Abstract
The acute respiratory distress syndrome (ARDS) is a common cause of acute respiratory failure, and is associated with substantial mortality and morbidity. Dozens of clinical trials targeting ARDS have failed, with no drug specifically targeting lung injury in widespread clinical use. Thus, the need for drug development in ARDS is great. Targeted proteomic studies in ARDS have identified many key pathways in the disease, including inflammation, epithelial injury, endothelial injury or activation, and disordered coagulation and repair. Recent studies reveal the potential for proteomic changes to identify novel subphenotypes of ARDS patients who may be most likely to respond to therapy and could thus be targeted for enrollment in clinical trials. Nontargeted studies of proteomics in ARDS are just beginning and have the potential to identify novel drug targets and key pathways in the disease. Proteomics will play an important role in phenotyping of patients and developing novel therapies for ARDS in the future.
Keywords: ARDS, therapy, phenotype, proteomics, inflammatory
Introduction
The acute respiratory distress syndrome (ARDS) is a common cause of respiratory failure in adults and is defined by the acute onset of bilateral lung infiltrates and a low arterial to fraction of inspired oxygen ratio (PaO2/FIO2 ≤ 300) absent isolated hydrostatic edema typical of congestive heart failure [1,2]. While the current definition is pragmatic, it continues to rely on nonspecific clinical criteria to group patients with a diverse array of pathophysiologies and very different prognoses.
ARDS occurs in the setting of a variety of risk factors, which are commonly characterized as ‘direct’ lung injury (e.g. pneumonia, aspiration, or chest trauma) or ‘indirect’ lung injury (e.g. sepsis, pancreatitis, or burns) [3]. The estimated incidence of ARDS is as high as 190,600 cases in the US per year, with >70,000 associated deaths [4].
Despite therapeutic advances in ARDS management, including widespread adaptation of low-tidal volume ventilation and restrictive fluid strategies, mortality from ARDS remains high, with estimates ranging from 20% to 40% [5,6]. Dozens of randomized clinical trials of drugs directly targeting ARDS have failed. The only drug therapy in widespread use for ARDS is neuromuscular blockade, which was shown to improve mortality in patients with moderate-to-severe ARDS in a multicenter RCT, but likely functions more as an adjuvant to lung protective ventilation than as a pharmacologic treatment of lung injury [7].
The reasons for failure in drug development in ARDS are likely multifactorial. As mentioned above, ARDS is a syndrome that is a final common pathway for various lung injury mechanisms (damage to lung endothelium, epithelium, cytokine injury, mechanical stretch by injurious ventilator settings, etc.) [5]. Most large-scale drug trials in ARDS enroll patients with many etiologies; if disease mechanisms vary with subclass, trials in all comers will be substantially underpowered. Patients with ARDS are by definition critically ill, and the primary cause of death in ARDS, especially in an era of lung protective ventilation, is often not respiratory failure, but multiorgan dysfunction [8]. Thus, mortality is often driven not by lung disease but other factors (family decisions to limit care, terminal illness prior to development of ARDS, etc.), which also leads to underpowered studies. However, established surrogate endpoints of ARDS severity are lacking. The empirically derived Berlin Definition found that measures of radiographic severity, respiratory system compliance, dead space, and level of positive end-expiratory pressure (PEEP) did not improve mortality prediction beyond classification by the PaO2/FIO2 ratio [2]. However, baseline PaO2/FiO2 ratio may not correlate with the degree of lung injury and substantial heterogeneity can occur in serial measurements of PaO2:FIO2 ratio depending on ventilator settings and degree of lung recruitability [9].
Timing of enrollment is also important in ARDS. ARDS is recognized to occur in several phases, including an early exudative/inflammatory phase (typically defined in the first 7 days) which may resolve, but in a subset of patients evolves into a later fibroproliferative phase [3]. Given profound changes in biomarker profiles even within the first few days of disease, more recent trials target ever earlier enrollments to limit this heterogeneity [7,10].
Given the focus of this review is on proteomics, we acknowledge that we cannot cover advances in genetic risk factors for ARDS nor focus on other genomic biomarkers, including gene expression and metabolomics. Instead, this review will discuss the role of proteomics in identifying biologic pathways important in the pathophysiology of ARDS. Targeted proteomics has already identified multiple potential ARDS biomarkers. We then review recent work incorporating multiple biomarkers into multivariable models that both improve ARDS prognostication and suggest the presence of novel ARDS subphenotypes, not always identifiable by clinical phenotyping. We emphasize the challenges inherent in differentiating protein signals of lung injury from underlying predisposing conditions of sepsis and trauma. Finally, we postulate a critical role for proteomics in informing drug development by enhancing phenotyping of clinical subgroups of ARDS subjects with the greatest potential to benefit from targeted pharmacologic therapy.
Individual proteomic derangements identify relevant biologic pathways in ARDS
Limitations of single-marker proteomic studies in ARDS to date
Several inherent challenges have limited the utility of protein biomarkers in ARDS relative to other diseases, and currently available biomarkers continue to lack sufficient validity to be incorporated into clinical practice for either the diagnosis or prognosis of ARDS. First, as noted above, ARDS is a syndrome that occurs in response to disparate pathophysiologic disturbances, limiting the value of individual biomarkers specific to one biologic pathway. Models combining multiple biomarkers from different biologic pathways may be needed to establish reliable biologic criteria for ARDS.
In addition to heterogeneity of underlying pathophysiologies, studies attempting to validate protein biomarkers have suffered from heterogeneity in the timing of sample collection relative to time of onset of ARDS. The underlying pathologies contributing to the development of ARDS are dynamic processes with rapidly changing protein signals between early versus late sepsis or early lung injury versus repair adding significant noise obscuring background signals of ARDS. Biobanks of reliably timed samples collected earlier in the development of ARDS may enhance efforts to derive a biologic signal of ARDS.
ARDS proteomics studies to date have overwhelmingly focused on plasma, which is relatively easily obtained and readily available in many large cohorts, but may poorly reflect conditions in the lung. Conversely, several nontargeted proteomic analyses have assessed bronchoalveolar lavage fluid (BAL) fluid which may be more relevant to lung pathology, but are relatively harder to obtain, and hard to obtain sequentially. We note that direct sampling of edema fluid is possible early in the course of ARDS [11]. Alternatively, as proteomic evaluations of exhaled breath improve [12], this may be an appealing option to enhance timing/longitudinal assessment for future studies.
Studies of biomarkers from unselected populations of ARDS suffer from poor specificity of markers of ARDS relative to underlying risk factors – notably sepsis and trauma – for ARDS. Future efforts will likely need to study more homogeneous subgroups of ARDS to identify biomarkers or therapeutic targets that may not be applicable to all patients with ARDS.
Finally, identifying proteomic changes specific to lung injury is limited by lack of specific clinical criteria for ARDS and also confounding of major biologic disturbances inherent to critical illness. Validity of biomarkers for the diagnosis of ARDS in current large multicenter populations is likely limited by misclassification of cases primarily with severe sepsis or trauma with relative minor or no diffuse lung injury. In the era of lung protective ventilation, risk for death in ARDS patients only partly relates their degree of ARDS severity. Therefore, in regard to prognostication, biomarkers that correctly identify more severe cases of ARDS may misclassify patients who lack other comorbidities and recover from their ARDS relative to patients with relatively minor ARDS who succumb from other significant comorbidities.
Individual proteomic changes in ARDS identify relevant pathways
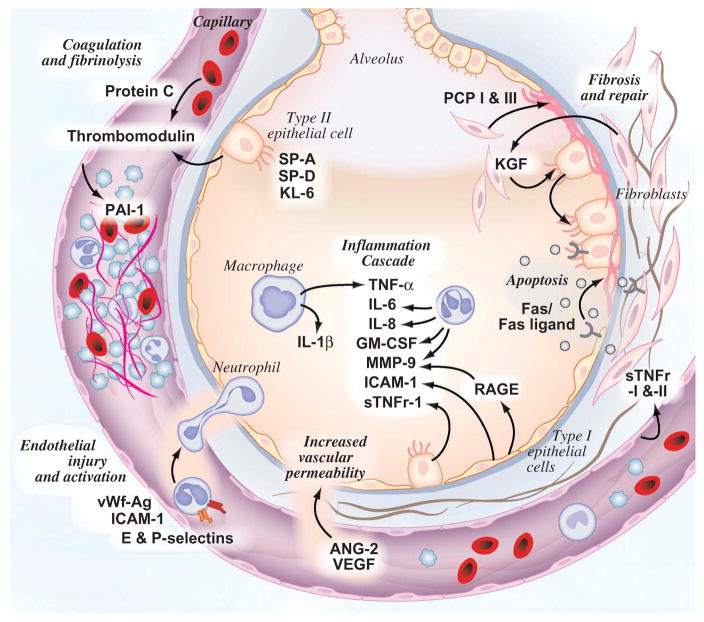
Despite the difficulties in identifying robust biomarkers in ARDS, targeted proteomics studies have identified important pathways in the disease. The protein biomarkers highlighted below are associated with either development of ARDS or mortality in patients with ARDS. Figure 1 summarizes the current knowledge of key biologic pathways and their associated proteomic biomarkers in the pathobiology of ARDS.
Figure 1.
Key pathways and known proteomic changes in ARDS pathogenesis.
