Abstract
Context
Low interobserver diagnostic agreement exists among high-grade endometrial carcinomas.
Objective
Evaluate diagnostic variability in FIGO grade 3 endometrioid adenocarcinoma (G3EC) in two different sign-out practices.
Design
Sixty-six G3EC cases were identified from pathology archives of Wayne State University, Detroit, MI (WSU) (general surgical pathology sign-out) and 65 from Memorial Sloan Kettering Cancer Center, New york, NY (MSK) (gynecologic pathology focused sign-out). Each case was reviewed together by two gynecologic pathologists, one from each institution, and classified into G3EC group or reclassified group. Clinicopathological parameters were compared.
Results
Twenty-five (38%) WSU cases were reclassified as undifferentiated (2), serous (4), mixed endometrioid and serous (12) carcinomas, and FIGO grade 2 endometrioid (G2EC) with focal marked nuclear atypia (7). Eleven (17 %) MSK cases were reclassified as undifferentiated (5), serous (1), mixed endometrioid and serous (4), mixed endometrioid and clear cell (1) carcinomas. Agreement rate between original and review diagnosis was 83% (54 of 65) at MSK and 62% (41 of 66) at WSU (P= .01) with overall rate of 73% (95 of 131). Undifferentiated carcinomas were higher at MSK than WSU (46 %, 5 of 11 vs. 8%, 2 of 25; P= .02). G2EC with focal marked nuclear atypia was higher in WSU (28%, 7 of 25) than MSK (0%) (P= .03). Mixed endometrioid and serous carcinoma was the most common misclassified subtype (44%, 16 of 36).
Conclusions
Moderate interobserver variability exists in the diagnosis of G3EC with significantly higher diagnostic agreement rate in gynecologic pathology focused sign-out than general sign-out practice.
INTRODUCTION
More than three decades ago, Bokhman identified two pathogenetic types of endometrial carcinomas (EC) based on clinicopathological features.1 Bokhman’s work served as the basis of broadly classifying these carcinomas as Type I and II endometrial carcinomas. Type I tumors are associated with unopposed estrogenic stimulation and endometrial hyperplasia and are seen in pre-, peri-, and postmenopausal women. Type II tumors are not estrogen driven and develop in the atrophic endometrium of postmenopausal women. The histological subtype correlates of Type I include endometrioid and mucinous adenocarcinomas, of which the prototype endometrioid carcinoma accounts for approximately 80% of endometrial carcinomas. Type II tumors include high-grade tumors such as serous carcinoma and carcinosarcoma.
Most women with endometrial carcinoma have Type I tumors, which include International Federation of Gynecology and Obstetrics (FIGO) grade 1 and 2 endometrioid or mixed endometrioid and mucinous carcinomas. These low-grade carcinomas are usually diagnosed at an early stage and are associated with favorable prognosis. In most cases, hysterectomy alone is the cure. Although these low-grade tumors, FIGO grade 1 and 2 endometrioid carcinomas, form the bulk of EC, it is the high-grade tumors including FIGO 3 endometrioid carcinoma (G3EC), serous carcinoma (SC), and clear cell carcinoma (CC) that account for a disproportionate number of deaths.2 While it may be relatively straightforward to distinguish high-grade tumors from low-grade tumors on the basis of morphology, differentiating high-grade tumors from each other can be challenging at times. While some authors describe a similar poor clinical outcome among all high-grade tumors,3–5 others report a significantly poorer prognosis for SC and CC compared to G3EC.2 Most recently, molecular characterization of endometrial carcinomas by The Cancer Genome Atlas Research Network (TCGA)6 found that about 25% of endometrial carcinomas classified as G3EC had a molecular profile similar to SC.
The foundation of all of the above-mentioned studies is accurate morphological classification and FIGO grading of various subtypes; such classification has prognostic value and influences clinical management.7–11 Furthermore, overlapping features among high-grade carcinomas warrant precise classification for diagnostic and research purposes. Endometrial carcinomas are graded using the 3-tiered 1988 FIGO grading system based on architecture, extent of non-squamous solid growth, and nuclear atypia.12 The semi-quantitative and qualitative nature of definitions and criteria make this a rather subjective system that often leads to interobserver variability and reproducibility issues in grading. Identifying and quantifying a non-squamous solid growth and assessing ‘notable’ nuclear atypia, which would indicate that it belongs in the next higher grade, are the most challenging areas to replicate. The moderate levels of interobserver agreement in FIGO grading of endometrial carcinoma have led to suggestions of a variety of binary grading systems, which have shown superior agreement strengths and similar or better prognostic value.13–16 Like grading, tumor subtype classification, especially of high-grade endometrial carcinomas, also presents diagnostic reproducibility challenges due to the subjectivity of interpretation of standard definitions and inherent morphological ambiguity. Studies have shown a moderate to poor level of agreement in diagnosing high-grade subtypes.7–9
We set out to examine interobserver variability in the diagnosis of G3EC and also to determine whether the setting of gynecologic (GYN) pathology focused sign-out and general sign-out practice has any impact on this variability. To do so, we compared rates of diagnostic reclassification given as initial diagnosis of G3EC in a general practice setting with rates in a sub-specialized practice setting. We also wanted to determine whether the sub-specialized group more reproducibly differentiated between types of high-grade endometrial carcinomas due to the fact that it has a GYN focused sign-out practice. We utilized cases diagnosed as G3EC as this is the more common high-grade endometrial carcinoma. Furthermore, its appropriate place in the traditional endometrial carcinoma classification has been questioned given the recent recognition that a variety of high-grade endometrial carcinomas may demonstrate either solid architecture or papillary or glandular architecture with diffusely distributed high nuclear grade.17,18
MATERIAL AND METHODS
Following Institutional Review Board approval for a retrospective study design, we identified 131 cases diagnosed as G3EC from two institutions. Of these, 66 cases (50.3%) were from the Wayne State University Pathology department in Detroit, MI (WSU), a general surgical pathology sign-out institution, from 2000 to 2012 and 65 cases (49.6%) were from Memorial Sloan Kettering Cancer Center, New York, NY (MSK), a subspecialty focused sign-out institution, from 2000 to 2009. The cases were retrieved through pathology database search at each institution. Clinical and pathological data were retrieved from patient medical records and surgical pathological reports. Variables included age; date of surgery for primary tumor removal; pathological stage; depth of myometrial invasion; cervical invasion; lymphovascular invasion; involvement of fallopian tube, ovary, omentum, and lymph node; date of last follow-up; and adjuvant therapy. Pathological TNM classification and staging was assigned to all cases based on the 2008 FIGO staging.10
Two experienced GYN pathologists (R.S. and R.A.F), one from each institution jointly reviewed an average of four hematoxylin eosin slides from each case. Immunohistochemistry was not utilized for review.
