Abstract
Antibodies (Abs) produced by immunoglobulin (IG) genes are the most diverse proteins expressed in humans. While part of this diversity is generated by recombination during B-cell development and mutations during affinity maturation, the germ-line IG loci are also diverse across human populations and ethnicities. Recently, proof-of-concept studies have demonstrated genotype–phenotype correlations between specific IG germ-line variants and the quality of Ab responses during vaccination and disease. However, the functional consequences of IG genetic variation in Ab function and immunological outcomes remain underexplored. In this opinion article, we outline interconnections between IG genomic diversity and Ab-expressed repertoires and structure. We further propose a strategy for integrating IG genotyping with functional Ab profiling data as a means to better predict and optimize humoral responses in genetically diverse human populations, with immediate implications for personalized medicine.
Trends
Genetic variation in human populations affects how individuals are able to mount functional antibody responses.
Different alleles can encode convergent binding motifs that result in successful Ab responses against specific infections and vaccinations.
Given the complexity of the IG loci and the diversity of the antibody repertoire, links between IG polymorphism and antibody repertoire variability have not been thoroughly explored.
We present a strategy to mine genotype–repertoire–disease associations.
The Molecular Basis for Antibody Diversity
Antibodies (Abs) have long been appreciated as key constituents of the adaptive immune response. Their function is to enable selective recognition and mediate immune responses to novel foreign antigens. This is accomplished through the somatic generation of vast repertoires of hundreds of millions of unique Ab receptors that can be selected, matured, and ultimately participate in the formation of long-term memory during B-cell development and activation. As a consequence of this diversity, even after nearly a century of research, the complexity of the Ab response within and between individuals is only beginning to be delineated at the molecular and genetic levels.
Hundreds of variable (V) and dozens of diversity (D) and joining (J) immunoglobulin (IG) germ-line gene segments across three primary loci in the human genome comprise the necessary building blocks of the expressed Ab heavy- and light-chain repertoires [1]. Whereas the heavy chain is encoded by genes at the IG heavy-chain locus (IGH), the light chain can be encoded by genes at either the IG kappa (IGK) or IG lambda (IGL) chain loci [1]. The naïve Ab repertoire is formed by assembling variants of these building blocks using a specialized V(D)J recombination process that somatically joins various V, D, and J segments (or V and J at IGK and IGL). The introduction and deletion of P and N nucleotides at V(D)J junctions and the pairing of different heavy and light chains dramatically increase diversity (Figure 1 ) [2]. Considering these processes alone, a given baseline or primary naïve repertoire can theoretically sample from 1015 different Abs [3]. The extraordinary diversity of the naïve repertoire ensures that it will likely contain a naïve Ab with at least weak initial binding against a vast array of antigens.
Figure 1.
A Basic Overview of Key Elements That Contribute to the Diversity of Naïve and Memory Repertoires. A basic schematic of the germ-line IGH locus is shown (not to scale), consisting of clusters of tandemly arrayed IGH V, D, J, and constant (C) gene segments. For a subset of these segments, multiple alleles are shown, representing population-level ‘allelic diversity’ (see Table 1). During the initial formation of the naïve repertoire, single IGH V, D and J gene segment on one of two chromosomes in a given B cell are somatically recombined; at each of these steps, P and N nucleotides are added at the D–J and V–D junctions (‘junctional diversity’), respectively. This process, known as V(D)J rearrangement, is the basis for ‘combinatorial diversity’. The recombined V (red), D (orange), and J (maroon) segments will then be transcribed, and following splicing, will be paired with a C gene (gray). The somatic recombination process also occurs at one of two loci encoding the Ab light-chain gene segments [IGK and IGL; except it involves only V (yellow), J (maroon), and C (light gray) gene segments]. Two identical heavy chains and two identical light chains are ultimately paired through disulfide bonds to form a functional Ab; thus, additional diversity in the expressed Ab repertoire comes from ‘heavy- and light-chain pairing’. Together, the V, D, and J segments depicted comprise the variable domain of the heavy chain of a functional antibody, and together with the variable domain of the light chain, encoded by V and J segments, are responsible for Ag binding. The C domains of both heavy and light chains provide structural and/or effector functions of the Ab. As shown here for the heavy chain, the variable domain is partitioned into four framework regions (FRs) and three complementarity-determining regions (CDRs). Following Ag stimulation, ‘somatic hypermutations’ introduce additional variation in the variable domain of the Ab (vertical purple bars), with the aim of improving binding affinity. Mutations that arise via SHM can occur across all FRs and CDRs, but these are most prevalent in CDRs, as illustrated by the hypothetical frequency histogram shown between the unmutated and mutated IG heavy-chain RNA. While the general molecular mechanisms outlined here have long been realized as the primary determinants of diversity within a given expressed Ab repertoire, there is a growing appreciation for the contribution of ‘allelic diversity’ as well, particularly as this pertains to repertoire differences observed between unrelated individuals. Ab, antibody; C, constant; D, diversity; IGH, immunoglobulin heavy-chain locus; IGK, immunoglobulin kappa; IGL, immunoglobulin lambda; J, joining; SHM, somatic hypermutation; V, variable.
Even so, this impressive baseline diversity can be subsequently augmented when a B cell encounters and is stimulated by an antigen to undergo somatic hypermutation (SHM; Figure 1), resulting in lineages of tens of thousands of clonally derived affinity maturation variants of the initial Ab. Specifically, SHM introduces somatic mutations throughout the variable portion of the Ab, including targeted hotspots residing within the antigen-contacting hypervariable complementarity-determining regions (CDRs). This process ultimately increases the affinity and specificity of the Ab for binding the target epitope, facilitating a highly focused antigen-specific response.
While the prevailing paradigm for investigating B-cell and Ab-mediated responses has placed emphasis on the importance of the unique molecular mechanisms cited earlier in the generation of key functional Abs, there is a growing appreciation for the fact that IG genes are highly variable at the germ-line level, exhibiting extreme allelic polymorphism and gene copy number variation (CNV) between individuals and across populations 4, 5, 6, 7, 8, 9. Recent studies have begun to highlight that, in addition to diversity introduced during V(D)J recombination, heavy- and light-chain pairing, and SHM, IG germ-line variation (e.g., allelic variation; Figure 1) plays a vital part in determining the development of the naïve repertoire, with downstream impacts on signatures observed in the memory compartment, and the capacity of an individual to mount an Ab response to specific epitopes 10, 11, 12, 13, 14, 15, 16.
