Abstract
Artemisinin (ART)-based combination therapies are the most efficacious treatment of non-complicated Plasmodium falciparum infection. Alarmingly, P. falciparum strains have acquired resistance to ART in Southeast Asia and Africa. ART creates widespread protein and lipid damage inside intra-erythrocytic parasites, necessitating macromolecule degradation. The proteasome is the main engine of Plasmodium protein degradation. Indeed, proteasome inhibition and ART synergized in ART-resistant parasites. Moreover, ubiquitin modification is associated with resistance to multiple antimalarials. Targeting the ubiquitin-proteasome system, therefore, is an attractive avenue to combat drug resistance. Here, we review recent advances leading to specific targeting of the Plasmodium proteasome. We also highlight the potential for targeting other non-proteasomal protein degradation systems as an additional strategy to disrupt protein homeostasis.
Keywords: Plasmodium, proteasome, Clp proteases, ubiquitin
Combating Drug-resistant Malaria: Upsetting the Proteostatic Balance
Malaria in humans is caused by five species of the Plasmodium eukaryotic parasite (P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi) and is transmitted by female Anopheles mosquitoes. Due to the range of the mosquito, half the world’s population is at risk of contracting a malaria infection. In 2015, an estimated 212 million cases were reported worldwide, leading to ~430,000 deaths [1]. The WHO recommends artemisinin-based combination therapy (ACT) for first-line treatment of non-complicated falciparum malaria. Alarmingly, resistance to artemisinin has arisen in geographic pockets in multiple countries in Southeast Asia [2–5], and one case of a Chinese migrant worker was recently reported in equatorial Guinea [6]. ART-resistant parasites are defined as parasites that clinically display delayed parasite clearance times in patients treated with an ART derivative or an ART-based combination therapy (ACT), and which in vitro can withstand short pulses of high ART concentrations (for recent reviews of artemisinin resistance, see [7] and [8]).
To maintain the gains we have made against malaria and to stem the spread of artemisinin resistance, we must discover novel targets and pathways that are not compromised by existing drug resistance. One such avenue is to target proteostatic pathways, as a dysregulation of these pathways leads to cellular death [9–18]. The major pathway for degradation in eukaryotes is undertaken by the 26S proteasome (for a comprehensive review of the proteasome, see [19] and [20]). Other compartmentalized proteolytic complexes whose activity is regulated by AAA ATPase chaperones include the archaeal 20S proteasome, the prokaryotic proteasome HslV, and the ClpP proteases, all of which exhibit similar architecture in which the chaperone is docked at either end of the proteolytic core. These chaperones recognize substrates, remove degradation tags, unfold and thread the substrate into the proteolytic core in an ATP-dependent manner, and allosterically activate gate opening into the channel where substrates are proteolytically processed [21]. Plasmodium are unique in that they possess the eukaryotic 26S proteasome in addition to a homolog of the prokaryotic proteasome HslV and a homolog of the cyanobacteria Clp protease. Maintaining multiple degradation systems points to the importance of this pathway. We discuss the potential of inhibiting degradation systems in the parasite as means to develop new anti-malarial therapy agents that may overcome some of the current issues with resistance.
Targeting the 26S Proteasome
The proteasome is a multi-subunit protein degradation complex that is necessary for protein turnover and cell differentiation in all eukaryotes. Several features render the Plasmodium proteasome an attractive therapeutic target. First, Plasmodium parasites require the proteasome to progress through their complex lifecycle and a number of different classes of inhibitors have been shown to block growth or kill parasite at all lifecycle stages including the transmissive gametocyte stages [9–18]. Second, proteins damaged by indiscriminate protein alkylation caused by the antimalarial action of endoperoxides such as ART and the newer synthetic ozonides (OZ277, OZ439) that mimic artemisinin action [22–24] must be degraded to maintain homeostasis. The proteasome carries the largest burden of protein degradation, and has been the best characterized of the various degradative systems in Plasmodium. Third, ART-resistant P. falciparum isolates demonstrate an increased cell stress response and reliance on the proteasome in the basal, non drug-treated state [25, 26]. Presumably, constitutive reliance on chaperones and degradative capacity allows these parasites to withstand short pulses of ART. However, this cellular re-wiring also exposes pathway vulnerabilities. An increased reliance on the proteasome pathway can result in increased potency of proteasome inhibitors in ART-resistant parasites [18]. Lastly, ART resistance is mediated by mutations in K13 (also known as Kelch13, PF3D7_1343700). Based on homology, K13 is suspected to be an adaptor protein for E3 ubiquitin ligases [27, 28]. Comparisons with a human kelch-like protein lead to the speculation that downstream effects on autophagy may rely on K13 and proteasome-mediated degradation [29].
ART and synthetic ozonides alkylate proteins that are involved in a wide variety of cellular processes, including hemoglobin degradation, antioxidant responses, and stress pathways [22–24]. Recent evidence suggests that ART-treated parasites accumulate ubiquitinated polypeptides [25], providing an increased load of proteins that require degradation. Parasite survival in the presence of ART, effectively rendering them drug-resistant, might depend upon the ability of the proteasome to dispose of these damaged proteins (Fig. 1). Indeed, transcriptomics studies of untreated ART-resistant parasites from Cambodia showed upregulation of the catalytic proteasome β-subunits as well as components of the Plasmodium reactive oxidative stress complex (PROSC) and T-complex protein-1 Ring Complex (TRiC) chaperone complexes [26]. Proteasome inhibitors were also reported to be more potent against ART-resistant parasites, and synergized with dihydroartemisinin, the active metabolite of ART, to mediate parasite killing [18, 25]. Together, the data suggest that increasing cellular damage, while decreasing the ability of the proteasome to process that workload, creates a homeostatic imbalance that is lethal to P. falciparum. Thus, specific targeting of the proteasome has the potential to overcome P. falciparum resistance to ART and mechanistically related drugs.
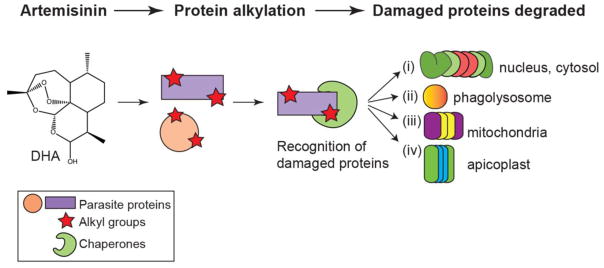
Fig. 1. Artemisinin, its derivatives, and the synthetic ozonides cause widespread protein damage that induce stress pathways.
Treatment with dihydroartemisinin (DHA), the active metabolite of artemisinin (ART), leads to non-specific protein alkylation. Damaged proteins are recognized by chaperones, and targeted for degradation by one of four pathways: (i) the ubiquitin-proteasome system (UPS), (ii) autophagy, (iii), the PfClpY/Q protease, or (iv) the PfClpC/P protease. The subcellular locations of these pathways are denoted to the right of each proteolytic system.
