Abstract
Background
Despite the introduction of effective novel agents, the outcome of patients with refractory multiple myeloma remains poor, particularly those refractory to both proteasome inhibitors (PIs) and immunomodulatory agents (IMiDs). Limited data is available on the role of autologous hematopoietic stem cell transplantation in this population.
Methods
We retrospectively analyzed refractory myeloma patients who underwent first auto-HCT between March 2000 and October 2015. Patients with primary refractory disease and those with relapsed and refractory disease were included. Patients with disease refractory to at least one PI and at least one IMiD were classified as double refractory (DR-MM).
Results
233 patients were identified: 105 (45%) had DR-MM while the remaining 128 (55%) patients were classified as non-double refractory (NDR-MM). With median follow up of 42 months for surviving patients, at least partial response was seen in 188 (81%) patients (DR-MM, 83 [79%]; NDR-MM, 105 [82%]; p=0.77). Near complete remission or better was seen in 52 (22%) patients (DR-MM, 25 [24%]; NDR-MM, 27 [21%]; p=0.77). The median progression-free survival (PFS) was 17.6 months (14.4 months in the DR-MM patients and 18.2 months in the NDR-MM patients) and the 2-year PFS rate was 38% (DR-MM, 35%; NDR-MM, 40%; p=0.40). Median overall survival (OS) was 48.0 months (38.9 months in DR-MM and 56.6 months in NDR-MM) and the 2-year OS rate was 74% (DR-MM, 71%; NDR-MM, 76%; p=0.27).
Conclusions
Our findings highlight that auto-HCT is an effective and safe therapy in patients with refractory multiple myeloma including those refractory to an IMiD and PI.
Keywords: multiple myeloma, autologous transplantation, refractory disease, proteasome inhibitor, immunomodulatory agent
BACKGROUND
Induction therapy with conventional chemotherapy agents in myeloma generally produced an overall response rate (ORR) of 50–60% 1. The landscape of myeloma therapy changed with the introduction of novel agents, e.g. proteasome inhibitors (PI) and immunomodulatory agents (IMiDs), which can yield a response in 80–90% of patients 2. The response rates are even more impressive with the new generation of PIs and IMiDs where ORR can exceed 90% 2. However, the prognostic value of the depth of response to induction therapy in patients who proceed to autologous hematopoietic stem cell transplantation (auto-HCT) is a topic of discussion. Before the introduction of novel agents, the depth of response in patients responding to conventional agents prior to auto-HCT was not clearly associated with longer survival 3–5. However, more recent data with novel agents suggests that depth of response to induction therapy correlates with superior progression free survival (PFS) 6 and overall survival (OS) after auto-HCT 7. On the other hand, patients refractory to induction therapy with either conventional therapy or with novel agents have poorer outcomes after auto-HCT versus responding patients 3,4,8–11. The subset of myeloma patients who fail to respond to induction therapy (primary refractory) or become refractory after an initial response (relapsed and refractory) have dismal outcomes. Many of these patients become refractory to both PIs and IMiDs (double-refractory). These constitute a higher-risk population with little data on the role of auto-HCT 8,12,13. In order to characterize the role of auto-HCT in patients with refractory multiple myeloma (MM), particularly those with double-refractory disease, we assessed the outcomes of patients who underwent auto-HCT at our center with a response status of less than partial response (PR) at the time of transplant.
METHODS
Patients
We retrospectively identified all patients with relapsed and refractory myeloma (defined as disease in patients that was nonresponsive while on salvage therapy or progression within 60 days of therapy in patients who had achieved a minimal response or better) and primary refractory myeloma (defined as disease that was nonresponsive in patients who never achieved a minimal response or better with any therapy) 14 who underwent first auto-HCT at the University of Texas MD Anderson Cancer Center between March 2000 and October 2015. A patient was deemed nonresponsive if they achieved less than a partial response (i.e. stable disease or progressive disease) to the therapy administered. The number of cycles administered before the patient was considered unresponsive to a particular regimen was at the discretion of the primary oncologist. The study population was divided into two groups: 1) double refractory MM (DR-MM) patients (i.e., being refractory to at least one IMiD and at least one PI 12 and 2) non-double refractory MM (NDR-RMM) patients (i.e., patients with refractory disease not classified as DR-MM). The institutional review board at MD Anderson Cancer Center approved this study.
Clinical and Outcome Measures
Cytogenetic risk was assessed based on cytogenetic analysis and interphase fluorescence in-situ hybridization (FISH) analysis results. Patients were defined as having high-risk MM if conventional cytogenetics in at least 2 metaphases performed at diagnosis or any time prior to auto-HCT demonstrated t(4;14), t(14;16), t(14;20), −13/del(13q), −17/del(17p), hypodiploidy (<45 chromosomes excluding –Y), or a chromosome 1 aberration (+1, −1, t(1;x) or if any FISH or conventional cytogenetics showed del(17p13), t(4;14), t(14;16), t(14;20) or chromosome 1 abnormalities.