All cases were categorized into two groups based on morphology. The first group was composed of G3EC where the reviewers agreed with the original diagnosis (non-reclassified cases) (Figure 1). The second group comprised cases that the reviewers reclassified as other histologic subtypes of endometrial carcinoma or a different grade of endometrioid carcinoma including undifferentiated carcinoma (Figure 2), serous carcinoma (Figure 3A–3D), mixed endometrioid and serous carcinoma (Figure 4A, 4B), mixed endometrioid and clear cell carcinoma, and, lastly, G2EC with focal marked nuclear atypia (reclassified cases) (Figure 5A, 5B). The histology subtype classification was based on World Health Organization (WHO) criteria,11 according to which endometrioid adenocarcinoma is composed of a varying proportion of glandular structures resembling normal endometrium with a spectrum of nuclear atypia; higher histologic grade tumors have less glandular differentiation and more solid sheets of cells with moderate to severe atypia. According to the FIGO grading system,12 grade 3 tumors have >50% non-squamous solid growth pattern and marked nuclear pleomorphism with prominent nucleoli and vesicular chromatin. Grade 1 and 2 tumors have </= 5% and 6–50% non-squamous growth pattern, respectively, with the potential for upgrade in the presence of ‘notable’ nuclear atypia, which does not have clearly defined criteria. For the purpose of our study, if ‘notable’ nuclear atypia was present and was seen in an arbitrarily set proportion of <10% of an endometrioid tumor, then reviewers classified the case as G2EC with focal marked nuclear atypia. Nuclear atypia was defined as ‘grade 3 nuclei’ in the presence of pleomorphic large nuclei with irregular nucleoli and coarse chromatin.19
Figure 1.
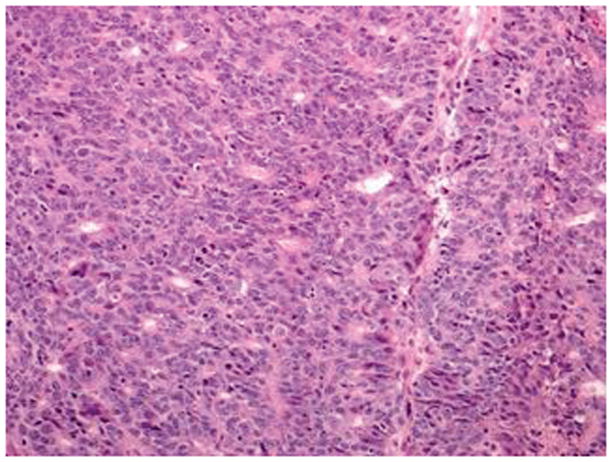
FIGO grade 3 endometrioid carcinoma has >50 % solid architecture with focal gland formation and moderate nuclear atypia (hematoxylin-eosin, original magnification ×20)
Figure 2.
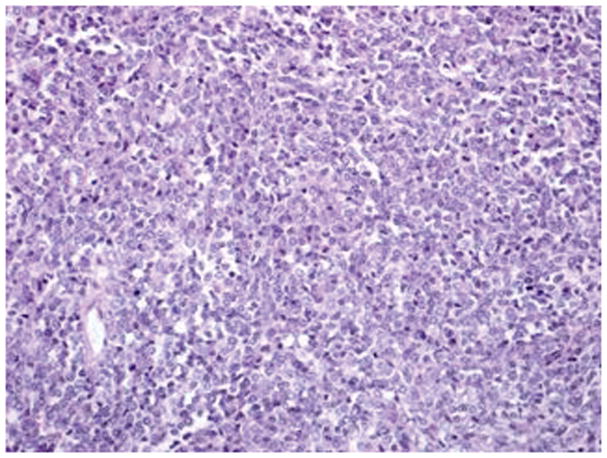
Undifferentiated carcinoma has monotonous proliferation of medium sized cells in solid and diffuse pattern (hematoxylin-eosin, original magnification ×20)
Figure 3.