IG Loci Haplotype Diversity in the Human Population
Recent genomic sequencing indicates that IG loci, specifically IGH, may be among the most polymorphic in the human genome [17]. Across IGH, IGK, and IGL, there are currently >420 alleles cataloged in the ImMunoGeneTics information system database (IMGT) 18, 19, 20, 21 that have been described from germ-line DNA in the human population, with an enrichment of nonsynonymous variants (Table 1 ). Although the validity of some alleles in IMGT has been called into question [22], the number of polymorphic alleles continues to grow 11, 23, 24, especially as IG gene sequencing is conducted in increasing numbers of non-Caucasian samples 7, 9, 25. A recent study conducted in 28 indigenous South Africans identified 122 non-IMGT IGHV alleles [9]. In addition to IG allelic variation and single nucleotide polymorphisms (SNPs), CNVs, including large deletions, insertions, and duplications (∼8–75 Kb in length), are also prevalent in IG regions (Table 1). Using IGH as an example, up to 29 of the 58 functional/open reading frame (ORF) IGHV genes may vary in genomic copy number 4, 6, 7, 11, 26, 27, 28; CNVs of IGH D (diversity) and constant (C) region genes are also known 11, 12, 29. Until recently, primarily due to technical difficulties associated with the complex genomic architecture of the IG loci, none of the known CNVs in IGHV had been sequenced at nucleotide resolution [7]; many likely remain undescribed at the genomic level.
Table 1.
Allelic, Copy Number, and Amino Acid Variation for IG Functional and Open Reading Frame Genes Cataloged in IMGTa
| Family | Genes | Alleles | NS variants | S variants | CDR-H1 NS variants | CDR-H2 NS variants | Genes in CNV |
|---|---|---|---|---|---|---|---|
| IGHV1 | 11 | 40 | 19 | 13 | 2 | 3 | 6 |
| IGHV2 | 4 | 23 | 26 | 9 | 3 | 1 | 1 |
| IGHV3 | 27 | 109 | 82 | 57 | 9 | 17 | 12 |
| IGHV4 | 10 | 78 | 92 | 71 | 11 | 8 | 8 |
| IGHV5 | 2 | 9 | 4 | 4 | 0 | 0 | 1 |
| IGHV6 | 1 | 2 | 0 | 1 | 0 | 0 | 0 |
| IGHV7 | 2 | 6 | 4 | 0 | 0 | 0 | 1 |
| Subtotal | 58 | 267 | 227 | 155 | 25 | 29 | 29 |
| IGKV1 | 20 | 35 | 33 | 17 | 4 | 1 | 1 |
| IGKV2 | 11 | 18 | 14 | 4 | 1 | 1 | 0 |
| IGKV3 | 8 | 18 | 24 | 9 | 2 | 1 | 0 |
| IGKV4 | 1 | 1 | NA | NA | NA | NA | 0 |
| IGKV5 | 1 | 1 | NA | NA | NA | NA | 0 |
| IGKV6 | 3 | 5 | 2 | 0 | 0 | 0 | 0 |
| IGKV7 | 0 | 0 | NA | NA | NA | NA | 0 |
| Subtotal | 44 | 78 | 73 | 30 | 7 | 3 | 1 |
| IGLV1 | 7 | 12 | 4 | 2 | 0 | 2 | 1 |
| IGLV2 | 6 | 20 | 13 | 8 | 2 | 3 | 0 |
| IGLV3 | 11 | 18 | 14 | 5 | 3 | 3 | 0 |
| IGLV4 | 3 | 6 | 2 | 1 | 0 | 0 | 0 |
| IGLV5 | 5 | 10 | 3 | 2 | 0 | 0 | 1 |
| IGLV6 | 1 | 2 | 2 | 0 | 0 | 0 | 0 |
| IGLV7 | 2 | 3 | 1 | 0 | 0 | 0 | 0 |
| IGLV8 | 1 | 3 | 1 | 1 | 0 | 0 | 1 |
| IGLV9 | 1 | 3 | 0 | 2 | 0 | 0 | 0 |
| IGLV10 | 1 | 3 | 4 | 1 | 1 | 0 | 0 |
| IGLV11 | 1 | 2 | 1 | 1 | 0 | 0 | 0 |
| Subtotal | 39 | 82 | 45 | 23 | 6 | 8 | 3 |
| Total | 141 | 427 | 345 | 208 | 38 | 40 | 33 |
Data accessed from IMGT February 2017. NS, nonsynonymous; S, synonymous.
The high prevalence of IG allelic and locus structural diversity translates into extreme levels of inter-individual haplotype variation 4, 5, 6, 7. For example, recent comparisons of the two available completed assemblies for the IGHV gene region (∼1 Mb in length) revealed that two human chromosomes can vary by >100 Kb of sequence, with >2,800 SNPs, and CNVs of 10 IGHV functional/ORF genes 7, 17. In population sequencing experiments, extreme examples of heterozygosity have been noted, with evidence of some individuals carrying more than one allele at every IGHV coding gene [9]. Supporting earlier genetic mapping data 4, 5, more recent analysis of inferred haplotypes from Ab repertoire data surveyed in nine individuals revealed that all 18 haplotypes characterized were unique [6]. Furthermore, at the population level, of the few SNPs and CNVs screened within IGH, allele and genotype frequencies have been shown to vary considerably between ethnic backgrounds 7, 8, 9, 15, with evidence of selection [7]. Despite the evidence for elevated germ-line diversity, genomic resources for IG loci continue to lag behind other regions of the genome [26]. Because of this, the comprehensive and accurate genotyping of IG polymorphisms remains a significant challenge 26, 30, and as a result, the full extent of IG polymorphism and the implications for human health are yet to be uncovered [26]. However, it is plausible that population-level diversity in the IG loci, particularly in IGH, will rival that of other complex immune gene families, such as the human leukocyte antigen (HLA) and killer cell IG-like receptor (KIR) genes. These genes are also characterized by extreme haplotype diversity, due to CNV and coding region variation 31, 32; HLA genes, for example, have thousands of known alleles [31]. In contrast to IG genes, HLA and KIR have been studied more extensively across human populations, and have demonstrated critical roles in disease 31, 32.