A summary of key biomarkers and their proposed biologic pathways is summarized in Table 1. Other reviews have provided a more comprehensive summary of the current literature [13–16]. Instead of recreating these efforts, we will highlight well-validated protein biomarkers representing critical pathways in the pathophysiology of ARDS. In particular, we will discuss markers of inflammation, epithelial injury, endothelial injury or activation, and disordered coagulation and repair with specific emphasis on their differential performance in subtypes of ARDS (e.g. with sepsis or trauma as their ARDS risk factor).
Table 1.
Proteomic pathways associated with ARDS.
| Pathway | Biomarker | Source | Role in ARDS | Reference |
|---|---|---|---|---|
| Endothelium injury and activation | VWF-Ag | Endothelial cells and platelets | Increase platelet binding to vascular endothelium | [17–22] |
| Ang-2 | Endothelial cells | Regulates capillary permeability | [21,23–26] | |
| ICAM-1 | Endothelial cells, type 1 alveolar cells, leukocytes | Promotes leukocyte binding and transmigration | [27–31] | |
| E-selectin & P-selectin | Endothelial cells, platelets & leukocytes | Promotes leukocyte binding and transmigration | [32–34] | |
| VEGF | Endothelial cells | Increases vascular permeability | [35–39] | |
| Epithelial injury | SP-A & SP-D | Type II alveolar cells | Impaired alveolar recruitment and innate immunity | [40–43] |
| RAGE | Type I epithelial cells | Receptor for inflammatory ligands | [44,45] | |
| KL-6 | Type II epithelial cells | Increased expression in injury or repair | [46] | |
| Inflammation | IL-6 | Leukocytes | Proinflammatory | [43,47–50] |
| IL-8 | Leukocytes | Proinflammatory | [43,47–50] | |
| IL-1β | Macrophages | Proinflammatory | [49] | |
| TNF-α | Macrophages | Proinflammatory | [49,51,52] | |
| sTNFr-I & II | Multiple tissues | Proinflammatory | [51–53] | |
| Neutrophils | Vascular migration | Promote phagocytosis and cellular injury | [54–56] | |
| MMP-9 | Neutrophils | Mediate neutrophil cellular injury | [57] | |
| Macrophages | Resident in lung and vascular migration | Promote immunity and repair | [58] | |
| GM-CSF | Leukocytes and endothelial cells | Promote immunity and repair | [59] | |
| Coagulation | Protein C | Liver | Prevents in-situ thrombosis | [23,30,60] |
| PAI-I | Vascular endothelial cells and platelets | Promotes thrombosis | [23,30,48,60–62] | |
| Thrombomodulin | Type II pneumocytes | Activates protein C | [19,60] | |
| Oxidative stress | Nitric oxide | Endothelial cells and leukocytes | Mediator of oxidative injury | [63] |
| Ferritin | Acute phase reactant and product of oxidative stress | Mediator of oxidative injury | [64,65] | |
| Antioxidants | Ubiquitous | Prevent oxidative injury | [66] | |
| Fibrosis | PCP I & III | Fibroblasts | Marker of collagen synthesis | [67–69] |
| Disordered repair | TGF-α | Ubiquitous | Endothelial cell migration and angiogenesis | [70,71] |
| KGF & HGF | Mesenchymal cells | Epithelial cell mitogens | ||
| Apoptosis | Fas/Fas ligand | Ubiquitous | Promote cell death | [72–74] |
| Perforin/granzyme | Ubiquitous | Promote cell death | [74] | |
| Extracellular matrix | Desmosine | Lung interstitium | Marker of disruption of extracellular matrix | [75,76] |
GM-CSF, granulocyte/macrophage colony stimulating factor; HGF, hepatocyte growth factor; ICAM-1, intercellular adhesion molecule 1; IL-1β, interleukin 1 beta; KGF, keratinocyte growth factor; PAI-I, plasminogen activator inhibitor-I; PCP I & III, procollagen peptides I & III; RAGE, receptor for advanced glycation end products; SPA & D, surfactant protein A & D; TGF-α transforming growth factor alpha; TNF-α, tumor necrosis factor alpha; vWF-Ag, von Willebrand factor antigen.
Endothelial injury and activation
Loss of integrity of the endothelium with efflux of protein rich edema fluid is an important step in the development of ARDS. Endothelial injury in response to inflammatory cytokines leads to increased capillary permeability while activation of endothelial binding proteins induces transmigration of inflammatory cells into alveoli controlling local infection but also propagating ongoing inflammation and alveolar epithelial injury [3].
von Willebrand factor antigen (VWf-Ag)
VWf is a large multimeric glycoprotein synthesized and stored in Weibel–Palade bodies in endothelial cells and megakaryocytes. VWf plays an important role in hemostasis by bridging platelet binding to vascular endothelium and serving as a carrier protein for factor VIII [77]. VWf is secreted by endothelial cells throughout the body in response to mediators of inflammation and clotting cascade including fibrin, histamine, thrombin, and trypsin [32,78,79]. However, this response is not specific to lung endothelium potentially limiting the role of VWf for identifying lung specific injury.
Studies of circulating levels of plasma VWf-Ag have produced mixed results for predicting both development of ARDS in at-risk patients and mortality in patients with ARDS [17–19]. Moss et al., studying both septic (pulmonary and nonpulmonary) and nonseptic (trauma, aspiration, massive transfusion, and pancreatitis) patients, found plasma VWf-Ag levels were elevated in both groups relative to normal controls but did not distinguish between patients who did and did not develop ARDS. Importantly, while the study separately evaluated both septic and nonseptic patients, both groups contained a mix of patients with potential for systemic endothelial activation (nonpulmonary sepsis in the septic group and trauma, pancreatitis, and massive transfusion in the nonseptic patients) versus lung-specific endothelial activation (pneumonia in the septic patients and aspiration in the nonseptic patients) which may have contributed to the lack of specificity of VWf-Ag for identifying patients who progressed to ARDS.
In a large multicenter analysis of 559 patients enrolled in the NHLBI ARDS Network multicenter trial of lower tidal volumes (ARMA) by Ware et al., higher baseline plasma VWF-Ag levels were associated with increased mortality despite controlling for severity of illness, sepsis, and ventilator strategy [20]. VWf-Ag levels were similar in septic and nonseptic patients but were lower in patients with trauma as a primary risk factor. Day 3 levels of VWF-Ag were also lower in survivors but were not different between the high and low tidal volume groups, suggesting that the beneficial effects of lower tidal volume ventilation may not be mediated primarily through effects on the lung endothelium. In analysis of 931 patients in the ARDS Network Fluid and Catheter Treatment Trial, Calfee et al. found a similar association of higher baseline VWF-Ag levels and mortality in both septic and nonseptic patients independent of severity of illness and fluid management strategy [21].
Angiopoietin-2 (Ang-2)
Ang-2 is a ligand for the Tie2 receptor, which blocks phosphorylation and promotes vessel destabilization [23]. In experimental models of ARDS, Ang-2 promotes alveolar epithelial cell death and extracellular gap formation in endothelial cells [23,24]. In humans, Ang-2 is elevated in sepsis and higher Ang-2 levels are associated with the development of ARDS in both septic and nonseptic patients, and with increased mortality in surgical patients with ARDS [23,25,26].
The complicated interaction of sepsis with Ang-2 levels in ARDS is highlighted by Calfee et al.’s analysis of the ARDS Network Fluid and Catheter Treatment Trial [21]. Higher levels of Ang-2 at baseline were associated with mortality only in nonseptic patients, suggesting a blunting of the signal for lung-specific endothelial injury with systemic increases of Ang-2 in sepsis. However, higher Ang-2 levels at day 3 were associated with mortality in both septic and nonseptic patients and rising Ang-2 levels in septic patients identified a particularly poor prognosis. In addition, a conservative fluid strategy was associated with lower Ang-2 levels in septic (particularly those not in shock and thus managed per protocol) but not nonseptic patients. In contrast, baseline VWf-Ag levels were lower in survivors independent of sepsis but change in VWf-Ag to day 3 was not associated with mortality, and VWf-Ag levels were not affected by fluid management strategy.
The response of Ang-2 levels to conservative fluid management in septic patients – which may simply reflect larger fluid balance shifts in patients who initially received volume loading during early goal-directed therapy – suggests that, in contrast to low tidal volume ventilation, the benefit of conservative fluid management may in part be mediated by decreased endothelial injury. Targeting patients with higher levels of Ang-2 could allow selection of patients with the most potential to benefit from future trials of fluid management in patients with or at-risk for ARDS. Interestingly, as with lower tidal volumes, fluid management did not impact VWf-Ag levels, and an explanation of this discordant effect on two makers of endothelial injury and activation is unclear.
Intercellular adhesion mollecule-1 (ICAM-1)
ICAM-1 is a cell surface glycoprotein expressed on leukocytes, vascular endothelial cells, and type 1 alveolar epithelial cells in response to inflammatory cytokines and is an important mediator for binding and transmigration of leukocytes into organs during inflammation or injury [80]. ICAM-1 levels appear to be elevated in BAL relative to serum in patients at-risk for ARDS or with ARDS but not with hydrostatic edema [27,28]. However, neither plasma nor BAL levels predict development of ARDS in at-risk patients nor distinguish patients with ARDS from at-risk patients [28].
ICAM-1 may have a greater role in prognosis of ARDS. Higher serum levels have been associated with worse outcomes in patients with ARDS [29–31]. In a large multicenter analysis of the ARDS Network ARMA trial, higher baseline plasma levels of ICAM-1 were independently associated with mortality and fewer ventilator- and organ-failure free days. As with Ang-2, rising levels of ICAM-1 on day 3 were associated with increased odds of death. Importantly, none of these studies specifically analyzed the interaction of sepsis on ICAM-1 levels, which may explain some of the lack of signal for identifying patients with ARDS. Also, the association with worse outcomes in ARDS may simply be a marker for more severe or nonresolving sepsis and not specifically worse lung injury.