Aside from the synergy displayed between proteasome inhibitors and ART derivatives in asexual blood stage parasites, proteasome inhibitors possess the additional benefit of being active throughout the parasite lifecycle: liver stages, asexual blood stages, sexual stages that are transmissible to Anopheles mosquitoes, and the mosquito stages. Proteasome inhibitor-treated P. berghei sporozoites fail to establish a liver stage infection and cannot develop into exoerythrocytic forms in hepatocytes both in vitro and in vivo [9]. Moreover, asexual blood stage parasite growth is potently inhibited when exposed to proteasome inhibitors at the ring, trophozoite, or schizont stages [9–11, 16, 30]. Proteasome inhibition can also block parasite transmission by inhibiting stage V gametocyte development [13] and can prevent development in the mosquito by interfering with oocyst formation in the midgut [9]. These studies, performed with inhibitors that also target the human proteasome, demonstrate that the Plasmodium counterpart is indispensable for parasite survival.
The 26S proteasome is a large, barrel-shaped multi-subunit protease complex composed of a 19S regulatory particle (RP) and a 20S core particle (CP). All subunits of the P. falciparum 26S proteasome have been identified [31], and are expressed throughout the lifecycle [32–34]. Recent characterization of the P. falciparum 26S proteasome and associated proteins recapitulate many of the lessons learned from yeast and mammals, with minor differences that can be exploited for therapeutic purposes [31]. The P. falciparum 20S CP exists freely, or is capped singly or doubly with a 19S RP [31], similar to that of other eukaryotes [35]. Subunits of the 19S RP recognize, unfold, and deubiqutinate degradation substrates. The 20S CP is responsible for proteolytic cleavage [19]. A recent cryo-EM structure of the P. falciparum 20S CP reveals four stacked heptameric rings, with two outer rings consisting of α1-α7, and the catalytic activity located in the two inner beta rings consisting of β1-β7 [18] (Fig. 2A,B). The catalytically-active β1, β2, and β5 subunits use a N-terminal threonine as the nucleophile [36] and have peptidylglutamyl-peptide (caspase)-like, trypsin-like, and chymotrypsin-like activities, cleaving after the carboxy-terminal side of acidic residues, tryptic residues, and hydrophobic residues respectively. These active sites face into the channel of the barrel to allow degradation of a substrate only after it has been actively inserted into the CP complex. The N-terminal tails of the α subunits prevent polypeptide access to the inner β core catalytic subunits. Proteasomal subunits in the 19S RP regulate gate opening of the 20S CP. [37]. Major structural differences between human and plasmodial proteasomes include an unusually open β2 active site in P. falciparum proteasomes [18]. To identify specific differences in the substrate specificities of the human and parasite enzymes Li et al. [18] performed an unbiased screening using diverse peptide substrates whose cleavage could be biochemically defined using mass spectrometry. This approach produced a map of substrates sequences that were accepted by the parasite but not by the human proteasome. While this information provided general specificity data for the full proteasome complex, it does not allow direct monitoring of specificities of each of the three individual β-subunit activities and thus the exact specificity profiles for each subunit remains to be determined. However, prior results using active site probes and subunit-selective inhibitors show that parasites are most sensitive to inhibition of the β5 subunit and that effective killing by β2-specific inhibitors requires inhibition of additional subunits [16]. Using the substrate specificity data, the authors generated peptide vinyl sulfone inhibitors containing the sequences preferred by the parasite enzyme. These inhibitors bound covalently to the β2 alone or to both the β2 and β5 proteasome subunits (Fig. 2C) resulting in potent killing of parasites (with IC50 values of 6 nM (Mu-WLL-vs) and 290 nM (Mu-WLW-vs) in 72 hour assays beginning with P. falciparum ring stages). Most importantly the compounds showed virtually no toxicity to the host cell and one was able to clear P. chaubaudi from infected mice without causing general toxicity [18]. The identification of these potent Plasmodium-specific proteasome inhibitors validates the parasite proteasome as a viable drug target, and has identified important leads for ongoing antimalarial drug discovery and development efforts. Currently, there are a number of different efforts to identify potent and selective proteasome inhibitors from multiple different classes of inhibitor scaffolds. We anticipate that many more reports of Plasmodium-specific proteasome inhibitors will appear in the literature in the near future. Below, we discuss other attractive targets of the ubiquitin-proteasome system (UPS), as well as other proteolytic systems in Plasmodium spp.
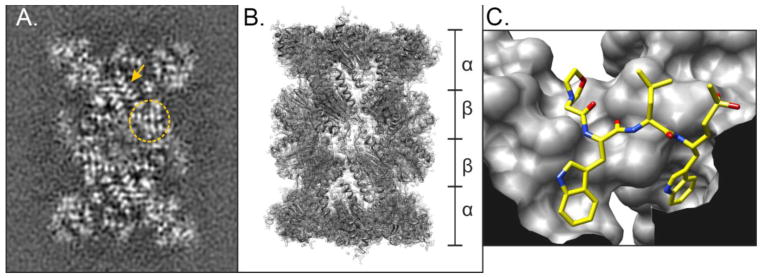
Fig. 2. Visualizing the Plasmodium falciparum 20S core particle.
(A) Cryo-EM image of the P. falciparum 20S core particle (CP). Image shows regions where β-sheet structures (circle) and α-helices (arrow) can be visualized. (B) Fitted model of the 20S core particle based on the cryo-EM images, denoting the stacked α and β rings. (C) Ball-and-stick representation of the parasite-specific peptide vinyl sulfone inhibitor Mu-WLW-vs [18] bound in the β2 active site of the 20S CP. The image shows the large S1 and S3 pockets that can accommodate the two tryptophan residues that do not fit in the human active site.
Targeting Enzymes Involved in the Ubiquitin Cascade
Considering that the vast majority of P. falciparum genes are tightly regulated so that transcripts are expressed according to the developmental lifecycle [38], post-translational modifications such as ubiquitination may provide a way for parasites to respond more dynamically to external stimuli such as temperature changes or antimalarial drugs. Indeed, the polyubiquitin gene was found to be upregulated in response to heat shock at 41°C [39], presumably to provide an abundance of ubiquitin molecules to tag damaged proteins for proteasome-mediated degradation. Thus, inhibiting ubiquitin conjugation or removal constitutes another potential strategy to kill parasites (Fig. 3A). Ubiquitin is a 76-amino acid protein that is highly conserved across prokaryotes and eukaryotes. The primary amino acid sequence of human and plasmodial ubiquitin differs by only one amino acid (E16 in Homo sapiens; D16 in P. falciparum) [39, 40]. In P. falciparum, ubiquitin can be derived from two sources: a polyubiquitin gene that encodes five tandem repeats of ubiquitin (PfpUb, PF3D7_1211800), or a gene fusion protein consisting of ubiquitin conjugated to the 60S ribosomal protein L40 (PF3D7_1365900) [39–41]. Both sources of ubiquitin are expressed in asexual blood stages, gametocytes and sporozoites. Polyubiquitin expression peaks during the intra-erythrocytic trophozoite and schizont stages [33, 39, 42], which are also the most susceptible to proteasome inhibitors [9, 16]. These findings suggest that the highly coordinated UPS is essential for parasite multiplication.