Influence of IG Germ-Line Diversity in the Expressed Ab Repertoire and Ab Function
Our limited knowledge of IG population diversity has hindered our ability to comprehensively test for direct connections between IG germ-line polymorphisms, variation in the repertoire generated after recombination, amino acid variation in the Ab produced, and ultimately Ab function. Advances in high-throughput sequencing technology now enable extensive characterization of the expressed Ab repertoire 33, 34, 35, creating opportunities for beginning to investigate the heritability of the Ab response at fine-scale resolution. Applications of these methods, collectively referred to as repertoire sequencing (‘IgSeq’ or ‘RepSeq’), have already led to a wealth of new discoveries in a range of contexts 33, 36. These include general observations that key features of the Ab repertoire show extensive variability between healthy individuals 10, 11, 13, 14, 37, and a limited overlap of B-cell receptor clones between individuals, even monozygotic (MZ) twins 10, 13, 14. However, RepSeq studies have also revealed that these inter-individual differences are not necessarily random, but likely have a strong underlying genetic component, providing initial support for the importance of germ-line IG polymorphism in determining the naïve and Ag-stimulated Ab repertoire. For example, several recent studies have revealed that V, D, and J gene usage in the naïve repertoire is much more highly correlated between MZ twins than between unrelated individuals 10, 13, 14, and that IG gene usage patterns are consistent across time points within a given individual [38]. A role for genetic factors can be seen for other repertoire features in twins as well, including the degree of SHM [13], and the distribution of CDR-H3 length and clone convergence 10, 13, 14. Intriguingly, although existing data suggest that features in the memory compartment are more stochastic, likely reflective of random recruitment and transient proliferation, certain genes and repertoire features exhibit predictable patterns even in memory B cells 10, 13, 14, 39.
Studies of repertoire heritability are consistent with a number of examples for which germ-line IG polymorphisms have been explicitly linked to features in the expressed Ab repertoire 12, 15, 40, 41, 42 (see Figure IA in Box 1 for hypothetical examples of IG genotype effects on the repertoire). Sasso et al. [40] reported the first direct connection to IG genotype, reporting that CNV of IGHV1-69 was tightly correlated with its relative usage in tonsillar B cells. Our own work has also demonstrated this relationship, but uncovered associations for IGHV1-69 coding and potentially noncoding polymorphism as well as CNV [15]. Inferred deletions of IGHD genes have also been shown to associate with variation in D–J pairing frequencies, demonstrating that germ-line effects on the repertoire extend beyond V genes [12]. An interesting aspect of IGH CNVs is that, in addition to observed effects of these variants on the genes within the CNV event, they also can impact the usage of genes elsewhere in the locus 12, 15. For example, we recently observed apparent long-range effects of IGHV1-69 CNV in the naïve and memory repertoire, in that individuals with fewer IGHV1-69 germ-line copies and reduced usage showed consistently higher usage of IGHV genes over 200 Kb away [15]. The mechanisms underlying the observed effects of CNVs in human IG loci remain technically difficult to assess experimentally, but it has been speculated that these large changes in locus architecture (i.e., deletions and insertions) could alter regulatory systems related to V(D)J recombination 12, 15, for example, by modifying the chromatin landscape, cis-regulatory elements and transcription factor binding, and/or the physical locations of the IG V, D, and J genes. All of these factors are known to be key determinants of IG gene accessibility and usage frequencies in mice 43, 44.
Figure I.
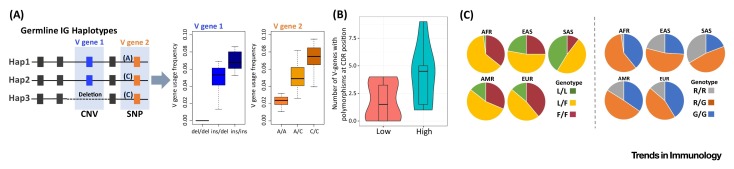
Impacts of IG Germ-Line Polymorphism on Ab Repertoire/Structural Diversity. (A) Hypothetical examples of associations between IG gene region CNV (V gene 1 insertion/deletion) and SNP (noncoding regulatory variant, A/C) genotypes and V gene usage frequencies in the expressed Ab repertoire. (B) Violin plot showing nonsynonymous polymorphism rates in CDR positions with high (>0.6; ‘high’, blue) or low (<0.25; ‘low’, red) frequency of contact with antigen, as labeled on the X axis. The Y axis records, for each CDR-H1 and CDR-H2 position, the number of IMGT IGHV genes that have alleles with nonsynonymous polymorphisms at that position. The positional probability of antigen contact was calculated for each CDR position as the percentage of 150 crystal structures of antibody–antigen complexes from the protein database (PDB) where any atom of that residue is within 5 Å of any antigen atom. Allelic variation is enriched in antigen-contact sites, in that the number of IGHV genes with alleles containing nonsynonymous polymorphisms is greater for high contact probability positions. (C) Genotype frequency differences between five human ethnic groups [Africans (AFR); East Asians (EAS); South Asians (SAS); Central/South American (AMR); and Europeans (EUR)], published by the 1000 Genomes Project [80]*, at two SNPs in IGHV1-69 that have been shown to encode functional residues critical for neutralizing Abs against the influenza HA stem (F54 and L54 amino acid-associated alleles; SNP rs55891010; left panel), and ‘NEAT2’ domain of Staphylococcus aureus (R50 and G50 alleles; SNP rs11845244; right panel). In the left panel, the F allele encodes the functional critical phenylalanine residue, and in the right panel, the primary glycine residue is encoded by the G allele. Interestingly, in both cases, the frequency of individuals lacking alleles encoding the critical residues varies among populations, with the L/L and R/R genotypes showing the lowest frequencies in Africans, and the highest frequencies in South Asians. rs55891010 and rs11845244 are in linkage disequilibrium, and thus R50 and L54 amino acids (and likewise, G50 and F54) tend to co-occur in alleles of IGHV1-69. This explains similarities in genotype frequency estimates between the two SNPs in each population. *Although these genotypes may contain error due to confounds of unrepresented CNV information, they can provide insight into potential population differences. Ab, antibody; CDR, complementarity-determining region; CNV, copy number variation; HA, hemagglutinin; IG, immunoglobulin; IMGT, ImMunoGeneTics information system database; SNP, single nucleotide polymorphism.
Box 1. Influence of IG Germ-Line Polymorphism on Ab Repertoire Variation and Functional Ab Structural Residues.
Although the roles of IG germ-line variants have not been comprehensively studied, there is now convincing evidence that they can influence Ab repertoire variation and function in two main ways (i and ii). In addition, known functional variants exhibit allele frequency variation between human populations (iii):
(i) Gene copy number changes and coding/noncoding SNPs in IGHV genes have been shown to correlate with gene usage patterns in the naïve repertoire, the memory repertoire, patterns of SHM, class-switch frequency, and circulating Ab titers (Figure IA).