Epithelial injury
Epithelial injury leading to sloughing of type 1 pneumocytes with loss of barrier function and reduced active resorption of edema fluid via ATPase-dependent sodium pumps is a hallmark of the pathophysiology of ARDS [3]. Protein biomarkers specific to alveolar epithelial injury have a theoretical benefit of less interaction with systemic inflammation and endothelial activation, making them highly sought as potentially more specific markers of lung injury, similar to troponin level for identifying myocardial injury. However, identification of a marker with sufficient sensitivity and specificity in peripheral blood for alveolar injury remains elusive.
Surfactant proteins
Surfactant proteins A through D are amphiphilic lipoproteins secreted by type II pneumocytes with important functions for lung homeostasis as highlighted by infantile respiratory distress syndrome which develops in premature infants born prior to development of sufficient surfactant production. Hydrophobic surfactant proteins B and C reduce alveolar surface tension at the air–fluid interface preventing alveolar collapse at expiratory lung volumes [81]. Hydrophilic surfactant proteins A and D (SP-A and SP-D) also contribute to innate immunity by coating bacteria and viruses, and promoting phagocytosis by macrophages [82].
Studies of surfactant proteins highlight the complexity of differing results depending on which surfactant proteins are measured and which compartments are sampled. Studies measuring SP-A and SP-D suggest levels of these proteins are decreased in the alveolar compartment in patients at-risk for and with ARDS while plasma levels are increased in ARDS [40,41]. In multicenter data from the ARDS Network ARMA trial, higher plasma levels SP-D but not SP-A were independently associated with death, and fewer ventilator- and organ-failure free days [42]. In contrast to VWf-Ag, analyzed in a separate analysis of the same trial, lower tidal volume ventilation attenuated the increase in SP-D levels on day 3 of mechanical ventilation. Together these results suggest surfactant proteins levels are reduced in alveoli due to impaired production or secretions by pneumocytes while increased plasma levels reflect loss of epithelial barrier integrity with systemic release.
More recently, plasma SP-D level was one of the best performing biomarkers for identifying cases of ARDS in patients with severe sepsis enrolled in the validating acute lung injury biomarkers for diagnosis (VALID) study and results were more predictive in analysis limited to more severe cases of ARDS [43]. This intriguing result suggests plasma SP-D may be particularly useful for diagnosing ARDS in sepsis since its presence in peripheral blood is likely more specific for alveolar epithelial injury rather than endothelial proteomic changes that may reflect systemic inflammation and injury in sepsis.
Receptor for advanced glycation end products (RAGE)
RAGE is a multiligand-binding transmembrane immunoglobulin that is heavily expressed in the lung and was previously thought to be specific to type 1 epithelial cells making it a compelling biomarker for ARDS [83]. However, RAGE expression has subsequently been found in a wide variety of cells including vascular endothelial cells [84]. Smaller studies have found higher plasma RAGE levels predict worse outcomes following lung transplant [44] and distinguished patients with ARDS from intubated patients with severe sepsis without ARDS [85].
In a multicenter analysis from the ARDS Network ARMA trial, higher baseline RAGE levels were only independently associated with worse outcomes in patients randomized to higher tidal volumes [86]. Also, RAGE levels declined more in patients randomized to lower tidal volumes and the mortality benefit of lower tidal volumes occurred in patients with the highest baseline RAGE levels.
These results suggest that the benefit of lower tidal volumes was mediated in part through decreased epithelial injury. RAGE was also one of the best performing biomarkers for diagnosing ARDS in the VALID cohort [43]. Thus, RAGE may have a role in identifying subgroups of patients most likely to benefit from future trials comparing strategies for lung protective ventilation, or as a surrogate endpoint for reduced epithelial injury in future phase II trials. However, in a separate analysis of the ARMA trial, RAGE was not associated with worse outcomes when the analysis was limited to patients with an APACHE II < 25 [87]. Along with the lack of association of baseline RAGE levels and outcomes in patients managed with lower tidal volumes in ARMA, this result questions the reliability of RAGE in less severely injured patients, especially in an era of lung protective ventilation.
Krebs von Lundgren-6 (KL-6)
KL-6 is a MUC1 mucin protein with increased expression on the surface of type II pneumocytes in response to injury or during repair [88]. In a recent meta-analysis of plasma biomarkers, KL-6 had the highest odds ratio (OR 6.1, 95%CI 3.3–12) for diagnosing ARDS and higher levels were also associated with increased mortality in patients with ARDS [15]. However, KL-6 has only been studied in relatively few patients and needs further validation in large, multicenter patient populations.
Inflammatory mediators of lung injury
Inflammation, either in response to primary lung injury or secondary to systemic inflammation, is an important mediator of ARDS. In addition, injurious mechanical ventilation can induce inflammation and contribute to multiorgan failure in ARDS regardless of the inciting etiology of the underlying lung injury.
Interlukin-6 and interlukin-8 (IL-6 and IL-8)
In the ARMA trial, higher baseline levels of proinflammatory cytokines, IL-6 and IL-8, were independently associated with fewer organ-failure and ventilator-free days but only IL-8 was associated with greater mortality [47]. Importantly, IL-6 and IL-8 were higher in sepsis and pneumonia relative to other causes of ARDS. However, lower tidal volumes were associated with a more rapid decline in both cytokines suggesting that change in IL-6 and IL-8 could serve as surrogate endpoints independent of the cause of lung injury. Importantly, IL-6 and IL-8 levels have also been found to be independently associated with subsequent studies of patients managed with lower tidal volume ventilation [30,48].
Tumor necrosis factor-alpha (TNF-α) and soluble tumor necrosis factor receptors I and II (s-TNFr-I and II)
As with IL-6 and IL-8, baseline levels of s-TNFr-I and II, but not TNF-α, were independently associated with worse outcomes in the ARMA trial [51]. However, only s-TNFr-I levels were significantly lower in patients treated with lower tidal volumes. Experimental human epithelial (A549) cells secrete s-TNFr-I, but not s-TNFr-II in response to inflammatory cytokines TNF-α, interlukin-1β (IL-1β), and interferon-γ, suggesting that s-TNFr-I may also serve as a surrogate endpoint for alveolar epithelial injury. In a single-center analysis, Meduri found baseline levels of IL-1β were also higher in nonsurvivors and, along with IL-6, persistent elevation of was a strong predictor of mortality [49]. Importantly, elevated cytokine levels were also associated with sepsis in this study, but were more highly associated with outcomes of ARDS than with underlying etiology.
Relevant to drug development, Meduri subsequently found that methylprednisolone improved mortality in a small trial of 24 patients with late (7–21 days), nonresolving ARDS [89]. However, in a subsequent multicenter clinical trial by the ARDS Network in a similar patient population, methylprednisolone reduced duration of mechanical ventilation but there was no improvement in mortality. In addition, there was a higher rate of re-intubation in patients treated with methylprednisolone and a trend toward worse mortality in patients enrolled between 14 and 21 days [90]. Importantly, cytokine profiles were not assessed as inclusion criteria for either trial. Also, a phase II trial of anti-TNF-α therapy with a fragment antigen-binding (Fab) dimer for patients with severe sepsis found rapid reduction in TNF-α levels and reduced ventilator- and ICU-free days [91]. However, a subsequent phase IIb trial of a related polyclonal Fab fragment found no clinical benefits despite efficient reduction in TNF-α concentrations [92]. Other targeted anti-inflammatory therapies have not been studied in ARDS.
Disordered coagulation
Activation of the coagulation cascade and impaired fibrinolytic activity leading to in-situ microvascular thrombosis are thought to contribute to organ failure in severe sepsis [93] and are specifically implicated in the pathogenesis of pneumonia and ARDS [94–97]. Activation of protein C by thrombo-modulin is an important step in inhibiting clot formation while plasminogen activator inhibitors released by endothelial cells and platelets are important inhibitors of fibrinolysis. Small studies have demonstrated lower plasma levels of activated protein C, and higher levels of plasminogen activator inhibitor-1 (PAI-1) and thombomodulin are associated with worse outcomes in ARDS [30,60,61].
In a large multicenter analysis from the ARMA trial, lower enrollment levels of protein C and higher levels of PAI-1 were independently associated with greater mortality and fewer organ-failure and ventilator-free days [98]. However, PAI-1 was not associated with outcomes in two separate multicenter analyses in patients with ARDS [48,62]. In a clinical trial of patients with ARDS but without severe sepsis, treatment with activated protein C reduced pulmonary dead space fraction but did not impact other clinically relevant of outcomes [99].
Apoptosis, fibrosis, and impaired healing
Activation of mesenchymal cells and collagen deposition is an important step in restoring the alveolar gas exchange apparatus following epithelial cell death in ARDS [3]. However, tight regulation of a complicated recovery process is needed and disordered repair and ongoing injury can lead to fibrosis and progressive loss of lung function [67]. The extent of fibrosis, either as a measure of the severity of injury or as a measure of disordered repair, correlates with outcome in ARDS [67].
Fas and Fas ligand
Apoptosis of epithelial cells occurs in both direct and indirect etiologies of ARDS [100]. The Fas/Fas ligand system is the best-studied mechanism regulating epithelial cell death. Soluble Fas and Fas ligand are elevated in pulmonary edema fluid relative to plasma levels in patients with early ARDS and relative to edema fluid from controls with hydrostatic edema [72,73]. Higher baseline Fas and Fas ligand levels in tissue and edema also correlate with increased morbidity and mortality [72,73]. Also, messenger RNA for Fas and Fas ligand were upregulated in BAL fluid during the acute phase of sepsis-related ARDS but not sepsis without ARDS or the late phase of sepsis-induced ARDS [74].
