Abstract
Rationale
Accumulating data supports a therapeutic role for mesenchymal stem cell (MSC) therapy; however, there is no consensus on the optimal route of delivery.
Objective
We tested the hypothesis that the route of MSC delivery influences the reduction in infarct size (IS) and improvement in left ventricular ejection fraction (LVEF).
Methods and Results
We performed a meta-analysis investigating the effect of MSC therapy in acute myocardial infarction (AMI) and chronic ischemic cardiomyopathy (ICM) preclinical studies (58 studies; n=1165 mouse, rat, swine) which revealed a reduction in IS and improvement of LVEF in all animal models. Route of delivery was analyzed in AMI swine studies and clinical trials (6 clinical trials; n=334 patients). In AMI swine studies, transendocardial stem cell injection (TESI) reduced IS (n=49, 9.4% reduction 95%CI −15.9, −3.0), whereas intramyocardial injection (DI), intravenous infusion (IV), and intracoronary infusion (IC) indicated no improvement. Similarly, TESI improved LVEF (n=65, 9.1% increase 95%CI 3.7, 14.5), as did DI and IV, while IC demonstrated no improvement. In humans, changes of LVEF paralleled these results, with TESI improving LVEF (n=46, 7.0% increase 95%CI 2.7, 11.3), as did IV, but again IC demonstrating no improvement.
Conclusions
MSC therapy improves cardiac function in animal models of both AMI and ICM. The route of delivery appears to play a role in modulating the efficacy of MSC therapy in AMI swine studies and clinical trials, suggesting the superiority of TESI due to its reduction in IS and improvement of LVEF, which has important implications for the design of future studies.
Keywords: Mesenchymal stem cell, route of delivery, cell therapy, myocardial infarction, meta-analysis
Subject Terms: Cell Therapy, Stem Cells, Myocardial Infarction, Translational Studies, Meta-Analysis
INTRODUCTION
Cardiovascular disease, which can lead to myocardial infarction (MI) and heart failure, is the leading cause of death worldwide1. Current standard therapies succeed only in temporarily managing the disease, illustrating the need for novel approaches to prevent and reverse cardiac dysfunction. Cell-based therapy displays remarkable regenerative promise for repairing cardiac damage post-MI2. Specifically, mesenchymal stem cells (MSCs) have produced significant and encouraging results for a variety of pathological conditions including MI in both preclinical studies2 and clinical trials3–8. MSC-based therapies are currently used to treat both acute MI (AMI) and chronic ischemic cardiomyopathy (ICM), which are thought to work by activating endogenous tissue repair through paracrine signaling as well as exhibiting immunomodulatory properties, reducing immune-mediated damage after MI9. For example, MSCs are antifibrotic and produce left ventricular reverse remodeling in preclinical models10. More importantly, MSCs improve patient functional status and quality of life8, 11, 12. Large animal models, specifically swine, are better predictors of response to MSC therapy in humans due to their longer life span and similarities in immune system properties13 and cardiac function14.
MSC translational research has focused mainly on AMI animal models, but there has been a shift toward also investigating ICM models10. MSC immunomodulatory properties and paracrine secretion reduce inflammation, protect compromised viable tissue and stimulate cellular growth, proliferation, and differentiation to help prevent and reverse ischemic injury in AMI15–17. MSCs prevent the initial cardiac damage of AMI before it progresses to pathologic remodeling of the heart17. MSC therapy for ICM focuses on reducing scar size and promoting endogenous tissue regeneration to reverse worsening cardiac dysfunction16. Reducing the infarct size (IS) improves the left ventricular ejection fraction (LVEF), because a smaller area of scarred and akinetic myocardium results in less ventricular remodeling18. A large IS with significant ventricular remodeling leads to increased chamber volume in an attempt to maintain cardiac output and compensate for the loss of viable myocardium. These effects are followed by an eventual decline in ejection fraction and poor long-term prognosis19.
In this meta-analysis, we discuss the beneficial effects of MSC therapy on AMI and ICM preclinical models and the implications for clinical intervention and therapies. We also assess the efficacy of different routes of MSC delivery, including transendocardial stem cell injection (TESI), intramyocardial injection (DI), intravenous infusion (IV), and intracoronary infusion (IC). TESI is a minimally invasive, catheter-based route of delivery, where cells are injected directly into the myocardium through the endocardium. DI is performed through a thoracotomy and cells are injected into the myocardium through the epicardium. IV is the least invasive route; cells are infused into the venous blood supply and allowed to migrate toward the injured myocardium. Lastly, in IC, cells are infused into the coronary artery that supplies the infarcted myocardium. A recent review by Golpanian et al.20 concluded that there is a lack of consensus as to the optimal route of stem cell delivery, illustrating the need for further examination of the efficacy of these different routes.
METHODS
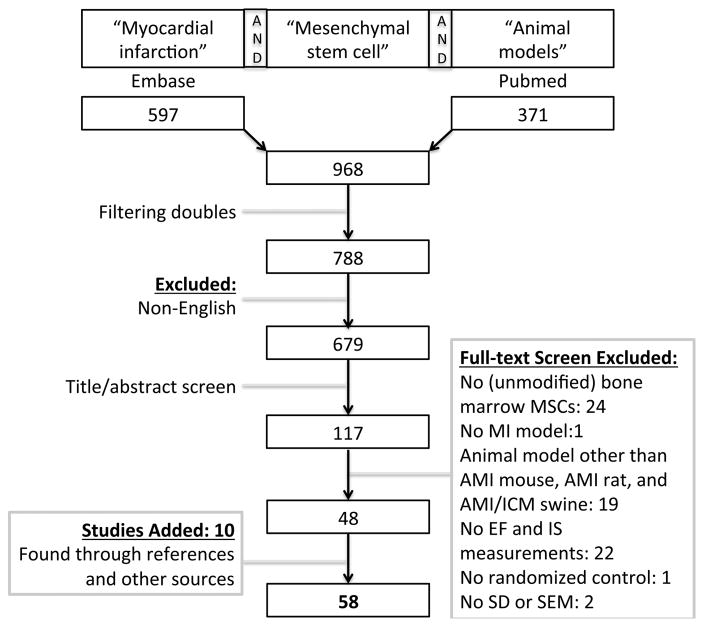
The research protocol was based on the meta-analysis conducted by Zwetsloot et al.21. Specifically, we performed a search of PubMed and Embase with the terms: myocardial infarction, mesenchymal stem cell, and animal models (Figure 1, flowchart). Clinical trials were found via clinicaltrials.gov as well as searching PubMed and Embase using the terms: myocardial infarction, mesenchymal stem cell, and clinical trials. Studies were screened by two independent investigators (A. Kanelidis, J. Lopez) in the title-abstract and full-text screen. A third investigator (C. Premer) was consulted in case of no consensus on inclusion. In addition, references and other sources were examined to find any other suitable studies based on the inclusion criteria. Only studies published in English were included. The studies were carefully examined to exclude overlapping data. Studies were included if they reported a placebo-controlled MI animal model (mouse, rat, or swine) where bone marrow-derived MSCs were administered and in which LVEF or IS was used as a parameter. We were interested in the effect of unmodified MSCs, so we excluded any pretreated, genetically engineered, or transfected cells. AMI mouse, rat, and swine models were analyzed, as well as AMI clinical trials. ICM was only analyzed in swine models due to the small number of trials in mice, rats, and humans. Stem cells were transplanted ≤1 week after MI in AMI animal models, and ≥1 month after MI in ICM animal models. Studies were excluded if the time from MI to stem cell transplantation was subacute (>1 week but <1 month).