(ii) There are now many examples that provide evidence for functional effects of germ-line variants encoded in CDR-H1 and CDR-H2, many of which are polymorphic and vary between human populations. Based on known IGHV alleles in the IMGT database, residues within CDR-H1/H2 that have a higher probability of making Ag contact are also more likely to be associated with a polymorphic allele (Figure IB).
(iii) Several positions in IGHV genes that encode residues critical for antigen binding are polymorphic and exhibit different genotype frequencies between human populations and ethnicities (Figure IC).
Alt-text: Box 1
A role for noncoding polymorphisms is also strongly supported by early work conducted in the human IGK region which directly showed that a variant associated with Haemophilus influenzae infection susceptibility in the recombination signal sequence (RSS) of IGKV2-29 significantly decreased gene rearrangement frequency [42]. RSSs, which are critical for the recruitment of RAG1/2 proteins, have also been demonstrated to impact IGHV gene usage in mice 43, 44. Moreover, extensive work in the murine IG gene loci has uncovered important roles for other key cis-regulatory sequences and transcription factors as well 45, 46. Such analyses have not yet been comprehensively conducted in humans, and as a result, our knowledge of the IG regulatory elements involved in the formation of the expressed Ab repertoire is restricted to canonical RSS, promoter, enhancer elements, and class switch regions. However, even for these well-known noncoding regulatory regions, limited data on human population-level variation exist, and thus the broader consequences of polymorphism in these elements on Ab repertoire variability have not been explored.
Although direct links between repertoire variability and human IG CNVs and noncoding polymorphisms remain limited to the few examples discussed above, additional evidence from expressed Ab repertoire studies in unrelated individuals also highlights the clear potential for these variants to have pervasive impacts on Ab repertoire features, particularly gene usage in the naïve compartment. Most demonstrable is the fact that many of the genes with the most variability in naïve repertoire usage across individuals are also known to be in CNV, including examples of the complete absence of genes in the expressed Ab repertoires of some donors 6, 10, 11, 12. In addition, allele-specific usage in the naïve Ab repertoires of individuals heterozygous at a given IGHV gene has been demonstrated, also clearly suggesting a role for noncoding variation and CNV [11]. Moreover, although effects of germ-line IG polymorphism may be most evident on a per gene basis, it is worth noting that findings from MZ twins demonstrated that certain CDR-H3 features are highly heritable 13, 14. This indicates that even strong genetically determined biases on individual V, D, and J gene usage [and thus their nonrandom combination during V(D)J rearrangement] could also be directly linked to variation observed within CDR-H3. This is an important point given that CDR-H3 variation has classically been considered independent of the germ line 13, 14.
In addition to effects of IG polymorphism on gene usage, functional CDR variants can also be directly encoded in the genome. For example, across the ∼267 coding alleles cataloged in IMGT for functional and ORF IGHV genes, 60% of the 382 polymorphisms are nonsynonymous (Table 1), including sites located in CDR-H1 and CDR-H2 with predicted relevance to Ab functional residue diversity (see Figure IB in Box 1). Although the CDR-H3 loop, formed at the V(D)J junction, is the most diverse region of an Ab and is a principal determinant of specificity 47, 48, there is a growing appreciation for the importance of residues outside of CDR-H3 in antigen recognition and binding 15, 49, 50, 51. For example, recent analyses have shown that the median length of CDR-H2, which is solely encoded by germ-line V gene sequence, is substantially longer than that of CDR-H3, and typically forms the same number of interactions with antigen [52]. Specifically, analyses of antigen-binding region (ABRs; which roughly correspond to CDRs, but differ slightly in their boundaries) have shown that Abs contain a median of six, six, and four contact residues in the heavy-chain CDR-H3, H2, and H1 ABR regions, respectively. In addition, the overall percentage of energetically important Ag-binding residues within each ABR follows the same rank order, with ∼31%, 23%, and 14% for H3, H2, and H1, respectively. Similar trends were noted for light-chain ABRs as well [52]. In addition, considering that many known nonsynonymous sites reside outside of CDRs (Table 1), it is worth highlighting the fact that there are also examples demonstrating indirect effects of framework region variants on Ag binding 53, 54.
The Identification of Shared Ab Immune Response Signatures across Individuals
A critical question is whether the germ-line effects on the repertoire outlined above can also partially account for inter-individual variation of the Ab-mediated response in disease and clinical phenotypes. The initial observation from RepSeq studies that essentially no Ab clones were shared among individuals, including MZ twins, posed a challenge to comparative Ab repertoire analysis: how could correlates of protection be identified in the Ab repertoire if every individual was responding with different Abs? However, an answer began to emerge with the observation that in multiple settings, including viral and bacterial infection, different individuals have been shown to respond to a given antigen with Abs that share convergent amino acid signatures 13, 49, 54, 55, 56, 57, 58. These convergent Abs are often encoded by common V genes or sets of V genes, and specific amino acid residues in their CDRs enable them to converge upon a common binding solution against a shared antigen. Critically, in some cases evaluated, convergent signatures include amino acid residues that are directly encoded in the germ line. The occurrence of such convergent Ab responses highlights the potential for tracking common immune responses across individuals, and understanding the role of genetic factors, even when each individual creates unique Abs. Importantly, the implications of this line of thinking could be broad, as IG gene biases have been observed in contexts other than infection, including autoimmunity and cancer 59, 60. Moreover, IG gene biases may also extend to usage patterns of D and J genes, light-chain genes, and heavy- and light-chain V gene pairing frequencies 56, 61, 62.
Structural Residues Critical for Ag Binding and Involved in Biased Gene Usage Are Encoded in the Germ Line and Exhibit Population Variability
There are now many instances for which functional contributions of biased IG genes have been traced back to specific germ-line-encoded residues, including sites that are polymorphic in the human population 15, 16, 50, 53, 54, 55, 63, 64, 65. These examples illuminate a direct role of the IG germ line in disease-associated Ab responses. In the case of stem-directed broadly neutralizing Abs (BnAbs) against influenza hemagglutinin (HA), the most prevalent Abs use the heavy-chain gene IGHV1-69 66, 67, 68, 69, 70. These IGHV1-69 BnAbs recognize an overlapping epitope of group 1 influenza A viruses and only amino acids from IGHV make contact with HA. Importantly, of the 14 known alleles at IGHV1-69, only those encoding a critical phenylalanine at position 54 (F54) within CDR-H2 have a major role in shaping the BnAbs response 16, 15, 55, 71. Although IGHV1-69 F54-encoding alleles are dominant, there is a growing list of additional HA-directed BnAbs that also show IG germ-line biases 51, 56, 72, 73, 74, including those also known to be polymorphic with respect to coding variants and CNVs.
