115 | 18% |
| 9 to <11 | 80 | 12% |
| Mean (SD) | 6.1 (2.0) | |
| Range | 3.0 to 10.9 | |
| Race/Ethnicity | ||
| White | 396 | 61% |
| Black/African American | 90 | 14% |
| Hispanic | 110 | 17% |
| Asian | 22 | 3% |
| Native Hawaiian/Other Pacific Islander | 2 | <1% |
| American Indian/Alaskan Native | 2 | <1% |
| More than one race | 18 | 3% |
| Unknown/not reported | 12 | 2% |
| Treatment Prior to Enrollment | ||
| Yes | 253 | 39% |
| No | 399 | 61% |
| Anisometropia | ||
| Yes | 88 | 13% |
| No | 564 | 87% |
Table 3.
Baseline Ocular Alignment and Stereoacuity
| Distance Assessment |
Near Assessment |
|||
|---|---|---|---|---|
| N | % | N | % | |
| Deviation Type | ||||
| No exodeviation | 0 | 0% | 18 | 3% |
| Exophoria | 0 | 0% | 133 | 20% |
| Intermittent exotropia | 571 | 88% | 499 | 77% |
| Constant exotropia | 81 | 12% | 2 | <1% |
| Exotropia Control Score | ||||
| 0 | 13 | 2% | 185 | 28% |
| 1 | 110 | 17% | 228 | 35% |
| 2 | 179 | 27% | 109 | 17% |
| 3 | 140 | 21% | 89 | 14% |
| 4 | 111 | 17% | 36 | 6% |
| 5 | 99 | 15% | 5 | <1% |
| Mean (SD) | 2.8 (1.4) | 1.4 (1.2) | ||
| Range | 0.0 to 5.0 | 0.0 to 5.0 | ||
| Exodeviation (Δ) by Prism and Alternate Cover Test | ||||
| <0 (esodeviation) | 0 | 0% | 1 | <1% |
| 0 | 0 | 0% | 15 | 2% |
| 1–9 | 0 | 0% | 54 | 8% |
| 10–14 | 12 | 2% | 131 | 20% |
| 16–18 | 99 | 15% | 123 | 19% |
| 20–25 | 327 | 50% | 191 | 29% |
| 30–35 | 176 | 27% | 108 | 17% |
| 40–50 | 38 | 6% | 29 | 4% |
| Mean | 25 (7) | 20 (10) | ||
| Range | 10 to 50 | −4 to 50 | ||
| Stereoacuity (seconds of arc) | ||||
| 40 | n/a | n/a | 183 | 28% |
| 60 | 214 | 33% | 146 | 22% |
| 100 | 127 | 19% | 136 | 21% |
| 200 | 94 | 14% | 70 | 11% |
| 400 | 86 | 13% | 71 | 11% |
| 800 | n/a | n/a | 14 | 2% |
| Nil | 131 | 20% | 32 | 5% |
| Median | 100 | 60 | ||
| Range | 60 to Nil | 40 to Nil | ||
Δ = Prism diopters
n/a refers to levels not assessed by distance stereoacuity
Associations between Stereoacuity, Control, and Angle of Deviation at Distance
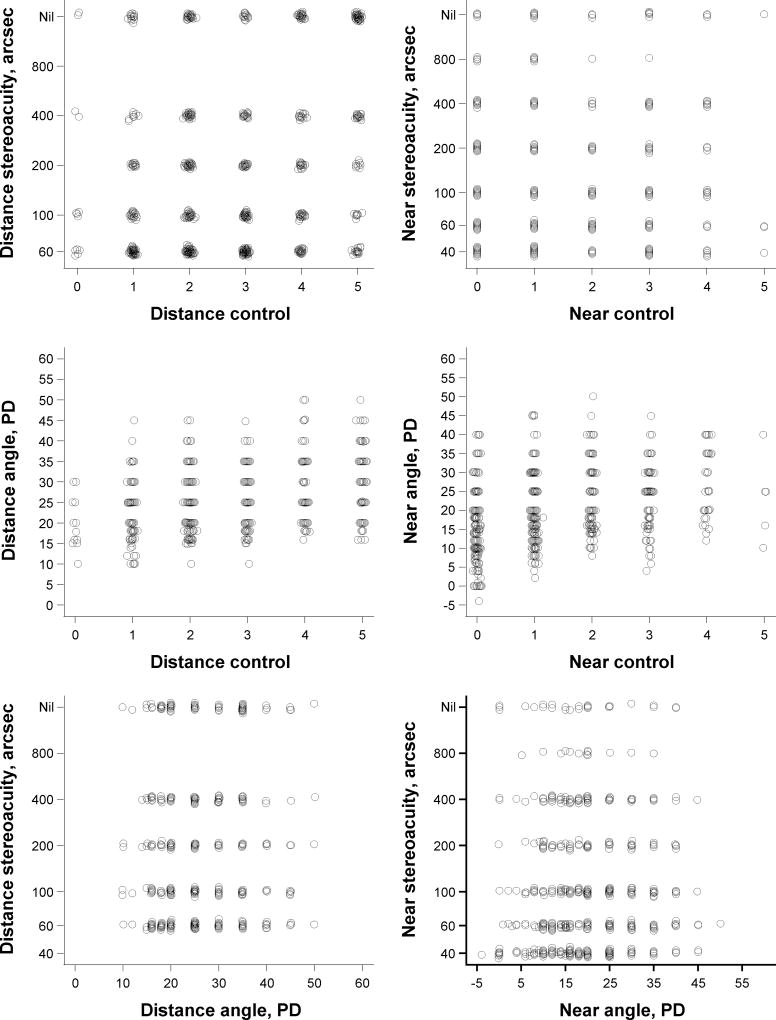
Worse distance control was weakly associated with poorer distance stereoacuity (R = 0.26; 99% CI, 0.17–0.36; Table 4) and with larger angles of distance deviation (R = 0.27; 99% CI, 0.17–0.36). There was no association between distance stereoacuity and angle of distance deviation (R = 0.07; 99% CI, −0.03 to 0.17). It is noteworthy that an individual could have any combination of distance stereoacuity, distance control, and angle of distance deviation (Figure 1).