Figure 1.
Flowchart of the systematic search, conducted on August 8, 2016.
We used LVEF and IS as our primary outcome measures, and therefore, excluded any studies that did not report an LVEF or IS measurement. Fractional shortening (FS), dose, and change (Δ) in IS (ΔIS) and LVEF (ΔLVEF) were used as secondary outcome measures. The studies measuring LVEF (% EF) used different methods such as echocardiography, MRI, left ventricular angiography, and single-photon emission computed tomography. We used the results provided by the studies and no distinction was made as to methodology. For the analysis of multiple measurements (LVEF, IS, FS), the time point furthest from cell transplantation was used, as we deemed this approach to be the best predictor of functional outcome. For ΔIS and ΔLVEF, the final time points were standardized to improve homogeneity and reduce bias between studies – up to 12 weeks for animal models and 24 weeks for clinical trials; baseline values were taken after MI. Studies with measurements of IS were determined by triphenyltetrazolium chloride stain, Masson’s Trichrome stain, or magnetic resonance imaging, which measured the scar volume as a percent of left ventricular volume. As with LVEF, we obtained the information provided by the studies and no distinction was made between different techniques. Subgroup analyses were conducted on routes of delivery to test for differences in efficacy. Differences in the number of administered MSCs were analyzed to control for dose as a confounding variable in regards to effect on IS and LVEF. In cases of missing data for our primary outcome measures, corresponding authors were contacted. Emails were sent to sixteen authors; eight responded.
Statistical analysis
Meta-analyses were performed and forest plots were generated using Revman v5.3 (Cochran Tech, London, UK). Random effects models were used throughout, due to the possible heterogeneity from sources such as the number of cells administered, autologous versus allogeneic stem cells, and differences in time for final end-points. If standard deviation (SD) was not provided by the studies, standard error of the mean (SEM) was used to calculate the standard deviation. If neither SD nor SEM were found, studies were excluded and deemed not estimable. Studies that were deemed not estimable due to missing data were displayed in the forest plots, but were excluded from the statistical analyses. The outcomes measured by this study are only continuous variables and as such are represented as a Mean Difference (MD) with 95% confidence interval (95%CI) between groups. For studies that contained more than two treatment arms, only control and MSC groups were analyzed. Studies conducting more than one experimental group containing MSCs had their values pooled together using mean, standard deviation, and size and were denoted by an asterisk (*).
Studies where P<0.05 were considered statistically significant and two-sided 95%CI were reported throughout the study. In addition, the I2 statistic was used to assess for heterogeneity within the different subgroups. We use I2 greater than 25% as moderate heterogeneity and greater than 75% as a high degree of heterogeneity. A sensitivity analysis was used to assess for risk of bias on significant results by excluding trials with unclear risk of performance bias, selection bias, or attrition bias. Studies that indicated a baseline value with no significant change were included in the analysis and noted with two asterisks (**). Studies that share the same first author and year were denoted with an up arrow (^). Meta-regression was conducted for dose analysis using Stata 13 (StataCorp LP, College Station, TX); P<0.05 was considered significant and 95% CI were used.
RESULTS
Our final search was performed on August 8, 2016 (Figure 1, flowchart). We identified 371 papers on PubMed and 597 on Embase. After removal of duplicates and title/abstract screening, 117 papers were selected for full-text screening. Fifty-eight papers were finally included and a meta-analysis was performed on mouse, rat, and swine studies investigating the effect of MSC therapy on AMI and ICM (n=1165 animals). Characteristics of the enrolled animal studies are presented in the Online Table I. A similar meta-analysis was performed on six AMI clinical trials (n=334 patients), characteristics of which are depicted in Online Table II.
MSC therapy reduces IS in animal models
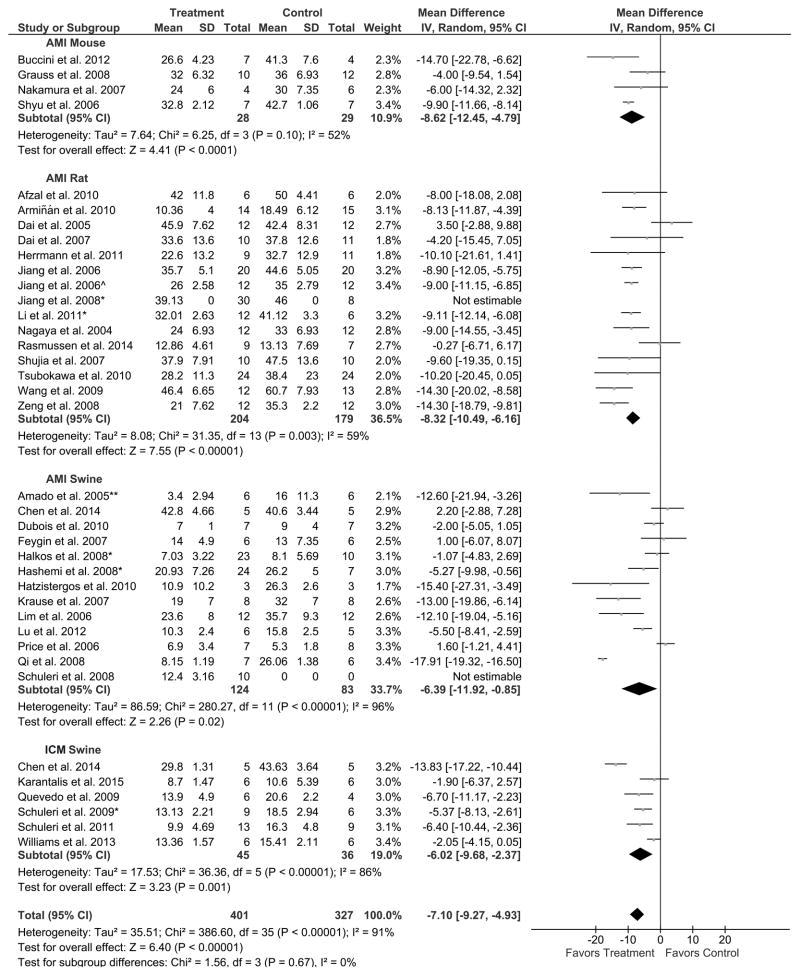
Nineteen rodent studies were examined to assess the efficacy of MSC therapy for reducing IS. Four mouse studies, comprised of 28 treated and 29 control mice were analyzed, and two of these studies favored MSC treatment while two did not indicate a difference between treatment and control. Meta-analysis of these mouse studies revealed an 8.6% reduction in IS (95% CI: −12.5, −4.8; Figure 2), thus favoring MSC treatment (P<0.0001). Fourteen rat studies (174 treated, 171 control rats) were analyzed for efficacy of MSC therapy in the reduction of IS. In seven of these studies, rats receiving MSC treatment exhibited improved IS, whereas in seven studies no difference was seen between the treated and control animals. Overall, there was an 8.3% greater reduction in IS (95% CI: −10.5, −6.2; Figure 2) in treated animals compared to control, favoring MSC treatment (P<0.00001) and paralleling the results of the mouse studies.