Interestingly, there are additional instances of biased IGHV1-69 allele usage in other disease contexts, with both overlapping and contrasting patterns to that observed for influenza. For example, F54 alleles are predominantly observed in IGHV1-69-expressing B cells associated with chronic lymphoid leukemia (CLL), whereas alleles encoding a leucine (L54) at this position are primarily used by non-neutralizing anti-gp41 Abs in HIV-1 63, 64. Moreover, it has been shown that IGHV1-69 F54 alleles, in comparison with L54 alleles, have lower usage in the memory B-cell pool 10, 15. This observation may be similar to trends noted for IGHV4-34, which is also significantly underrepresented in the memory compartment of healthy individuals [10], and presumes to reflect a selective pressure against autoreactive Abs 75, 76.
Other polymorphic positions in the framework regions of IGHV1-69, in conjunction with CDR-H2 54, have also recently been shown to influence Ab binding of Middle East respiratory syndrome coronavirus (MERS-CoV) [53] and the Staphylococcus aureus NEAr iron transporter 2 (NEAT2) domain [54]. In the example of NEAT2, neutralizing Abs encoded by IGHV1-69 alleles carrying an arginine (R) at position 50 in place of glycine (G) showed significantly reduced NEAT2 binding [54]. Interestingly, based on publicly available data, the frequencies of critical alleles within polymorphic positions of IGHV1-69 vary across populations (see Figure IC in Box 1).
A Strategy for Defining Relationships between IG Polymorphisms, Expressed Ab Signatures, and Functional Outcomes
Considering the aforementioned evidence, we argue that the antigen-specific Ab repertoire is likely influenced by the host genotype. Although the genetic bases for repertoire and germ-line gene biases have not been comprehensively investigated, several recent studies provide a strategy for systematically integrating data on IG polymorphism and Ab responses at the population and molecular levels to provide unique insight into Ab signatures associated with disease.
We have begun to explore this idea in detail at the IGHV1-69 locus in the context of influenza vaccination [15]. Providing strong proof-of-concept, by initially focusing on observed IGHV1-69 allelic usage bias against a critical broadly neutralizing epitope, we genotyped the IGHV1-69 F54/L54 allele and copy number frequencies in a cohort of 85 H5N1 vaccines, including 18 individuals with accompanying Ab repertoire data [15]. Drawing directly on aspects of repertoire heritability reviewed above, we found robust connections between these polymorphisms and repertoire gene usage in both the unmutated IgM (naïve) and IgG memory repertoires, with IGHV1-69 germ-line gene usage increasing with the number of copies of F54 alleles. In addition to usage frequencies, IGHV1-69 genotype also associated with IGHV1-69 B-cell expansion, SHM, and Ig class switching. It is important to note that these genotype effects extended to levels of circulating anti-HA stem BnAbs postvaccination, with individuals carrying only germ-line-encoded CDR-H2 L54 alleles having lower IGHV1-69 BnAbs. Furthermore, with direct repertoire sequencing, we were able to specifically demonstrate that only carriers of the IGHV1-69 F54 alleles expressed convergent anti-BnAb signatures. These results are bolstered by similar observations recently made by two other groups that also carried out IGHV1-69 F54/L54 allele genotyping in their cohorts 16, 55. Altogether, these data demonstrate that genetically determined baseline differences in the Ab repertoire can set the stage for disease-related responses.
A crucial aspect of this story (which is expected to emerge in other cases as well) is that the frequency of IGHV1-69 F54 alleles and CNV varies considerably across populations 7, 15. Specifically, the number of individuals that would be predicted to lack the capacity to generate effective IGHV1-69 BnAbs was much higher in some populations. However, we and others have shown that individuals lacking IGHV1-69 F54 alleles likely utilize other germ-line genes in place of IGHV1-69 51, 55. This finding in particular both highlights the complexity of the Ab response and demonstrates that the integration of genotyping information can help provide a more nuanced interpretation of the signatures discovered in the expressed repertoire. Moreover, it suggests that efforts should be made to study these complex responses in larger and more diverse cohorts, including individuals from presently understudied populations.
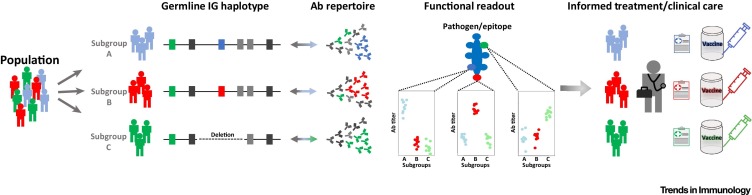
Building on findings in these initial studies 15, 16, 55, we propose a framework for integrating genotypic information into future studies of the Ab response in wellness and disease (Figure 2 , Key Figure). The general strategy is as follows: (i) identify IG gene biases observed in a disease-related or epitope-specific response; (ii) characterize this response at the population level by performing comprehensive genotyping of coding, noncoding, and gene copy number variants at and around the locus of interest (and others if there is rationale); (iii) perform repertoire sequencing and analysis of the response in all relevant B-cell subsets to identify all Ab convergence groups with allele bias; and (iv) evaluate genotype–phenotype linkages of the functional Ab response and specific Ab convergence groups.
Figure 2.
Key Figure: A New Paradigm for Integrating Genotypic Information into the Study of the Ab-Mediated Response in Disease and Clinical Phenotypes
In the proposed paradigm, a population cohort is partitioned into subgroups based on functional genotypes/haplotypes that are directly associated with subgroup-specific signatures in the expressed repertoire and other relevant phenotypes (e.g., Ab titer; clinical outcome) associated with the Ab response to a given antigen/epitope. This partitioning can be used to inform tailored clinical care and treatment (e.g., vaccination regime). Ab, antibody.