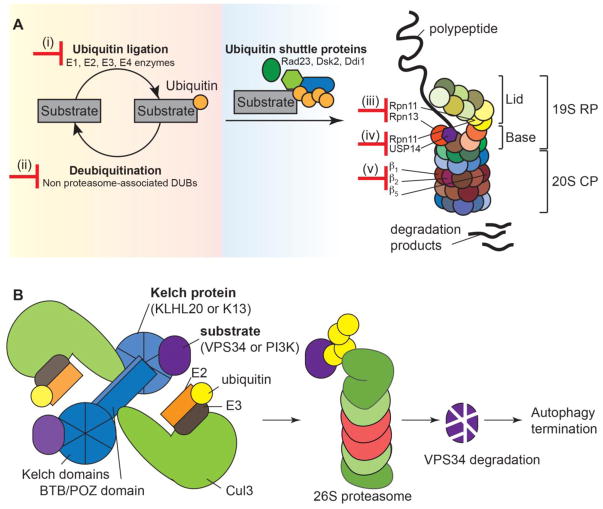
Fig. 3. The ubiquitin-proteasome system facilitates cellular homeostasis.
(A) There are multiple ways to target the ubiquitin-proteasome system. Inhibiting enzymes involved in (i) attachment or (ii) removal of ubiquitin will affect homeostasis of protein degradation. Targeting (iii) proteasomal regulation, (iv) proteasome-associated de-ubiquitinating enzymes, or (v) the catalytic subunits will impair the ability of the 26S proteasome to degrade proteins. (B) A hypothetical relationship between K13 and PI3K in regulating autophagy. Kelch-like proteins dimerize via their BTB/POZ domains and bind substrate via their Kelch domains. Theoretically, each Kelch-like protein dimer can bind two different substrates. Here, we show a model in which human KLHL20 binds Cul3 to mediate ubiquitination of the class III PI3K VPS34. VPS34 then undergoes proteasome-mediated degradation. This, and degradation of other proteins in the VPS34 complex, turns off autophagy. In a similar manner, K13 could mediate ubiquitination and proteasomal degradation of PI3K, leading to autophagy termination. Artemisinin-resistant parasites, harboring mutations in the propeller domain of K13, are unable to bind and target PI3K for proteasomal degradation. Prolonged autophagy may confer these parasites with an enhanced ability to dispose of damaged proteins upon artemisinin treatment. CP, core particle (20S) of the 26S proteasome; DUBs, deubiquitinating enzymes; K13, Kelch 13; PI3K, phosphatidylinositol 3 kinase; RP, regulatory particle (19S) of the 26S proteasome.
Ubiquitin is attached in a controlled and step-wise manner. First, ubiquitin is proteolytically processed from its precursor form by ubiquitin-specific proteases (USPs) to reveal a di-glycine motif. Then, in an ATP-dependent step, an E1 ubiquitin activating enzyme forms a thioester bond between its active site cysteine and the C-terminus of ubiquitin. Ubiquitin is then transferred via a transthioesterification reaction to the active site cysteine of an E2 ubiquitin-conjugating enzyme. Finally, E3 ligases belonging to the Homologous to E6-associated protein C-terminus (HECT) family bind ubiquitin prior to transfer onto a substrate, while those in the Really Interesting New Gene (RING) and U-box families facilitate transfer of ubiquitin directly from a E2 onto the substrate [43]. E4 ubiquitin chain elongation factor can further polyubiquitinate polypeptides [44]. Ubiquitin and its E1, E2, E3, and E4 enzymes are expressed throughout the asexual blood stages [32, 38, 41, 45]. Molecular characterization of a set of E1, E2, and E3 enzymes revealed significant functional conservation in Plasmodium. The P. falciparum homologs of E1 UBA (PfUBA1, PF3D7_1225800), E2 UBC7 (PfUBC, PF3D7_1203900) and E3 RING ligase HRD1 (PfHRD1, PF3D7_1422500) were shown to have in vitro ubiquitinating activities. Furthermore, these proteins were found by immunofluorescence assays to reside in the expected subcellular localizations (PfUBA1 and PfUBC in the cytosol, PfHRD1 at the ER membrane), indicating that these enzymes likely function in endoplasmic reticulum-associated degradation (ERAD) [46]. Ubiquitin itself has seven lysine residues (K6, K11, K27, K29, K33, K48, and K63) that can be mono- or poly-ubiquitinated, either in single or multiple types of ubiquitin branches. In addition, ubiquitin can be attached to its first amino acid (M1), thereby forming linear chains [47]. Ubiquitin conjugation promotes a variety of cellular processes [43], including targeting proteins for proteasome-mediated degradation. Proteins destined for destruction are modified with K48-linked polyubiquitin chains, although the proteasome is also able to associate with and process all other non-K63-linked polyubiquitin chains, albeit less efficiently [48]. Proteomic analysis of P. falciparum parasites have revealed that K48-linkage is found in ~80% of ubiquitin conjugates [42]. Inhibiting ubiquitin conjugation will affect the homeostasis of the UPS and could negatively impact parasite growth. Inhibitors to human ubiquitin E1, E2, and E3 enyzmes have been approved for hematological malignancies or are in clinical trials (ClinicalTrials.gov identifier: NCT02045095) [49, 50], demonstrating the feasibility of targeting these enzymes.
De-ubiquitinating enzymes (DUBs) are required for maturation of C termini of ubiquitin precursors, ubiquitin chain editing, and removal of ubiquitin tags prior to polypeptide threading into the proteasome for protein degradation. Five classes are papain-type cysteine proteases: ubiquitin C-terminal hydrolases (UCH), ubiquitin specific proteases (USP), otubains (OTU), ataxin-3/Josephin ubiquitin proteases (MJD), and the predicted family permuted papain fold peptidases of dsRNA viruses and eukaryotes (PPPDE). A sixth class of Jab1/Mov34/Mpr1 Pad1 N-terminal (MPN+) (JAMM) isopeptidases is comprised of zinc-dependent metalloproteases [51]. Members of each class have been found in Plasmodium spp. [41]. Proteasome-associated DUBs and their regulators are potential drug targets. One such candidate is the DUB USP14, which removes polyubiquitin chains from degradation substrates [52] to recycle ubiquitin molecules, and regulates Rpn11-mediated unfolding of substrates targeted for proteasome-mediated degradation, thereby controlling the timing of protein degradation [53, 54]. The P. falciparum homolog PfUSP14 (PF3D7_0527200) was shown to bind plasmodial proteasomes, and small-molecule inhibition (b-AP15) of this enzyme led to accumulation of proteasomal substrates and P. falciparum death [31]. Inhibitors of the human USP14 are currently in clinical trials to treat multiple myeloma [55, 56]. Recently, multiple natural and synthetic compounds have been identified to inhibit the deubiquitinating activity of the 19S subunit Rpn11 [57–59].
In addition to targeting the catalytic activities of DUBs, targeting DUB regulators has been shown to be effective in inhibiting proteasomal activity. Inhibitors of human Rpn13 inhibited proteasome function without inhibiting 20S catalytic activity or 19S deubiquitinating activity [60]. Rpn13 is a proteasome subunit that resides in the base of the 19S RP, binds K48-linked di-ubiquitin polypeptides via a pleckstrin-like receptor for ubiquitin (Pru) domain [61], and activates the proteasome-associated DUB UCH37 [62–64]. This strategy could potentially work in P. falciparum. The Pru domain of PfRpn13 (PF3D7_1414000) expressed as a recombinant protein was unable to bind ubiquitin in vitro [31], but ubiquitin binding might depend upon conformational changes that were lacking without full protein expression. PfUCH54 (PF3D7_1117100), the P. falciparum homolog of UCH37, was found in a complex with the P. falciparum 26S proteasome in 3 of 4 mass spectrometry runs in the presence of formaldehyde crosslinking [31], and demonstrated to have in vitro deubiquitinating and deneddylating activities [65].