The time to neutrophil engraftment was the first of 3 consecutive days with absolute neutrophil count (ANC) ≥ 0.5 × 109/L after post-transplantation nadir 15. Platelet engraftment was defined as the first of seven consecutive days with platelet count 20 × 109/L in the absence of platelet transfusion for the preceding seven days 15.
The International Myeloma Working Group (IMWG) criteria were used to define treatment response, disease progression and relapse 14. Complete response (CR) included patients with stringent CR (i.e., having a negative immunofixation on the serum and urine, disappearance of any soft tissue plasmacytoma, <5% plasma cells in the bone marrow biopsy and a normal free light chain ratio) 14 and patients with near CR (i.e., only having immunofixation electrophoresis positive)16.
Statistical Methods
Summaries for patient demographics and clinical characteristics were produced for all patients and by MM group. Associations between MM group and categorical measures were assessed using either Fisher’s exact test or generalized Fisher’s exact test while differences in continuous measures between groups were evaluated using Wilcoxon rank sum test. PFS was computed from date of transplant to date of disease progression or death (if died without disease progression) or the last evaluation date. Patients who were alive and did not experience progression of disease at the last follow-up date were censored. OS was computed from the date of transplant to the last known vital sign. Patients alive at the last follow-up date were censored. The Kaplan-Meier method was used to estimate OS and PFS and group differences were assessed using the log-rank test. The association between OS and PFS and patient subgroups was determined using Cox proportional hazards regression models. Factors significantly associated with OS and PFS in univariate models (p<0.05) were included in a multivariable model. The cumulative incidence of non-relapse mortality (NRM) was determined using the competing risks method. The competing risk for NRM included relapse; patients who were still alive at the last follow-up date were censored. Differences in NRM between groups were assessed using Gray’s test17. All statistical analysis were performed using SAS 9.3 for Windows (Copyright © 2011 by SAS Institute Inc., Cary, NC). All statistical tests used a significant level of 5%. No adjustments for multiple testing were made.
RESULTS
Patient and Disease Characteristics
The study population consisted of 233 patients: 105 (45%) had DR-MM and the remaining 128 (55%) were NDR-MM. Patient characteristics are summarized in Table 1. The median age at auto-HCT was 59 years, with patients with DR-MM being significantly older than those with NDR-MM (60 vs. 56 years; p=0.005). Compared to patients with NDR-MM, those with DR-MM had higher rates of chemomobilization (51% vs. 31%; p=0.002), were more likely to be treated with a triple regimen induction regimen (52% vs. 21%; p<0.001) and underwent more lines of therapy prior to transplantation (median 2 lines vs. 1 line; p<0.001). In addition, patients with DR-MM were more likely to have relapsed refractory disease (45% vs. 26%; p=0.003). To further characterize the nature of disease in relapsed/refractory patients, we further evaluated these patients based on time from initial best response until time to progression. Of the 71 patients with available data, 44 (62%) relapsed within six months of initial best response to therapy and 27 (38%) relapsed after 6 months. There was no significant difference in OS or PFS between the two populations.
Table 1.
