Abstract
Background and Purpose
Preclinical studies suggest that exercise can enhance cognition after cerebral ischemia but often employ long training regiments and test cognition during or acutely after training. The cognitive changes may result from enhanced physical fitness and may only provide acute benefit. We sought to determine if a short period of exercise after cerebral ischemia could improve cognitive outcomes when measured days after completion of exercise training in two cerebral ischemia models.
Methods
Focal or global cerebral ischemia was induced in Sprague-Dawley rats. Rats recovered (3–4 days) then were subject to no exercise (0 m/min), mild (6 m/min), moderate (10 m/min), or heavy (15–18 m/min) treadmill exercise (5–6 days). Cognition was tested 8–10 days after last exercise session with hippocampal-dependent contextual fear conditioning.
Results
A short training period of moderate exercise enhanced cognitive function over a week after exercise completion in both models of cerebral ischemia.
Conclusions
Utilization of this exercise paradigm can further the elucidation of exercise-mediated factors involved in cognitive recovery independent of changes in physical fitness.
Keywords: Stroke, Brain Ischemia, Exercise, Cognition
Survivors of stroke will be at an enhanced risk for accelerated cognitive decline, yet there remains no well-accepted therapy for cognitive recovery1. Physical exercise (PE) after stroke improves motor functions in patients2 and animal models.3 A recent meta-analysis suggested that PE improves cognitive performance in models of acquired brain injuries4. However, studies that examine the ability of PE to promote cognitive recovery after cerebral ischemia employ weeks of PE training and test cognition during or immediately after the PE period4. These study designs cannot distinguish if the effects of PE are due to enhancements in overall animal fitness or an acute effect of exercise intervention. It remains unclear if a short bout of PE after cerebral ischemia has prolonged positive effects.
In this proof-of-concept study, we aimed to determine if a short bout of PE training initiated after cerebral ischemia can improve hippocampal-dependent contextual fear memory tested over a week after the last exercise session.
Materials and Methods
Animal Care and Ischemia Surgeries
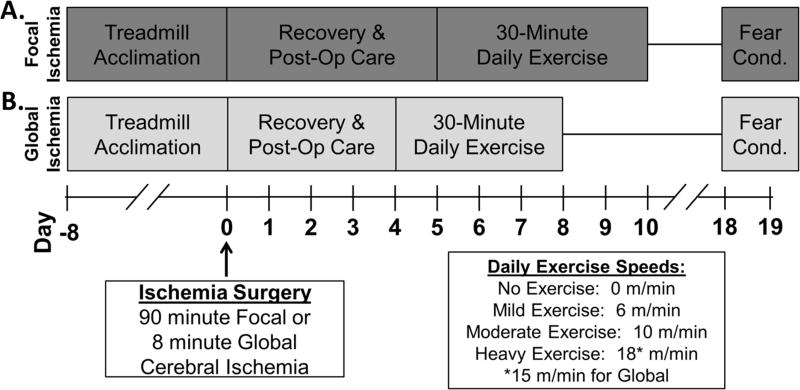
All experiments were performed in accordance with the Guide for Care and Use of Laboratory Animals and approved by the University of Miami’s Institutional Animal Care and Use Committee. Experimental timeline is indicated in Figure 1. Detailed experimental methods including randomization, blinding, and allocation concealment are in Online Supplement (please see http://stroke.ahajournals.org). Focal cerebral ischemia was achieved with intraluminal suture blockage of the middle cerebral artery for 90 minutes5, 6. Global cerebral ischemia was induced with 8-minute asphyxia cardiac arrest5, 7. Physiological parameters were maintained within normal ranges during surgeries (Online Supplement).
Figure 1. Experimental timeline.
Adult, male Sprague-Dawley rats were acclimated to treadmill walking (5 m/min for 5 minutes) over 8 days prior to surgery. (A) Rats were subject to focal ischemia, recovered for four full days, then randomized to exercise speed. Daily exercise consisted of a 2-minute warm-up at 5 m/min followed by 30 minutes at randomized speed. Completing 6 days of exercise, rats were undisturbed until contextual fear conditioning. (B) Rats subject to global cerebral ischemia received a similar training paradigm but began exercise after 3 full days of recovery and exercised for 5 days. Unable to maintain the higher speed, rats randomized to heavy exercise after global ischemia ran at 15 m/min.