These results suggest epithelial apoptosis occurs early in ARDS and, at least in the alveolar compartment, Fas and Fas ligand may be specific markers for ARDS in patients with sepsis. However, the Fas/Fas ligand system is not unique to lung epithelial cells likely limiting the sensitivity and specificity of Fas and Fas ligand as plasma biomarkers. However, edema fluid from patients with ARDS can induce apoptosis in cultures of epithelial cells [72]. This process was blocked by inhibiting the Fas/Fas ligand pathway, suggesting a potential novel therapeutic target for the prevention or treatment of ARDS.
Procollagen peptides
Procollagen peptide III (PCP III), a marker of collagen synthesis, is elevated in edema fluid sampled during intubation in patients with ARDS compared to those with hydrostatic edema and higher levels of PCP III are highly associated with mortality, suggesting the fibroproliferative response occurs early and may play an active role in the progression of ARDS [57,68]. Meduri also found plasma levels of PCP I and PCP III were increased at baseline in patients with ARDS and rising levels predicted worse outcomes [69]. Treatment with methylprednisolone led to rapid and sustained reduction in plasma and BAL levels of both proteins. However, as above, the benefits of methylprednisolone for treatment of late, nonresolving ARDS were not confirmed in a multicenter clinical trial by the ARDS Network [89,90].
Keratinocyte growth factor (KGF)
KGF is a member of the fibroblast growth factor family and is a potent mitogen for type II pneumocytes and may be an important mediator of epithelial repair following lung injury [101,102]. In addition to enhancing alveolar epithelial cell migration and repair in wound healing studies of isolated alveolar epithelial cells, KGF protected against lung injury in experimental models of ARDS from hyperoxia, acid aspiration, mechanical ventilation, bacterial pneumonia, radiation injury, and bleomycin-induced lung injury [103]. In ex vivo human lung studies, KGF enhanced resolution of alveolar edema by restoring normal alveolar edema fluid clearance and enhanced monocyte phagocytosis of E. coli, via a KGF receptor found on monocytes [104]. A phase II study of KGF for the prevention of ARDS in patients with severe sepsis has recently been completed but published results are pending (ISRCTN95690673).
Proteomics studies incorporating multiple biomarkers
In addition to the targeted single biomarker studies described above, which point to multiple distinct mechanisms involved in ARDS pathogenesis, several groups have tested multiple biomarkers within the same cohort to either identify novel aspects of ARDS pathogenesis or improve identification and prediction of ARDS.
Novel ARDS subphenotypes
Calfee et al. [105] used latent class analysis (LCA) to identify subtypes of ARDS using data from two large clinical trials incorporating patients with diverse etiology of ARDS [47,106]. More than 30 baseline clinical and biomarker variables were considered as class-defining variables. In both large clinical trials, the populations were best defined using two classes, or sub-phenotypes. The smaller class (~30% of both ARDS populations) was consistent with a more inflammatory type – higher levels of IL-6 and IL-8, more frequent baseline vasopressor requirement, and comprised of a higher proportion of patients with sepsis as the underlying ARDS risk factor. Importantly, this class of patients could not be inferred by clinical categories, but was associated with both higher mortality and a differential response to therapy with high PEEP. A parsimonious model incorporating two biomarkers (IL-6 and soluble TNFr-I) and the clinical variable of vasopressor use at baseline correctly classified the phenotype with >90% accuracy in both populations. This suggests that this phenotype could potentially be assessed as an enriched target population for enrollment in future clinical trials.
Direct versus indirect lung injury
Calfee et al. [107] examined how biomarkers differ by lung injury mechanism (e.g. pneumonia or aspiration for direct injury vs. pancreatitis or nonpulmonary sepsis for indirect injury). Five biomarkers were tested simultaneously in two cohorts (a single-center cohort [108] and a multicenter clinical trial [109]), including markers of epithelial injury (SP-D and RAGE), endothelial injury (Ang-2), and inflammation (IL-6 and IL-8). As expected, SP-D was significantly higher and Ang-2 significantly lower in patients with direct versus indirect lung injury. However, the prognostic value of the biomarkers for association with mortality was similar regardless of lung injury etiology, with IL-6, IL-8, and Ang-2 associated with increased odds of death in both populations. RAGE showed a trend toward significance only in patients with direct lung injury (OR 2.3 vs. 1.0 in the single-center cohort and 1.5 vs. 1.2 in the clinical trial, though neither interaction term was significant).
Multimarker protein panels in identifying ARDS
Fremont et al. examined seven biomarkers representing multiple biologic pathways (including RAGE, PCP III, Ang-2, BNP, IL-10, TNFα, and IL-8) in 192 patients admitted to the trauma ICU, of whom 107 developed ARDS [110]. The 7-biomarker panel differentiated ARDS with an AUC of 0.82. In a population of sepsis patients, Ware et al. examined 11 biomarkers in 100 patients with sepsis and ARDS versus 100 with sepsis and no ARDS. They identified a panel of five biomarkers [SP-D, RAGE, IL-6, IL-8, and club cell secretory protein (CC-16)] representing multiple types of injury out-performed any single biomarker, and performed particularly well at identifying severe ARDS [43].
Multimarker protein panels in improving ARDS mortality prediction
Calfee et al. tested multiple biomarkers for risk prediction for mortality in comparison to the widely used APACHE 2 score [111]. They found that a parsimonious 3-biomarker panel (IL-8, TNF-α, and SP-D) substantially improved risk prediction in two independent ARDS cohorts.
Nontargeted proteomics
While the studies above all focused on a single or several individual proteins with an a priori hypothesis for involvement in lung injury using highly quantitative techniques like ELISA to measure a specific protein, nontargeted proteomics instead examines many analytes in an unbiased fashion. The benefit of this approach is the potential to identify novel ARDS mechanisms and potential therapeutic targets.
A detailed discussion of the methodology of nontargeted proteomics is beyond the scope of this article; fortunately, multiple outstanding reviews have been published previously [112,113]. Briefly, mass spectrometry is the most commonly used method of proteomic profiling, which was made possible on a large scale because of advances in electrospray ionization of peptides. The two most commonly used methods of reliable protein identification are also those most widely used in ARDS proteomics studies today. The first is difference in gel electrophoresis (DIGE), in which gel spots are identified and then excised for further characterization by mass spectrometry, often termed a ‘bottom-down approach’. In contrast, a ‘bottom-up approach’ involves proteolysis of the sample to peptides followed by liquid chromatography-mass spectroscopy (LC-MS-MS). Each method has its strengths, with DIGE able to detect a more limited range of high-abundance proteins, while LC-MS-MS was previously thought to be slightly less quantitative. This latter deficit is somewhat overcome with stable isotope tagging (e.g. isobaric tags for relative and absolute quantification (iTRAQ), below).
In Table 2, we have summarized the ARDS nontargeted proteomic literature to date. We note several aspects to these studies. First, no two studies use identical methods for sample handling, let alone proteomic profiling, with some using DIGE, others LC MS-MS, yet others iTRAQ labeling. Thus, given that different methods are optimized to identify different proteins, it is unsurprising that their major conclusions vary widely.
Table 2.
Nontargeted proteomics studies in acute respiratory distress syndrome (ARDS).
| First author/year | N | Control population | Fluid type | N proteins | Profiling method | Major conclusions |
|---|---|---|---|---|---|---|
| Bowler [116] | 16 ARDS | 12 healthy controls | Plasma and pulmonary edema/BAL | 158 | 2-D gel electrophoresis – MALDI TOF | ↑Surfactant protein A isoforms in patients with ARDS & many protein modifications/truncations |
| De Torre[118] | 11 ARDS | 33 healthy controls + endotoxin challenge | BAL | SELDI-TOF mass spectrometry | ↑ApoA1, S100 Ca-binding proteins in both ARDS and inflammatory response | |
| Schnapp [115] | 3 ARDS | 6 healthy controls | BAL | 226–659 per patient | Shotgun proteomics, LC-MS-MS | Many proteins differ; focus on IGF pathway changes, validated in independent BAL set |
| Chang [114] | ARDS by time: D1 (N = 7), D3 (N = 8), D7 (N = 5) | 9 healthy controls | BAL | 37 | 2-D gel electrophoresis-MALDI TOF | Proteins in inflammation, infection, and injury differ in ARDS vs. healthy and differ over time |
| Gessner [120] | 24 ARDS | 10 healthy 6 pneumonia (no ARDS) |
Exhaled breath | 3 | 2-D gel electrophoresis – MALDI TOF | Cytokeratins 2, 9, and 10 present in ARDS. Higher levels correlated with PEEP, PIP, and ARDS |
| Chen [119] | Direct (6) & indirect (5) ARDS | 15 healthy controls | Plasma | 132 | iTRAQ (isoberic tags for relative absolute quantification) LC-MS-MS | Substantial overlap in ARDS differentially expressed proteins, regardless of direct/indirect; involve 8 canonical pathways |
| Bhargava[117] | Early ARDS survivors (7) vs. nonsurvivors (8) Late ARDS (7) |
N/A | BALF | 499 | iTRAQ LC MS/MS | Early survivors: ↑ injury response incl. immune response & cation/iron homeostasis; vs. nonsurvivors: collagen synthesis/catabolism |
BAL: bronchoalveolar lavage fluid; IGF: insulin-like growth factor; iTRAQ: isobaric tags for relative and absolute quantification; MALDI TOF: matrix-assisted laser desorption/ionization TOF (time of flight); PEEP: positive end-expiratory pressure; PIP: peak inspiratory pressure; SELDI-TOF: surface-enhanced laser desorption/ionization-TOF (time of flight).