Figure 2. Endpoint: Infarct size (IS; % of left ventricle [LV]. Grouped by: Animal model. Result: Favors MSC treatment.
Mean effect ± standard deviation (SD) of mesenchymal stem cell (Treatment) or placebo/no treatment (Control) on the reduction of IS (% of LV) for acute myocardial infarction (AMI) mouse, rat, and swine studies and chronic ischemic cardiomyopathy (ICM) swine studies. Number of animals in each arm of the study (Total). Relative weight of each study (Weight). Mean difference between Treatment and Control with a 95% confidence interval (95% CI), using inverse variance (IV) and random effects model (Random).
Twelve AMI swine studies were analyzed for efficacy of MSC therapy in the reduction of IS. Seven of the studies favored MSC treatment, while five revealed no difference between treatment and control. Out of a total of 114 treated and 83 control swine, there was a 6.4% reduction in IS (95% CI: −11.9, −0.9; Figure 2), demonstrating an overall improvement in MSC treated animals compared to control (P=0.02). Six ICM swine studies (45 treated, 36 control swine) were analyzed for efficacy of MSC therapy in the reduction of IS; four favored MSC treatment, while two studies did not reveal a difference between treatment and control. There was a 6.0% reduction in IS in treated animals (95% CI: −9.7, −2.4; Figure 2), thus favoring MSC treatment (P=0.001). Ultimately, there was a 7.1% reduction in IS for all MSC treated animal models compared to control (361 treated, 319 control animals; 95% CI: −9.3, −4.9; P<0.00001; Figure 2), favoring MSC therapy.
MSC therapy improves LVEF in animal models
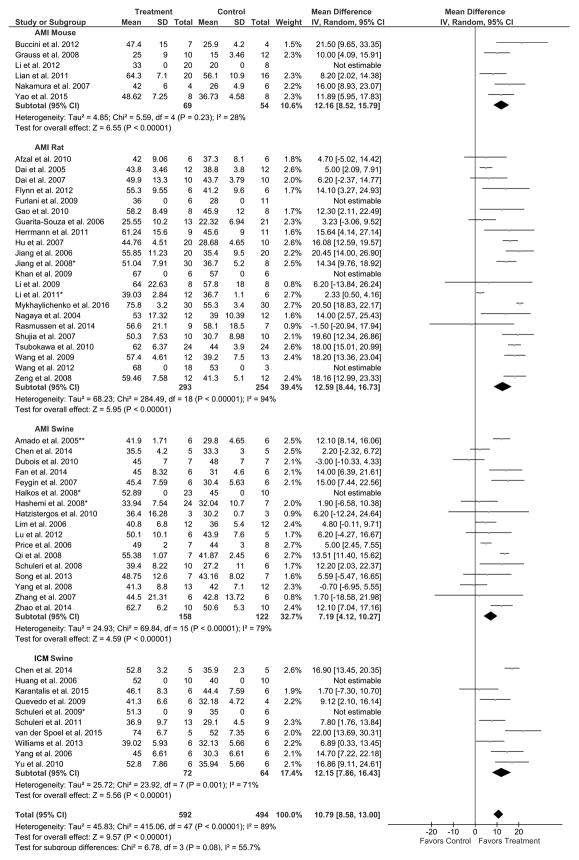
Twenty-four rodent studies were examined to assess the efficacy of MSC therapy for improving LVEF. All five mouse studies analyzed favored MSC treatment. There was an improvement in LVEF for MSC treated mice compared to control (49 treated, 46 control mice) by 12.2% (95% CI: 8.5, 15.8; P<0.00001; Figure 3). In the nineteen rat studies analyzed for efficacy of MSC therapy (263 treated, 234 control rats), fourteen favored MSC treatment, whereas five studies resulted in no difference between treatment and control. There was a 12.6% improvement in LVEF in treated animals compared to control (95% CI: 8.4, 16.7; P<0.00001; Figure 3).
Figure 3. Endpoint: Left ventricular ejection fraction (LVEF; %). Grouped by animal model. Result: Favors MSC treatment.
Mean effect ± standard deviation (SD) of mesenchymal stem cells (Treatment) or placebo/no treatment (Control) on the improvement of LVEF (%) for acute myocardial infarction (AMI) mouse, rat, and swine studies and chronic ischemic cardiomyopathy (ICM) swine studies. Number of animals in each arm of the study (Total). Relative weight of each study (Weight). Mean difference between Treatment and Control with a 95% confidence interval (95% CI), using inverse variance (IV) and random effects model (Random).
Sixteen AMI swine studies (135 treated, 112 control swine) were analyzed for efficacy of MSC therapy in the improvement of LVEF. Seven studies favored MSC treatment, while the other nine studies did not demonstrate a difference between treatment and control. There was a 7.2% improvement in LVEF (95% CI: 4.1, 10.3; Figure 3) in treated animals, thus favoring MSC treatment (P<0.00001). Furthermore, eight ICM swine studies (53 treated, 48 control swine) were analyzed, seven of which favored MSC treatment, whereas only one study did not reveal a difference between treatment and control. Accordingly, there was a 12.2% improvement in LVEF (95% CI: 7.9, 16.4) with a preference toward MSC treatment (P<0.00001; Figure 3). Lastly, there was a 10.8% improvement of LVEF for all MSC treated animal models compared to control (500 treated, 440 control animals; 95% CI: 8.6, 13.0; P<0.00001; Figure 3), again favoring MSC therapy.
Route of delivery affects IS reduction in AMI swine studies
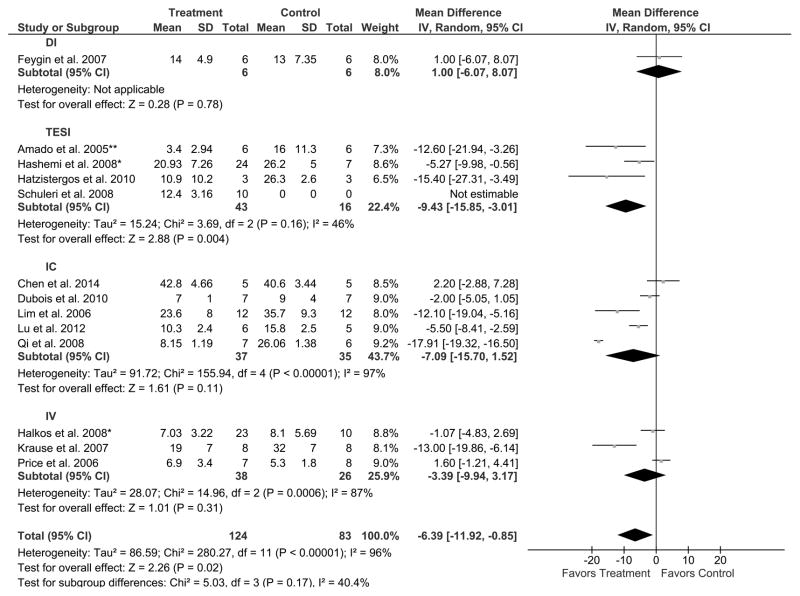
We next assessed the influence of route of delivery on the therapeutic efficacy of MSCs in the reduction of IS in AMI swine studies. Twelve studies using four different cell delivery routes were examined: DI, TESI, IC, and IV. One study assessed the efficacy of the DI route of MSC delivery for the reduction of IS, concluding that MSC treatment did not reduce IS compared to control (6 treated, 6 control swine); rather, it increased the IS by 1.0% (95% CI: −6.1, 8.1; Figure 4), suggesting that DI is not a favorable route of delivery (P=0.78). All three studies using TESI favored MSC treatment compared to control. These studies revealed a 9.4% reduction in IS (95% CI: −15.9, −3.0; Figure 4) in the 33 treated swine compared to the 16 controls, thus favoring TESI (P=0.004). Five studies were analyzed for the efficacy of the IC route of delivery, with three studies demonstrating that MSC treatment reduced IS compared to control, while two studies did not (37 treated, 35 control swine). There was a 7.1% reduction in IS (95% CI: −15.7, 1.5; Figure 4) suggesting that IC is not a favorable route (P=0.11). Two out of three studies that used IV did not reveal a difference between MSC treatment and control (38 treated, 26 control swine). There was a 3.4% decrease in IS (95% CI: −9.9, 3.2; Figure 4), which did not favor IV route of delivery (P=0.31).