The Roles of Ubiquitin Ligases and Deubiquitinating Enzymes in P. falciparum Drug Resistance
Field-based studies have shown an association between delayed parasite clearance times in artesunate or ACT-treated patients and mutations in the propeller domain of P. falciparum K13 [3, 6, 27]. In vitro k13 gene editing of several K13 mutations demonstrated that they mediate high levels of survival following exposure of early ring stages to short pulses of dihydroartemisinin [66, 67]. K13 contains a BTB/POZ (Broad-Complex, Tramtrack and Bric a Brac/Poxvirus or Zinc-finger) domain as well as a six-bladed Kelch propeller domain [7, 27]. BTB domains dimerize, and each can associate with Cullin3 substrate adaptors in the Cullin-Ring E3 ubiquitin ligase (CRL) complex, whereas Kelch domains associate with substrate to be ubiquitinated [28]. The function of K13 may include being an adaptor for an as-yet-unidentified E3 ubiquitin ligase.
K13 might potentially be involved in regulating autophagy by modulating levels of phosphatidylinositol (3)-phosphate (PI3P), which is essential for autophagosome formation [68, 69]. P. falciparum phosphatidylinositol 3 kinase (PI3K, PF3D7_0515300), most homologous to the class III PI3K VPS34 (first identified in vesicle-mediated vacuolar protein sorting in yeast), phosphorylates phosphatidylinositol to generate PI3P. Recent evidence suggests that PI3K is in a complex with wild-type K13, but not with mutant K13 that confers ART resistance [70]. ART-resistant parasites had slightly increased levels of PI3K, leading the authors to speculate that PI3K escapes K13-mediated proteasomal degradation in ART-resistant lines. The molecular mechanism demonstrating how increased levels of PI3P would lead to ART resistance has not been reported. In human cells, a Cul3-KLHL20 ubiquitin ligase complex promotes ubiquitination and proteasome-mediated degradation of VPS34, leading to termination of autophagy [29]. K13 is homologous to KLHL20 in the propeller domains, with 29% identity. This level of identity is similar to that of KLHL12 (30%), KLHL2 (27%), and Keap1 (28%), three proteins reported to be homologous to K13 [27]. K13 could similarly associate with PI3K and promote its degradation. In ART-resistant parasites harboring mutations in the K13 propeller domains, K13 cannot bind and target PI3K for proteasome-mediated degradation. One possibility is that prolonged autophagy might help in removing proteins damaged by ART treatment, thereby allowing survival of short pulses of ART (Fig. 3B). Details of autophagy in Plasmodium are still being elucidated. Using the ubiquitin-like protein ATG8 (PF3D7_1019900) as a marker, researchers have demonstrated punctate staining and partial co-localization with the apicoplast marker acyl carrier protein (ACP, PF3D7_0208500). In these studies, ART treatment decreased PfATG8 levels as evidenced by a decrease in intensity in immunofluorescence assays and Western blot, while the number of vesicles remained the same [71, 72]. However, treatment with the lysosomal Na+/H+ pump inhibitor Bafilomycin A1 or lysosomal cysteine protease inhibitor E64d did not lead to accumulation of ATG8. Accumulation of ATG8 in the presence of these inhibitors would indicate efficient autophagic efflux [73]. Therefore, these drugs may have affected other cellular processes.
Variants of E3 ligases and DUBs have been associated with parasite susceptibility to a range of antimalarial drugs, presumably via altered ubiquitin modifications of particular substrates. Multiple studies have shown an association between mutations in the DUB ubiquitin carboxy-terminal hydrolase 1 (UBP1) and reduced parasite susceptibility to a number of antimalarials. Resistance selection and genetic mapping experiments followed by whole-genome sequencing of the rodent parasite P. chabaudi identified two distinct mutations (V2697F and V2728F) in PcUBP1 (PCHAS_0207200) that were associated with ART resistance, as measured by the speed of parasite recrudescence after ART treatment. The UBP1 V2728F mutation appears to contribute to a multi-drug resistance phenotype: this mutation was also identified in P. chabaudi parasites subjected to chloroquine or mefloquine drug pressure (but were not exposed to ART pressure) [74, 75]. The contribution of UBP1 mutations to reduced susceptibility to artemisinin derivatives was also seen in ex vivo studies. Culture-adapted P. falciparum Kenyan isolates subjected to whole-genome sequencing and half-maximal inhibitory concentrations (IC50) of standard antimalarial drugs associated the K873R mutation in PfUBP1 (Pf3D7_0104300) with reduced DHA susceptibility [76]. In yeast, Ubp1 plays a role in the endocytic pathway [77], consistent with the known role of ubiquitin in the internalization step of endocytosis at the plasma membrane [78]. Variants of UBP1 might interfere with endocytosis of host products such as hemoglobin, thus allowing reduced susceptibilities to drugs that interact with host heme moieties.
Mutations in other components of the endocytosis pathway have also been proposed to modulate parasite susceptibility to ART. Whole-genome sequencing of ART-pressured P. chaubaudi parasites referred to above [74] identified a single mutation: I568T in the mu chain of the AP2 adaptor complex (PCHAS_143590) [79]. In humans the AP2 complex is involved in clathrin-mediated endocytosis [80]. Homology modeling studies predict that the I568T mutation alters binding affinity of the PcAP2 μchain to cargo protein undergoing endocytosis [79]. Analysis of parasite isolates from ACT-treated Kenyan children suggested an increased prevalence of S160N/T mutations in PfAP2-μ (PF3D7_1218300) or a E1528D mutation in PfUBP1 in parasites surviving ACT treatment [81]. Expression of an extra copy of pfap2-mu harboring a S160N mutation increased P. falciparum in vitro sensitivity to chloroquine and quinine [82]. A separate study associated the HECT E3 ligase P. falciparum ubiquitin transferase (PfUT) (PF3D7_0704600) variant harboring a Y1387F mutation, in the presence of the K76T mutation in the P. falciparum chloroquine resistance transporter (PfCRT), with quinine resistance [83]. The molecular mechanisms underlying how mutations in PfUT could contribute to quinine resistance remain to be identified. Since ART can be activated by Fe2+-heme released by hemoglobin digestion, one possibility is that a decrease in hemoglobin endocytosis caused by mutations in UBP1 or AP2-μ might allow parasites to escape ART action due to a lower amount of activated drug. Alternatively, a decrease in endocytosis might be a general mechanism of survival by reducing the uptake of antimalarial drugs. These data suggest that inhibition of endocytic trafficking might be one strategy to allow parasites to escape drug-mediated cell death.