The nontargeted proteomic studies in ARDS conducted to date differ in important ways from the targeted plasma biomarker studies highlighted above. First, proteomic studies in ARDS to date have typically examined a small number of samples (Table 2). Given the extensive heterogeneity in ARDS discussed earlier in this review, small sample sizes will likely contribute to heterogeneous conclusions. Also notable is the fact that most nontargeted proteomics studies have focused on comparing the protein levels in the alveolar compartment, usually BAL, undiluted pulmonary edema fluid or exhaled breath. While most studies to date compared ARDS samples to healthy controls, Bhargava et al. examined BAL fluid from different subtypes of ARDS patients – specifically 15 in early phase (days 1–7), including 7 survivors and 8 non-survivors; and 7 in late phase (day 8–35). They identified 792 proteins, and found that 161 were differentially expressed in early-phase ARDS between survivors and nonsurvivors. Pathways including activation of immune response, wound healing, and multiple pathways involving coagulation are more abundant in survivors, while collagen synthesis and carbohydrate catabolism are more abundant in nonsurvivors. While these results are exploratory given small numbers, they suggest the promise of nontargeted proteomic study in identifying novel mechanisms and thus future drug targets in ARDS.
Proteomics and the future of drug discovery in ARDS
As highlighted above, targeted proteomics has already identified multiple mechanistic pathways that are involved in ARDS, including endothelial and epithelial injury, inflammation, disordered coagulation and proteins involved in lung healing (including apoptosis and fibrosis pathways). The importance of these pathways has been confirmed in human disease, as many of the proteins can serve as biomarkers either in identifying subphenotypes of ARDS or associated with disease prognosis.
In contrast, nontargeted proteomics has been applied to much smaller datasets in ARDS to date, often comparing ARDS edema fluid to that of healthy controls as opposed critically ill or at-risk patients without ARDS. Because of differing proteomic profiling techniques and patient populations, the role of these newly identified proteins as potential biomarkers or as novel ARDS therapeutic mechanisms is less certain.
Despite these advances in ARDS proteomics to date, none of the pathways have specifically been targeted in novel drug trials. We nonetheless argue that proteomics has substantial potential to advance drug development in ARDS.
Proteomics for identifying endophenotypes of ARDS for therapeutic trials
While the vast majority of ARDS clinical trials to date have largely included all patients (mild and severe ARDS, direct and indirect lung injury, and septic and nonseptic patients), proteomics will likely play a major role in identifying enriched subphenotypes of ARDS with the highest likelihood of benefiting from specific targeted therapy. A 2-biomarker panel of IL-6, soluble TNFr-I, and need for vasopressors identified a subgroup of ‘inflammatory ARDS’ patients who benefited from the intervention of higher levels of PEEP but could not be identified based on clinical factors alone [105]. Thus, a trial targeting this enriched population of 300 patients may have shown a significant benefit where the larger trial of 1000 patients did not. We anticipate that collecting a plasma sample will be the default in future therapeutic clinical trials to test whether therapies are effective in a subset of patients. If a subphenotype is identified repeatedly in clinical trials as particularly likely to benefit from therapeutic interventions, these patients may specifically be targeted in novel therapeutic trials. Equally importantly, patients unlikely to benefit can be excluded from such trials and thus are spared the risk of potential side effects. To enable this important advance, point-of-care tests of proteomic biomarkers would need to be developed to enable identification of eligible subjects at the time of trial enrollment.
Nontargeted proteomics for identifying novel ARDS mechanisms
As reviewed above, nontargeted proteomics studies to date have been limited by small sample size and use of healthy controls as a comparison group. As proteomics technologies improve and become less costly, we anticipate that larger-scale nontargeted proteomics studies of both plasma and BAL will identify novel mechanisms of ARDS pathogenesis, which could themselves serve as targets for future drug development. Of particular interest are therapies with the potential to convert patients from a phenotype of collagen synthesis and carbohydrate catabolism seen in nonsurvivors to one of an activated but regulated immune response, wound healing, and coagulation homeostasis seen in survivors.
Proteomic change as intermediate endpoint in clinical trials
While large, multicenter trials in ARDS often target mortality as an endpoint, as mortality of ARDS has improved from 40% to 50% 10 years ago to 20% to 25% in recent clinical trials [121], powering trials for a mortality endpoint is becoming increasingly expensive. In addition, patients with ARDS are by definition critically ill, and death is often attributed to multisystem organ failure rather or other comorbidities rather than refractory ARDS. In this review, we have highlighted protein biomarkers that have been associated with important endpoints like ARDS mortality, development of ARDS, and even response to fluid management strategy. The discriminatory ability of these biomarkers has not yet been sufficiently established; thus, they have yet to be incorporated into clinical practice or ARDS trial design. However, if proteomic biomarkers (from plasma or BAL) are repeatedly found to be associated with key pulmonary outcomes, these could serve as important intermediate variables and surrogate endpoints in clinical trials.
Trials targeting multiple pathways
Targeted proteomics has shown that ARDS involves multiple pathways leading to a distinct clinical syndrome. Therapeutic trials in ARDS usually target only a single pathway (e.g. statins or corticosteroids for anti-inflammatory properties, or beta-agonists to enhance alveolar fluid clearance). Given the many dysregulated pathways, it seems reasonable to consider that a multipronged therapeutic approach, using multiple agents (e.g. similar to the approach used in cancer or HIV, among other diseases) may be necessary to effect meaningful change in ARDS pathogenesis. A ‘personalized medicine’ approach to ARDS, in which the most dysregulated pathways for a given individual could be targeted by specific therapies, is likely years away, but may ultimately improve outcomes of ARDS.
Summary
In summary, ARDS is a disease that carries substantial mortality and morbidity, and therapeutic options are lacking. Targeted proteomic approaches have identified multiple proteins that can serve individually and together as biomarkers of both subphenotypes of disease and disease prognosis. We anticipate that in the future, proteomics will play a major role in ARDS by identifying novel targets for drug development and distinguishing enriched subphenotypes of patients with the highest likelihood of benefitting from specific target therapies.
Expert commentary
ARDS is an important disease with substantial attributable morbidity and mortality and very limited therapeutic options. Proteomics has already improved our understanding of ARDS pathophysiology in important ways. Targeted proteomics has confirmed the importance of various pathways of lung injury (notably inflammation, epithelial injury, endothelial injury or activation, and disordered coagulation and repair). Protein changes, alone or in models that combine multiple proteomic changes with clinical features, can serve as potential biomarkers for ARDS pathogenesis. Most excitingly, protein alterations can help to identify novel subphenotypes of disease that may differentiate patients most likely to benefit from novel therapeutic options.
Five-year view
We anticipate that proteomics will advance our understanding of ARDS pathophysiology in several important ways in the next 5 years. First, nontargeted proteomic studies of ARDS are just beginning; as proteomic methods improve and become cheaper, larger studies of carefully phenotyped patients will identify novel pathophysiology in the disease. These novel pathways could serve as fruitful targets for drug development. Second, we anticipate that proteomic biomarkers will improve ARDS phenotyping and enable identification of subsets of patients most likely to benefit from novel therapeutic options. This should in turn enable better-powered ARDS trials because of a lack of misclassified subject enrollment, and hopefully maximize our likelihood of identifying novel therapeutic options for this devastating disease.
Key issues.
ARDS is an important disease with substantial attributable morbidity and mortality and very limited therapeutic options.
Marked heterogeneity in pathogenesis and prognosis of existing populations of ARDS have challenge the identification of protein biomarkers with sufficient validity for clinical use.
While no proteomic biomarkers are currently in widespread clinical practice, targeted proteomics has confirmed the importance of several pathways of lung injury, including inflammation, epithelial injury, endothelial injury or activation, and disordered coagulation and repair
Nontargeted proteomic studies in ARDS have used small sample sizes and methods between studies (including phenotype investigated) has varied, limiting the reproducibility between studies to date.