Figure 4. Endpoint: IS (% of LV) in AMI swine studies. Grouped by route of cell delivery. Result: Favors TESI.
Mean effect ± standard deviation (SD) of mesenchymal stem cells (Treatment) or placebo/no treatment (Control) on the reduction of IS (% of LV) based on the route of delivery: intramyocardial injection (DI), transendocardial stem cell injection (TESI), intracoronary infusion (IC), and intravenous infusion (IV), in acute myocardial infarction (AMI) swine studies illustrating that TESI favors MSC treatment while DI, IC, and IV does not. Number of animals in each arm of the study (Total). Relative weight of each study (Weight). Mean difference between Treatment and Control with a 95% confidence interval (95% CI), using inverse variance (IV) and random effects model (Random).
Route of delivery affects LVEF improvement in AMI swine studies
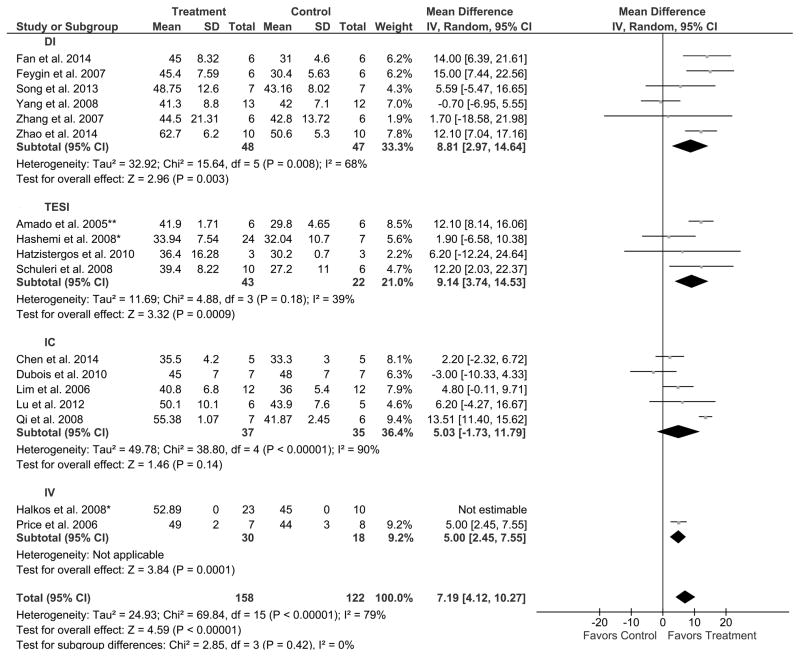
Similarly, we assessed the effect of route of delivery on improvement of LVEF in a total of sixteen AMI swine studies. Six studies used DI, and three of these studies favored MSC treatment compared to control, whereas three studies did not reveal a difference. Out of 48 treated swine compared to 47 controls, there was an 8.8% improvement in LVEF (95% CI: 3.0, 14.6; Figure 5), highlighting a preference toward DI (P=0.003). Four studies were analyzed for efficacy of TESI as a route of delivery. Two studies favored MSC treatment compared to control, whereas the other two did not reveal a difference. Out of 43 treated swine compared to 22 controls, there was a 9.1% improvement of LVEF (95% CI: 3.7, 14.5; Figure 5), therefore favoring TESI (P=0.0009). Five studies assessed the IC route of delivery of MSCs. Only one study favored MSC treatment versus control (37 treated, 35 control swine), while the remaining four studies did not reveal a difference. There was a 5.0% increase in LVEF (95% CI: −1.7, 11.8; Figure 5), which did not favor IC (P=0.14). Only one study was analyzed in AMI swine models using IV administration of cells and it indicated a preference toward MSC treatment compared to control (7 treated, 8 control swine) with a 5.0% improvement in LVEF (95% CI: 2.5, 7.6; Figure 5), thus favoring IV (P=0.0001).
Figure 5. Endpoint: LVEF (%) in AMI swine studies. Grouped by route of cell delivery. Result: Favors TESI, DI, and IV.
Mean effect ± standard deviation (SD) of mesenchymal stem cells (Treatment) or placebo/no treatment (Control) on the improvement of LVEF (%) based on the route of delivery: intramyocardial injection (DI), transendocardial stem cell injection (TESI), intracoronary infusion (IC), and intravenous infusion (IV), in acute myocardial infarction (AMI) swine studies illustrating that TESI, DI, and IV favors MSC treatment while IC does not. Number of animals in each arm of the study (Total). Relative weight of each study (Weight). Mean difference between Treatment and Control with a 95% confidence interval (95% CI), using inverse variance (IV) and random effects model (Random).
Route of delivery affects LVEF improvement in AMI clinical trials
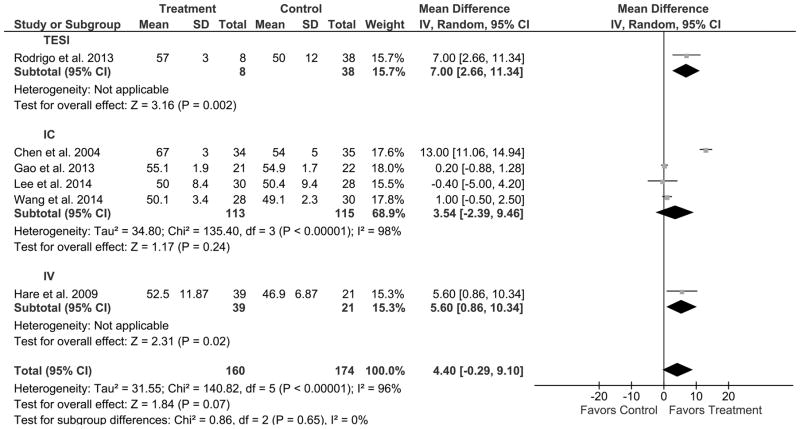
The route of MSC delivery on improvement of LVEF was examined in six AMI clinical trials, none of which utilized DI. The efficacy of TESI administration of MSCs was analyzed in one clinical trial, which favored MSC treatment compared to control. Out of a total of 8 treated patients compared to 30 controls, there was a 7.0% improvement in LVEF (95% CI: 2.7, 11.3; Figure 6), thus favoring TESI (P=0.002). Four clinical trials were analyzed for efficacy of the IC route of delivery. One favored MSC treatment compared to control, whereas 3 clinical trials revealed no difference. Out of 113 treated patients compared to 115 controls, there was a 3.5% improvement in LVEF (95% CI: −2.4, 9.5; Figure 6), a difference that was not statistically significant, suggesting that IC is not favorable (P=0.24). The efficacy of the IV route of delivery was assessed in one clinical trial, which demonstrated a favorable effect of MSC treatment (39 patients) compared to control (21 patients). Specifically, there was a 5.6% improvement in LVEF (95% CI: 0.9, 10.3; Figure 6), favoring IV (P=0.02).