Patient Baseline Characteristics and Outcomes
| Characteristic | R-MM (n=233) | NDR-MM (n=128) | DR-MM (n=105) | p-value* |
|---|---|---|---|---|
|
| ||||
| Median age, years (range) | 59 (23–79) | 56 (23–75) | 60 (35–79) | 0.005 |
|
| ||||
| Male sex | 138 (59%) | 73 (57%) | 65 (62%) | 0.50 |
|
| ||||
| Race | ||||
| White | 141 (61%) | 76 (59%) | 65 (62%) | 0.49 |
| Black | 57 (24%) | 36 (28%) | 21 (20%) | |
| Hispanic | 19 (8%) | 8 (6%) | 11 (10%) | |
| Other | 11 (5%) | 5 (4%) | 6 (6%) | |
| Unknown | 5 (2%) | 3 (2%) | 2 (2%) | |
|
| ||||
| Histologic Subtype | ||||
| IgA | 37 (16%) | 23 (18%) | 14 (13%) | 0.28 |
| IgG | 136 (58%) | 71 (55%) | 65 (62%) | |
| Light chain only | 37 (16%) | 24 (19%) | 13 (12%) | |
| Other | 23 (10%) | 10 (8%) | 13 (12%) | |
|
| ||||
| ISS Stage | ||||
| I | 54 (23%) | 29 (23%) | 25 (24%) | 0.58 |
| II | 40 (17%) | 26 (20%) | 14 (13%) | |
| III | 46 (20%) | 24 (19%) | 22 (21%) | |
| Unknown | 93 (40%) | 49 (38%) | 44 (42%) | |
|
| ||||
| Disease Status | ||||
| Primary Refractory | 153 (66%) | 95 (74%) | 58 (55%) | 0.003 |
| Relapsed Refractory | 80 (34%) | 33 (26%) | 47 (45%) | |
|
| ||||
| Response prior to transplant | ||||
| Stable Disease | 188 (81%) | 101 (79%) | 87 (83%) | 0.51 |
| Progressive Disease | 45 (19%) | 27 (21%) | 18 (17%) | |
| Partial response or better | 0 | 0 | 0 | |
|
| ||||
| KPS at auto-HCT, median (range) | 90 (40–100) | 90 (40–100) | 90 (60–100) | 0.28 |
|
| ||||
| High Risk Cytogenetics (high risk/patients tested) | 67/140 (48%) | 32/51 (63%) | 35/89 (39%) | 0.009 |
|
| ||||
| Bone marrow plasma cell (%), median (range) | 40 (0–100) | 40 (0–100) | 43 (0–95) | 0.20 |
|
| ||||
| Hemoglobin (g/dL), median (range) | 10.5 (4.2–17.0) | 10.6 (4.2–15.8) | 10.3(5.5–17.0) | 0.54 |
|
| ||||
| Lactate dehydrogenase (U/L), median (range) | 412 (68–1024) | 399 (83–1024) | 418 (68–962) | 0.78 |
|
| ||||
| Calcium (mg/dL), median (range) | 9.5 (6.8–16.9) | 9.6 (8.1–16.9) | 9.3 (6.8–16.1) | 0.021 |
|
| ||||
| Creatinine (mg/dL), median (range) | 1.1 (0.5–14.5) | 1.1 (0.5–14.5) | 1.1 (0.6–10) | 0.43 |
|
| ||||
| Beta-2-microglobulin (mg/L), median (range) | 3.4 (0.9–42.7) | 3.4 (0.9–33.7) | 3.6 (1.4–42.7) | 0.87 |
|
| ||||
| Induction Regimen | ||||
| Doublet | 117 (50%) | 75 (59%) | 42 (40%) | <0.001 |
| Triplet | 82 (35%) | 27 (21%) | 55 (52%) | |
| Other | 34 (15%) | 26 (20%) | 8 (8%) | |
|
| ||||
| Number of lines of prior therapy, median (range) | ||||
| Primary Refractory | 1 (1–5) | 1 (1–5) | 2 (1–5) | <0.001 |
| Relapsed/Refractory | 2 (1–7) | 2 (1–5) | 3 (1–7) | |
|
| ||||
| Time from diagnosis to auto-HCT (months), median (range) | 9.4 (2.2–309.6) | 8.1 (2.2–309.6) | 11.8 (3.2–220.8) | <0.001 |
|
| ||||
| Year of Transplantation | ||||
| 2000–2004 | 76 (32%) | 70 (55%) | 6 (6%) | NA |
| 2005–2009 | 55 (24%) | 24 (19%) | 31 (29%) | |
| 2010–2015 | 102 (44%) | 34 (26%) | 68 (65%) | |
|
| ||||
| Induction Regimen | ||||
| IMID+PI | NA | |||
| VRD | 38 (16 %) | 0 | 38 | |
| VTD | 11 (5%) | 0 | 11 | |
| VTD-PACE | 2 | 0 | 2 | |
| Other | 10 | 0 | 10 | |
| PI Based | ||||
| CyBorD | 20 | 13 | 7 | |
| VD | 14 | 9 | 5 | |
| mCBAD | 2 | 2 | 0 | |
| IMiD Based | ||||
| RD | 10 | 4 | 6 | |
| TD | 56 | 42 | 14 | |
| Other | ||||
| CVAD | 5 | 5 | 0 | |
| VAD | 16 | 14 | 2 | |
| Dexamethasone | 17 | 15 | 2 | |
| Melphalan/Prednisone | 11 | 9 | 2 | |
| Other | 21 | 13 | 8 | |
|
| ||||
| Chemomobilization | 94 (40%) | 40 (31%) | 54 (51%) | 0.002 |
|
| ||||
| Conditioning regimen | ||||
| Melphalan alone | 168 (72%) | 99 (77%) | 69 (66%) | 0.057 |
| Melphalan-based combination | 65 (28%) | 29 (23%) | 36 (34%) | |
|
| ||||
| Engraftment (days) | ||||