Subacute Treadmill Exercise
After 3–4 days of recovery, adult (3-month old), male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were exercised on a treadmill (0.59 mA aversion grid; Columbus Instruments, Columbus, OH) for 5–6 days (Figure 1). A blinded investigator randomized rats to speeds selected based on our experience and other reports8, 9: no exercise (0 m/min); mild (6 m/min); moderate (10 m/min); or heavy exercise (15–18 m/min) with sample sizes for each speed: n= 11, n=11, n=10, and n=9 respectively for focal ischemia; and n=10, n=5, n=7, and n=5 respectively for global ischemia. After focal ischemia, heavy exercise was set at 18 m/min. However, rats were unable to run at 18 m/min after global ischemia so 15 m/min was used for heavy exercise. Daily PE consisted of a 2-minute warm-up at 5 m/min then 30 minutes of exercise at designated speed. Rats randomized to the 0 m/min group remained on a stationary treadmill for 30 minutes. Animals that refused to run were excluded from analysis (n=2, global ischemia with heavy exercise).
Contextual Fear Conditioning
Eighteen days after surgery (8–10 days after exercise), hippocampal-dependent contextual fear conditioning was performed7. Rats were placed into the operant conditioning chamber (Coulbourn Instruments, Whitehall, PA) for 370 seconds with a 2 second 1.5 mA shock administered at second 340. The following day, rats remained in the chamber for 480 seconds with no shock administered. Amount of time freezing was quantified (FreezeFrame, Coulbourn Instruments) prior to the shock on the first day (Baseline) and for the entire duration on the second day (Contextual). Animals that froze more than 50% on the first day were excluded from further analysis (n=1, global cerebral ischemia with moderate exercise).
Statistical Analysis
All data are represented as mean ± S.E.M. Sample size calculations are detail in the Online Supplement. Data was analyzed with One-Way ANOVA and comparisons between individual exercise groups quantified with Bonferroni Post-hoc tests. Significance was assessed as p<0.05.
Results
At baseline, there were no differences in the amount of time freezing between groups subject to focal (p=0.6597, Figure 2A) or global (p=0.2635, Figure 2C) cerebral ischemia. Contextual freezing was significantly different among groups (p=0.0078 for focal, p=0.0037 for global, Figure 2B,D) with animals randomized to no exercise (0 m/min) freezing the least suggesting worse memory. After focal ischemia, rats subjected to moderate exercise (10 m/min) had a significant enhancement in duration of freezing compared to non-exercised rats (p<0.01, Figure 2B). Moderate exercise had a similar effect after global cerebral ischemia enhancing contextual fear memory as compared to the non-exercised controls (p<0.05, Figure 2D). Heavy exercise (15 m/min) after global cerebral ischemia also enhanced percent time freezing (p<0.01).
Figure 2. Short period of moderate exercise enhances contextual fear memory.
(A, C) There were no significant differences at baseline between groups. (B, D) As compared to non-exercised animals (0 m/min), rats subject to moderate (10 m/min) exercise had enhanced fear memory after focal and global cerebral ischemia. Heavy exercise (15 m/min) after global cerebral ischemia also significantly improved hippocampal-dependent contextual fear memory. *p<0.05, **p< 0.01 Bonferroni Post-hoc.
Discussion
While there is no treatment for cognitive dysfunction after stroke, prolonged PE can improve cognitive recovery after cerebral ischemia in animal models (reviewed in4). Using two models of cerebral ischemia and three speeds of treadmill exercise, we show that a brief bout of moderate exercise initiated after cerebral ischemia can enhance memory tested over a week after the last exercise session. This suggests that post-ischemia, exercise-mediated cognitive benefits are unlikely a result of changes in physical fitness or acute exercise effects. While future studies are needed to determine the duration of enhanced fear memory, the fact that this enhancement was evident over a week after the last exercise session suggests it is long lasting. Additionally, our finding that the fastest treadmill speed (18 m/min) was unable to improve cognition is similar to that of others where exercise at 8 m/min, but not 20 m/min, resulted in an acute improvement in spatial memory.8, 9 Future studies that aim to understand exercise-mediated cognitive improvements after cerebral ischemia should use speeds between 8–15 m/min.