Figure 6. Endpoint: LVEF (%) in AMI clinical trials. Grouped by route of cell delivery. Result: Favors TESI and IV.
Mean effect ± standard deviation (SD) of mesenchymal stem cells (Treatment) or placebo/no treatment (Control) on the improvement of LVEF (%) based on the route of delivery: transendocardial stem cell injection (TESI), intracoronary infusion (IC), and intravenous infusion (IV), in acute myocardial infarction (AMI) clinical trials illustrating that TESI and IV favors MSC treatment while IC does not. Number of patients in each arm of the study (Total). Relative weight of each study (Weight). Mean difference between Treatment and Control with a 95% confidence interval (95% CI), using inverse variance (IV) and random effects model (Random).
Meta-analysis of secondary outcomes
For all secondary outcomes (fractional shortening [FS], ΔIS, ΔLVEF, and dose) similar trends were observed, all favoring MSC therapy. FS was analyzed in sixteen rat and swine studies (170 treated, 168 control), twelve of which favored MSC treatment, whereas four revealed no difference between treatment and control. Accordingly, there was an 8.2% improvement in FS (95% CI: 3.1, 13.0) favoring MSC treatment (P<0.00001; Online Figure I). For ΔIS, eight swine studies (58 treated, 49 control) were analyzed, six of which favored MSC treatment, whereas two did not. Thus, there was a 6.4% reduction in ΔIS (95% CI: −8.6, −4.3) favoring MSC treatment (P<0.00001; Online Figure II). For ΔLVEF, twenty-two mouse, rat, and swine studies (184 treated, 172 control) were assessed, eight of which favored MSC treatment. Overall, there was a 7.5% increase in ΔLVEF (95% CI: 4.5, 10.6) favoring MSC treatment (P<0.00001; Online Figure III). Furthermore, preclinical studies used between 5×104–4.4×108 cells, with generally more cells used for larger animals (swine>rat>mouse). Meta-regression showed no statistically significant difference for IS and LVEF when dose was taken into account for all preclinical studies (P=0.06 and P=0.15, respectively; data not shown). Meta-regression specifically for AMI swine studies, which compared all four routes of delivery, also indicated that dose was not a significant predictor for IS and LVEF (P=0.54 and P=0.62, respectively; data not shown).
DISCUSSION
We performed a meta-analysis of MSC therapy for three animal models: mouse, rat and swine. Our primary outcomes (IS and LVEF) revealed a reduction in IS and improvement of LVEF in all animal models treated with MSCs (Figures 2 and 3). We also calculated ΔIS and ΔLVEF to normalize potential confounding factors, such as differences in baseline between control and treatment. Both ΔIS and ΔLVEF (Online Figure II and III, respectively) revealed a similar improvement after MSC therapy. The AMI studies were also analyzed based on routes of delivery, including DI, TESI, IV and IC to identify differences. The route of delivery modulated the efficacy of MSC therapy in both AMI swine models and clinical trials. In AMI swine models TESI produced more favorable results, revealing a reduction in IS, while DI, IC, and IV indicated no improvement (Figure 4). Similarly, TESI improved LVEF, as did DI and IV, while IC delivery revealed no improvement (Figure 5). In AMI clinical trials, changes of LVEF paralleled these results, with TESI again improving LVEF, as well as IV, while IC indicated no improvement (Figure 6). DI route of delivery has not been studied in AMI clinical trials. Our meta-analysis confirms that MSCs are an effective therapy for preserving cardiac function by reducing IS and improving LVEF in all three animal models (mouse, rat, and swine). Moreover, the meta-analysis compared AMI and ICM swine studies for improvement of IS and LVEF. AMI swine studies were analyzed based on route of delivery; however, there were not sufficient numbers of ICM swine studies to do a similar comparison. Likewise, there are few ICM clinical trials; therefore, a similar comparison for route of delivery is currently not possible.
Results of Phase I/II clinical trials illustrate that stem cell therapy is safe and efficacious for both AMI and ICM, and furthermore, that MSC therapy favorably affects patients’ functional capacity, ventricular remodeling, and quality of life8, 11, 12, 22. Importantly, we demonstrated that the route of cell delivery modulated the efficacy of MSC therapy. While the reasons for these differences are unclear, the advantages and disadvantages of each route of delivery are highlighted in Online Table III.
DI is the most direct, precise, and accurate epicardial approach of injecting stem cells in and around the infarcted area of the heart. However, a swine study by Grossman et al.23 revealed that despite direct injection into the myocardium, there was a lower total cell retention rate when compared to TESI, due to leakage from the injection site during and after the DI procedure. The invasive nature of a thoracotomy is the biggest drawback for DI, with greater risks for complications and increased morbidity and mortality24. The gold standard of treatment for an acute myocardial infarction is percutaneous coronary intervention (PCI) or medical management, not thoracotomy. Therefore, DI has not been investigated as a route of delivery in AMI clinical trials. DI can be performed during open-heart surgery in coronary artery bypass grafting for heart failure, which explains why it has been investigated in ICM clinical trials.
TESI is a minimally invasive procedure that is feasible and safe, with continuous advancements in imaging and catheterization techniques. There are at least five different TESI catheter designs and three imaging platforms to guide the injections. In 2005, Amado et al.25 used the corkscrew-shaped needle Helix (Biocardia). The studies conducted from 2008–201026–29 utilized the straight needle Stiletto (Boston Scientific). All of these studies used conventional two-dimensional projection X-ray fluoroscopy for imaging. The catheter delivery system has since progressed to the straight needle Myostar (Biosense Webster), which has been utilized in both translational and clinical trials from 2013–20156, 10, 18, 30. The imaging technique also progressed to 3-dimensional electromechanical mapping of the left ventricle using the NOGA system (Biosense Webster). With TESI, the stem cells are injected directly into the myocardium through the endocardium. As with all techniques that require injection into the myocardium, there is a small risk of perforation as well as induction of arrhythmias. However, the benefits of TESI outweigh the risks compared to more invasive procedures like DI, and many swine studies have shown TESI to be a very efficacious route of delivery25, 28, 29, 31.
IV cell delivery is the most convenient and least invasive route, used more often after AMI because of the preponderance of physiological homing signals which allow the cells to migrate toward the injured myocardium24. The biggest concern for the IV route is the lack of implantation and retention in the infarcted region of the heart, since cells are delivered through the systemic circulation, possibly accounting for IV treatment not reducing IS compared to control. There is also an increased likelihood of the MSCs lodging and engrafting in other organs, particularly the lungs, or being eliminated by the reticuloendothelial system, including the spleen32.