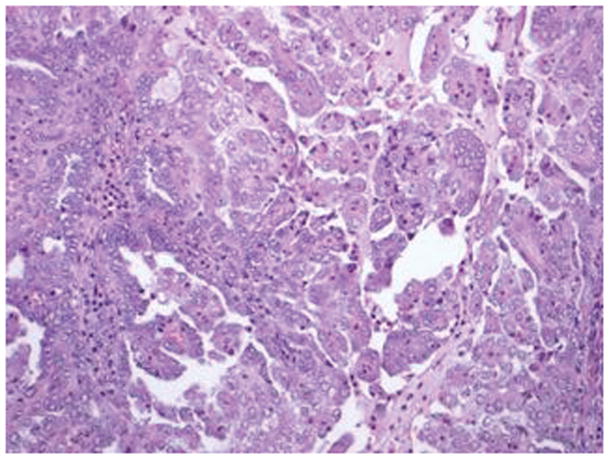
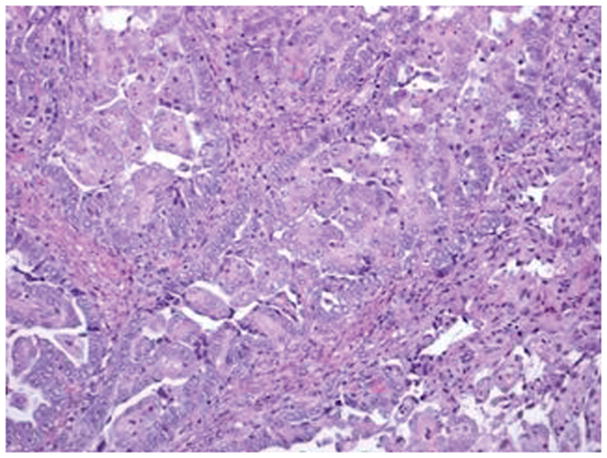
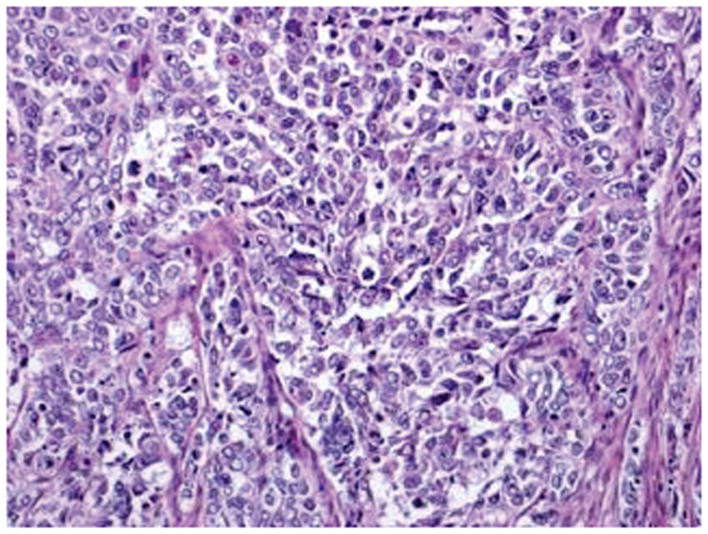
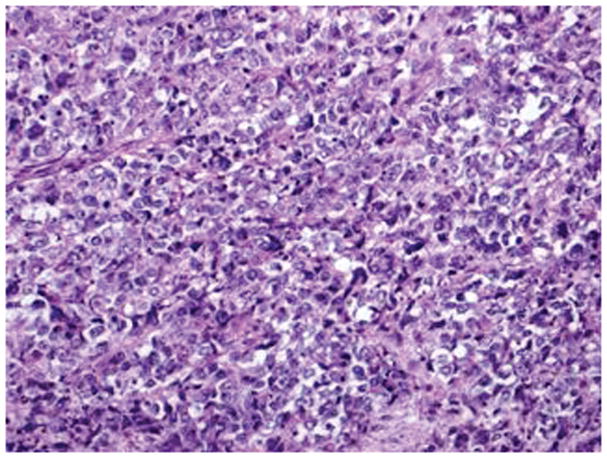
Serous carcinoma features a papillary component with slit like spaces (A, B) and/or a solid component (C, D) with high nuclear grade. Pleomorphic nuclei with prominent nucleoli, hyperchromatic nuclei with smudged chromatin and increased mitotic activity are readily observed (hematoxylin-eosin, original magnifications ×20 [A, B, C and D]
Figure 4.
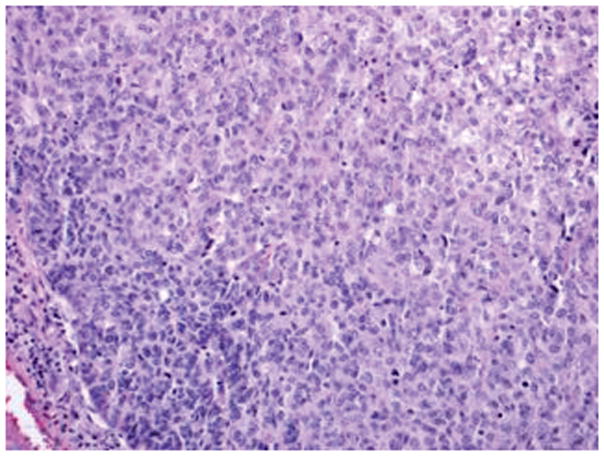
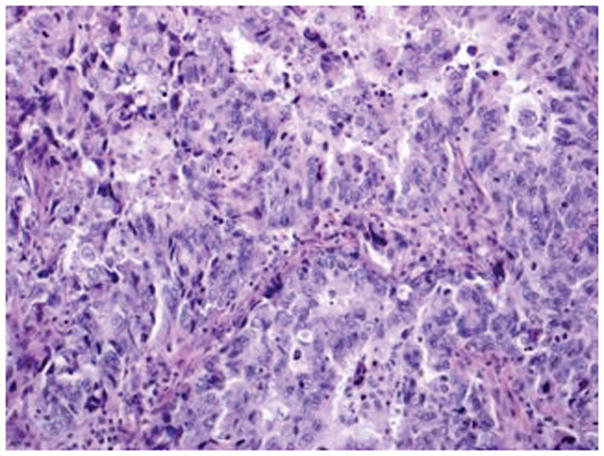
Mixed epithelial carcinoma: Areas of high-grade endometrioid carcinoma showing predominantly solid growth, focal evidence of gland formation and moderate nuclear atypia (A). Other areas in the same case have serous carcinoma (B) with papillary/glandular architecture, high nuclear grade, increased mitosis and necrotic luminal debris (hematoxylin-eosin, original magnifications ×20 [A, B]).
Figure 5.