Single and multi-proteomic biomarker studies are enabling identification of novel subphenotypes of disease, which may enable enrollment of homongenous populations of ARDS patients who are particularly likely to respond to therapy in future clinical trials
Footnotes
Financial & competing interests disclosure
AJ Rogers has been supported by grant number K23 HL 125663-01. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson ND, Fan E, Camporota L, et al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med. 2012 Oct;38(10):1573–1582. doi: 10.1007/s00134-012-2682-1. [DOI] [PubMed] [Google Scholar]
- 3.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000 May 4;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 4.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005 Oct 20;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 5.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012 Aug 1;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villar J, Sulemanji D, Kacmarek RM. The acute respiratory distress syndrome: incidence and mortality, has it changed? Curr Opin Crit Care. 2014 Feb;20(1):3–9. doi: 10.1097/MCC.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 7.Papazian L, Forel J-M, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010 Sep 16;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 8.Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest. 2005 Aug;128(2):525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 9.Villar J, Pérez-Méndez L, Blanco J, et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting–a prospective, multicenter validation study. Intensive Care Med. 2013 Apr;39(4):583–592. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 10.Guérin C, Reignier J, Richard J-C. Prone positioning in the acute respiratory distress syndrome. N Engl J Med. 2013 Sep 5;369(10):980–981. doi: 10.1056/NEJMc1308895. [DOI] [PubMed] [Google Scholar]
- 11.Olman MA, White KE, Ware LB, et al. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 beta-induced IL-6 expression. J Immunol. 2004 Feb 15;172(4):2668–2677. doi: 10.4049/jimmunol.172.4.2668. [DOI] [PubMed] [Google Scholar]
- 12.Kurova VS, Anaev EC, Kononikhin AS, et al. Proteomics of exhaled breath: methodological nuances and pitfalls. Clin Chem Lab Med. 2009;47(6):706–712. doi: 10.1515/CCLM.2009.166. [DOI] [PubMed] [Google Scholar]
- 13.Levitt JE, Gould MK, Ware LB, et al. The pathogenetic and prognostic value of biologic markers in acute lung injury. J Intensive Care Med. 2009 May-Jun;24(3):151–167. doi: 10.1177/0885066609332603. [DOI] [PubMed] [Google Scholar]
- 14.Pittet JF, Mackersie RC, Martin TR, et al. Biological markers of acute lung injury: prognostic and pathogenetic significance. Am J Respir Crit Care Med. 1997 Apr;155(4):1187–1205. doi: 10.1164/ajrccm.155.4.9105054. [DOI] [PubMed] [Google Scholar]
- 15.Terpstra ML, Aman J, van Nieuw Amerongen GP, et al. Plasma biomarkers for acute respiratory distress syndrome: a systematic review and meta-analysis*. Crit Care Med. 2014 Mar;42(3):691–700. doi: 10.1097/01.ccm.0000435669.60811.24. [DOI] [PubMed] [Google Scholar]
- 16•.Walter JM, Wilson J, Ware LB. Biomarkers in acute respiratory distress syndrome: from pathobiology to improving patient care. Expert Rev Respir Med. 2014 Oct;8(5):573–586. doi: 10.1586/17476348.2014.924073. Recent review of ARDS biomarkers with additional detail about several protein biomarkers not emphasized in this current work. [DOI] [PubMed] [Google Scholar]
- 17.Rubin DB, Wiener-Kronish JP, Murray JF, et al. Elevated von Willebrand factor antigen is an early plasma predictor of acute lung injury in nonpulmonary sepsis syndrome. J Clin Invest. 1990 Aug;86(2):474–480. doi: 10.1172/JCI114733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moss M, Ackerson L, Gillespie MK, et al. von Willebrand factor antigen levels are not predictive for the adult respiratory distress syndrome. Am J Respir Crit Care Med. 1995 Jan;151(1):15–20. doi: 10.1164/ajrccm.151.1.7812545. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj MS, Tricomi SM. Plasma levels of the three endothelial-specific proteins von Willebrand factor, tissue factor pathway inhibitor, and thrombomodulin do not predict the development of acute respiratory distress syndrome. Intensive Care Med. 1999 Nov;25(11):1259–1266. doi: 10.1007/s001340051054. [DOI] [PubMed] [Google Scholar]
- 20.Ware LB, Eisner MD, Thompson BT, et al. Significance of von Willebrand factor in septic and nonseptic patients with acute lung injury. Am J Respir Crit Care Med. 2004 Oct 1;170(7):766–772. doi: 10.1164/rccm.200310-1434OC. [DOI] [PubMed] [Google Scholar]
- 21.Calfee CS, Gallagher D, Abbott J, et al. Plasma angiopoietin-2 in clinical acute lung injury: prognostic and pathogenetic significance. Crit Care Med. 2012 Jun;40(6):1731–1737. doi: 10.1097/CCM.0b013e3182451c87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ware LB, Conner ER, Matthay MA. von Willebrand factor antigen is an independent marker of poor outcome in patients with early acute lung injury. Crit Care Med. 2001 Dec;29(12):2325–2331. doi: 10.1097/00003246-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Parikh SM, Mammoto T, Schultz A, et al. Excess circulating angio-poietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006 Mar;3(3):e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhandari V, Choo-Wing R, Lee CG, et al. Hyperoxia causes angio-poietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006 Nov;12(11):1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallagher DC, Parikh SM, Balonov K, et al. Circulating angiopoietin 2 correlates with mortality in a surgical population with acute lung injury/adult respiratory distress syndrome. Shock. 2008 Jun;29(6):656–661. doi: 10.1097/shk.0b013e31815dd92f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Heijden M, van Nieuw Amerongen GP, Koolwijk P, et al. Angiopoietin-2, permeability oedema, occurrence and severity of ALI/ARDS in septic and non-septic critically ill patients. Thorax. 2008 Jun 17;63:903–909. doi: 10.1136/thx.2007.087387. [DOI] [PubMed] [Google Scholar]
- 27.Conner ER, Ware LB, Modin G, et al. Elevated pulmonary edema fluid concentrations of soluble intercellular adhesion molecule-1 in patients with acute lung injury: biological and clinical significance. Chest. 1999 Jul;116(1 Suppl):83S–84S. doi: 10.1378/chest.116.suppl_1.83s. [DOI] [PubMed] [Google Scholar]
- 28.Agouridakis P, Kyriakou D, Alexandrakis MG, et al. The predictive role of serum and bronchoalveolar lavage cytokines and adhesion molecules for acute respiratory distress syndrome development and outcome. Respir Res. 2002;3:25. doi: 10.1186/rr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flori HR, Ware LB, Glidden D, et al. Early elevation of plasma soluble intercellular adhesion molecule-1 in pediatric acute lung injury identifies patients at increased risk of death and prolonged mechanical ventilation. Pediatr Crit Care Med. 2003 Jul;4(3):315–321. doi: 10.1097/01.PCC.0000074583.27727.8E. [DOI] [PubMed] [Google Scholar]
- 30.McClintock D, Zhuo H, Wickersham N, et al. Biomarkers of inflammation, coagulation and fibrinolysis predict mortality in acute lung injury. Crit Care. 2008;12(2):R41. doi: 10.1186/cc6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008 Dec;63(12):1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ribes JA, Francis CW, Wagner DD. Fibrin induces release of von Willebrand factor from endothelial cells. J Clin Invest. 1987 Jan;79(1):117–123. doi: 10.1172/JCI112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okajima K, Harada N, Sakurai G, et al. Rapid assay for plasma soluble E-selectin predicts the development of acute respiratory distress syndrome in patients with systemic inflammatory response syndrome. Transl Res. 2006 Dec;148(6):295–300. doi: 10.1016/j.trsl.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Donnelly SC, Haslett C, Dransfield I, et al. Role of selectins in development of adult respiratory distress syndrome. Lancet. 1994 Jul 23;344(8917):215–219. doi: 10.1016/s0140-6736(94)92995-5. [DOI] [PubMed] [Google Scholar]
- 35.Abadie Y, Bregeon F, Papazian L, et al. Decreased VEGF concentration in lung tissue and vascular injury during ARDS. Eur Respir J. 2005 Jan;25(1):139–146. doi: 10.1183/09031936.04.00065504. [DOI] [PubMed] [Google Scholar]
- 36.Maitre B, Boussat S, Jean D, et al. Vascular endothelial growth factor synthesis in the acute phase of experimental and clinical lung injury. Eur Respir J. 2001 Jul;18(1):100–106. doi: 10.1183/09031936.01.00074701. [DOI] [PubMed] [Google Scholar]
- 37.Thickett DR, Armstrong L, Christie SJ, et al. Vascular endothelial growth factor may contribute to increased vascular permeability in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001 Nov 1;164(9):1601–1605. doi: 10.1164/ajrccm.164.9.2011071. [DOI] [PubMed] [Google Scholar]
- 38.Thickett DR, Armstrong L, Millar AB. A role for vascular endothelial growth factor in acute and resolving lung injury. Am J Respir Crit Care Med. 2002 Nov 15;166(10):1332–1337. doi: 10.1164/rccm.2105057. [DOI] [PubMed] [Google Scholar]
- 39.Ware LB, Kaner RJ, Crystal RG, et al. VEGF levels in the alveolar compartment do not distinguish between ARDS and hydrostatic pulmonary oedema. Eur Respir J. 2005 Jul;26(1):101–105. doi: 10.1183/09031936.05.00106604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng IW, Ware LB, Greene KE, et al. Prognostic value of surfactant proteins A and D in patients with acute lung injury. Crit Care Med. 2003 Jan;31(1):20–27. doi: 10.1097/00003246-200301000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Greene KE, Wright JR, Steinberg KP, et al. Serial changes in surfactant-associated proteins in lung and serum before and after onset of ARDS. Am J Respir Crit Care Med. 1999 Dec;160(6):1843–1850. doi: 10.1164/ajrccm.160.6.9901117. [DOI] [PubMed] [Google Scholar]