IC delivery has the main advantage of delivering MSCs proximal to the infarcted myocardial regions through the appropriate coronary vessel. After the catheter is in position, a balloon is inflated to block the blood flow, which helps MSCs to adhere and transmigrate to the infarcted region of the myocardium2. An intrinsic disadvantage of this route is the difficulty of delivering cells into an area that is not well perfused, possibly explaining the lack of reduction of IS. Additionally, there is a concern of inducing further ischemia by occluding the coronary artery. There is also a threshold for the number of cells that can be delivered before the possibility of embolization in the small coronary arteries and vascular microinfarcts33.
In AMI swine models, TESI was the most favorable route of delivery for reduction of IS. TESI also revealed an improvement in LVEF, consistent with the decrease in IS. The results of TESI for IS and LVEF are promising and support conducting clinical trials using this modality for stem cell delivery. It is important to note that not only is TESI efficacious, but it is also a direct and minimally invasive procedure; therefore, TESI is the route of delivery that is most promising for clinical trials. While DI revealed an improvement in LVEF, it did not demonstrate a reduction in IS, which may be due to lower total cell retention rate23 when compared to TESI. The IV route of delivery also indicated an improvement in LVEF, but similar to DI, it did not demonstrate a difference in IS. Of note, only one study investigated the IV route of delivery, which makes it difficult to make firm conclusions. However, one potential explanation for these results is that the IV route of delivery leads to a migration of MSCs toward the injured heart, but the MSCs do not engraft into the infarcted area. Instead, the MSCs may engraft onto the surrounding viable myocardial tissue, a less hypoxic environment, which may work to reduce ventricular remodeling and improve LVEF without necessarily reducing the IS. For IC delivery, no change in LVEF or reduction in IS was seen.
Although there have been few AMI clinical trials, we explored comparisons to the AMI swine studies. As previously stated, DI has not been investigated in AMI clinical trials. One clinical trial demonstrated that TESI was a favorable and efficacious route of delivery6. IC did not seem to be an efficacious route of delivery in clinical trials. In three of four studies no difference between treatment and control was seen, while Chen et al.3 indicated a difference; albeit a significant outlier, which we chose not to exclude due to the already low number of clinical trials conducted. It is possible that Chen et al. found such a striking difference due to the large number of cells injected, 6 ml containing 8–10×109 BMSCs/ml, compared to an average of 1×106–1×108 cells/ml in the other clinical trials. Therefore, the effect of dose on MSC therapy should be further explored, as seen with TESI in the POSEIDON clinical trial11. The IV route appears to be an effective method of delivery, revealing an improvement of the patients’ LVEF, similar to the translational data for LVEF in AMI swine models. This result lends support to the hypothesis that the IV route helps reduce ventricular remodeling and recovers functional aspects of the heart, such as LVEF, without necessarily reducing the IS.
To take dose into account for preclinical studies we conducted a meta-regression, which indicated that the number of MSCs delivered was not a significant predictor for IS and LVEF in the animal models, including specifically AMI swine models, which compared all four routes of delivery. Golpanian et al.20 concluded that the field of cell therapy lacks consistent and reliable evidence for dosage, with conflicting data for “low” versus “high” dose. While some preclinical and clinical studies report that the number of cells administered is proportional to the observed clinical effect, other studies have yielded paradoxical results. Schuleri et al.34 studied a ICM swine model where MSCs were administered via DI and reported a significant reduction in IS with a “high” dose (200 million MSCs) compared to a “low” dose (20 million MSCs). In contrast to these findings, Hashemi et al.28 studied an AMI swine model with TESI as the route of delivery, which found that the “lower” doses (24 and 240 million MSCs) exhibited a significant decrease in IS, whereas the “higher” dose (440 million MSCs) did not. Future studies should use dose escalations with the different routes of delivery to assess the optimal number of MSCs to administer.
Additionally, studies with fewer animals per group may be underpowered, whereas studies with more animals per group are likely more informative. Therefore, for purposes of this meta-analysis, the forest plots were weighted in terms of standard deviation and sample size so that larger studies held more weight, to account for the studies that may have been underpowered. Even in the presence of underpowered studies, MSC therapy showed a favorable effect on reduction of IS and improvement of LVEF.
Furthermore, many animal studies showed large improvements of LVEF and IS, while other studies including some clinical trials showed improvements, but to a lesser extent. These parameters were analyzed as the primary endpoints because they were shared between the translational studies and the clinical trials. More importantly, however, is the goal of increasing cardiac function, quality of life, and survival in patients. Several clinical trials, including POSEIDON (Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis)11 and TAC-HFT (Transendocardial Autologous Cells in Ischemic Heart Failure Trial)12, reported that MSC therapy did not greatly improve LVEF 12-months post-stem cell injection; however, significant improvements were seen in the clinical status of the patients, as measured by 6-min walk test and Minnesota Living with Heart Failure questionnaire score10.
While our study analyzed both a structural and a functional parameter, IS and LVEF respectively, ischemic cardiomyopathy is a complex disease with multiple mechanisms contributing to its pathology. Following an MI, the heart is in an inflammatory state with microvascular disease, dysfunctional viable myocardium, wall stress, mitochondrial dysfunction, oxidative stress, increased apoptosis, and fibrosis. All of these changes lead to progressive, adverse left ventricular remodeling35. MSCs, via their paracrine signaling and immunomodulatory properties, can attenuate the multiple pathways contributing to adverse left ventricular remodeling. Therefore, we also analyzed FS, another measure of contractility, to help address the remodeling of the heart in ischemic cardiomyopathy. Similar to LVEF, there was an improvement in FS following MSC therapy. Further studies are needed to identify the mechanism(s) by which MSCs attenuate disease progression.
Based on the translational and clinical data, TESI appears to be a favorable method for administration of MSCs. The swine studies using TESI as the route of delivery revealed both a reduction in IS and improvement of LVEF25–27, a result reinforced by TESI providing an improvement of LVEF seen in the AMI clinical trials6. A future application would be to provide MSC therapy via TESI to patients who present with an AMI and undergo PCI, which, as our results suggest, will lead to a better clinical outcome.
Limitations
Our statistical analysis consisted of forest plots, which depicted differences between treatment and control. This type of analysis enabled us to assess if certain routes of delivery were favorable, but it did not allow us to analyze the differences between groups. Also, as in all meta-analyses, heterogeneity must be taken into account. To eliminate some of the heterogeneity, we looked at subgroups, however, this approach reduced the number of studies analyzed per group, at times leaving only one study. Furthermore, not every study provided the SEM/SD, which did not allow us to include them in the analysis.
Also, the infarct sizes showed great variability across some studies, specifically in rat models, which may have impacted the results because larger infarcts are more detrimental to cardiac function. To control for this variability, we calculated ΔIS to account for differences in baseline IS. We analyzed swine studies since they were the only ones to provide enough data for the calculations. Our analysis showed that MSC therapy had an equivalent reduction for ΔIS and IS at the final endpoints, giving us confidence in our results.
While comparison of all four routes of delivery was conducted in acute swine studies, our analysis was limited in the murine and rodent studies since only DI and IV were performed, as well as in human AMI trials where DI was not performed. In addition, due to the low number of ICM swine and human studies, we were not able to do subgroup analyses for the routes of delivery. Therefore, additional studies are needed to verify the best delivery route(s).