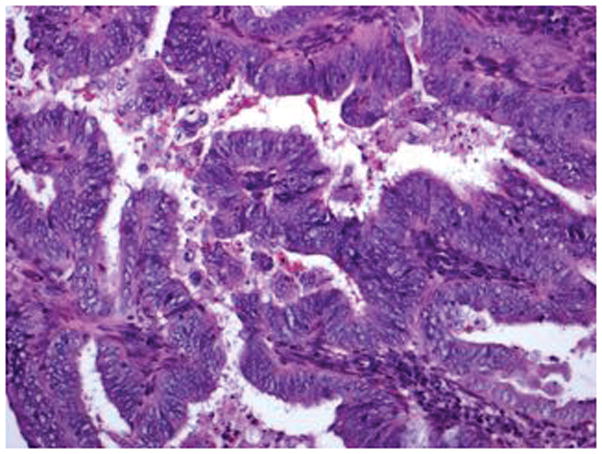
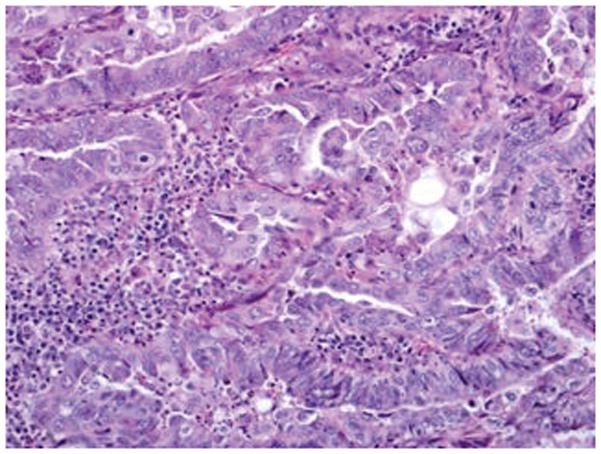
FIGO grade 2 endometrioid carcinoma with focal nuclear atypia: Area of endometrioid carcinoma showing low nuclear grade with oval nuclei and inconspicuous nucleoli (A) and foci in the same case showing higher nuclear grade with rounded nuclei, open chromatin, and distinct nucleoli (B) (hematoxylin-eosin, original magnifications ×20 [A, B]).
The agreement rate was defined as the concordance of an originally rendered diagnosis with the review diagnosis, which was calculated for each institution. Various clinicopathological parameters as mentioned above were compared overall and within each institution to determine any significant differences. Statistical tools utilized to analyze variances and associations of clinicopathological parameters included T-test, Fisher exact test, and Chi-square tests. A P-value of less than .05 was considered statistically significant. All tests were performed using IBM SPSS Statistics 19.0 for Windows (IBM Corp., Armonk, NY, 2010).
RESULTS
A total of 131 cases with a diagnosis of G3EC were included in the study. The 66 cases from WSU had a median patient age of 61 years and a median follow-up period of 39 months (range, 0.7–212 months). The 65 cases from MSK had a median patient age of 62 years and a median follow-up period of 55 months (range, 3.3–130 months). Table 1 compares the clinicopathological parameters of WSU and MSK cohorts. A significant difference was noted in tumor size, adnexal involvement, omentectomy and, lastly, choice of adjuvant treatment. The WSU cohort presented with larger tumor size than the MSK cohort (P< .001). The WSU cohort had a significantly higher proportion of cases with adnexal involvement (15%, 10 of 66 vs. 3%, 2 of 65; P= .03) and had more cases with omentum sampling (50%, 33 of 66 vs. 26%, 17 of 65 P= .01). Administration of adjuvant therapy, specifically radiation therapy, differed between institutions wherein more patients at MSK received radiation therapy than at WSU (62%, 40 of 65 vs. 40%, 26 of 66; P< .02).
Table 1.
Comparison of WSU and MSK cohorts.
| Total cases =131 | WSU 66 (50.3%) |
MSK 65 (49.6%) |
P value |
|---|---|---|---|
| Median age in years (range) | 61 (27–90) | 62 (33–90) | .92 |
| FIGO Stage | |||
| I | 39(59) | 46(70) | .31 |
| II | 6(9) | 3(5) | |
| III | 15(23) | 14(22) | |
| IV | 6(9) | 2(3) | |
| Reclassification status | |||
| Not reclassified | 41(62) | 54(83) | .01 |
| Reclassified histology subtype | 25(38) | 11(17) | |
| Undifferentiated carcinoma | 2 (8) | 5 (46) | .02 |
| Mixed endometrioid & serous carcinoma | 12 (48) | 4 (36) | >.99 |
| Mixed endometrioid & clear cell carcinoma | 0 (0) | 1 (9) | |
| FIGO 2 endometrioid with focal marked nuclear atypia | 7 (28) | 0 (0) | .03 |
| Serous carcinoma | 4(16) | 1 (9) | >.99 |
| Tumor size in cm (range)* | 5 (1–14) | 3 (0.3–11) | <. 001 |
| Myometrial invasion | |||
| Absent | 4 (6) | 8 (12) | .14 |
| Inner half | 27 (41) | 33 (51) | |
| Outer half | 35(53) | 24 (37) | |
| Lymphovascular invasion | |||
| Absent | 21 (32) | 28 (43) | .21 |
| Present | 45 (68) | 37 (57) | |
| Cervical stromal invasion | |||
| Absent | 49 (74) | 56 (86) | .12 |
| Present | 17 (26) | 9 (14) | |
| Adnexal involvement | |||
| Absent | 56 (85) | 63 (97) | .03 |
| Present | 10 (15) | 2 (3) | |
| Lymph node sampled | |||
| No | 11 (17) | 4 (6) | .10 |
| Yes | 55(83) | 61 (94) | |
| Lymph node involved** | |||
| No | 41(75) | 47(77) | .80 |
| Yes | 14(25) | 14(23) | |
| Omentum sampled | |||
| No | 33(50) | 48(74) | .01 |
| Yes | 33(50) | 17(26) | |
| Omentum involved** | |||
| No | 29(88) | 16(94) | .70 |
| Yes | 4(12) | 1(6) | |
| Adjuvant Therapy | |||
| None | 13 (20) | 7 (11) | .22 |
| Chemotherapy | 15 (22) | 8 (12) | .17 |
| Radiation | 26(40) | 40 (62) | .02 |
| Chemoradiation | 12(18) | 10 (15) | .82 |
| Recurrence | |||
| No | 50 (76) | 57 (88) | .11 |
| Yes | 16 (24) | 8 (12) |
Number in parentheses represents % except for median age and tumor size
missing data in 2 cases, information not available
subset of cases where sampling was performed
Wayne State University Pathology department in Detroit, MI (WSU)
Memorial Sloan Kettering Cancer Center, New York, NY (MSK)
Histology subtype reclassification and diagnostic agreement
Upon slide review of WSU cases, the two experts reclassified 38% of cases (25 of 66) into a different histology subtype, which included undifferentiated carcinoma (8%, 2 of 25), serous carcinoma (16%, 4 of 25), mixed endometrioid and serous carcinoma (48%, 12 of 25), and G2EC with focal marked nuclear atypia (28%, 7 of 25). Among the MSK cases, 17% (11of 65) were reclassified as undifferentiated carcinoma (45%, 5 of 11), serous carcinoma (9%, 1 of 11), mixed endometrioid and serous carcinoma (36%, 4 of 11), and mixed endometrioid and clear cell carcinoma (9%, 1 of 11). The diagnostic agreement in subtype classification was higher at MSK (83%, 54 of 65) than at WSU (62%, 41 of 66) and was statistically significant (P= .01). Both institutions had a high proportion of mixed serous and endometrioid carcinoma as a reclassified diagnosis (48%, 12 of 25 and 36%, 4 of 11). MSK had a significantly higher proportion of undifferentiated carcinoma than WSU (46 %, 5 of 11 vs. 8%, 2 of 25; P= .02). WSU had a high proportion of G2EC with focal marked nuclear atypia (28%, 7 of 25) while MSK did not have any (P= .03). Overall, mixed endometrioid and serous carcinoma was the most common subtype (44%, 16 of 36) (Table 1).