Targeting PfClpY/Q, the Plasmodium homolog of the ClpY/ClpQ prokaryotic proteasome
In addition to the eukaryotic proteasome, Plasmodium homologs of the catalytic subunit of the prokaryotic proteasome caseinolytic protease Q (ClpQ), also known as heat shock locus V (HslV), and its regulatory chaperone ClpY/HslU have been identified in P. falciparum (PfClpQ, PF3D7_1230400, and PfClpY, PF3D7_0907400) [84, 85]. PfClpY and PfClpQ proteins are expressed at the trophozoite, schizont, and merozoite stages [84–86]. Bioinformatics analysis predicted mitochondrial localization of PfClpQ, which was confirmed by immunofluorescence assays and immuno-electron microscopy of an enhanced yellow fluorescent protein (EYFP)-tagged PfClpQ [87]. Thus, this protease may be responsible for degrading proteins specifically in the mitochondria. By homology to HslV, PfClpQ is predicted to consist of two stacked homohexameric rings [88]. In vitro fluorometric assays of recombinant PfClpQ demonstrated that this enzyme displays threonine protease-like, caspase-like and chymotrypsin-like activity, which were inhibited by lactacystin, chymostatin, and MG132, respectively [86]. The caspase-like and chymotrypsin-like activities are reminiscent of the proteolytic activity seen in the β1 and β5 subunits of the 26S proteasome respectively. Minimal PfClpQ activity is greatly enhanced upon activation by the AAA-ATPase chaperone PfClpY, which forms a complex with PfClpQ via its C-terminal domain [85, 89]. Like the 19S RP of the 26S proteasome, PfClpY recognizes, unfolds, and translocates substrates into PfClpQ. A peptide-based inhibitor that disrupts PfClpY/Q complex formation caused a loss of mitochondria membrane potential, activation of caspase-like cysteine proteases, and DNA fragmentation characteristic of apoptosis, leading to inhibition of parasite growth and an inability to form mature schizonts [85]. The PfClpY/Q protease resembles the 26S proteasome structurally and functionally. It would be interesting to test whether the recently identified Plasmodium-specific 20S proteasome inhibitors can also inhibit the PfClpY/Q system. Indeed, the absence of a homolog in humans makes this a particularly attractive target (Fig. 4).
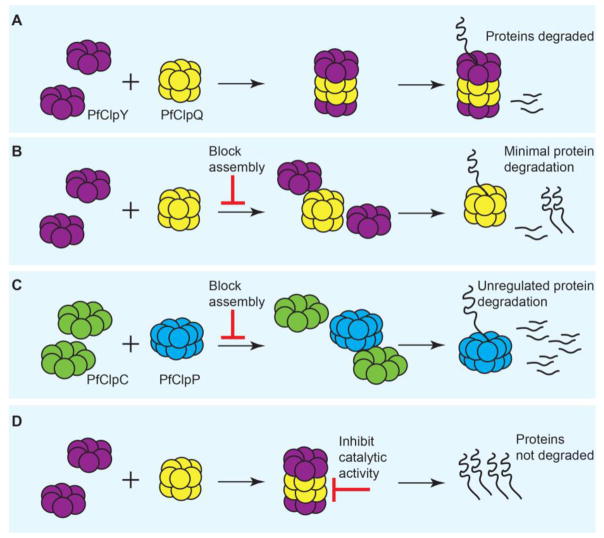
Fig. 4. Inhibition of PfClpY/Q and PfClpC/P in the mitochondria and apicoplast, respectively.
(A) Assembly of the chaperone (PfClpY or PfClpC) and the protease (PfClpQ or PfClpP) allow regulated proteolytic cleavage of degradation substrates. Shown here is a representation for the PfClpY/Q complex. (B) Inhibition of PfClpY assembly with a short peptide prevents complex formation. Unassembled PfClpQ has shown minimal degradation activities. Through an unknown mechanism, incubation with the short inhibitory peptide causes loss of mitochondrial membrane potential and eventual parasite death. (C) An antibiotic that prevented closure of the Staphylococcus aureus ClpP proteolytic chamber led to non-specific protein degradation and bacterial death. Similar strategies can be envisioned for Plasmodium. (D) Inhibiting the catalytic sites in PfClpQ (depicted here) or PfClpP will prevent substrate cleavage, leading to dysregulation of protein homeostasis.
Targeting PfClpC/P, the Plasmodium homolog of the cyanobacterial ClpP
P. falciparum homologs to the cyanobacterial ClpP (PfClpP, PF3D7_0307400) and its associated chaperone ClpC (PfClpC, PF3D7_1406600) have been identified and characterized [90, 91]. PfClpC/P proteins are expressed during the late trophozoite and early schizont stages of the asexual blood stage cycle [90, 91]. The PfClpC/P complex is structurally and functionally analogous to the 26S proteasome and the ClpY/ClpQ systems. Complementing the protein degradation machinery in the cytosol and mitochondria, PfClpC/P functions in the apicoplast [91]. This organelle is responsible for biosynthesis of fatty acid metabolism, heme, and isoprenoids, and is essential for asexual blood stages [92, 93]. Bioinformatics identified apicoplast-targeting sequences in PfClpP and PfClpC [90, 91]. Immunofluorescence assays examining a streptavidin-3xhemagluttinin-tagged PfClpC [91] and a construct expressing the amino-terminus of PfClpP harboring the apicoplast-targeting sequences fused to GFP [90] confirmed co-localization with the apicoplast marker acyl carrier protein (ACP). Immuno-electron microscopy further confirmed that the PfClpP-GFP fusion construct resided in the apicoplast [90]. Size-exclusion chromatography, analytical ultracentrifugation, and electron microscopy revealed that amino-terminally truncated PfClpP primarily form homoheptameric rings. Under the conditions studied, only a small percentage of PfClpP existed as an oligomeric complex consisting of two stacked heptamers [90, 91].
PfClpP is thought to complex with and be activated by PfClpC via a ClpP binding loop [91]. Presumably, the protease subunits are docked at either end of the cylinder by the AAA ATPase PfClpC chaperone that recognizes, unfolds, and threads substrates into the proteolytic core. The amino-truncated PfClpP demonstrated chymotrypsin-like serine protease activity as measured by Suc-LLVY-AMC. Accordingly, PfClpP protease activity was inhibited by serine protease inhibitors (chymostatin and phenylmethylsulfonyl fluoride (PMSF)), but not cysteine protease inhibitors (E-64 and leupeptin), or an aspartic protease inhibitor (pepstatin) [90, 91]. PfClpP demonstrates substrate preferences at the P1 position that are distinct from Escherichia coli and Homo sapiens [94], which could be exploited for drug discovery purposes. The β-lactone compound U1 bound PfClpP, affected apicoplast growth and segregation, and resulted in a delayed death phenotype [90], which is defined as parasites dying in the second generation following drug exposure and which is typically associated with inhibition of apicoplast protein translation [93, 95–97]. Antibacterial research has identified an antibiotic (acyldepsipeptide, ADEP4) that mediates over-activation of Staphylococcus aureus ClpP. In these antibiotic-treated cultures, ADEP4 bound ClpP and kept the proteolytic chamber open, preventing the chaperone from regulating substrate access to the ClpP protease, thus allowing the protease to non-specifically degrade proteins. Dysregulation of this proteolytic system resulted in death of bacteria that were actively-growing or in the stationary phase [98, 99] (Fig. 4C). Similar methods of proteolytic perturbations could be explored as a novel antimalarial approach in targeting non-replicating forms of Plasmodium.
