Abstract
Presently, evidence for the efficacy of medications for the treatment of juvenile fibromyalgia syndrome (JFMS) is limited. While there are medications approved by the US Food and Drug Administration (duloxetine, milnacipran and pregabalin) for adults with fibromyalgia syndrome, there are none for the treatment of JFMS. A variety of medications have been prescribed for the treatment of JFMS, including (but not limited to) non-opioid analgesics, opioids, anticonvulsants, antidepressants, and muscle relaxants. Psychological therapies, most prominently cognitive-behavioral therapy, are the most evidenced-based treatment modalities for JFMS. A multidisciplinary approach, combining pharmacological, behavioral and exercise-based modalities is currently the standard of care for JFMS. In the future, more stringent randomized, controlled trials with longer follow-up periods are needed in order to determine the long-term efficacy and safety of medications in the treatment of JFMS. Additionally, improved recognition of JFMS will allow for better patient recruitment to permit for adequately powered study designs.
1. Introduction
Juvenile fibromyalgia syndrome (JFMS) is a non-inflammatory musculoskeletal chronic pain condition with a high prevalence [1–4]. The lack of validated diagnostic criteria, evidence-based consensus guidelines and limited knowledge regarding the epidemiology and natural history of JFMS make treating these children challenging. Our purpose is to provide an update on the current treatments for the management of JFMS as well as to highlight recent advancements. Various studies have reported on the use of a variety of medications for the treatment of JFMS but there are few modern literature reviews of the treatment approaches for JFMS. We seek to provide an evidence-based, critical review of both the pharmacological and non-pharmacological management of chronic pediatric non-inflammatory musculoskeletal pain. Although there are no medications approved by the US Food and Drug Administration (FDA) for use in JFMS there are a plethora of medications that have been explored which we will summarize.
2. Background and Epidemiology
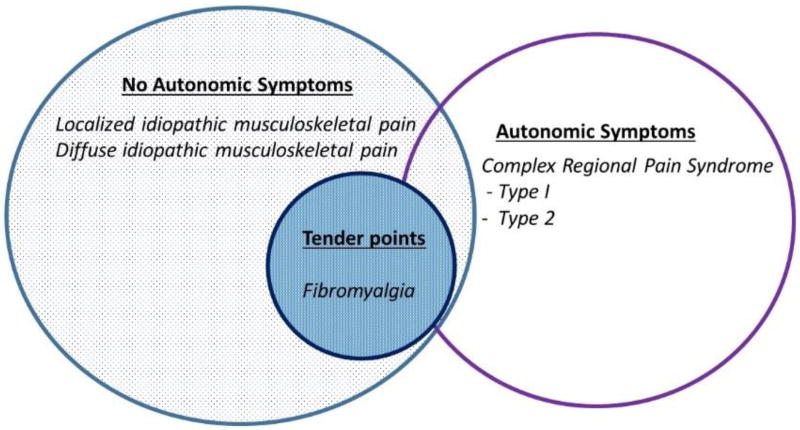
Chronic widespread pain is estimated to be common among children and young adults with prevalence rates ranging from 2–6% among school aged children [5–7]. There are a number of different chronic, non-inflammatory pain conditions in children and it is worth explaining the various terminology used. Amplified musculoskeletal pain syndrome (AMPS) is a descriptive term for syndromes involving central and/or peripheral sensory amplification that encompass the entire spectrum of manifestations of chronic pediatric musculoskeletal pain. The subsets of AMPS can be separated into those with overt autonomic signs (including complex regional pain syndrome types I and II); without autonomic signs (including diffuse idiopathic pain and localized amplified pain); or with tender points or widespread pain with multiple associated symptoms (fibromyalgia syndrome [FMS]) (Figure 1) [8].
Figure 1. Spectrum of Amplified Musculoskeletal Pain Syndromes.
Legend. There is significant overlap between the various pain manifestations. Along with pain, all patients are likely to have some degree of fatigue, muscle aches, sleep and mood disturbances along with a host of other possible somatic complaints (see 2010/2011 fibromyalgia criteria, Table 3).