Concluding Remarks
We see a growing body of evidence to support the link between IG polymorphism and phenotype that may have important clinical applications (see Outstanding Questions). The most obvious of these correlations include potential effects of CNV and SNPs in non-translated and translated IG gene regions on expressed repertoire variability in naïve and memory B cell subsets. Some of these polymorphisms could be expected to more broadly impact variation in protective Ab responses [77] and quality of the memory B-cell pool. We anticipate that IG polymorphism will contribute to differences in expression of common (public) and unique (private) antibody signatures that are associated with protective responses in disease and in response to vaccination. We propose a model for the future in which cataloging these public signatures for biased gene use, V(D)J associations, SHMs, and heavy-light chain pairing in the context of IG germ-line variation should begin to provide us with information to advance our understanding of the immunogenetic potential of an individual’s baseline naïve repertoire (Figure 2), particularly when more complete data sets of biased Ab signatures to specific epitopes become available. Based on existing genetic data, it is probable that similar IG haplotypes will associate with overlapping signatures in baseline repertoire profiles, even if not to the degree of repertoire similarity observed in MZ twins. This IG polymorphism, as we and others have begun to show, may further influence the evolution of antigen-experienced B cells and plasma cells, where other genetic polymorphisms in the IG loci and environmental exposures come into play in continuing to shape affinity, epitope specificity, and fate. In addition, class-switched memory B-cell compartments will vary over time [37], and could be quantitated in the type and size of clonotypes with both public and private signatures against immunodominant epitopes.
Together, this knowledge should pave the way to using molecular and genetic signatures for mapping an individual’s exposure history, current wellness state, and immune potential against future antigenic threats. For example, characterization of genotypes that specifically lead to common BnAb signatures in the repertoire should be useful for tailoring vaccines to responsive genotypes with the goal of achieving 100% ‘universal vaccine’ responsiveness at the population level (Figure 2). In addition, such information could lead to advances in the use of anti-idiotypic antibody and chimeric antigen receptor T-cell therapies that are directed against germ-line gene expressing B-cell clonotypes that are directly involved in autoimmune disease and hematologic malignancies 78, 79. We face tall hurdles to moving this paradigm forward, the greatest being the completion of a comprehensive catalogue of human IG haplotype variation [26]. However, with ever expanding advances in immunologic and genomic technologies, we believe that such integrative approaches are within our reach, and have the potential to transform our understanding of Ab-mediated immune responses in the clinical and research arenas.
Outstanding Questions.
How large of an effect does IG polymorphism have on the development of the baseline naïve repertoire, and what types of genetic variation (CNV, coding variants, regulatory variants) matter most?
Do effects of IG genetic variants on the Ab repertoire correspond to known biases in disease and/or clinically relevant Ab responses?
What can population-level data on genetic and expressed Ab repertoire signatures tell us about an individual’s exposure history, current wellness state, and immune potential against future antigenic threats?
Can we leverage integrated population-level data sets to inform clinical care, and more effective vaccine and therapeutic strategies?
Acknowledgments
This work was supported by the National Institute of Allergy & Infectious Disease of the US National Institutes of Health (NIH) under awards U01-AI074518, R56-AI109223, and R01-AI121285 to W.A.M.
Contributor Information
Corey T. Watson, Email: corey.watson@louisville.edu.
Jacob Glanville, Email: jake@distributedbio.com.
Wayne A. Marasco, Email: wayne_marasco@dfci.harvard.edu.
References
- 1.Lefranc M.-P., Lefranc G. Academic Press; 2001. The Immunoglobulin Factsbook. [Google Scholar]
- 2.Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder H.W. Similarity and divergence in the development and expression of the mouse and human antibody repertoires. Dev. Comp. Immunol. 2006;30:119–135. doi: 10.1016/j.dci.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Chimge N.-O. Determination of gene organization in the human IGHV region on single chromosomes. Genes Immun. 2005;6:186–193. doi: 10.1038/sj.gene.6364176. [DOI] [PubMed] [Google Scholar]
- 5.Li H. Genetic diversity of the human immunoglobulin heavy chain VH region. Immunol. Rev. 2002;190:53–68. doi: 10.1034/j.1600-065x.2002.19005.x. [DOI] [PubMed] [Google Scholar]
- 6.Kidd M.J. The inference of phased haplotypes for the immunoglobulin H chain V region gene loci by analysis of VDJ gene rearrangements. J. Immunol. 2012;188:1333–1340. doi: 10.4049/jimmunol.1102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson C.T. Complete haplotype sequence of the human immunoglobulin heavy-chain variable, diversity, and joining genes and characterization of allelic and copy-number variation. Am. J. Hum. Genet. 2013;92:530–546. doi: 10.1016/j.ajhg.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sasso E.H. Ethnic differences in polymorphism of an immunoglobulin VH3 gene. J. Clin. Invest. 1995;96:1591–1600. doi: 10.1172/JCI118198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheepers C. Ability to develop broadly neutralizing HIV-1 antibodies is not restricted by the germline IG gene repertoire. J. Immunol. 2015;194:4371–4378. doi: 10.4049/jimmunol.1500118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glanville J. Naive antibody gene-segment frequencies are heritable and unaltered by chronic lymphocyte ablation. Proc. Natl. Acad. Sci. U. S. A. 2011;108:20066–20071. doi: 10.1073/pnas.1107498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyd S.D. Individual variation in the germline Ig gene repertoire inferred from variable region gene rearrangements. J. Immunol. 2010;184:6986–6992. doi: 10.4049/jimmunol.1000445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidd M.J. DJ pairing during VDJ recombination shows positional biases that vary among individuals with differing IGHD locus immunogenotypes. J. Immunol. 2015;196:1158–1164. doi: 10.4049/jimmunol.1501401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C. B-cell repertoire responses to varicella-zoster vaccination in human identical twins. Proc. Natl. Acad. Sci. U. S. A. 2015;112:500–505. doi: 10.1073/pnas.1415875112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubelt F. Individual heritable differences result in unique lymphocyte receptor repertoires of naïve and antigen-experienced cells. Nat. Commun. 2016;6:1–12. doi: 10.1038/ncomms11112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avnir Y. IGHV1-69 polymorphism modulates anti-influenza antibody repertoires, correlates with IGHV utilization shifts and varies by ethnicity. Sci. Rep. 2016;6:20842. doi: 10.1038/srep20842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheatley A.K. H5N1 vaccine-elicited memory B cells are genetically constrained by the IGHV locus in the recognition of a neutralizing epitope in the hemagglutinin stem. J. Immunol. 2015;195:602–610. doi: 10.4049/jimmunol.1402835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watson C.T. Sequencing of the human IG light chain loci from a hydatidiform mole BAC library reveals locus-specific signatures of genetic diversity. Genes Immun. 2014;16:24–34. doi: 10.1038/gene.2014.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pallarès N. The human immunoglobulin heavy variable genes. Exp. Clin. Immunogenet. 1999;16:36–60. doi: 10.1159/000019095. [DOI] [PubMed] [Google Scholar]