Concluding remarks
The recent identification of Plasmodium-specific inhibitors of the catalytic subunits of the 26S proteasome provides a promising new avenue for antimalarial drug discovery. Small-molecule inhibitors of the proteasome have demonstrated that this catalytic multi-protease subunit is essential for Plasmodium parasite viability. Identification of multiple candidate drug targets within this system could lead to antimalarial combination therapies that are not compromised by existing mechanisms of multidrug resistance. Activators and regulatory subunits of the proteasome also constitute attractive drug targets, in addition to the proteasome catalytic subunits. Enzymes involved in the attachment and removal of ubiquitin have been shown to be viable targets in humans, and should be explored in Plasmodium. Additionally, the PfClp proteases remain attractive targets, especially since the mitochondria and apicoplast organelles are vital to Plasmodium survival and cell stress responses. The necessity of these degradation pathways and the abundance of targets within the pathways suggest that this is a good general strategy for development of novel anti-malarial drugs (see Outstanding Questions).
Outstanding Questions Box.
Will it be possible to develop inhibitors against substrate ubiquitination and deubiquitination to block specific key events during the parasite lifecycle?
Are other non-proteasome targets in the UPS viable drug targets in P. falciparum?
Will parasites treated with proteasome inhibitors develop resistance?
Will parasite-specific proteasome inhibitors work equally well on parasites resistant to multiple drugs?
Can combining proteasome inhibitors with ART treatment suppress ART resistance?
Will it be possible to generate safe and orally available malaria proteasome inhibitors?
Is it possible to develop inhibitors against PfClp proteases based on inhibitors active against other organisms?
Trends Box.
Proteasome inhibitors are effective against Plasmodium spp., but past inhibitors had a low therapeutic index due to inhibition of the host enzyme complex.
Recent cryo-EM data have illuminated differences between the human and P. falciparum 20S proteasome core particles, allowing the generation of P. falciparum-specific proteasome inhibitors.
The recent characterization of Plasmodium proteins involved in the UPS has identified several enzymes involved in attachment and removal of ubiquitin that could be viable drug targets.
Inhibitors of the human proteasome, ubiquitin E1, E2, and E3 enzymes, as well as DUBs have been FDA-approved or are in clinical trials, demonstrating the therapeutic potential of inhibitors against these enzymes.
Inhibitors to PfClp proteases are attractive since there is no human homolog to the mitochondrial-based PfClpY/Q, while PfClpC/P resides in the apicoplast, a cyanobacterial relic organelle that is not present in humans.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. World Malaria Report. 2016. [Google Scholar]
- 2.Dondorp AM, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–67. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashley EA, et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2014;371:411–23. doi: 10.1056/NEJMoa1314981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takala-Harrison S, et al. Independent emergence of artemisinin resistance mutations among Plasmodium falciparum in Southeast Asia. J Infect Dis. 2015;211:670–9. doi: 10.1093/infdis/jiu491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tun KM, et al. Spread of artemisinin-resistant Plasmodium falciparum in Myanmar: a cross-sectional survey of the K13 molecular marker. Lancet Infect Dis. 2015;15:415–21. doi: 10.1016/S1473-3099(15)70032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu F, et al. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med. 2017 doi: 10.1056/NEJMc1612765. [DOI] [PubMed]
- 7.Tilley L, et al. Artemisinin action and resistance in Plasmodium falciparum. Trends Parasitol. 2016;32:682–96. doi: 10.1016/j.pt.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paloque L, et al. Plasmodium falciparum: multifaceted resistance to artemisinins. Malar J. 2016;15:149. doi: 10.1186/s12936-016-1206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gantt SM, et al. Proteasome inhibitors block development of Plasmodium spp. Antimicrob Agents Chemother. 1998;42:2731–8. doi: 10.1128/aac.42.10.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindenthal C, et al. The proteasome inhibitor MLN-273 blocks exoerythrocytic and erythrocytic development of Plasmodium parasites. Parasitology. 2005;131:37–44. doi: 10.1017/s003118200500747x. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds JM, et al. Antimalarial activity of the anticancer and proteasome inhibitor bortezomib and its analog ZL3B. BMC Clin Pharmacol. 2007;7:13. doi: 10.1186/1472-6904-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prudhomme J, et al. Marine actinomycetes: a new source of compounds against the human malaria parasite. PLoS One. 2008;3:e2335. doi: 10.1371/journal.pone.0002335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czesny B, et al. The proteasome inhibitor epoxomicin has potent Plasmodium falciparum gametocytocidal activity. Antimicrob Agents Chemother. 2009;53:4080–5. doi: 10.1128/AAC.00088-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, et al. Validation of the proteasome as a therapeutic target in Plasmodium using an epoxyketone inhibitor with parasite-specific toxicity. Chem Biol. 2012;19:1535–45. doi: 10.1016/j.chembiol.2012.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tschan S, et al. Broad-spectrum antimalarial activity of peptido sulfonyl fluorides, a new class of proteasome inhibitors. Antimicrob Agents Chemother. 2013;57:3576–84. doi: 10.1128/AAC.00742-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, et al. Assessing subunit dependency of the Plasmodium proteasome using small molecule inhibitors and active site probes. ACS Chem Biol. 2014;9:1869–76. doi: 10.1021/cb5001263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, et al. Identification of potent and selective non-covalent inhibitors of the Plasmodium falciparum proteasome. J Am Chem Soc. 2014;136:13562–5. doi: 10.1021/ja507692y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, et al. Structure- and function-based design of Plasmodium-selective proteasome inhibitors. Nature. 2016;530:233–6. doi: 10.1038/nature16936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finley D. Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem. 2009;78:477–513. doi: 10.1146/annurev.biochem.78.081507.101607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finley D, et al. Gates, channels, and switches: elements of the proteasome machine. Trends Biochem Sci. 2016;41:77–93. doi: 10.1016/j.tibs.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Striebel F, et al. Controlled destruction: AAA+ ATPases in protein degradation from bacteria to eukaryotes. Curr Opin Struct Biol. 2009;19:209–17. doi: 10.1016/j.sbi.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Robert A, et al. The antimalarial drug artemisinin alkylates heme in infected mice. Proc Natl Acad Sci U S A. 2005;102:13676–80. doi: 10.1073/pnas.0500972102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jourdan J, et al. Monoclonal antibodies that recognize the alkylation signature of antimalarial ozonides OZ277 (arterolane) and OZ439 (artefenomel) ACS Infect Dis. 2016;2:54–61. doi: 10.1021/acsinfecdis.5b00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ismail HM, et al. A click chemistry-based proteomic approach reveals that 1,2,4-trioxolane and artemisinin antimalarials share a common protein alkylation profile. Angew Chem Int Ed Engl. 2016;55:6401–5. doi: 10.1002/anie.201512062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dogovski C, et al. Targeting the cell stress response of Plasmodium falciparum to overcome artemisinin resistance. PLoS Biol. 2015;13:e1002132. doi: 10.1371/journal.pbio.1002132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mok S, et al. Population transcriptomics of human malaria parasites reveals the mechanism of artemisinin resistance. Science. 