Clinicopathological parameters of reviewed histology: G3EC and reclassified subtypes
Patients with G3EC had a mean age of 62 years and follow-up period of 1 to 209 months (mean, 66 months). Most of the patients presented at stage I (64%, 60 of 95), followed by stage III (24%, 23 of 95). Ninety percent (86 of 95) had myometrial invasion, 67% (64 of 95) had lymphovascular invasion, and 20% (19 of 95) had cervical stromal invasion (Table 2). Disease recurrence was seen in 22 of 95 patients (23%); of these, 15 died. A total of 60 patients (63%, 60 of 95) died; stage I=39 (65%), stage II=3 (5%), stage III=14 (23%), and stage IV=4 (7%) (Table 3).
Table 2.
Clinicopathological features of various histology subtypes
| G3EC n =95 | Undifferentiated carcinoma n=7 | Mixed carcinoma n=17 | FIGO 2 with focal marked nuclear atypia n=7 | Serous carcinoma n=5 | ||
|---|---|---|---|---|---|---|
| Mean Age in years (range) | 61(27–90) | 57(42–69) | 64(49–80) | 58(50–65) | 64(55–68) | |
| FIGO Stage | I | 60 (64) | 3(42) | 13(77) | 6(86) | 3(60) |
| II | 6(6) | 0 | 1(6) | 0 | 2(40) | |
| III | 23(24) | 2(29) | 3(17) | 1(14) | 0 | |
| IV | 6(6) | 2(29) | 0 | 0 | 0 | |
| Tumor Size in cm (range)* | 4.3 (0.3–14) | 5.0 (0.4–11) | 4.4 (1.5–8) | 5.0 (3.5–8) | 2.8(0.4–6.5) | |
| Myometrial invasion | Absent | 9(10) | 1(14) | 1(6) | 0(0) | 1(20) |
| Inner half | 39(40) | 3(43) | 10(59) | 5(72) | 3(60) | |
| Outer half | 47(50) | 3(43) | 6(35) | 2(28) | 1(20) | |
| Lymphovascular invasion | Absent | 31 (33) | 4(57) | 8(47) | 4(57) | 3(60) |
| Present | 64(67) | 3(43) | 9(53) | 3(43) | 2(40) | |
| Cervix stromal invasion | Absent | 76(80) | 5(71) | 15(88) | 6(86) | 3(60) |
| Present | 19(20) | 2(29) | 2(12) | 1(14) | 2(40) | |
| Adnexal involvement | Absent | 87(92) | 4(57) | 16(94) | 7(100) | 5(100) |
| Present | 8(8) | 3(43) | 1(6) | 0 | 0 | |
| Lymph node sampled | No | 9(9) | 1(14) | 4(24) | 0 | 1(20) |
| Yes | 86(91) | 6(86) | 13(76) | 7(100) | 4(80) | |
| Lymph node involved** | No | 63(73) | 4(67) | 11(85) | 6(86) | 4(100) |
| Yes | 23(27) | 2(33) | 2(15) | 1(14) | 0 | |
| Omentum sampled | No | 73(77) | 2(29) | 14(82) | 2(72) | 1(20) |
| Yes | 22(23) | 5(71) | 3(18) | 5(28) | 4(80) | |
| Omentum involved** | No | 19(86) | 3(60) | 3(100) | 5(100) | 4(100) |
| Yes | 3(14) | 2(40) | 0 | 0 | 0 | |
| Adjuvant Therapy | None | 15(16) | 1(13) | 2(12) | 1(14) | 1(20) |
| Chemo | 18(19) | 2(29) | 3(17.5) | 0 | 0 | |
| Radiation | 47(49) | 2(29) | 9(53) | 5(72) | 3(60) | |
| Chemoradiation | 15(16) | 2(29) | 3(17.5) | 1(14) | 1(20) | |
| Recurrence | No | 73(77) | 7(100) | 16(88) | 7(100) | 4(80) |
| Yes | 22(23) | 0 | 1(12) | 0 | 1(20) | |
| Survival | Alive | 35(37) | 4(57) | 10(59) | 5(71) | 3(60) |
| Deceased | 60(63) | 3(43) | 7(41) | 2(29) | 2(40) | |
Number in parentheses represents % except for median age and tumor size
missing data in 2 cases, information not available
subset of cases where sampling was performed
Table 3.