Table 4.
Associations between Stereoacuity, Exotropia Control, and Angle of Deviation
| Baseline Factor 1a | Baseline Factor 2a | Correlation (99% CI)b |
|---|---|---|
| Distance Fixation | ||
| Distance Stereoacuity | Distance Control | 0.26 (0.17 to 0.36) |
| Distance Stereoacuity | Distance Angle | 0.07 (−0.03 to 0.17) |
| Distance Control | Distance Angle | 0.27 (0.17 to 0.36) |
| Near Fixation | ||
| Near Stereoacuity | Near Control | 0.17 (0.07 to 0.27) |
| Near Stereoacuity | Near Angle | 0.02 (−0.08 to 0.12) |
| Near Control | Near Angle | 0.37 (0.28 to 0.45) |
Stereoacuity analyzed using log arcseconds. Angle of deviation analyzed in prism diopters.
Correlation strengths were interpreted using Dancey’s categorization: R≤0.10 indicated no association, 0.10<R≤0.30 indicated a weak association, 0.30<R≤0.60 indicated a moderate association, and R>0.60 indicated a strong association.
FIG 1.
Relationships between stereoacuity, control of exotropia, and angle of deviation between parameters measured at the same distance.
Associations between Stereoacuity, Control, and Angle of Deviation at Near
Worse near control was weakly associated with poorer near stereoacuity (R = 0.17; 99% CI, 0.07–0.27; Table 4) and moderately associated with larger angle of near deviation (R = 0.37; 99% CI, 0.28–0.45). There was no association between near stereoacuity and angle of near deviation (R = 0.02; 99% CI, −0.08 to 0.12). It is noteworthy that an individual could have any combination of near stereoacuity, near control, and angle of near deviation (Figure 1).
Sensory Monofixation
Sensory monofixation was associated with poorer exotropia control scores at distance and near. On average, participants with distance sensory monofixation had worse distance control scores (difference = 0.73 point; 99% CI, 0.40–1.07) than bifoveal participants, and those with near sensory monofixation had worse near control scores (difference = 0.55 point; 99% CI, 0.24–0.86) than bifoveal participants. The odds of being monofixational at distance, compared with being bifoveal, were greater when distance control was worse (OR = 1.49; 99% CI, 1.24–1.80; Table 5). A similar relationship was observed at near (OR = 1.42; 99% CI, 1.16–1.73; Table 5). Sensory monofixation was not associated with angle of deviation at distance or at near (P > 0.01).
Table 5.