| Median neutrophil engraftment | 10 (0–19) | 10 (0–19) | 11 (9–13) | < 0.001 |
| Median platelet engraftment | 11 (0–34) | 10 (0–23) | 11 (0–34) | 0.016 |
|
| ||||
| Best Response to auto-HCT | ||||
| CR | 52 (22%) | 27 (21%) | 25 (24%) | 0.77 |
| VGPR | 43 (18%) | 27 (21%) | 16 (15%) | |
| PR | 93 (40%) | 51 (40%) | 42 (40%) | |
| SD | 31 (13%) | 15 (12%) | 16 (15%) | |
| PD | 13 (6%) | 7 (5%) | 6 (6%) | |
|
| ||||
| Day 100 response to auto-HCT | ||||
| CR | 28 (12%) | 17 (13%) | 11 (10%) | 0.45** |
| VGPR | 36 (15%) | 24 (19%) | 12 (11%) | |
| PR | 106 (45%) | 55 (43%) | 51 (49%) | |
| SD | 43 (18%) | 20 (16%) | 23 (22%) | |
| PD | 17 (7%) | 10 (8%) | 7 (7%) | |
| Unknown | 3 (1%) | 2 (2%) | 1 (1%) | |
|
| ||||
| Maintenance Therapy | ||||
| Lenalidomide | 55 | 26 | 29 | NA |
| Thalidomide/Dexamethasone | 15 | 13 | 2 | |
| Lenalidomide/Dexamethasone | 10 | 6 | 4 | |
| Bortezomib | 8 | 5 | 3 | |
| Lenalidomide/Ixazomib | 5 | 4 | 1 | |
| Thalidomide | 5 | 4 | 1 | |
| Dexamethasone | 3 | 2 | 1 | |
| Interferon | 5 | 4 | 1 | |
| Pomalidomide | 3 | 1 | 2 | |
| VRD | 2 | 1 | 1 | |
| Carfilzomib | 1 | 1 | 0 | |
| Other | 2 | 1 | 1 | |
R-MM: refractory multiple myeloma; NDR-MM: non-double refractory multiple myeloma; DR-MM: double refractory multiple myeloma; ISS: international staging system; KPS: karnofsky performance status; auto-HCT: autologous hematopoietic stem cell transplantation; VRD: bortezomib, lenalidomide, dexamethasone; VTD: bortezomib, thalidomide, dexamethasone; VTD-PACE: bortezomib, thalidomide, dexamethasone, cisplatin, doxorubicin, cyclophosphamide, etoposide; CyBorD: cyclophosphamide, bortezomib, dexamethasone; VD: bortezomib, dexamethasone; mCBAD: modified cyclophosphamide, bortezomib, doxorubicin, dexamethasone; RD: lenalidomide and dexamethasone; TD: thalidomide and dexamethasone; CVAD: cyclophosphamide, vincristine, doxorubicine and dexamethasone; VAD: vincristine, doxorubicin and dexamethasone; CR: complete response; VGPR: very good partial response; PR: partial response; SD: stable disease; PD: progressive disease
P-value represents comparative analysis of the DR-MM and NDR-MM groups.
Comparison was PR or better versus SD and PD.
Though data were not available for all patients, 48% (67 of 140 tested) had high risk cytogenetics (DR-MM: 39% (35/89) vs. NDR-MM: 63% (32/51; p=0.009).
Engraftment and NRM
The median number of infused CD34+ cells was 4.8 × 106 cells/kg, with 4.7 × 106 in the patients with DR-MM and 4.8 × 106 in the NDR-MM patients (p=0.25). The median (range) time to neutrophil engraftment was 11 (9–13) days in the DR-MM group and 10 (0–19) days in the NDR-MM group (p<0.001). Similarly, the median days to platelet engraftment was 11 days (0–34) and 10 (0–23) days, respectively (p=0.016). The cumulative incidence of NRM was very low and similar between MM groups (DR-MM group: Day 100=0%, Month 6=1%; NDR-MM group: Day 100=2%, Month 6=2%; p=0.56).
Treatment after Auto-HCT and Response to Auto-HCT
Sixteen patients (7%) received consolidation therapy after transplantation: 8 in the DR-MM group and 8 in the NDR-MM group (p=0.80). Conversely, about half of the patients (113/229) received maintenance therapy where a significantly higher percentage of DR-MM patients (61%) received treatment compared with the NDR-MM group (40%, p=0.001). Distribution of maintenance regimens for all patients is presented in Table 1. The most common maintenance regimen was lenalidomide.
The overall response rate for all patients was 80% (188/233) (CR=22%, VGPR= 18% and PR=40%). DR-MM patients had similar response rates to NDR-MM with 79% and 82%, respectively, (p=0.77).