2015;347:431–5. doi: 10.1126/science.1260403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ariey F, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–5. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canning P, et al. Structural basis for Cul3 protein assembly with the BTB-Kelch family of E3 ubiquitin ligases. J Biol Chem. 2013;288:7803–14. doi: 10.1074/jbc.M112.437996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu CC, et al. Cul3-KLHL20 ubiquitin ligase governs the turnover of ULK1 and VPS34 complexes to control autophagy termination. Mol Cell. 2016;61:84–97. doi: 10.1016/j.molcel.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Prasad R, et al. Blocking Plasmodium falciparum development via dual inhibition of hemoglobin degradation and the ubiquitin proteasome system by MG132. PLoS One. 2013;8:e73530. doi: 10.1371/journal.pone.0073530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, et al. Characterization of the 26S proteasome network in Plasmodium falciparum. Sci Rep. 2015;5:17818. doi: 10.1038/srep17818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Roch KG, et al. Discovery of gene function by expression profiling of the malaria parasite life cycle. Science. 2003;301:1503–8. doi: 10.1126/science.1087025. [DOI] [PubMed] [Google Scholar]
- 33.Lopez-Barragan MJ, et al. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genomics. 2011;12:587. doi: 10.1186/1471-2164-12-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lasonder E, et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res. 2016;44:6087–101. doi: 10.1093/nar/gkw536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomko RJ, Jr, Hochstrasser M. Molecular architecture and assembly of the eukaryotic proteasome. Annu Rev Biochem. 2013;82:415–45. doi: 10.1146/annurev-biochem-060410-150257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Groll M, Huber R. Inhibitors of the eukaryotic 20S proteasome core particle: a structural approach. Biochim Biophys Acta. 2004;1695:33–44. doi: 10.1016/j.bbamcr.2004.09.025. [DOI] [PubMed] [Google Scholar]
- 37.Groll M, et al. A gated channel into the proteasome core particle. Nat Struct Biol. 2000;7:1062–7. doi: 10.1038/80992. [DOI] [PubMed] [Google Scholar]
- 38.Bozdech Z, et al. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003;1:E5. doi: 10.1371/journal.pbio.0000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horrocks P, Newbold CI. Intraerythrocytic polyubiquitin expression in Plasmodium falciparum is subjected to developmental and heat-shock control. Mol Biochem Parasitol. 2000;105:115–25. doi: 10.1016/s0166-6851(99)00174-7. [DOI] [PubMed] [Google Scholar]
- 40.Ozkaynak E, et al. The yeast ubiquitin genes: a family of natural gene fusions. EMBO J. 1987;6:1429–39. doi: 10.1002/j.1460-2075.1987.tb02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ponts N, et al. Deciphering the ubiquitin-mediated pathway in apicomplexan parasites: a potential strategy to interfere with parasite virulence. PLoS One. 2008;3:e2386. doi: 10.1371/journal.pone.0002386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ponts N, et al. Unraveling the ubiquitome of the human malaria parasite. J Biol Chem. 2011;286:40320–30. doi: 10.1074/jbc.M111.238790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–79. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 44.Koegl M, et al. A novel ubiquitination factor, E4, is involved in multiubiquitin chain assembly. Cell. 1999;96:635–44. doi: 10.1016/s0092-8674(00)80574-7. [DOI] [PubMed] [Google Scholar]
- 45.Aminake MN, et al. The proteasome of malaria parasites: A multi-stage drug target for chemotherapeutic intervention? Int J Parasitol Drugs Drug Resist. 2012;2:1–10. doi: 10.1016/j.ijpddr.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chung DW, et al. Characterization of the ubiquitylating components of the human malaria parasite’s protein degradation pathway. PLoS One. 2012;7:e43477. doi: 10.1371/journal.pone.0043477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yau R, Rape M. The increasing complexity of the ubiquitin code. Nat Cell Biol. 2016;18:579–86. doi: 10.1038/ncb3358. [DOI] [PubMed] [Google Scholar]
- 48.Xu P, et al. Quantitative proteomics reveals the function of unconventional ubiquitin chains in proteasomal degradation. Cell. 2009;137:133–45. doi: 10.1016/j.cell.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ito T, Handa H. Cereblon and its downstream substrates as molecular targets of immunomodulatory drugs. Int J Hematol. 2016;104:293–9. doi: 10.1007/s12185-016-2073-4. [DOI] [PubMed] [Google Scholar]
- 50.Kojima K, et al. Pharmacological activation of wild-type p53 in the therapy of leukemia. Exp Hematol. 2016;44:791–8. doi: 10.1016/j.exphem.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reyes-Turcu FE, et al. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–97. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee BH, et al. USP14 deubiquitinates proteasome-bound substrates that are ubiquitinated at multiple sites. Nature. 2016;532:398–401. doi: 10.1038/nature17433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aufderheide A, et al. Structural characterization of the interaction of Ubp6 with the 26S proteasome. Proc Natl Acad Sci U S A. 2015;112:8626–31. doi: 10.1073/pnas.1510449112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bashore C, et al. Ubp6 deubiquitinase controls conformational dynamics and substrate degradation of the 26S proteasome. Nat Struct Mol Biol. 2015;22:712–9. doi: 10.1038/nsmb.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang X, et al. The proteasome deubiquitinase inhibitor VLX1570 shows selectivity for ubiquitin-specific protease-14 and induces apoptosis of multiple myeloma cells. Sci Rep. 2016;6:26979. doi: 10.1038/srep26979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hewings DS, et al. Activity-based probes for the ubiquitin conjugation-deconjugation machinery: new chemistries, new tools, and new insights. FEBS J. 2017 doi: 10.1111/febs.14039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez C, et al. Discovery of an Inhibitor of the Proteasome Subunit Rpn11. J Med Chem. 2017;60:1343–1361. doi: 10.1021/acs.jmedchem.6b01379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li J, et al. Capzimin is a potent and specific inhibitor of proteasome isopeptidase Rpn11. Nat Chem Biol. 2017;13:486–493. doi: 10.1038/nchembio.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lauinger L, et al. Thiolutin is a zinc chelator that inhibits the Rpn11 and other JAMM metalloproteases. Nat Chem Biol. 2017 doi: 10.1038/nchembio.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song Y, et al. Targeting proteasome ubiquitin receptor Rpn13 in multiple myeloma. Leukemia. 2016;30:1877–86. doi: 10.1038/leu.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Husnjak K, et al. Proteasome subunit Rpn13 is a novel ubiquitin receptor. Nature. 2008;453:481–8. doi: 10.1038/nature06926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiao L, et al. Mechanism of the Rpn13-induced activation of Uch37. Protein Cell. 2014;5:616–30. doi: 10.1007/s13238-014-0046-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.VanderLinden RT, et al. Structural basis for the activation and inhibition of the UCH37 deubiquitylase. Mol Cell. 2015;57:901–11. doi: 10.1016/j.molcel.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Randles L, et al. The Proteasome Ubiquitin Receptor hRpn13 and Its Interacting Deubiquitinating Enzyme Uch37 Are Required for Proper Cell Cycle Progression. J Biol Chem. 2016;291:8773–83. doi: 10.1074/jbc.M115.694588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Artavanis-Tsakonas K, et al. Identification by functional proteomics of a deubiquitinating/deNeddylating enzyme in Plasmodium falciparum. Mol Microbiol. 2006;61:1187–95. doi: 10.1111/j.1365-2958.2006.05307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ghorbal M, et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR-Cas9 system. Nat Biotechnol. 2014;32:819–21. doi: 10.1038/nbt.2925. [DOI] [PubMed] [Google Scholar]