Outcomes of histology subtypes by FIGO stage
| Histology subtype, No. | Deceased in each Stage, No. | Recurrence, No. | Deceased with recurrence, No. | |||
|---|---|---|---|---|---|---|
| I | II | III | IV | |||
| FIGO 3 endometrioid adenocarcinoma, 95 | 39 | 3 | 14 | 4 | 22 | 15 |
| Undifferentiated carcinoma, 7 | 0 | 0 | 1 | 2 | 0 | 0 |
| Mixed carcinoma, 17 | 5 | 0 | 2 | 0 | 1 | 1 |
| FIGO 2 endometrioid adenocarcinoma with focal marked nuclear atypia, 7 | 1 | 0 | 1 | 0 | 0 | 0 |
| Serous carcinoma, 5 | 1 | 1 | 0 | 0 | 1 | 1 |
| Total, 131 | 46 | 4 | 18 | 6 | 24 | 17 |
For the 7 patients with undifferentiated carcinomas, the mean age was 57 years and the follow-up period ranged from 1 to 111 months (mean, 50 months). Three (42%, 3 of 7) presented with stage I, 2 with stage III (29%, 2 of 7), and 2 (29%, 2 of 7) with stage IV disease. Eighty-six percent (6 of 7) had myometrial invasion. None of the patients had recurrent disease (Table 2). Of the 3 who died, 1 had stage III and 2 had stage IV disease (Table 3). Patients with mixed epithelial carcinomas had a mean age of 64 years and the follow-up period was 3 to 135 months (mean, 50 months). The majority presented with stage I disease (77%, 13 of 17) and had myometrial invasion (94%, 16 of 17). Only 2 of 17 (12%) had cervical stromal invasion and 1 of 16 (6%) had adnexal involvement (Table 2). A total of 7 (41%, 7 of 17) patients died (5 stage I, and 2 stage III). One patient had recurrent disease (stage I) and was among those who died (Table 3). The 7 patients with G2EC with focal marked nuclear atypia had a mean age of 58 years and a follow-up period of 30 to 210 months (mean, 82 months). Six patients (86%, 6 of 7) presented with stage I disease and one patient had stage III (14%, 1 of 7). All had myometrial invasion, none had adnexal involvement, and 14% (1 of 7) had cervical stromal invasion (Table 2). Of the 2 (29%, 2 of 7) patients who died, 1 was stage I and 1 was stage III. None had recurrent disease (Table 3). Among the 5 patients with SC, the mean age was 64 years and the follow-up period was 51 months (range, 21–86 months). All had early-stage disease: 3 (60%) stage I and 2 (40%) stage II. Four of five (80%) had myometrial invasion, one of which was deep invasion. Lymph node, omental, and adnexal involvement were absent in those sampled (Table 2). Two patients died (40%, 2 of 5), one of who had recurrent disease. None of the others experienced recurrence (Table 3).
DISCUSSION
Grading and subtype classification of endometrial carcinomas has prognostic and therapeutic relevance. Therefore, reproducibility of these systems is essential for consistent clinical care. Many studies have observed a moderate level of agreement among reviewers for FIGO grading.13,15 Poor interobserver reproducibility has also been observed in the diagnosis of high-grade endometrial carcinoma. An earlier study showed good levels of agreement in diagnosing endometrial carcinoma types, but pathologists without GYN pathology experience found it more difficult to diagnose serous and clear cell carcinomas.9 Gilks et al demonstrated only 62.5% agreement among three pathologists with special interest in GYN pathology when distinguishing between subtypes of high-grade endometrial carcinomas. There was a similar level of agreement between the reviewers (κ=0.57–0.68).7 Han et al reported similar interobserver agreement (κ=0.575) for high-grade endometrial carcinomas reviewed by 11 GYN pathologists.8 Studying the variability enables us to identify and address areas that should improve diagnostic agreement.
In our study, we examined the collective performance of two groups of pathologists, general and GYN focused, by comparing their original diagnoses with those of two experienced pathologist reviewers and assessing the concordance between them as a means of gauging variability in grading and histology subtyping. The diagnostic agreement was higher at MSK (83%, 54 of 65) compared to WSU (62%, 41 of 66) and the difference was statistically significant (P=. 01). In our study, the reviewing GYN pathologists disagreed with the original diagnosis of G3EC in 36 of 131 cases (28%). Most of the discrepant cases (81%, 29 of 36) were interpreted as another subtype of high-grade endometrial carcinoma (undifferentiated, serous, mixed serous and endometrioid or mixed clear cell and endometrioid). The rest was ‘downgraded’ from G3EC to G2EC with focal marked nuclear atypia (19%, 7 of 36).
Mixed serous and endometrioid carcinoma was the most commonly reclassified diagnosis in both institutions (44%, 16 of 36). According to the recent WHO classification16 of tumors of female reproductive organs, the entity of mixed epithelial carcinoma is defined as the presence of two or more different histological types of endometrial, at least one of which is from the type II category; each type must comprise at least 5% of the tumor. The mixed epithelial category is poorly understood and represents a heterogeneous group of tumors. Although it has been reported that even a 5% component of serous carcinoma in a mixed endometrial carcinoma imparts a worse prognosis in early-stage disease,20 fewer patients with a serous component (40%, 2 of 5) died compared to G3EC patients (63%, 60 of 95) in our study. A formal statistical comparison was not possible due to small numbers.