Survival
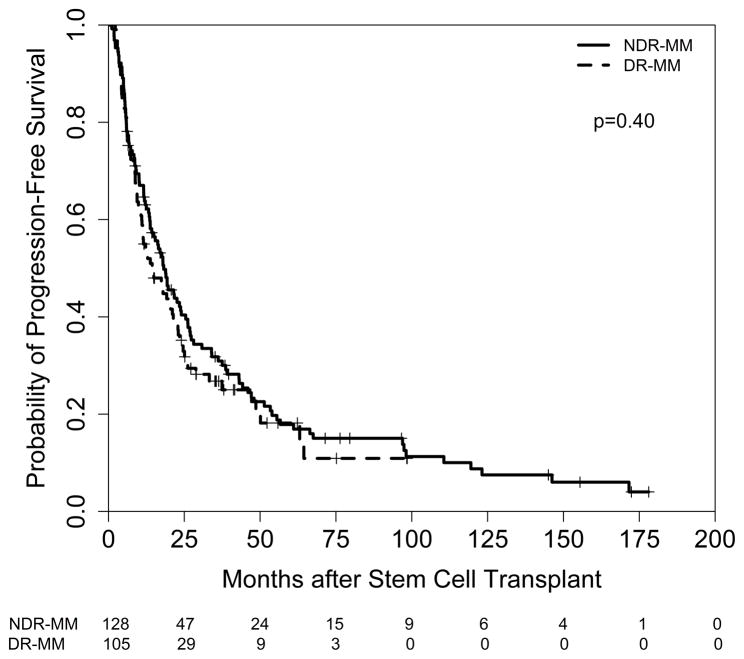
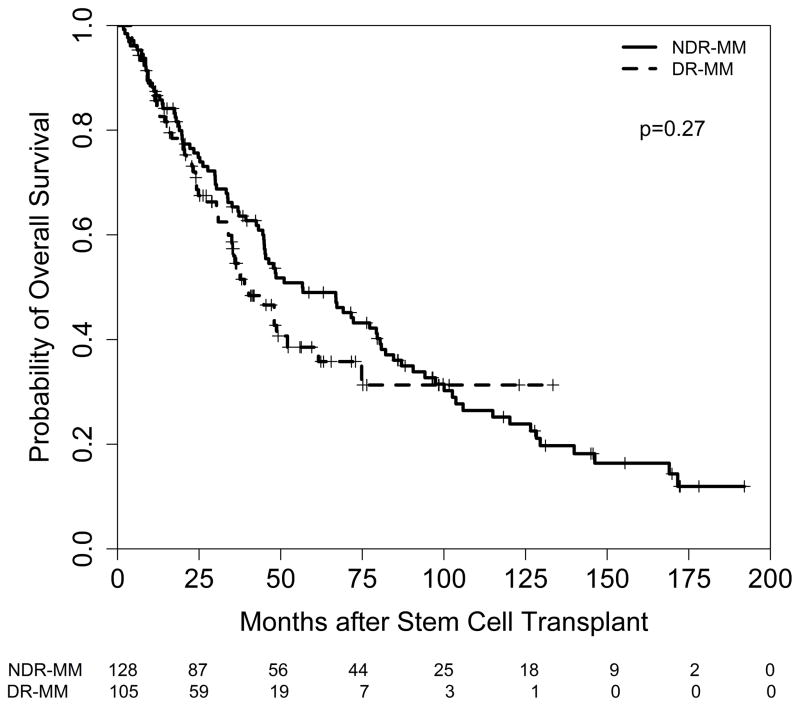
The median follow up after auto-HCT for surviving patients was 42 months (range 6–192 months). Seventy-five percent of the patients progressed in the study and 60% died. The median PFS was 17.6 months and the median OS was 48.0 months for all refractory patients (Figure 1 and 2, respectively). Although PFS and OS were longer for NDR-MM patients (PFS=18.2 months; OS 56.6 months) compared with DR-MM patients (PFS=14.4 months; OS=38.9 months), the differences were not statistically significant (p≥ 0.27). In contrast, a significant association between PFS and type of refractory disease (relapsed and refractory vs. primary refractory), hemoglobin level, cytogenetic risk, number of lines of prior chemotherapy, and prior disease status was noted. Patients with relapsed refractory MM (hazard ratio (HR)=1.9; p<0.001), those with hemoglobin levels < 10 g/dL (HR=1.6; p=0.004), high-risk cytogenetic patients (HR=2.2; p<0.001), those receiving more lines of prior treatment (HR=1.2; p=0.004), and patients with progressive disease prior to auto-HCT (HR=2.1; p<0.001) experienced worse outcomes. The international staging system (ISS) score did not have a significant impact on PFS at the 0.05 level. Taken together, only cytogenetic risk and number of lines of prior chemotherapy remained independently associated with PFS.
Figure 1.
PFS in NDR-MM versus DR-MM patients after auto-HCT
Figure 2.