- 67.Straimer J, et al. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science. 2015;347:428–31. doi: 10.1126/science.1260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Farrell F, et al. Phosphoinositide 3-kinases as accelerators and brakes of autophagy. FEBS J. 2013;280:6322–37. doi: 10.1111/febs.12486. [DOI] [PubMed] [Google Scholar]
- 69.Proikas-Cezanne T, et al. WIPI proteins: essential PtdIns3P effectors at the nascent autophagosome. J Cell Sci. 2015;128:207–17. doi: 10.1242/jcs.146258. [DOI] [PubMed] [Google Scholar]
- 70.Mbengue A, et al. A molecular mechanism of artemisinin resistance in Plasmodium falciparum malaria. Nature. 2015;520:683–7. doi: 10.1038/nature14412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cervantes S, et al. The multifunctional autophagy pathway in the human malaria parasite, Plasmodium falciparum. Autophagy. 2014;10:80–92. doi: 10.4161/auto.26743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Navale R, et al. Characterization of the autophagy marker protein Atg8 reveals atypical features of autophagy in Plasmodium falciparum. PLoS One. 2014;9:e113220. doi: 10.1371/journal.pone.0113220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Barth S, et al. Autophagy: assays and artifacts. J Pathol. 2010;221:117–24. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hunt P, et al. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol Microbiol. 2007;65:27–40. doi: 10.1111/j.1365-2958.2007.05753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hunt P, et al. Experimental evolution, genetic analysis and genome re-sequencing reveal the mutation conferring artemisinin resistance in an isogenic lineage of malaria parasites. BMC Genomics. 2010;11:499. doi: 10.1186/1471-2164-11-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borrmann S, et al. Genome-wide screen identifies new candidate genes associated with artemisinin susceptibility in Plasmodium falciparum in Kenya. Sci Rep. 2013;3:3318. doi: 10.1038/srep03318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmitz C, et al. The deubiquitinating enzyme Ubp1 affects sorting of the ATP-binding cassette-transporter Ste6 in the endocytic pathway. Mol Biol Cell. 2005;16:1319–29. doi: 10.1091/mbc.E04-05-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCann AP, et al. Deubiquitylating enzymes in receptor endocytosis and trafficking. Biochem J. 2016;473:4507–4525. doi: 10.1042/BCJ20160826. [DOI] [PubMed] [Google Scholar]
- 79.Henriques G, et al. Artemisinin resistance in rodent malaria--mutation in the AP2 adaptor mu-chain suggests involvement of endocytosis and membrane protein trafficking. Malar J. 2013;12:118. doi: 10.1186/1475-2875-12-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol. 2011;12:517–33. doi: 10.1038/nrm3151. [DOI] [PubMed] [Google Scholar]
- 81.Henriques G, et al. Directional selection at the pfmdr1, pfcrt, pfubp1, and pfap2mu loci of Plasmodium falciparum in Kenyan children treated with ACT. J Infect Dis. 2014;210:2001–8. doi: 10.1093/infdis/jiu358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Henriques G, et al. The Mu subunit of Plasmodium falciparum clathrin-associated adaptor protein 2 modulates in vitro parasite response to artemisinin and quinine. Antimicrob Agents Chemother. 2015;59:2540–7. doi: 10.1128/AAC.04067-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sanchez CP, et al. A HECT ubiquitin-protein ligase as a novel candidate gene for altered quinine and quinidine responses in Plasmodium falciparum. PLoS Genet. 2014;10:e1004382. doi: 10.1371/journal.pgen.1004382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mordmuller B, et al. Plasmodia express two threonine-peptidase complexes during asexual development. Mol Biochem Parasitol. 2006;148:79–85. doi: 10.1016/j.molbiopara.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 85.Rathore S, et al. Disruption of a mitochondrial protease machinery in Plasmodium falciparum is an intrinsic signal for parasite cell death. Cell Death Dis. 2011;2:e231. doi: 10.1038/cddis.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramasamy G, et al. Characterization and localization of Plasmodium falciparum homolog of prokaryotic ClpQ/HslV protease. Mol Biochem Parasitol. 2007;152:139–48. doi: 10.1016/j.molbiopara.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 87.Tschan S, et al. Mitochondrial localization of the threonine peptidase PfHslV, a ClpQ ortholog in Plasmodium falciparum. Int J Parasitol. 2010;40:1517–23. doi: 10.1016/j.ijpara.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 88.Bochtler M, et al. The structures of HsIU and the ATP-dependent protease HsIU-HsIV. Nature. 2000;403:800–5. doi: 10.1038/35001629. [DOI] [PubMed] [Google Scholar]
- 89.Subramaniam S, et al. Molecular modeling studies of the interaction between Plasmodium falciparum HslU and HslV subunits. J Biomol Struct Dyn. 2009;26:473–9. doi: 10.1080/07391102.2009.10507262. [DOI] [PubMed] [Google Scholar]
- 90.Rathore S, et al. A cyanobacterial serine protease of Plasmodium falciparum is targeted to the apicoplast and plays an important role in its growth and development. Mol Microbiol. 2010;77:873–90. doi: 10.1111/j.1365-2958.2010.07251.x. [DOI] [PubMed] [Google Scholar]
- 91.El Bakkouri M, et al. The Clp chaperones and proteases of the human malaria parasite Plasmodium falciparum. J Mol Biol. 2010;404:456–77. doi: 10.1016/j.jmb.2010.09.051. [DOI] [PubMed] [Google Scholar]
- 92.Ralph SA, et al. Tropical infectious diseases: metabolic maps and functions of the Plasmodium falciparum apicoplast. Nat Rev Microbiol. 2004;2:203–16. doi: 10.1038/nrmicro843. [DOI] [PubMed] [Google Scholar]
- 93.Goodman CD, et al. The effects of anti-bacterials on the malaria parasite Plasmodium falciparum. Mol Biochem Parasitol. 2007;152:181–91. doi: 10.1016/j.molbiopara.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Lin W, et al. Atypical caseinolytic protease homolog from Plasmodium falciparum possesses unusual substrate preference and a functional nuclear localization signal. Parasitol Res. 2009;105:1715–22. doi: 10.1007/s00436-009-1612-9. [DOI] [PubMed] [Google Scholar]
- 95.Fichera ME, et al. In vitro assays elucidate peculiar kinetics of clindamycin action against Toxoplasma gondii. Antimicrob Agents Chemother. 1995;39:1530–7. doi: 10.1128/aac.39.7.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fichera ME, Roos DS. A plastid organelle as a drug target in apicomplexan parasites. Nature. 1997;390:407–9. doi: 10.1038/37132. [DOI] [PubMed] [Google Scholar]
- 97.He CY, et al. A plastid segregation defect in the protozoan parasite Toxoplasma gondii. EMBO J. 2001;20:330–9. doi: 10.1093/emboj/20.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brotz-Oesterhelt H, et al. Dysregulation of bacterial proteolytic machinery by a new class of antibiotics. Nat Med. 2005;11:1082–7. doi: 10.1038/nm1306. [DOI] [PubMed] [Google Scholar]
- 99.Conlon BP, et al. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature. 2013;503:365–70. doi: 10.1038/nature12790. [DOI] [PMC free article] [PubMed] [Google Scholar]