Yunus et al first proposed criteria for FMS in adults in 1981 and in children in 1985 (Table 1) [9, 10]. While the Yunus & Masi criteria have not been validated, they are the only criteria created specifically for children and are standardly used in this patient population. Tables 2–4 outlines the various revisions of the American College of Rheumatology (ACR) criteria for FMS. Both the Yunus et al and ACR criteria of 1990 utilize tender point counts along with widespread pain lasting at least 3 months [11]. Criteria for tender point count in adults is satisfied if 11 of 18 sites are affected, whereas in children involvement of only 5 of 18 sites satisfies the criteria. Adult criteria were further redefined by the ACR in 2010/2011 in which trigger points are not counted but rather number of painful regions of the body as well as a severity score accounting for both severity and number of somatic complaints [12, 13]. These were validated in adolescent girls by Ting et al [14]. The adult criteria have been further revised to decrease the number of somatic symptoms needed [15].
Table 1.
Yunus & Masi Suggested Criteria for Childhood Fibromyalgia Syndrome [10]
| (All Four Major and Three Minor or the First Three Major and Four Painful Sites and Five Minor Needed) |
|---|
Major:
|
Minor
|
the original paper listed 31 tender points but after 1990 ACR criteria paper most people used the 18 tender points listed on Table 2.
Table 2.
The 1990 American College of Rheumatology (ACR) Criteria for Fibromyalgia Syndrome [11]
| 1. Widespread pain of at least 3 months* |
| 2. Pain and 11 of 18 tender points** (approximate 4 kg of pressure) |
| 3. The presence of a second diagnosis does not exclude a diagnosis of fibromyalgia |
Widespread pain diagnosed as pain neck, back, or chest along with pain and at least half of the body (left side, right side, above the waist, below the waist)
tender points include those at the occiput, lateral neck, trapezius, supraspinatus, second costochondral chondral junction, lateral epicondyles, gluteal fold, posterior greater trochanter, medial knee fat pad.
Table 4.
The 2016 Revision to the 2010/2011 American College of Rheumatology (ACR) Criteria for Fibromyalgia Syndrome [15]
| 1. Generalized pain, defined as pain in at least 4 of 5 regions*, is present. |
| 2. Symptoms have been present at a similar level for at least 3 months. |
| 3. Widespread pain index** (WPI) ≥7 and symptom severity scale score*** (SSS) score ≥ 5 or WPI 4-6 and SS scale ≥ 9 |
| 4. A diagnosis of fibromyalgia is valid irrespective of other diagnosis. A diagnosis of fibromyalgia does not exclude the presence of other clinically important illnesses. |
Generalized pain is ascertained by reported pain in any of the following body regions over the last week: left upper region (shoulder, upper arm or lower arm), right upper region (shoulder, upper arm or lower arm), left lower region (left buttocks, hip, upper leg or lower leg), right lower region (right buttocks, hip, upper leg or lower leg), axial region (neck, upper back and lower back) for a total possible score of 5. Jaw, chest and abdominal pain are not included in the generalized pain definition.
Widespread pain index calculated by adding number of body regions in pain within the last week including: jaw, shoulder, upper arm, lower arm, buttocks, upper leg, lower leg (2 points if bilateral) and single points for neck, chest, abdomen, upper back and lower back for a total possible score of 19.
Symptom severity scale score may be as high as 12 and is calculated by adding the sum of the self-reported severity scores for 3 core symptoms: fatigue, waking unrefreshed and thinking difficulty (0 = no problem, 1 = mild problem, 2 = moderate problem, 3 = severe problem for a total possible score of 9 and a symptom checklist score of 1 point each for headache, pain or cramps in the lower abdomen, and depression.
Notably, depending on the physical exam findings at the time of the medical appointment, a patient may or may not meet criteria for JFMS on that specific date. Therefore this diagnosis is dynamic and fluctuates over time. As made clear in the most recent criteria, a diagnosis of fibromyalgia does not preclude the presence of other, potentially more acute and severe medical problems. Therefore, a child with JFMS may still have an inflammatory chronic pain condition or neurologic deficit in addition to his JFMS; this must not be dismissed by the medical provider nor the patient and the patient’s family. Additionally, a child with JFMS may experience an evolution of his or her symptoms and thereby develop a new diagnosis of complex regional pain syndrome or another manifestation of AMPS.