- 19.Lefranc M.-P. IMGT®, the international ImMunoGeneTics information system® 25 years on. Nucleic Acids Res. 2014;43:D413–D422. doi: 10.1093/nar/gku1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pallarés N. The human immunoglobulin lambda variable (IGLV) genes and joining (IGLJ) segments. Exp. Clin. Immunogenet. 1998;15:8–18. doi: 10.1159/000019054. [DOI] [PubMed] [Google Scholar]
- 21.Barbié V., Lefranc M.P. The human immunoglobulin kappa variable (IGKV) genes and joining (IGKJ) segments. Exp. Clin. Immunogenet. 1998;15:171–183. doi: 10.1159/000019068. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y. Many human immunoglobulin heavy-chain IGHV gene polymorphisms have been reported in error. Immunol. Cell Biol. 2008;86:111–115. doi: 10.1038/sj.icb.7100144. [DOI] [PubMed] [Google Scholar]
- 23.Gadala-Maria D. Automated analysis of high-throughput B-cell sequencing data reveals a high frequency of novel immunoglobulin V gene segment alleles. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E862–E870. doi: 10.1073/pnas.1417683112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corcoran M.M. Production of individualized V gene databases reveals high levels of immunoglobulin genetic diversity. Nat. Commun. 2016;7:13642. doi: 10.1038/ncomms13642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y. Genomic screening by 454 pyrosequencing identifies a new human IGHV gene and sixteen other new IGHV allelic variants. Immunogenetics. 2011;63:259–265. doi: 10.1007/s00251-010-0510-8. [DOI] [PubMed] [Google Scholar]
- 26.Watson C.T., Breden F. The immunoglobulin heavy chain locus: genetic variation, missing data, and implications for human disease. Genes Immun. 2012;13:363–373. doi: 10.1038/gene.2012.12. [DOI] [PubMed] [Google Scholar]
- 27.Milner E.C. Polymorphism and utilization of human VH genes. Ann. N. Y. Acad. Sci. 1995;764:50–61. doi: 10.1111/j.1749-6632.1995.tb55806.x. [DOI] [PubMed] [Google Scholar]
- 28.Shin E.K. Polymorphism of the human immunoglobulin variable region segment V1-4.1. Immunogenetics. 1993;38:304–306. doi: 10.1007/BF00188810. [DOI] [PubMed] [Google Scholar]
- 29.Bottaro A. Pulsed-field electrophoresis screening for immunoglobulin heavy-chain constant-region (IGHC) multigene deletions and duplications. Am. J. Hum. Genet. 1991;48:745–756. [PMC free article] [PubMed] [Google Scholar]
- 30.Luo S. Estimating copy number and allelic variation at the immunoglobulin heavy chain locus using short reads. PLoS Comput. Biol. 2016;12:1–21. doi: 10.1371/journal.pcbi.1005117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trowsdale J., Knight J.C. Major histocompatibility complex genomics and human disease. Annu. Rev. Genomics Hum. Genet. 2013;14:301–323. doi: 10.1146/annurev-genom-091212-153455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parham P., Moffett A. Variable NK cell receptors and their MHC class I ligands in immunity, reproduction and human evolution. Nat. Rev. Immunol. 2013;13:133–144. doi: 10.1038/nri3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Georgiou G. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat. Biotechnol. 2014;32:158–168. doi: 10.1038/nbt.2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyd S.D., Joshi S.A. High-throughput DNA sequencing analysis of antibody repertoires. Microbiol. Spectr. 2014;2:1–13. doi: 10.1128/microbiolspec.AID-0017-2014. [DOI] [PubMed] [Google Scholar]
- 35.Yaari G., Kleinstein S.H. Practical guidelines for B-cell receptor repertoire sequencing analysis. Genome Med. 2015;7:121. doi: 10.1186/s13073-015-0243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson K.J.L. The shape of the lymphocyte receptor repertoire: lessons from the B cell receptor. Front. Immunol. 2013;4:1–12. doi: 10.3389/fimmu.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galson J.D. In-depth assessment of within-individual and inter-individual variation in the B cell receptor repertoire. Front. Immunol. 2015;6:1–13. doi: 10.3389/fimmu.2015.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laserson U. High-resolution antibody dynamics of vaccine-induced immune responses. Proc. Natl. Acad. Sci. U. S. A. 2014;111:4928–4933. doi: 10.1073/pnas.1323862111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vollmers C. Genetic measurement of memory B-cell recall using antibody repertoire sequencing. Proc. Natl. Acad. Sci. U. S. A. 2013;110:13463–13468. doi: 10.1073/pnas.1312146110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sasso E.H. Expression of the immunoglobulin VH gene 51p1 is proportional to its germline gene copy number. J. Clin. Invest. 1996;97:2074–2080. doi: 10.1172/JCI118644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharon E. Genetic variation in MHC proteins is associated with T cell receptor expression biases. Nat. Genet. 2016;48:995–1002. doi: 10.1038/ng.3625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Feeney A.J. A defective Vkappa A2 allele in Navajos which may play a role in increased susceptibility to Haemophilus influenzae type b disease. J. Clin. Invest. 1996;97:2277–2282. doi: 10.1172/JCI118669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feeney A.J. Genetic and epigenetic control of V gene rearrangement frequency. Adv. Exp. Med. Biol. 2009;650:73–81. doi: 10.1007/978-1-4419-0296-2_6. [DOI] [PubMed] [Google Scholar]
- 44.Choi N.M. Deep sequencing of the murine IgH repertoire reveals complex regulation of nonrandom V gene rearrangement frequencies. J. Immunol. 2013;191:2393–2402. doi: 10.4049/jimmunol.1301279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Volpi S.A. Germline deletion of Igh 3′ regulatory region elements hs 5, 6, 7 (hs5-7) affects B cell-specific regulation, rearrangement, and insulation of the Igh locus. J. Immunol. 2012;188:2556–2566. doi: 10.4049/jimmunol.1102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Verma-Gaur J. Noncoding transcription within the Igh distal VH region at PAIR elements affects the 3D structure of the Igh locus in pro-B cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17004–17009. doi: 10.1073/pnas.1208398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu J.L., Davis M.M. Diversity in the CDR3 region of V H is sufficient for most antibody specificities. Immunity. 2000;13:37–45. doi: 10.1016/s1074-7613(00)00006-6. [DOI] [PubMed] [Google Scholar]