Conclusions
We demonstrated that MSC therapy leads to a reduction in IS and an increase in LVEF and cardiac function in both AMI and ICM animal models, and AMI clinical trials, supporting the use of MSC therapy. Furthermore, the route of delivery influences the efficacy of MSC therapy in preclinical and clinical studies. TESI appears to be the most favorable route of delivery due to its reduction in IS and improvement of LVEF in AMI preclinical and clinical trials, which has important implications for the design of future studies.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
There is a therapeutic role for mesenchymal stem cell (MSC) therapy in acute myocardial infarction (AMI) and chronic ischemic cardiomyopathy (ICM) preclinical studies and clinical trials.
Many questions remain on how to optimize MSC therapy, such as, which route of delivery is the most efficacious.
What New Information Does This Article Contribute?
Route of delivery modulates the efficacy of MSC therapy in AMI swine studies and clinical trials.
MSCs administered via transendocardial stem cell injection (TESI) improved cardiac function and appeared to be superior to intramyocardial injection (DI), intravenous infusion (IV), and intracoronary infusion (IC).
MSCs are a promising therapy for treating both AMI and ICM in preclinical studies and clinical trials. Our meta-analysis examined fifty-eight preclinical studies that included 1165 animals and six clinical trials with 334 patients, and confirmed the therapeutic efficacy of MSC therapy. However, many questions remain as to optimizing treatment. Currently, there are four different routes of delivery for MSC therapy: TESI, DI, IV, and IC. We tested the hypothesis that the route of MSC delivery influences the reduction in infarct size (IS) and improvement in left ventricular ejection fraction (LVEF). We discovered that the route of delivery did indeed play an important role in the efficacy of MSC therapy. TESI appears to be the most favorable route of delivery due to both its reduction in IS and improvement of LVEF in AMI preclinical and clinical trials, which has important implications for the design of future studies.
Acknowledgments
We thank Irene S. Margitich for critical reading of the manuscript.
SOURCES OF FUNDING
This research is supported by a US National Institutes of Health (NIH) grant to Joshua M. Hare R01 HL084275. JM Hare is also supported by NIH grants UM1 HL113460, R01 HL107110, and R01 HL110737. A. Kanelidis was supported by a Summer Fellowship from the American Heart Association. C. Premer is supported by a Predoctoral Fellowship from the American Heart Association.
Nonstandard Abbreviations and Acronyms
- MSC
Mesenchymal stem cell
- IS
infarct size
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- TESI
transendocardial stem cell injection
- DI
intramyocardial injection
- IV
intravenous infusion (IV)
- IC
intracoronary infusion
- AMI
acute myocardial infarction
- ICM
chronic ischemic cardiomyopathy
- PCI
percutaneous coronary intervention
- FS
fractional shortening
- 95% CI
95% confidence interval
Footnotes
DISCLOSURES
Dr. Hare reports having a patent for cardiac cell-based therapy; owning equity in Vestion Inc; member of the scientific advisory board and consultant of Vestion, Inc.; Dr. Hare reports being a board member of Vestion Inc. Vestion Inc. did not participate in funding this work.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jimenez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Heart Disease and Stroke Statistics-2016 Update: A Report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. [DOI] [PubMed] [Google Scholar]
- 2.Williams AR, Hare JM. Mesenchymal Stem Cells: Biology, Pathophysiology, Translational Findings, and Therapeutic Implications for Cardiac Disease. Circ Res. 2011;109:923–940. doi: 10.1161/CIRCRESAHA.111.243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen SL, Fang WW, Ye F, Liu YH, Qian J, Shan SJ, Zhang JJ, Chunhua RZ, Liao LM, Lin S, Sun JP. Effect on Left Ventricular Function of Intracoronary Transplantation of Autologous Bone Marrow Mesenchymal Stem Cell in Patients with Acute Myocardial Infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 4.Gao LR, Pei XT, Ding QA, Chen Y, Zhang NK, Chen HY, Wang ZG, Wang YF, Zhu ZM, Li TC, Liu HL, Tong ZC, Yang Y, Nan X, Guo F, Shen JL, Shen YH, Zhang JJ, Fei YX, Xu HT, Wang LH, Tian HT, Liu da Q, Yang Y. A Critical Challenge: Dosage-Related Efficacy and Acute Complication Intracoronary Injection of Autologous Bone Marrow Mesenchymal Stem Cells in Acute Myocardial Infarction. Int J Cardiol. 2013;168:3191–3199. doi: 10.1016/j.ijcard.2013.04.112. [DOI] [PubMed] [Google Scholar]
- 5.Lee JW, Lee SH, Youn YJ, Ahn MS, Kim JY, Yoo BS, Yoon J, Kwon W, Hong IS, Lee K, Kwan J, Park KS, Choi D, Jang YS, Hong MK. A Randomized, Open-Label, Multicenter Trial for the Safety and Efficacy of Adult Mesenchymal Stem Cells after Acute Myocardial Infarction. J Korean Med Sci. 2014;29:23–31. doi: 10.3346/jkms.2014.29.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodrigo SF, van Ramshorst J, Hoogslag GE, Boden H, Velders MA, Cannegieter SC, Roelofs H, Al Younis I, Dibbets-Schneider P, Fibbe WE, Zwaginga JJ, Bax JJ, Schalij MJ, Beeres SL, Atsma DE. Intramyocardial Injection of Autologous Bone Marrow-Derived Ex Vivo Expanded Mesenchymal Stem Cells in Acute Myocardial Infarction Patients Is Feasible and Safe up to 5 Years of Follow-Up. J Cardiovasc Transl Res. 2013;6:816–825. doi: 10.1007/s12265-013-9507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang X, Xi WC, Wang F. The Beneficial Effects of Intracoronary Autologous Bone Marrow Stem Cell Transfer as an Adjunct to Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction. Biotechnol Lett. 2014;36:2163–2168. doi: 10.1007/s10529-014-1589-z. [DOI] [PubMed] [Google Scholar]