Odds of Having Sensory Monofixation (versus Bifoveal Fixation) According to Baseline Factors
| Independent Factor | Odds Ratio (99% CI)a | P-value |
|---|---|---|
| Associations at Distance | ||
| Distance Controlbd | 1.49 (1.24 to 1.80) | <0.001 |
| Distance Angle (Δ)c | 1.02 (0.99 to 1.06) | 0.15 |
| Anisometropia (Yes vs. No) | 1.72 (0.84 to 3.54) | 0.05 |
| Associations at Near | ||
| Near Controlbd | 1.42 (1.16 to 1.73) | <0.001 |
| Near Angle (Δ)c | 1.01 (0.99 to 1.04) | 0.26 |
| Anisometropia (Yes vs. No) | 1.43 (0.71 to 2.89) | 0.19 |
Table displays the odds of having sensory monofixation versus bifoveal fixation, computed using logistic regression models that adjust for prior treatment and the presence of anisometropia, where applicable.
Results displayed represent the change in the odds of having sensory monofixation associated with a 1 point increase (worsening) in exotropia control score
Results displayed represent the change in the odds of having monofixation associated with a 10PD increase in angle of deviation
Associations considered statistically significant (p≤0.01).
Δ=Prism diopters
Additional Analyses
Age was weakly associated with distance stereoacuity (R = −0.19; 99% CI, −0.29 to −0.10; Table 6) and moderately associated with near stereoacuity (R = −0.32; 99% CI, −0.41 to −0.23; Table 6). Older participants had better stereoacuity at distance and at near. Age was not associated with control score or angle of deviation at distance or at near (Table 6). The presence of anisometropia was not associated with stereoacuity, control score, angle of deviation (data not shown), or sensory monofixation at distance or at near (Table 5). Sex was not associated with stereoacuity, control score, angle of deviation, or sensory monofixation at distance or at near (data not shown).
Table 6.
Correlations between Baseline Characteristics and Age
| Baseline Factor 1 | Baseline Factor 2 | Correlation (99% CI)a |
|---|---|---|
| Distance Fixation | ||
| Distance Stereoacuity (log arcseconds) | Age | −0.19 (−0.29 to −0.10) |
| Distance Control | Age | −0.05 (−0.15 to 0.05) |
| Distance Angle (Δ) | Age | −0.07 (−0.17 to 0.03) |
| Near Fixation | ||
| Near Stereoacuity (log arcseconds) | Age | −0.32 (−0.41 to −0.23) |
| Near Control | Age | 0.01 (−0.09 to 0.11) |
| Near Angle (Δ) | Age | −0.07 (−0.17 to 0.03) |
Correlation strengths were interpreted using Dancey’s categorization: R≤0.10 indicated no association, 0.10<R≤0.30 indicated a weak association, 0.30<R≤0.60 indicated a moderate association, and R>0.60 indicated a strong association.
Δ=Prism diopter
Discussion
This study evaluated the relationships between stereoacuity, control of exotropia, and angle of deviation in children 3–10 years of age with IXT. Our results support our hypotheses that worse control is associated with poorer stereoacuity and larger angles of exodeviation. However, we did not find that poorer stereoacuity was associated with larger angles of exodeviation. In fact, we found that an individual child could have any combination of stereoacuity, control, and angle of deviation.
One possible explanation for the lack of association between angle magnitude and stereoacuity is that in participants with IXT both stereoacuity and angle of deviation can vary considerably during the day, not necessarily worsening or improving over the course of the day.11,12 A participant who appears to have sensory monofixation at one moment may appear to have sensory bifoveal fusion a few moments later.9
Another potential explanation for observing no relationship between stereoacuity and angle of deviation in our study could have been the order of testing. Stereoacuity was tested first, followed by control, and finally angle of deviation. It is possible that the act of breaking fusion by measuring control in a participant with good stereoacuity could have caused a change in the angle of deviation. If stereoacuity and ocular alignment had been tested consecutively, we might have had different results.
From a clinical standpoint, our findings do not allow us to make generalizations or to predict one measurement based on other measurements for an individual patient with IXT. For example, some participants with exceptionally good control of exotropia at distance had no measurable stereoacuity, and some participants with excellent stereoacuity had poor control of their exotropia. The clinician should therefore not assume that if the angle of deviation is large or the control is poor, the stereoacuity will be poor. Each clinical characteristic should be measured and considered separately for an individual patient.