OS in NDR-MM versus DR-MM patients after auto-HCT
Consistent with PFS, a significant association between OS and type of refractory disease, hemoglobin level, cytogenetic risk, number of lines of prior chemotherapy, and prior disease status was observed. Moreover, disease stage and induction treatment were significantly associated with OS. Patients with relapsed refractory MM (HR=2.0; p<0.001), those with hemoglobin levels < 10 g/dL (HR=1.8; p<0.001), high-risk cytogenetic patients (HR=2.3; p<0.001), those receiving more lines of prior treatment (HR=1.2; p=0.013), patients with progressive disease prior to auto-HCT (HR=2.4; p<0.001), and triplet induction treatment (triplet vs. doublet: HR=1.6; p=0.014) experienced an increased risk of death, while patients receiving maintenance treatment (HR=0.7; p=0.027) experienced a decreased risk of death. Number of lines of prior chemotherapy, and induction treatment were independent predictors of OS in the multivariable analysis.
DISCUSSION
Myeloma patients with refractory disease present a unique treatment challenge where the clinical outcomes remain suboptimal despite deployment of novel agents. The induction regimens using PIs and/or IMiDs are extremely effective and induce responses in >80% of patients, so the patients who fail to respond or become refractory to these highly effective drugs represent a particularly aggressive form of disease. The ideal treatment algorithm for these patients who have a suboptimal response to induction therapy is not clear. In the current study, we report our institutional experience in patients who have less than PR prior to auto-HCT and demonstrate that HDT followed by auto-HCT is an effective therapy to induce response, even in patients refractory to novel agents.
In our study, the overall response rate for all patients was 80%, with 22% achieving a CR. In the NDR-MM cohort, 82% of patients achieving at least PR or better and 21% achieved CR. Although the majority of patients in the NDR-MM group were not exposed to a PI, 53% (n=68) did demonstrate refractoriness to an IMiD. In the DR-MM cohort, 79% achieved a least a PR with 24% achieving a CR. These response rates are similar to other studies evaluating patients with refractory disease who underwent auto-HCT demonstrating clear role and effectiveness of high dose melphalan in this patient population. Combination therapy for patients with refractory myeloma with newer agents including daratumumab, carfilzomib, elotuzumab or panobinostat have demonstrated ORR of 60–90% with 11–20% of patients achieving a CR 18–22. As demonstrated, auto-HCT yields impressive response rates with a number of CRs and will likely complement therapies with newer agents.
Despite impressive response rates, the PFS and OS are lower than what would be expected in patients who have at least a PR. This correlates with results in other studies. Gertz et al. evaluated patients based on response to an IMiD prior to auto-HCT and found that those who did not achieve PR prior to auto-HCT had a shorter PFS and OS (13.1 months and 30.4 months, respectively) than those who demonstrated response prior to transplantation (22.1 months versus 73.5 months, respectively) 10. Similarly, Lee et al. evaluated the impact of pre-transplant response to novel based regimens (majority were bortezomib based) and found that those achieving less than PR had a median PFS of 4.7 months and median OS of 11.6 months which was significantly lower than those demonstrating response prior to transplantation (patients with CR+VGPR had median PFS of 26.6 months and median OS was not reached) 9. The outcomes of this study demonstrate poorer PFS and OS than shown in our study; however there is no mention of the utilization of maintenance therapy in this study which may account, at least in part, for our improved outcomes.
The DR-MM cohort in our group had shorter PFS and OS compared with those in the NDR-MM group, although not statistically significant. However, it is important to point out that the groups were not matched and there were important differences in the patient characteristics. For instance, significantly more patients in DR-MM group received induction with triplet regimens (52% vs. 21% in NDR-MM, p<0.001). Similarly, chemomobilization was used more often in DR-MM (51% vs. 31% in NDR-MM, p=0.002) and significantly more patients in DR-MM received maintenance therapy (61% vs. 40% in NDR-MM, p=0.001). It is also important to note that the majority of patients in the DR-MM group (65%), underwent auto-HCT after 2010, while the majority of patients in the NDR-MM (74%) were transplanted prior to 2010. With lenalidomide and thalidomide being approved by the Food and Drug Administration (FDA) for MM in 2006 and bortezomib in 2008, many of the patients in the NDR-MM did not have access to these agents and clearly represent a distinct population. Nevertheless, the median PFS of 14.4 months and median OS of 38.9 months in patients refractory to both IMiDs and PIs is quite encouraging. For example a large analysis of the U.S. patients from two independent databases by Usmani et al. showed that the median OS was only 6.7 months in double-refractory patients 12. Overall, our data highlight that auto-HCT is an effective therapy to induce response and possibly prolong survival in double-refractory patients. However, the role of auto-HCT in this scenario will continue to evolve as more effective IMiDs, PIs, and other agents become available. It is likely that these agents will augment the outcome of auto-HCT in these high-risk patients.