After stroke, there is a period of enhanced plasticity with extensive remodeling occurring in the peri-infarct cortex10, 11. Targeted, task-specific motor training can improve motor function and mold this redeveloping motor network12. The ability to translate this approach to improving cognitive functioning after stroke has been limited. Several brain regions are involved in cognition necessitating a multi-targeted rehabilitation intervention. Additionally, ischemia-induced enhancement of plasticity may be limited further from the infarct13. Here, we tested cognition with hippocampal-dependent contextual fear conditioning7. Our previous studies find no gross neuronal loss in the hippocampus after focal ischemia5 and extensive hippocampal neuronal loss after global ischemia14. Despite these injury differences, PE improved hippocampal-dependent cognitive functioning suggesting that PE may be a multi-targeted therapy that can improve cognitive centers regardless of ischemic insult.
Summary
In this proof-of-concept study, we find that an early, short bout of moderate exercise in two models of cerebral ischemia promotes an enhancement of cognitive function over a week after the last exercise session. Without the confounding effects of prolonged exercise training on overall fitness or acute effects of recent exercise, our experimental paradigm can be utilized for identification of biomarkers and/or soluble mediators of lasting exercise benefits.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by the American Heart Association Fellowship 15PRE2236000 (HMS-C); American Heart Association/American Stroke Association Bugher Foundation 14BFSC17690007 (RLS,CBW,MAP-P); NIH/NINDS R01 NS34773-15 and NS45676-08 (MAP-P); Lois Pope LIFE Fellows Program (HMS-C); and the McKnight Brain Foundation.
Footnotes
Disclosures: None.
References
- 1.Levine DA, Galecki AT, Langa KM, Unverzagt FW, Kabeto MU, Giordani B, et al. Trajectory of cognitive decline after incident stroke. Jama. 2015;314:41–51. doi: 10.1001/jama.2015.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoller O, de Bruin ED, Knols RH, Hunt KJ. Effects of cardiovascular exercise early after stroke: Systematic review and meta-analysis. BMC neurology. 2012;12:45. doi: 10.1186/1471-2377-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmidt A, Wellmann J, Schilling M, Strecker JK, Sommer C, Schabitz WR, et al. Meta-analysis of the efficacy of different training strategies in animal models of ischemic stroke. Stroke. 2014;45:239–247. doi: 10.1161/STROKEAHA.113.002048. [DOI] [PubMed] [Google Scholar]
- 4.Wogensen E, Mala H, Mogensen J. The effects of exercise on cognitive recovery after acquired brain injury in animal models: A systematic review. Neural plasticity. 2015;2015:830871. doi: 10.1155/2015/830871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin HW, Saul I, Gresia VL, Neumann JT, Dave KR, Perez-Pinzon MA. Fatty acid methyl esters and solutol hs 15 confer neuroprotection after focal and global cerebral ischemia. Translational stroke research. 2014;5:109–117. doi: 10.1007/s12975-013-0276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. discussion 1623. [DOI] [PubMed] [Google Scholar]
- 7.Cohan CH, Neumann JT, Dave KR, Alekseyenko A, Binkert M, Stransky K, et al. Effect of cardiac arrest on cognitive impairment and hippocampal plasticity in middle-aged rats. PloS one. 2015;10:e0124918. doi: 10.1371/journal.pone.0124918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimada H, Hamakawa M, Ishida A, Tamakoshi K, Nakashima H, Ishida K. Low-speed treadmill running exercise improves memory function after transient middle cerebral artery occlusion in rats. Behavioural brain research. 2013;243:21–27. doi: 10.1016/j.bbr.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Shih PC, Yang YR, Wang RY. Effects of exercise intensity on spatial memory performance and hippocampal synaptic plasticity in transient brain ischemic rats. PloS one. 2013;8:e78163. doi: 10.1371/journal.pone.0078163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy TH, Corbett D. Plasticity during stroke recovery: From synapse to behaviour. Nature reviews. Neuroscience. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- 11.Harrison TC, Silasi G, Boyd JD, Murphy TH. Displacement of sensory maps and disorganization of motor cortex after targeted stroke in mice. Stroke. 2013;44:2300–2306. doi: 10.1161/STROKEAHA.113.001272. [DOI] [PubMed] [Google Scholar]
- 12.Nishibe M, Urban ET, 3rd, Barbay S, Nudo RJ. Rehabilitative training promotes rapid motor recovery but delayed motor map reorganization in a rat cortical ischemic infarct model. Neurorehabilitation and neural repair. 2015;29:472–482. doi: 10.1177/1545968314543499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4101–4109. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dave KR, Raval AP, Prado R, Katz LM, Sick TJ, Ginsberg MD, et al. Mild cardiopulmonary arrest promotes synaptic dysfunction in rat hippocampus. Brain research. 2004;1024:89–96. doi: 10.1016/j.brainres.2004.07.050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.