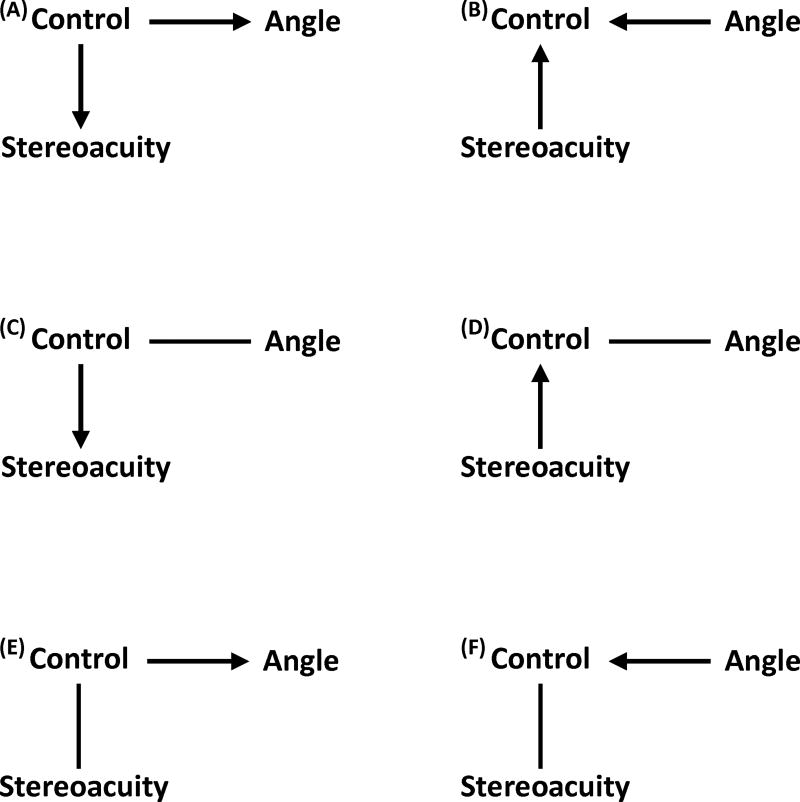
Our data suggest weak relationships between stereoacuity, angle of deviation, and control of exotropia, indicating a number of clinically plausible hypotheses that might be explored in future studies (Figure 2): (1) control may influence both stereoacuity and angle of deviation, (2) both stereoacuity and angle of deviation may influence control, (3) either stereoacuity or angle of deviation may influence control and the other is noncausally associated with control, or (4) either stereoacuity or angle of deviation may be influenced by control and the other is noncausally associated with control. Statistical analysis does not allow us to determine which of these possibilities is most likely. If central control is the primary driver, a patient who loses her ability to control their exotropia would be expected to have diminished stereoacuity and possibly an increased angle of deviation. Alternatively, if central stereoacuity is primary, a patient with longstanding poor stereoacuity would be expected to have poorer control of their exotropia and possibly a larger angle of deviation.
FIG 2.
Suggested relationships between control, stereoacuity, and angle of deviation. The figure displays 6 potential relationships between stereoacuity, control of exotropia (control), and angle of deviation (angle) based on the results. A, Control may influence stereoacuity and angle. B, Angle and stereoacuity may influence control. C, Control may influence stereoacuity and may be noncausally associated with angle. D, Stereoacuity influences control. Control is noncausally associated with angle. E, Control influences angle and is noncausally associated with stereoacuity. F, Angle influences control. Control is noncausally associated with stereoacuity. Lines represent noncausal associations while arrows display causal associations.
Additional analyses found that age was weakly associated with distance stereoacuity and moderately associated with near stereoacuity. These results are consistent with the common finding that stereoacuity improves with age, likely due to maturation.6,8 Consequently, failure to improve from below age-normal values might be a factor that alerts the clinician to the possibility of disease progression, as long as the observer accounts for test-retest variability of the measurement.
The visual characteristics evaluated in the present study did not appear to be worse in females, despite reports of IXT being more prevalent in females.13 Similarly, the presence of anisometropia was not associated with stereoacuity, control, or angle of deviation at distance or near in this cohort, however the definition of anisometropia must be considered when interpreting these results. Only 13% of participants had a difference in SE refractive error of ≥ 0.5 D. The absolute difference in SE refractive error ranged from 0 to 2.5 D. The low prevalence of anisometropia in this cohort, coupled with the limited range of difference in refractive error, may have affected our ability to find an association if one exists.
Our study has some limitations. Measurements of stereoacuity, ocular alignment, and control are variable.11,12 Only one measurement of exodeviation control was obtained at each distance. Recent studies have suggested that the mean of three separate measures of control, performed at 3 nonconsecutive times during a single office visit, provides a more representative assessment of an individual’s IXT control.14,15 The completion of assessments at varying distances may have also influenced our results. At near, control and ocular alignment were assessed at 33 cm, whereas stereoacuity was assessed at 40 cm. At distance, control and ocular alignment were assessed at 6 m; stereoacuity, at 3 m. In addition, the order of testing may have contributed to our failure to find a relationship between stereoacuity and angle of deviation; nevertheless, Smith and colleagues16 found that stereoacuity thresholds were not influenced by brief periods of dissociation during clinical testing such as measuring visual acuity. Finally, our results are not generalizable to all exotropia patients, because the participants we describe had to meet enrollment criteria to participate in the IXT studies, and, for example, we enrolled few patients with large amounts of anisometropia.