McConechy et al hypothesized that some mixed serous and endometrioid carcinomas may represent progression from low-grade endometrioid tumors21 and in some cases may retain the clinical characteristics of a low-grade tumor. Furthermore, a subset of cases with “mixed serous and endometrioid carcinomas morphology” has been described in association with POLE exonuclease domain mutations,22 a recently described genomic group by The Cancer Genome Atlas6 with favorable clinical outcomes. Finally, the term “mixed epithelial carcinoma” has in some cases been used to describe tumors with ambiguous features of both endometrioid and serous carcinomas; there are indications that many of these are instead microsatellite instability - high endometrioid carcinomas or those with POLE mutations.23
A notable number of undifferentiated carcinomas were diagnosed as G3EC (19%, 7 of 36), which has a predominant solid sheet-like growth pattern with high-grade nuclear, cellular dyshesion, and increased mitotic activity and can overlap morphologically with G3EC. Undifferentiated carcinoma is defined in the WHO11 as a malignant epithelial neoplasm with no differentiation. Recently authors have attempted to refine this definition as a tumor composed of medium to large size cells with complete absence of glandular differentiation and with absent or minimal (<10%) neuroendocrine differentiation.24, 25 Focal gland formation or nested and trabecular growth of cohesive cells favor a diagnosis of G3EC rather than undifferentiated carcinoma. However, when undifferentiated carcinoma is associated with a low-grade endometrioid carcinoma, as in dedifferentiated carcinoma,26 features such as well-demarcated areas of glandular differentiation with abrupt transition to sheet-like growth favor a de-differentiated endometrial carcinoma while seamless transition from glandular to solid areas and comparable cytology in the two areas favor a diagnosis of G3EC.25,27 Undifferentiated carcinoma shows variable positivity with cytokeratin and a more consistent labeling with EMA and CK18. In contrast, G3EC shows strong and diffuse staining patterns with cytokeratin and substantially more cellular cohesion.24,27
There was only one case in our series that was reclassified due to the presence of a clear cell component. Fadare et al demonstrated that there was a moderate level of interobserver variability in diagnosing endometrial carcinomas with clear cells based on morphology alone.28 Interobserver variability for a diagnosis of a pure clear cell carcinoma was least when features included papillary or tubulocystic architecture, clear cytoplasm, absence of nuclear pseudostratification, hyalinized papillary cores, and hyperchromatic nuclei, but the presence of an almost exclusively solid growth pattern was problematic.28 Such areas in a mixed epithelial carcinoma could mimic an endometrioid carcinoma with markedly glycogenated squamous differentiation or endometrioid carcinoma with clear cell features.29 In good examples of mixed endometrioid and clear cell carcinoma, the clear cell carcinoma areas typically have nuclear features distinct from the endometrioid components.
The FIGO grading system defines grade 3 carcinomas as having >50% non-squamous solid growth pattern. However, notable nuclear atypia in grade 1 or 2 tumors raises the grade by 1. The lack of defined criteria and subjectivity regarding what constitutes ‘notable nuclear atypia’ could explain lenient upgrading of otherwise grade 2 endometrioid carcinoma by general pathologists who had 11% (7 of 66) of their G3ECs downgraded to G2EC with focal marked nuclear atypia in this series. This was not encountered with the GYN focused pathologists. In considering an upgrade based on nuclear atypia, Zaino et al19 recommend assigning grade 3 nuclei to those with ‘nuclear atypia’ being present in a majority of the tumor cells. In our study, finding grade 3 nuclei in <10% downgraded the tumor from G3EC to a G2EC.
Irrespective of the institution or diagnosis, 65% (85 of 131) of patients presented with early-stage disease (stage I, II). MSK had a significantly higher rate of lymph node sampling (94%, 61 of 65) than WSU (83%, 55 of 66). In the subset that had lymphadenectomy, pelvic nodes were always sampled and para-aortic nodes were sampled with varying frequencies. Even with this differential practice, lymph node positivity was comparable between the 2 cohorts (23%, 14 of 61 and 25%, 14 of 55); this was expected since most patients in both institutions with positive lymph nodes (24%, 28 of 116) presented with tumor sizes >2cm (93%, 26 of 28), which is reportedly associated with pelvic nodal disease in high-grade endometrial cancers.30
Variability in management guidelines of G3EC within and between each institution is reflected in the proportion of patients receiving adjuvant therapy. Radiation therapy was the most common modality delivered in both institutions, but MSK had a higher proportion of patients treated with radiation. Among the histology subtypes, G3EC had a higher proportion of patients with lymphovascular invasion and recurrent disease. The proportion of patients who died was higher given a consensus diagnosis of GE3C, compared to tumors with a reclassified diagnosis. Some of the limitations we encountered were small sample and insufficient follow-up, which restricts the use of overall survival and disease-free interval parameters in evaluating the impact of reclassification on clinical outcome. Using immunohistochemistry in the reclassified cases would have added strength by supporting the expert interpretation.
In summary, our findings suggest that a moderate level of interobserver variability exists in the diagnosis of high-grade endometrial carcinomas and also that GYN focused pathologists had a significantly higher degree of concordance than general surgical pathologists in diagnosing G3EC. The discrepancies arose predominantly from morphological overlap of G3EC with other high-grade endometrial carcinomas and secondarily from assessment of the type and distribution of nuclear atypia sufficient to upgrade G2EC to G3EC. We conclude that better diagnostic agreement by the latter group of pathologists can be attributed to focused experience in GYN pathology, especially when there is frequent exposure to oncology specimens. We can only strive to maximize reproducibility and minimize variations through continued education, peer review, and consensus.