In the multivariable analysis, cytogenetic risk and number of lines of prior chemotherapy were independently associated with PFS. Poorer outcomes in patients with high risk cytogenetics have been previously described 23,24. Similarly, the current literature suggests that giving additional lines of therapy prior to auto-HCT to patients who do not respond to the first line therapy may not result in longer survival. For instance, a Center for International Blood and Marrow Research (CIBMTR) analysis found that further salvage therapy prior to auto-HCT in patients not achieving optimal response to induction may improve depth of response prior to auto-HCT, but did not influence PFS or OS 25. Consistent results were seen in a study of primary refractory myeloma patients from Mayo Clinic where the 3-year OS from the start of initial treatment for those going to auto-HCT directly versus additional chemotherapy was similar 8. The above studies suggest that the patients eligible for transplant may proceed to auto-HCT without further attempts to achieve a deeper response after failing initial induction therapy. However, this notion is likely going to be challenged in the future with the availability of newer and more potent anti-myeloma agents.
Limitations to this study include its retrospective nature and non-standardized induction and maintenance regimens, as well as reflecting old patterns of care (most patients in the NDR-MM group did not receive a PI before SCT). There is also possible selection bias on patients with nonresponsive disease that proceeded to transplantation, specifically in those patients with relapsed/refractory disease. To attempt to capture the aggressiveness of the disease we evaluated the relapsed/refractory subgroup into two groups based on if they progressed within six months of initial response versus after six months of initial response. No significant difference was found in the OS and PFS between these groups. Noting that the treatment paradigms and supportive care guidelines have changed over the course of time in our cohort of patients, we evaluated the year of transplantation in respect to transplantation outcomes. There was no statistically significant difference in year of transplantation for OS or PFS in the univariable or multivariable model. The strengths include the large sample size to demonstrate the effectiveness of HDT and auto-HCT in this difficult patient population with the majority of patients able to achieve a response and a proportion of those able to achieve a complete response. With the current treatment options, HDT with auto-HCT stands as an effective tool to implement response in patients with refractory myeloma, however further studies should be performed to evaluate the role of auto-HCT in combination with newer agents.
Acknowledgments
Sources of Funding: This research is supported in part by the National Institutes of Health through MD Anderson’s Cancer Center Support Grant (CCSG PA30 CA016672).
Footnotes
- Nina Shah, MD: Membership on an entity’s Board of Directors or advisory committees and Research Funding for Celgene and Takeda
- Stefan O. Ciurea, MD: Equity Ownership of Cyto-Sen Therapeutics and Advisory Board member for Spectrum Pharmaceuticals
- Lauren W. Veltri, MD: data curation, investigation, original draft
- Denai R. Milton: formal analysis, original draft
- Ruby Delgado: data curation
- Nina Shah, MD: review and editing
- Krina Patel, MD: review and editing
- Yago Nieto, MD, PhD: review and editing
- Partow Kebriaei, MD: review and editing
- Uday R. Popat, MD: review and editing
- Simrit Parmar, MD: review and editing
- Betul Oral, MD: review and editing
- Stefan O. Ciurea, MD: review and editing
- Chitra Hosing, MD: review and editing
- Hans Lee, MD: review and editing
- Elizabet E. Manasanch, MD: review and editing
- Robert Z. Orlowski, PhD, MD: review and editing
- Elizabeth J. Sphall, MD: review and editing
- Richard E. Champlin, MD: review and editing
- Muzaffar H. Qazilbash, MD: review and editing
- Qaiser Bashir, MD: conceptualization, funding acquisition, investigation, methodology, project administration, supervision, original draft, review and editing
References
- 1.Combination chemotherapy versus melphalan plus prednisone as treatment for multiple myeloma: An overview of 6,633 patients from 27 randomized trials. myeloma trialists’ collaborative group. J Clin Oncol. 1998;16(12):3832–3842. doi: 10.1200/JCO.1998.16.12.3832. [DOI] [PubMed] [Google Scholar]
- 2.Dhakal B, Girnius S, Hari P. Recent advances in understanding multiple myeloma. F1000Res. 2016;5 doi: 10.12688/f1000research.8777.1. eCollection 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosinol L, Garcia-Sanz R, Lahuerta JJ, et al. Benefit from autologous stem cell transplantation in primary refractory myeloma? different outcomes in progressive versus stable disease. Haematologica. 2012;97(4):616–621. doi: 10.3324/haematol.2011.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blade J, Lopez-Guillermo A, Bosch F, et al. Impact of response to treatment on survival in multiple myeloma: Results in a series of 243 patients. Br J Haematol. 1994;88(1):117–121. doi: 10.1111/j.1365-2141.1994.tb04986.x. [DOI] [PubMed] [Google Scholar]