Although weak and moderate associations were found between stereoacuity, control, and angle of deviation, a child may exhibit any combination of these. Since the specific roles of control, stereoacuity, and angle of deviation in the diagnosis, management, and pathogenesis of IXT are unclear, and each appears to yield somewhat independent information, it is reasonable to collect data on all three factors as we continue to study and manage this condition.
Supplementary Material
Acknowledgments
Supported by National Eye Institute of National Institutes of Health, Department of Health and Human Services EY011751, EY023198, and EY018810, and an unrestricted grant to Department of Ophthalmology, Mayo Clinic, from Research to Prevent Blindness, New York, New York. The funding organizations had no role in the design or conduct of this research.
References
- 1.Govindan M, Mohney BG, Diehl NN, Burke JP. Incidence and types of childhood exotropia: a population-based study. Ophthalmology. 2005;112:104–8. doi: 10.1016/j.ophtha.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 2.Cotter SA, Mohney BG, Chandler DL, et al. Pediatric Eye Disease Investigator Group. A randomized trial comparing part-time patching with observation for children 3 to 10 years of age with intermittent exotropia. Ophthalmology. 2014;121:2299–310. doi: 10.1016/j.ophtha.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohney BG, Cotter SA, Chandler DL, et al. Pediatric Eye Disease Investigator Group. A randomized trial comparing part-time patching with observation for intermittent exotropia in children 12 to 35 months of age. Ophthalmology. 2015;122:1718–25. doi: 10.1016/j.ophtha.2015.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fu VL, Birch EE, Holmes JM. Assessment of a new Distance Randot stereoacuity test. J AAPOS. 2006;10:419–23. doi: 10.1016/j.jaapos.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 5.Holmes JM, Birch EE, Leske DA, Fu VL, Mohney BG. New tests of distance stereoacuity and their role in evaluating intermittent exotropia. Ophthalmology. 2007;114:1215–20. doi: 10.1016/j.ophtha.2006.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Birch E, Williams C, Drover J, et al. Randot Preschool Stereoacuity Test: normative data and validity. J AAPOS. 2008;12:23–6. doi: 10.1016/j.jaapos.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohney BG, Holmes JM. An office-based scale for assessing control in intermittent exotropia. Strabismus. 2006;14:147–50. doi: 10.1080/09273970600894716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang J, Hatt SR, O’Connor AR, et al. Final version of the Distance Randot Stereotest: normative data, reliability, and validity. J AAPOS. 2010;14:142–6. doi: 10.1016/j.jaapos.2009.12.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatt SR, Leske DA, Mohney BG, Brodsky MC, Holmes JM. Classification and misclassification of sensory monofixation in intermittent exotropia. Am J Ophthalmol. 2010;150:16–22. doi: 10.1016/j.ajo.2010.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dancey CP. Statistics without maths for psychology: using SPSS for Windows. 6. Philadelphia, PA: Trans-Atlantic Publications; 2014. [Google Scholar]
- 11.Hatt SR, Mohney BG, Leske DA, Holmes JM. Variability of stereoacuity in intermittent exotropia. Am J Ophthalmol. 2008;145:556–61. doi: 10.1016/j.ajo.2007.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatt SR, Leske DA, Liebermann L, Mohney BG, Holmes JM. Variability of angle of deviation measurements in children with intermittent exotropia. J AAPOS. 2012;16:120–24. doi: 10.1016/j.jaapos.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nusz KJ, Mohney BG, Diehl NN. Female predominance in intermittent exotropia. Am J Ophthalmol. 2005;140:546–7. doi: 10.1016/j.ajo.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Hatt SR, Liebermann L, Leske DA, Mohney BG, Holmes JM. Improved assessment of control in intermittent exotropia using multiple measures. Am J Ophthalmol. 2011;152:872–6. doi: 10.1016/j.ajo.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatt SR, Leske DA, Liebermann L, Holmes JM. Quantifying variability in the measurement of control in intermittent exotropia. J AAPOS. 2015;19:33–7. doi: 10.1016/j.jaapos.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith SJ, Leske DA, Hatt SR, Holmes JM. Stereoacuity thresholds before and after visual acuity testing. Ophthalmology. 2012;119:164–9. doi: 10.1016/j.ophtha.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.