- 48.Mahon C.M. Comprehensive interrogation of a minimalist synthetic CDR-H3 library and its ability to generate antibodies with therapeutic potential. J. Mol. Biol. 2013;425:1712–1730. doi: 10.1016/j.jmb.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 49.Thomson C.A. Germline V-genes sculpt the binding site of a family of antibodies neutralizing human cytomegalovirus. EMBO J. 2008;27:2592–2602. doi: 10.1038/emboj.2008.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bryson S. Structures of preferred human IgV genes-based protective antibodies identify how conserved residues contact diverse antigens and assign source of specificity to CDR3 loop variation. J. Immunol. 2016;196:4723–4730. doi: 10.4049/jimmunol.1402890. [DOI] [PubMed] [Google Scholar]
- 51.Fu Y. A broadly neutralizing anti-influenza antibody reveals ongoing capacity of haemagglutinin-specific memory B cells to evolve. Nat. Commun. 2016;7:12780. doi: 10.1038/ncomms12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kunik V., Ofran Y. The indistinguishability of epitopes from protein surface is explained by the distinct binding preferences of each of the six antigen-binding loops. Protein Eng. Des. Sel. 2013;26:599–609. doi: 10.1093/protein/gzt027. [DOI] [PubMed] [Google Scholar]
- 53.Ying T. Junctional and allele-specific residues are critical for MERS-CoV neutralization by an exceptionally potent germline-like antibody. Nat. Commun. 2015;6:8223. doi: 10.1038/ncomms9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeung Y.A. Germline-encoded neutralization of a Staphylococcus aureus virulence factor by the human antibody repertoire. Nat. Commun. 2016;7:13376. doi: 10.1038/ncomms13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pappas L. Rapid development of broadly influenza neutralizing antibodies through redundant mutations. Nature. 2014;516:418–422. doi: 10.1038/nature13764. [DOI] [PubMed] [Google Scholar]
- 56.Joyce M.G. Vaccine-induced antibodies that neutralize group 1 and group 2 influenza A viruses. Cell. 2016;166:609–623. doi: 10.1016/j.cell.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parameswaran P. Article convergent antibody signatures in human dengue. Cell Host Microbe. 2013;13:691–700. doi: 10.1016/j.chom.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strauli N.B., Hernandez R.D. Statistical inference of a convergent antibody repertoire response to influenza vaccine. Genome Med. 2016;8:60. doi: 10.1186/s13073-016-0314-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johansen J.N. Intrathecal BCR transcriptome in multiple sclerosis versus other neuroinflammation: equally diverse and compartmentalized, but more mutated, biased and overlapping with the proteome. Clin. Immunol. 2015;160:211–225. doi: 10.1016/j.clim.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 60.Bomben R. Expression of mutated IGHV3-23 genes in chronic lymphocytic leukemia identifies a disease subset with peculiar clinical and biological features. Clin. Cancer Res. 2010;16:620–628. doi: 10.1158/1078-0432.CCR-09-1638. [DOI] [PubMed] [Google Scholar]
- 61.Forconi F. The IGHV1-69/IGHJ3 recombinations of unmutated CLL are distinct from those of normal B cells. Blood. 2013;119:2106–2109. doi: 10.1182/blood-2011-08-375501. [DOI] [PubMed] [Google Scholar]
- 62.Zhu D. Biased immunoglobulin light chain use in the Chlamydophila psittaci negative ocular adnexal marginal zone lymphomas. Am. J. Hematol. 2013;88:379–384. doi: 10.1002/ajh.23416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hwang K.K. IGHV1-69 B cell chronic lymphocytic leukemia antibodies cross-react with HIV-1 and hepatitis C virus antigens as well as intestinal commensal bacteria. PLoS One. 2014;9:e90725. doi: 10.1371/journal.pone.0090725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams W.B. HIV-1 vaccines. Diversion of HIV-1 vaccine-induced immunity by gp41-microbiota cross-reactive antibodies. Science. 2015;349:aab1253. doi: 10.1126/science.aab1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu L., Lucas A.H. IGH V3-23*01 and its allele V3-23*03 differ in their capacity to form the canonical human antibody combining site specific for the capsular polysaccharide of Haemophilus influenzae type b. Immunogenetics. 2003;55:336–338. doi: 10.1007/s00251-003-0583-8. [DOI] [PubMed] [Google Scholar]
- 66.Throsby M. Heterosubtypic neutralizing monoclonal antibodies cross-protective against H5N1 and H1N1 recovered from human IgM+ memory B cells. PLoS One. 2008;3:e3942. doi: 10.1371/journal.pone.0003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wrammert J. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ekiert D.C. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kashyap A.K. Combinatorial antibody libraries from survivors of the Turkish H5N1 avian influenza outbreak reveal virus neutralization strategies. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5986–5991. doi: 10.1073/pnas.0801367105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Corti D. A neutralizing antibody selected from plasma cells that binds to group 1 and group 2 influenza A hemagglutinins. Science. 2011;333:850–856. doi: 10.1126/science.1205669. [DOI] [PubMed] [Google Scholar]
- 71.Lingwood D. Structural and genetic basis for development of broadly neutralizing influenza antibodies. Nature. 2012;489:566–570. doi: 10.1038/nature11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nakamura G. An in vivo human-plasmablast enrichment technique allows rapid identification of therapeutic influenza A antibodies. Cell Host Microbe. 2013;14:93–103. doi: 10.1016/j.chom.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 73.Kallewaard N.L. Structure and function analysis of an antibody recognizing all influenza A subtypes. Cell. 2016;166:596–608. doi: 10.1016/j.cell.2016.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Y. A potent broad-spectrum protective human monoclonal antibody crosslinking two haemagglutinin monomers of influenza A virus. Nat. Commun. 2015;6:7708. doi: 10.1038/ncomms8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pugh-Bernard A.E. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J. Clin. Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cappione A.J. Lupus IgG VH4.34 antibodies bind to a 220-kDa glycoform of CD45/B220 on the surface of human B lymphocytes. J. Immunol. 2004;172:4298–4307. doi: 10.4049/jimmunol.172.7.4298. [DOI] [PubMed] [Google Scholar]
- 77.Lee J. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat. Med. 2016;22:1456–1464. doi: 10.1038/nm.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fesnak A.D. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chang D.K. Humanized mouse G6 anti-idiotypic monoclonal antibody has therapeutic potential against IGHV1-69 germline gene-based B-CLL. MAbs. 2016;8:787–798. doi: 10.1080/19420862.2016.1159365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Auton A. A global reference for human genetic variation. Nature. 2015;526:68–74. doi: 10.1038/nature15393. [DOI] [PMC free article] [PubMed] [Google Scholar]