JFMS is more prevalent in Caucasians and girls are affected approximately 4 times more than boys [10, 16, 17]. Although described in patients as young as 2 years old, the majority of patients with JFMS present in late childhood and early adolescence with a reported mean age of onset of 12 to 13 years [18]. Common findings associated with JFMS include increasing pain over time, allodynia (pain with light touch), disproportional dysfunction, widely varying symptoms, an incongruent affect and conversion symptoms. The etiology of JFMS is unknown, but frequently it is seen in the setting of injury, illness, or psychological distress. Hormonal and genetic factors may also play a role [19].
3. Pharmacologic Management
There is a paucity of evidence supporting the use of pharmacological treatment of JFMS. In fact, while there are FDA approved medications (duloxetine, milnacipran and pregabalin) for adult FMS, there are no FDA approved medications for the treatment of JFMS [20, 21]. Additionally, medications that have been used in adults, have for the most part, not been studied in well-controlled trials in JFMS.
3.1 Non-Opioid Analgesics and Anti-inflammatory Medications
The majority of children with JFMS and their parents commonly seek out the use of over the counter medications. These include topical and oral analgesics and non-steroidal anti-inflammatories (NSAIDs), all of which have been used in the treatment of JFMS but overall are not effective [20]. This lack of efficacy is felt to be due to their peripheral action which provides little benefit for fibromyalgia syndrome given the underlying centrally mediated pain mechanisms [19]. There are no controlled studies reporting the effects of salicylates or other NSAIDs in either adult or JFMS [22]. A cross-sectional cohort study from 1991 examined the presenting features, medications and diagnostic testing of 15 children presenting to a private rheumatology practice over the course of two years [23]. In this study, none of the patients responded to a salicylate or other anti-inflammatory medication. In our experience, the lack of response to anti-inflammatory medications is a key clue to the diagnosis.
A study from 1986 compared the effectiveness of amitriptyline versus naproxen in the treatment of adults with FMS [22]. In this 6-week, double-blind trial, 62 adult patients were randomly assigned to one of four treatment arms: 1) amitriptyline 25 mg nightly; 2) naproxen 500 mg twice daily; 3) amitriptyline plus naproxen; or 4) placebo. Amitriptyline was associated with improvements in all outcome parameters (including patient and physician global assessments, patient pain, sleep difficulties, fatigue, and tender point score with p<0.001 for all variables of interest). Evaluation of these parameters among those who received naproxen did not reveal significant improvements. Additionally, naproxen together with amitriptyline was not determined to have a synergistic effect.
Prednisone, likewise, has not been found to be effective in the treatment of adult FMS, although data in the pediatric population is lacking. Clark et al performed a double blinded crossover trial to compare the effects of prednisone (dosed at 15 mg/day) versus placebo [24]. Treatment was administered for 14 days before subjects were crossed-over to the alternative regimen. There was no significant improvement in pain, sleep disturbances, morning stiffness, fatigue or dolorimetry readings.
3.2 Anticonvulsants
The gabapentinoids, including pregabalin and gabapentin, were originally designed as anti-epileptics but are commonly used for the treatment of chronic pain. Pregabalin and gabapentin both function by binding to the alpha2-delta subunit of voltage-gated calcium channels in the central nervous system and the former has FDA approval for the treatment of FMS [25]. Additionally, the use of pregabalin is recommended in published treatment guidelines for adult FMS [26–29].