- 42.Eisner MD, Parsons P, Matthay MA, et al. Plasma surfactant protein levels and clinical outcomes in patients with acute lung injury. Thorax. 2003 Nov;58(11):983–988. doi: 10.1136/thorax.58.11.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Ware LB, Koyama T, Zhao Z, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17(5):R253. doi: 10.1186/cc13080. Study of multiple protein biomarkers reflecting disparate mechanisms of disease. Identified a 5-biomarker panel particularly good at identifying severe sepsis-induced ARDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calfee CS, Budev MM, Matthay MA, et al. Plasma receptor for advanced glycation end-products predicts duration of ICU stay and mechanical ventilation in patients after lung transplantation. J Heart Lung Transplant. 2007 Jul;26(7):675–680. doi: 10.1016/j.healun.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida T, Shirasawa M, Ware LB, et al. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006 May 1;173(9):1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ishizaka A, Matsuda T, Albertine KH, et al. Elevation of KL-6, a lung epithelial cell marker, in plasma and epithelial lining fluid in acute respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2004 Jun;286(6):L1088–L1094. doi: 10.1152/ajplung.00420.2002. [DOI] [PubMed] [Google Scholar]
- 47••.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. Pivotal trial in ARDS establishing the importance of low tidal volume ventilation in improving mortality. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal A, Zhuo H, Brady S, et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: results from two clinical trials. Am J Physiol Lung Cell Mol Physiol. 2012 Oct 15;303(8):L634–L639. doi: 10.1152/ajplung.00195.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meduri GU, Headley S, Kohler G, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS. Plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995 Apr;107(4):1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 50.Calfee CS, Eisner MD, Ware LB, et al. Trauma-associated lung injury differs clinically and biologically from acute lung injury due to other clinical disorders. Crit Care Med. 2007 Jul 17;35:2243–2250. doi: 10.1097/01.ccm.0000280434.33451.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsons PE, Matthay MA, Ware LB, et al. Elevated plasma levels of soluble TNF receptors are associated with morbidity and mortality in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2005 Mar;288(3):L426–L431. doi: 10.1152/ajplung.00302.2004. [DOI] [PubMed] [Google Scholar]
- 52.Parsons PE, Eisner MD, Thompson BT, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Crit Care Med. 2005 Jan;33(1):1–6. doi: 10.1097/01.ccm.0000149854.61192.dc. discussion 230–232. [DOI] [PubMed] [Google Scholar]
- 53.Pugin J, Ricou B, Steinberg KP, et al. Proinflammatory activity in bronchoalveolar lavage fluids from patients with ARDS, a prominent role for interleukin-1. Am J Respir Crit Care Med. 1996 Jun;153(6 Pt 1):1850–1856. doi: 10.1164/ajrccm.153.6.8665045. [DOI] [PubMed] [Google Scholar]
- 54.Miller EJ, Cohen AB, Nagao S, et al. Elevated levels of NAP-1/interleukin-8 are present in the airspaces of patients with the adult respiratory distress syndrome and are associated with increased mortality. Am Rev Respir Dis. 1992 Aug;146(2):427–432. doi: 10.1164/ajrccm/146.2.427. [DOI] [PubMed] [Google Scholar]
- 55.Fowler AA, Hyers TM, Fisher BJ, et al. The adult respiratory distress syndrome. Cell populations and soluble mediators in the air spaces of patients at high risk. Am Rev Respir Dis. 1987 Nov;136(5):1225–1231. doi: 10.1164/ajrccm/136.5.1225. [DOI] [PubMed] [Google Scholar]
- 56.Weiland JE, Davis WB, Holter JF, et al. Lung neutrophils in the adult respiratory distress syndrome. Clinical and pathophysiologic significance. Am Rev Respir Dis. 1986 Feb;133(2):218–225. doi: 10.1164/arrd.1986.133.2.218. [DOI] [PubMed] [Google Scholar]
- 57.Pugin J, Verghese G, Widmer MC, et al. The alveolar space is the site of intense inflammatory and profibrotic reactions in the early phase of acute respiratory distress syndrome. Crit Care Med. 1999 Feb;27(2):304–312. doi: 10.1097/00003246-199902000-00036. [DOI] [PubMed] [Google Scholar]
- 58.Sibille Y, Reynolds HY. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990 Feb;141(2):471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 59.Matute-Bello G, Liles WC, Radella F, 2nd, et al. Modulation of neutrophil apoptosis by granulocyte colony-stimulating factor and granulocyte/macrophage colony-stimulating factor during the course of acute respiratory distress syndrome. Crit Care Med. 2000 Jan;28(1):1–7. doi: 10.1097/00003246-200001000-00001. [DOI] [PubMed] [Google Scholar]
- 60.Ware LB, Fang X, Matthay MA. Protein C and thrombomodulin in human acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003 Sep;285(3):L514–L521. doi: 10.1152/ajplung.00442.2002. [DOI] [PubMed] [Google Scholar]
- 61.Prabhakaran P, Ware LB, White KE, et al. Elevated levels of plasminogen activator inhibitor-1 in pulmonary edema fluid are associated with mortality in acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2003 Jul;285(1):L20–L28. doi: 10.1152/ajplung.00312.2002. [DOI] [PubMed] [Google Scholar]
- 62.Jalkanen V, Yang R, Linko R, et al. SuPAR and PAI-1 in critically ill, mechanically ventilated patients. Intensive Care Med. 2013 Mar;39(3):489–496. doi: 10.1007/s00134-012-2730-x. [DOI] [PubMed] [Google Scholar]
- 63.Zhu S, Ware LB, Geiser T, et al. Increased levels of nitrate and surfactant protein a nitration in the pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med. 2001 Jan;163(1):166–172. doi: 10.1164/ajrccm.163.1.2005068. [DOI] [PubMed] [Google Scholar]
- 64.Sharkey RA, Donnelly SC, Connelly KG, et al. Initial serum ferritin levels in patients with multiple trauma and the subsequent development of acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999 May;159(5 Pt 1):1506–1509. doi: 10.1164/ajrccm.159.5.9809027. [DOI] [PubMed] [Google Scholar]
- 65.Connelly KG, Moss M, Parsons PE, et al. Serum ferritin as a predictor of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1997 Jan;155(1):21–25. doi: 10.1164/ajrccm.155.1.9001283. [DOI] [PubMed] [Google Scholar]
- 66.Bowler RP, Velsor LW, Duda B, et al. Pulmonary edema fluid anti-oxidants are depressed in acute lung injury. Crit Care Med. 2003 Sep;31(9):2309–2315. doi: 10.1097/01.CCM.0000085090.06078.8C. [DOI] [PubMed] [Google Scholar]
- 67.Martin C, Papazian L, Payan MJ, et al. Pulmonary fibrosis correlates with outcome in adult respiratory distress syndrome. A study in mechanically ventilated patients. Chest. 1995 Jan;107(1):196–200. doi: 10.1378/chest.107.1.196. [DOI] [PubMed] [Google Scholar]
- 68.Chesnutt AN, Matthay MA, Tibayan FA, et al. Early detection of type III procollagen peptide in acute lung injury. Pathogenetic and prognostic significance. Am J Respir Crit Care Med. 1997 Sep;156(3 Pt 1):840–845. doi: 10.1164/ajrccm.156.3.9701124. [DOI] [PubMed] [Google Scholar]
- 69.Meduri GU, Tolley EA, Chinn A, et al. Procollagen types I and III aminoterminal propeptide levels during acute respiratory distress syndrome and in response to methylprednisolone treatment. Am J Respir Crit Care Med. 1998 Nov;158(5 Pt 1):1432–1441. doi: 10.1164/ajrccm.158.5.9801107. [DOI] [PubMed] [Google Scholar]
- 70.Madtes DK, Rubenfeld G, Klima LD, et al. Elevated transforming growth factor-alpha levels in bronchoalveolar lavage fluid of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1998 Aug;158(2):424–430. doi: 10.1164/ajrccm.158.2.9711112. [DOI] [PubMed] [Google Scholar]
- 71.Chesnutt AN, Kheradmand F, Folkesson HG, et al. Soluble transforming growth factor-alpha is present in the pulmonary edema fluid of patients with acute lung injury. Chest. 1997 Mar;111(3):652–656. doi: 10.1378/chest.111.3.652. [DOI] [PubMed] [Google Scholar]
- 72.Matute-Bello G, Liles WC, Steinberg KP, et al. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999 Aug 15;163(4):2217–2225. [PubMed] [Google Scholar]
- 73.Albertine KH, Soulier MF, Wang Z, et al. Fas and fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002 Nov;161(5):1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hashimoto S, Kobayashi A, Kooguchi K, et al. Upregulation of two death pathways of perforin/granzyme and FasL/Fas in septic acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000 Jan;161(1):237–243. doi: 10.1164/ajrccm.161.1.9810007. [DOI] [PubMed] [Google Scholar]
- 75.Tenholder MF, Rajagopal KR, Phillips YY, et al. Urinary desmosine excretion as a marker of lung injury in the adult respiratory distress syndrome. Chest. 1991 Nov;100(5):1385–1390. doi: 10.1378/chest.100.5.1385. [DOI] [PubMed] [Google Scholar]
- 76.Fill JA, Brandt JT, Wiedemann HP, et al. Urinary desmosine as a biomarker in acute lung injury. Biomarkers. 2006 Jan-Feb;11(1):85–96. doi: 10.1080/13547500500343225. [DOI] [PubMed] [Google Scholar]
- 77.Fay PJ, Coumans JV, Walker FJ. von Willebrand factor mediates protection of factor VIII from activated protein C-catalyzed inactivation. J Biol Chem. 1991 Feb 5;266(4):2172–2177. [PubMed] [Google Scholar]
- 78.Hamilton KK, Sims PJ. Changes in cytosolic Ca2+ associated with von Willebrand factor release in human endothelial cells exposed to histamine. Study of microcarrier cell monolayers using the fluorescent probe indo-1. J Clin Invest. 1987 Feb;79(2):600–608. doi: 10.1172/JCI112853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Collins PW, Macey MG, Cahill MR, et al. von Willebrand factor release and P-selectin expression is stimulated by thrombin and trypsin but not IL-1 in cultured human endothelial cells. Thromb Haemost. 1993 Aug 2;70(2):346–350. [PubMed] [Google Scholar]