- 8.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Jr, Reisman MA, Schaer GL, Sherman W. A Randomized, Double-Blind, Placebo-Controlled, Dose-Escalation Study of Intravenous Adult Human Mesenchymal Stem Cells (Prochymal) after Acute Myocardial Infarction. J Am Coll Cardiol. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma S, Xie N, Li W, Yuan B, Shi Y, Wang Y. Immunobiology of Mesenchymal Stem Cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales A, Hatzistergos K, Natsumeda M, Margitich I, Schulman IH, Gomes SA, Mushtaq M, DiFede DL, Fishman JE, Pattany P, Zambrano JP, Heldman AW, Hare JM. Synergistic Effects of Combined Cell Therapy for Chronic Ischemic Cardiomyopathy. J Am Coll Cardiol. 2015;66:1990–1999. doi: 10.1016/j.jacc.2015.08.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of Allogeneic Vs Autologous Bone Marrow-Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients with Ischemic Cardiomyopathy: The Poseidon Randomized Trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy: The Tac-Hft Randomized Trial. JAMA. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harding J, Roberts RM, Mirochnitchenko O. Large Animal Models for Stem Cell Therapy. Stem Cell Res Ther. 2013;4:23. doi: 10.1186/scrt171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milani-Nejad N, Janssen PM. Small and Large Animal Models in Cardiac Contraction Research: Advantages and Disadvantages. Pharmacol Ther. 2014;141:235–249. doi: 10.1016/j.pharmthera.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elnakish MT, Hassan F, Dakhlallah D, Marsh CB, Alhaider IA, Khan M. Mesenchymal Stem Cells for Cardiac Regeneration: Translation to Bedside Reality. Stem Cells Int. 2012;2012:646038. doi: 10.1155/2012/646038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baraniak PR, McDevitt TC. Stem Cell Paracrine Actions and Tissue Regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van den Akker F, Deddens JC, Doevendans PA, Sluijter JP. Cardiac Stem Cell Therapy to Modulate Inflammation Upon Myocardial Infarction. Biochim Biophys Acta. 2013;1830:2449–2458. doi: 10.1016/j.bbagen.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 18.Williams AR, Suncion VY, McCall F, Guerra D, Mather J, Zambrano JP, Heldman AW, Hare JM. Durable Scar Size Reduction Due to Allogeneic Mesenchymal Stem Cell Therapy Regulates Whole-Chamber Remodeling. J Am Heart Assoc. 2013;2:e000140. doi: 10.1161/JAHA.113.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakker R, Yang P. Mesenchymal Stem Cell Therapy for Cardiac Repair. Curr Treat Options Cardiovasc Med. 2014;16:323. doi: 10.1007/s11936-014-0323-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golpanian S, Schulman IH, Ebert RF, Heldman AW, DiFede DL, Yang PC, Wu JC, Bolli R, Perin EC, Moye L, Simari RD, Wolf A, Hare JM Cardiovascular Cell Therapy Research N. Concise Review: Review and Perspective of Cell Dosage and Routes of Administration from Preclinical and Clinical Studies of Stem Cell Therapy for Heart Disease. Stem Cells Transl Med. 2016;5:186–191. doi: 10.5966/sctm.2015-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zwetsloot PP, Vegh AM, Jansen Of Lorkeers SJ, van Hout GP, Currie GL, Sena ES, Gremmels H, Buikema JW, Goumans MJ, Macleod MR, Doevendans PA, Chamuleau SA, Sluijter JP. Cardiac Stem Cell Treatment in Myocardial Infarction: A Systematic Review and Meta-Analysis of Preclinical Studies. Circ Res. 2016;118:1223–1232. doi: 10.1161/CIRCRESAHA.115.307676. [DOI] [PubMed] [Google Scholar]
- 22.Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous Mesenchymal Stem Cells Produce Concordant Improvements in Regional Function, Tissue Perfusion, and Fibrotic Burden When Administered to Patients Undergoing Coronary Artery Bypass Grafting: The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (Prometheus) Trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grossman PM, Han Z, Palasis M, Barry JJ, Lederman RJ. Incomplete Retention after Direct Myocardial Injection. Catheter Cardiovasc Interv. 2002;55:392–397. doi: 10.1002/ccd.10136. [DOI] [PubMed] [Google Scholar]
- 24.Sheng CC, Zhou L, Hao J. Current Stem Cell Delivery Methods for Myocardial Repair. Biomed Res Int. 2013;2013:547902. doi: 10.1155/2013/547902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amado LC, Saliaris AP, Schuleri KH, St John M, Xie JS, Cattaneo S, Durand DJ, Fitton T, Kuang JQ, Stewart G, Lehrke S, Baumgartner WW, Martin BJ, Heldman AW, Hare JM. Cardiac Repair with Intramyocardial Injection of Allogeneic Mesenchymal Stem Cells after Myocardial Infarction. Proc Natl Acad Sci U S A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schuleri KH, Amado LC, Boyle AJ, Centola M, Saliaris AP, Gutman MR, Hatzistergos KE, Oskouei BN, Zimmet JM, Young RG, Heldman AW, Lardo AC, Hare JM. Early Improvement in Cardiac Tissue Perfusion Due to Mesenchymal Stem Cells. Am J Physiol Heart Circ Physiol. 2008;294:H2002–2011. doi: 10.1152/ajpheart.00762.2007. [DOI] [PubMed] [Google Scholar]
- 27.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and Differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashemi SM, Ghods S, Kolodgie FD, Parcham-Azad K, Keane M, Hamamdzic D, Young R, Rippy MK, Virmani R, Litt H, Wilensky RL. A Placebo Controlled, Dose-Ranging, Safety Study of Allogenic Mesenchymal Stem Cells Injected by Endomyocardial Delivery after an Acute Myocardial Infarction. Eur Heart J. 2008;29:251–259. doi: 10.1093/eurheartj/ehm559. [DOI] [PubMed] [Google Scholar]
- 29.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic Mesenchymal Stem Cells Restore Cardiac Function in Chronic Ischemic Cardiomyopathy Via Trilineage Differentiating Capacity. Proc Natl Acad Sci U S A. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Spoel TIG, Gathier WA, Koudstaal S, van Slochteren F, Jansen Lorkeers S, Sluijter JPG, Hoefer IE, Steendijk P, Cramer MJM, Doevendans PA, van Belle E, Chamuleau SAJ. Autologous Mesenchymal Stem Cells Show More Benefit on Systolic Function Compared to Bone Marrow. Mononuclear Cells in a Porcine Model of Chronic Myocardial Infarction. Journal of Cardiovascular Translational Research. 2015;8:393–403. doi: 10.1007/s12265-015-9643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Spoel TI, Jansen of Lorkeers SJ, Agostoni P, van Belle E, Gyongyosi M, Sluijter JP, Cramer MJ, Doevendans PA, Chamuleau SA. Human Relevance of Pre-Clinical Studies in Stem Cell Therapy: Systematic Review and Meta-Analysis of Large Animal Models of Ischaemic Heart Disease. Cardiovasc Res. 2011;91:649–658. doi: 10.1093/cvr/cvr113. [DOI] [PubMed] [Google Scholar]
- 32.Kurtz A. Mesenchymal Stem Cell Delivery Routes and Fate. Int J Stem Cells. 2008;1:1–7. doi: 10.15283/ijsc.2008.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vulliet PR, Greeley M, Halloran SM, MacDonald KA, Kittleson MD. Intra-Coronary Arterial Injection of Mesenchymal Stromal Cells and Microinfarction in Dogs. Lancet. 2004;363:783–784. doi: 10.1016/S0140-6736(04)15695-X. [DOI] [PubMed] [Google Scholar]
- 34.Schuleri KH, Feigenbaum GS, Centola M, Weiss ES, Zimmet JM, Turney J, Kellner J, Zviman MM, Hatzistergos KE, Detrick B, Conte JV, McNiece I, Steenbergen C, Lardo AC, Hare JM. Autologous Mesenchymal Stem Cells Produce Reverse Remodelling in Chronic Ischaemic Cardiomyopathy. Eur Heart J. 2009;30:2722–2732. doi: 10.1093/eurheartj/ehp265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kelkar AA, Butler J, Schelbert EB, Greene SJ, Quyyumi AA, Bonow RO, Cohen I, Gheorghiade M, Lipinski MJ, Sun W, Luger D, Epstein SE. Mechanisms Contributing to the Progression of Ischemic and Nonischemic Dilated Cardiomyopathy: Possible Modulating Effects of Paracrine Activities of Stem Cells. J Am Coll Cardiol. 2015;66:2038–2047. doi: 10.1016/j.jacc.2015.09.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.