- 5.Singhal S, Powles R, Sirohi B, Treleaven J, Kulkarni S, Mehta J. Response to induction chemotherapy is not essential to obtain survival benefit from high-dose melphalan and autotransplantation in myeloma. Bone Marrow Transplant. 2002;30(10):673–679. doi: 10.1038/sj.bmt.1703717. [DOI] [PubMed] [Google Scholar]
- 6.Moreau P, Attal M, Pegourie B, et al. Achievement of VGPR to induction therapy is an important prognostic factor for longer PFS in the IFM 2005-01 trial. Blood. 2011;117(11):3041–3044. doi: 10.1182/blood-2010-08-300863. [DOI] [PubMed] [Google Scholar]
- 7.Tan D, Lao Z, Loh Y, et al. Attainment of at least a very good partial response after induction treatment is an important surrogate of longer survival for multiple myeloma. Bone Marrow Transplant. 2010;45(11):1625–1630. doi: 10.1038/bmt.2010.25. [DOI] [PubMed] [Google Scholar]
- 8.Majithia N, Vincent Rajkumar S, Lacy MQ, et al. Outcomes of primary refractory multiple myeloma and the impact of novel therapies. Am J Hematol. 2015;90(11):981–985. doi: 10.1002/ajh.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SE, Yoon JH, Shin SH, et al. Impact of failed response to novel agent induction in autologous stem cell transplantation for multiple myeloma. Ann Hematol. 2014;93(4):627–634. doi: 10.1007/s00277-013-1911-1. [DOI] [PubMed] [Google Scholar]
- 10.Gertz MA, Kumar S, Lacy MQ, et al. Stem cell transplantation in multiple myeloma: Impact of response failure with thalidomide or lenalidomide induction. Blood. 2010;115(12):2348–2353. doi: 10.1182/blood-2009-07-235531. [DOI] [PubMed] [Google Scholar]
- 11.Oivanen TM, Kellokumpu-Lehtinen P, Koivisto AM, Koivunen E, Palva I. Response level and survival after conventional chemotherapy for multiple myeloma: A finnish leukaemia group study. Eur J Haematol. 1999;62(2):109–116. doi: 10.1111/j.1600-0609.1999.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 12.Usmani S, Ahmadi T, Ng Y, et al. Analysis of real-world data on overall survival in multiple myeloma patients with >/=3 prior lines of therapy including a proteasome inhibitor (PI) and an immunomodulatory drug (IMiD), or double refractory to a PI and an IMiD. Oncologist. 2016 doi: 10.1634/theoncologist.2016-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: A multicenter international myeloma working group study. Leukemia. 2012;26(1):149–157. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajkumar SV, Harousseau JL, Durie B, et al. Consensus recommendations for the uniform reporting of clinical trials: Report of the international myeloma workshop consensus panel 1. Blood. 2011;117(18):4691–4695. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallington-Beddoe CT, Gottlieb DJ, Garvin F, Antonenas V, Sartor MM. Failure to achieve a threshold dose of CD34+CD110+ progenitor cells in the graft predicts delayed platelet engraftment after autologous stem cell transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2009;15(11):1386–1393. doi: 10.1016/j.bbmt.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Lee CK, Barlogie B, Munshi N, et al. DTPACE: An effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma. J Clin Oncol. 2003;21(14):2732–2739. doi: 10.1200/JCO.2003.01.055. [DOI] [PubMed] [Google Scholar]
- 17.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of Statistics. 1988;16(3):1141–1154. [Google Scholar]
- 18.Palumbo A, Chanan-Khan A, Weisel K, et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(8):754–766. doi: 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Oriol A, Nahi H, et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med. 2016;375(14):1319–1331. doi: 10.1056/NEJMoa1607751. [DOI] [PubMed] [Google Scholar]
- 20.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373(7):621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 21.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17(1):27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 22.San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 23.Kazmi SM, Nusrat M, Gunaydin H, et al. Outcomes among high-risk and standard-risk multiple myeloma patients treated with high-dose chemotherapy and autologous hematopoietic stem-cell transplantation. Clin Lymphoma Myeloma Leuk. 2015;15(11):687–693. doi: 10.1016/j.clml.2015.07.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scott EC, Hari P, Sharma M, et al. Post-transplant outcomes in high-risk compared with non-high-risk multiple myeloma: A CIBMTR analysis. Biol Blood Marrow Transplant. 2016;22(10):1893–1899. doi: 10.1016/j.bbmt.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vij R, Kumar S, Zhang MJ, et al. Impact of pretransplant therapy and depth of disease response before autologous transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2015;21(2):335–341. doi: 10.1016/j.bbmt.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]