- 80.Hordijk PL. Endothelial signalling events during leukocyte transmigration. The FEBS Journal. 2006 Oct;273(19):4408–4415. doi: 10.1111/j.1742-4658.2006.05440.x. [DOI] [PubMed] [Google Scholar]
- 81.Chroneos ZC, Sever-Chroneos Z, Shepherd VL. Pulmonary surfactant: an immunological perspective. Cell Physiol Biochem. 2010;25(1):13–26. doi: 10.1159/000272047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pastva AM, Wright JR, Williams KL. Immunomodulatory roles of surfactant proteins A and D: implications in lung disease. Proc Am Thorac Soc. 2007 Jul;4(3):252–257. doi: 10.1513/pats.200701-018AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liliensiek B, Weigand MA, Bierhaus A, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004 Jun;113(11):1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie J, Méndez JD, Méndez-Valenzuela V, et al. Cellular signalling of the receptor for advanced glycation end products (RAGE) Cell Signal. 2013 Nov;25(11):2185–2197. doi: 10.1016/j.cellsig.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 85.Jabaudon M, Futier E, Roszyk L, et al. Soluble form of the receptor for advanced glycation end products is a marker of acute lung injury but not of severe sepsis in critically ill patients. Crit Care Med. 2011 Mar;39(3):480–488. doi: 10.1097/CCM.0b013e318206b3ca. [DOI] [PubMed] [Google Scholar]
- 86.Calfee CS, Ware LB, Eisner MD, et al. Plasma receptor for advanced glycation end-products and clinical outcomes in acute lung injury. Thorax. 2008 Jun 19;63:1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Agrawal A, Matthay MA, Kangelaris KN, et al. Plasma angiopoietin-2 predicts the onset of acute lung injury in critically ill patients. Am J Respir Crit Care Med. 2013 Apr 1;187(7):736–742. doi: 10.1164/rccm.201208-1460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hirasawa Y, Kohno N, Yokoyama A, et al. KL-6, a human MUC1 mucin, is chemotactic for human fibroblasts. Am J Respir Cell Mol Biol. 1997 Oct;17(4):501–507. doi: 10.1165/ajrcmb.17.4.2253. [DOI] [PubMed] [Google Scholar]
- 89.Meduri GU, Headley AS, Golden E, et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. JAMA. 1998 Jul 8;280(2):159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 90.National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med. 2006 Apr 20;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 91.Rice TW, Wheeler AP, Morris PE, et al. Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection (CytoFab) in severe sepsis. Crit Care Med. 2006 Sep;34(9):2271–2281. doi: 10.1097/01.CCM.0000230385.82679.34. [DOI] [PubMed] [Google Scholar]
- 92.Bernard GR, Francois B, Mira J-P, et al. Evaluating the efficacy and safety of two doses of the polyclonal anti-tumor necrosis factor-α fragment antibody AZD9773 in adult patients with severe sepsis and/or septic shock: randomized, double-blind, placebo-controlled phase IIb study*. Crit Care Med. 2014 Mar;42(3):504–511. doi: 10.1097/CCM.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 93.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001 Mar 8;344(10):699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 94.Choi G, Schultz MJ, van Till JW, et al. Disturbed alveolar fibrin turnover during pneumonia is restricted to the site of infection. Eur Respir J. 2004 Nov;24(5):786–789. doi: 10.1183/09031936.04.00140703. [DOI] [PubMed] [Google Scholar]
- 95.Günther A, Mosavi P, Heinemann S, et al. Alveolar fibrin formation caused by enhanced procoagulant and depressed fibrinolytic capacities in severe pneumonia. Comparison with the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2000 Feb;161(2 Pt 1):454–462. doi: 10.1164/ajrccm.161.2.9712038. [DOI] [PubMed] [Google Scholar]
- 96.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003 Apr;31(4 Suppl):S213–S220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 97.Schultz MJ, Millo J, Levi M, et al. Local activation of coagulation and inhibition of fibrinolysis in the lung during ventilator associated pneumonia. Thorax. 2004 Feb;59(2):130–135. doi: 10.1136/thorax.2003.013888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ware LB, Matthay MA, Parsons PE, et al. Pathogenetic and prognostic significance of altered coagulation and fibrinolysis in acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2007 Aug;35(8):1821–1828. doi: 10.1097/01.CCM.0000221922.08878.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu KD, Levitt J, Zhuo H, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008 Jun 19;178:618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galani V, Tatsaki E, Bai M, et al. The role of apoptosis in the pathophysiology of acute respiratory distress syndrome (ARDS): an up-to-date cell-specific review. Pathol Res Pract. 2010 Mar 15;206(3):145–150. doi: 10.1016/j.prp.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 101.Verghese GM, McCormick-Shannon K, Mason RJ, et al. Hepatocyte growth factor and keratinocyte growth factor in the pulmonary edema fluid of patients with acute lung injury. Biologic and clinical significance. Am J Respir Crit Care Med. 1998 Aug;158(2):386–394. doi: 10.1164/ajrccm.158.2.9711111. [DOI] [PubMed] [Google Scholar]
- 102.Ware LB, Matthay MA. Keratinocyte and hepatocyte growth factors in the lung: roles in lung development, inflammation, and repair. Am J Physiol Lung Cell Mol Physiol. 2002 May;282(5):L924–L940. doi: 10.1152/ajplung.00439.2001. [DOI] [PubMed] [Google Scholar]
- 103.Lee JW, Krasnodembskaya A, McKenna DH, et al. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. 2013 Apr 1;187(7):751–760. doi: 10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Atabai K, Ishigaki M, Geiser T, et al. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002 Jul;283(1):L163–L169. doi: 10.1152/ajplung.00396.2001. [DOI] [PubMed] [Google Scholar]
- 105••.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014 Aug;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. Using latent class analysis, the authors identified 2 distinct classes of ARDS patients, including an inflammatory phenotype that seemed to improve on higher PEEP strategy in 2 large clinical trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004 Jul 22;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 107.Calfee CS, Janz DR, Bernard GR, et al. Distinct molecular phenotypes of direct vs indirect ARDS in single-center and multicenter studies. Chest. 2015 Jun;147(6):1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.O’Neal HR, Jr, Koyama T, Koehler EAS, et al. Prehospital statin and aspirin use and the prevalence of severe sepsis and acute lung injury/acute respiratory distress syndrome. Crit Care Med. 2011 Jun;39(6):1343–1350. doi: 10.1097/CCM.0b013e3182120992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wiedemann HP, Wheeler AP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006 Jun 15;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 110.Fremont RD, Koyama T, Calfee CS, et al. Acute lung injury in patients with traumatic injuries: utility of a panel of biomarkers for diagnosis and pathogenesis. J Trauma. 2010 May;68(5):1121–1127. doi: 10.1097/TA.0b013e3181c40728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111•.Calfee CS, Ware LB, Glidden DV, et al. Use of risk reclassification with multiple biomarkers improves mortality prediction in acute lung injury. Crit Care Med. 2011 Apr;39(4):711–717. doi: 10.1097/CCM.0b013e318207ec3c. Incorporating protein biomarkers improves risk reclassification in ARDS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aebersold R, Mann M. Mass spectrometry-based proteomics. Nature. 2003 Mar 13;422(6928):198–207. doi: 10.1038/nature01511. [DOI] [PubMed] [Google Scholar]
- 113.Ong S-E, Mann M. Mass spectrometry-based proteomics turns quantitative. Nat Chem Biol. 2005 Oct;1(5):252–262. doi: 10.1038/nchembio736. [DOI] [PubMed] [Google Scholar]
- 114.Chang DW, Hayashi S, Gharib SA, et al. Proteomic and computational analysis of bronchoalveolar proteins during the course of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2008 Oct 1;178(7):701–709. doi: 10.1164/rccm.200712-1895OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schnapp LM, Donohoe S, Chen J, et al. Mining the acute respiratory distress syndrome proteome: identification of the insulin-like growth factor (IGF)/IGF-binding protein-3 pathway in acute lung injury. Am J Pathol. 2006 Jul;169(1):86–95. doi: 10.2353/ajpath.2006.050612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bowler RP, Duda B, Chan ED, et al. Proteomic analysis of pulmonary edema fluid and plasma in patients with acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004 Jun;286(6):L1095–L1104. doi: 10.1152/ajplung.00304.2003. [DOI] [PubMed] [Google Scholar]
- 117.Bhargava M, Becker TL, Viken KJ, et al. Proteomic profiles in acute respiratory distress syndrome differentiates survivors from non-survivors. PLoS One. 2014;9(10):e109713. doi: 10.1371/journal.pone.0109713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.De Torre C, Ying S-X, Munson PJ, et al. Proteomic analysis of inflammatory biomarkers in bronchoalveolar lavage. Proteomics. 2006 Jul;6(13):3949–3957. doi: 10.1002/pmic.200500693. [DOI] [PubMed] [Google Scholar]
- 119.Chen X, Shan Q, Jiang L, et al. Quantitative proteomic analysis by iTRAQ for identification of candidate biomarkers in plasma from acute respiratory distress syndrome patients. Biochem Biophys Res Commun. 2013 Nov 8;441(1):1–6. doi: 10.1016/j.bbrc.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 120.Gessner C, Dihazi H, Brettschneider S, et al. Presence of cytokeratins in exhaled breath condensate of mechanical ventilated patients. Res Med. 2008;102(2):299–306. doi: 10.1016/j.rmed.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 121.Erickson SE, Martin GS, Davis JL, et al. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009 May;37(5):1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]