Acknowledgments
Funding: Dr. Soslow is supported in part by the MSK Cancer Center Support Grant P30 CA008748
References
- 1.Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15(1):10–17. doi: 10.1016/0090-8258(83)90111-7. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton CA, Cheung MK, Osann K, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006;94(5):642–646. doi: 10.1038/sj.bjc.6603012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voss MA, Ganesan R, Ludeman L, et al. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and pathological evaluation. Gynecol Oncol. 2012;124(1):15–20. doi: 10.1016/j.ygyno.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 4.Soslow RA, Bissonnette JP, Wilton A, et al. Clinicopathologic analysis of 187 high-grade endometrial carcinomas of different histologic subtypes: similar outcomes belie distinctive biologic differences. Am J Surg Pathol. 2007;31(7):979–987. doi: 10.1097/PAS.0b013e31802ee494. [DOI] [PubMed] [Google Scholar]
- 5.Creasman WT, Kohler MF, Odicino F, et al. Prognosis of papillary serous, clear cell, and grade 3 stage I carcinoma of the endometrium. Gynecol Oncol. 2004;95(3):593–596. doi: 10.1016/j.ygyno.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Research Network. Kandoth C, Schultz N, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol. 2013;37(6):874–881. doi: 10.1097/PAS.0b013e31827f576a. [DOI] [PubMed] [Google Scholar]
- 8.Han G, Sidhu D, Duggan MA, et al. Reproducibility of histological cell type in high-grade endometrial carcinoma. Mod Pathol. 2013;26(12):1594–1604. doi: 10.1038/modpathol.2013.102. [DOI] [PubMed] [Google Scholar]
- 9.Nedergaard L, Jacobsen M, Andersen JE. Interobserver agreement for tumour type, grade of differentiation and stage in endometrial carcinomas. APMIS. 1995;103(7–8):511–518. doi: 10.1111/j.1699-0463.1995.tb01399.x. [DOI] [PubMed] [Google Scholar]
- 10.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103–104. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 11.Kurman RJ, Carcangiu ML, Herrington S, Young RH. WHO Classification of Tumors of Female Reproductive Organs. 4. Lyon, France: IARC; 2014. [Google Scholar]
- 12.Announcements. Gynecol Oncol. 1989;35(1):125–127. [Google Scholar]
- 13.Lax SF, Kurman RJ, Pizer ES, et al. A binary architectural grading system for uterine endometrial endometrioid carcinoma has superior reproducibility compared with FIGO grading and identifies subsets of advance-stage tumors with favorable and unfavorable prognosis. Am J Surg Pathol. 2000;24(9):1201–1208. doi: 10.1097/00000478-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Scholten AN, Smit VT, Beerman H, et al. Prognostic significance and interobserver variability of histologic grading systems for endometrial carcinoma. Cancer. 2004;100(4):764–772. doi: 10.1002/cncr.20040. [DOI] [PubMed] [Google Scholar]
- 15.Alkushi A, Abdul-Rahman ZH, Lim P, et al. Description of a novel system for grading of endometrial carcinoma and comparison with existing grading systems. Am J Surg Pathol. 2005;29(3):295–304. doi: 10.1097/01.pas.0000152129.81363.d2. [DOI] [PubMed] [Google Scholar]
- 16.Conlon N, Leitao MM, Jr, Abu-Rustum NR, Soslow RA. Grading uterine endometrioid carcinoma: a proposal that binary is best. Am J Surg Pathol. 2014;38(12):1583–1587. doi: 10.1097/PAS.0000000000000327. [DOI] [PubMed] [Google Scholar]
- 17.Hoang LN, McConechy MK, Kobel M, et al. Histotype-genotype correlation in 36 high-grade endometrial carcinomas. Am J Surg Pathol. 2013 Sep;37:1421–1432. doi: 10.1097/PAS.0b013e31828c63ed. [DOI] [PubMed] [Google Scholar]
- 18.Hussein YR, Broaddus R, Weigelt B, Levine DA, Soslow RA. The Genomic Heterogeneity of FIGO Grade 3 Endometrioid Carcinoma Impacts Diagnostic Accuracy and Reproducibility. Int J Gynecol Pathol. 2015 Jul 9; doi: 10.1097/PGP.0000000000000212. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaino RJ, Kurman RJ, Diana KL, Morrow CP. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer. 1995;75(1):81–86. doi: 10.1002/1097-0142(19950101)75:1<81::aid-cncr2820750114>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 20.Quddus MR, Sung CJ, Zhang C, et al. Minor serous and clear cell components adversely affect prognosis in “mixed-type” endometrial carcinomas: a clinicopathologic study of 36 stage-I cases. Reprod Sci. 2010;17(7):673–678. doi: 10.1177/1933719110368433. [DOI] [PubMed] [Google Scholar]
- 21.McConechy MK, Ding J, Cheang MC, et al. Use of mutation profiles to refine the classification of endometrial carcinomas. J Pathol. 2012;228(1):20–30. doi: 10.1002/path.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussein YR, Weigelt B, Levine DA, et al. Clinicopathological analysis of endometrial carcinomas harboring somatic POLE exonuclease domain mutations. Mod Pathol. 2015;28(4):505–514. doi: 10.1038/modpathol.2014.143. [DOI] [PubMed] [Google Scholar]
- 23.Broaddus R, Hussein YR, Weigelt B, et al. FIGO grade 3 endometrioid carcinoma versus serous carcinoma: Morphologic mimicry involves only specific genomically defined subsets of endometrial carcinoma. Mod Pathol. 2014;27(Suppl 2):277A. [Google Scholar]
- 24.Silva EG, Deavers MT, Malpica A. Undifferentiated carcinoma of the endometrium: a review. Pathology. 2007;39(1):134–138. doi: 10.1080/00313020601159494. [DOI] [PubMed] [Google Scholar]
- 25.Altrabulsi B, Malpica A, Deavers MT, et al. Undifferentiated Carcinoma of the Endometrium. Am J Surg Pathol. 2005;29(10):1316–1321. doi: 10.1097/01.pas.0000171003.72352.9a. [DOI] [PubMed] [Google Scholar]
- 26.Silva EG, Deavers MT, Bodurka DC, Malpica A. Association of Low-Grade Endometrioid Carcinoma of the Uterus and Ovary With Undifferentiated Carcinoma:A New Type of Dedifferentiated Carcinoma? Int J Gynecol Pathol. 2006;25(1):52–58. doi: 10.1097/01.pgp.0000183048.22588.18. [DOI] [PubMed] [Google Scholar]
- 27.Al-Loh A, Maysa Al-Hussaini M. Undifferentiated Endometrial Carcinoma A Diagnosis Frequently Overlooked. Arch Pathol Lab Med. 2013;137(3):438–442. doi: 10.5858/arpa.2011-0461-RS. [DOI] [PubMed] [Google Scholar]
- 28.Fadare O, Parkash V, Dupont WD, et al. The diagnosis of endometrial carcinomas with clear cells by gynecologic pathologists: an assessment of interobserver variability and associated morphologic features. Am J Surg Pathol. 2012;36(8):1107–1118. doi: 10.1097/PAS.0b013e31825dd4b3. [DOI] [PubMed] [Google Scholar]
- 29.Silva EG, Young RH. Endometrioid neoplasms with clear cells: a report of 21 cases in which the alteration is not of typical secretory type. Am J Surg Pathol. 2007;31(8):1203–1208. doi: 10.1097/PAS.0b013e3180339ed7. [DOI] [PubMed] [Google Scholar]
- 30.Doll KM, Tseng J, Denslow SA, Fader AN, Gehrig PA. High-grade endometrial cancer: revisiting the impact of tumor size and location on outcomes. Gynecol Oncol. 2001;132(1):44–49. doi: 10.1016/j.ygyno.2013.10.023. [DOI] [PubMed] [Google Scholar]