Arnold et al recently examined the safety and efficacy of pregabalin in adolescents with fibromyalgia [30]. The study was a randomized, double-blind, placebo-controlled trial with a 6-month open-label extension. It was conducted in 4 different countries (36 centers in total) in subjects between 12 and 17 years of age and they defined JFMS by the Yunus and Masi criteria [10]. Pain alleviating medications were discontinued prior to the start of the trial, however, acetaminophen (maximum dose of 3 grams per day) was allowed as a rescue medication with no medication restrictions enforced during the open-label study. Additionally, non-pharmacological therapy started more than 30 days before the onset of the study was permitted. Randomization to pregabalin or matched placebo was performed 1:1. Medication was administered twice daily starting at a dose of 75 mg/day and titrated up over 4 weeks to an optimal dose (maximum of 450 mg/day). This dose regimen is slightly lower than that approved for studies in adult FMS (300–450 mg/day; starting dose 150 mg/day) [2]. Subjects in the double-blind study received their optimized dose for the 12-week maintenance phase while subjects in the open-label study could have their dose adjusted throughout the following 6 months. The primary study outcome was change from baseline in mean pain score at study endpoint. The pain score was determined from the subjects’ daily pain diaries with entries performed daily in the afternoon and evening with a 24 hour recall period.
A total of 107 subjects were included in this study with 54 randomized to pregabalin and 53 to placebo. The majority were female and the mean age was 14 years. Regarding the primary study outcome, there was no statistically significant improvement among those treated with pregabalin. The treatment difference was −0.66 (95% CI: −1.51, 0.18) with a p-value of 0.121. In sensitivity analyses, the primary outcome showed the same trend towards improvement but this was not statistically significant. However, the study’s secondary outcomes showed the change in weekly mean pain score was greater with pregabalin for 10 of the 15 weeks assessed (p<0.05) and there was also a significantly greater change in pain score at week 15 (treatment difference −0.87 [95% CI: −1.68, −0.05]) with a p-value of 0.04. The main adverse events observed included dizziness and nausea. It is notable, however, that the incidence of nausea and fatigue was higher in this study compared to studies of adults (22.2% vs ~8% and14.8% vs. ~7.5% respectively); 21.6% had significant weight gain and 1 had major depression. Thus, this study did not demonstrate clear efficacy of pregabalin in the treatment of JFMS and demonstrated a slightly worse safety profile compared to adults.
There is a paucity of data regarding the efficacy of gabapentin in the treatment of JFMS and no controlled studies; however, given the similar mechanism of action as pregabalin, it has been administered for the treatment of JFMS. In 2007, Arnold and colleagues published the results of a 12-week, randomized, double-blind study comparing gabapentin (1200–2400 mg/day) to placebo in adults [31]. The primary outcome was the Brief Pain Inventory (BPI) average pain severity score (range 0–10, where 0 = no pain and 10 = pain as bad as you can imagine). Subjects treated with gabapentin had a significantly greater improvement in the BPI average pain severity score with a difference of −0.92 (95% CI: −1.75, −0.71) (p= 0.015). The mean pain score fell from 5.8 to 3.2 in those on gabapentin and from 6.0 to 4.6 in those on placebo. The medication was well-tolerated and there were also positive findings regarding the secondary study outcomes.
A recent study examined the potential benefit of a novel form of extended-release gabapentin in a 15-week, open-label, single-arm, single-center study in adult FMS [32]. Those who had failed gabapentin and pregabalin due to side effects, and did not have autoimmune conditions and were not taking opioids, were eligible. Subjects were prescribed an extended-release gabapentin for a total of 12 weeks. Gabapentin extended release, utilizes gastric retention technology so that it can be taken once a day. Additionally, the titration period is shorter (2 weeks versus 6 weeks). 34 subjects were enrolled in this study and 29 completed it. The primary outcome was pain relief as measured by the Numeric Pain Rating System (NPRS) scores. Patients on therapy reported significant pain relief according to the NPRS by the end of 4 weeks (p<0.001). This study was limited, however, by its small sample size, short treatment duration (15 weeks) and lack of a control group. For these reasons, subsequent larger studies in children are needed in order to make any recommendations regarding gabapentin extended-release.
3.3 Anti-depressants
There are three main classes of anti-depressants employed in the treatment of FMS with some limited data supporting their use in the pediatric population. These include serotonin-norepinephrine reuptake inhibitors (SNRIs), selective serotonin reuptake inhibitors (SSRIs) and tricyclic anti-depressants (TCAs). The two main SNRIs include duloxetine and milnacipran, both of which are FDA approved but neither of which is approved by the European Medicines Agency for the management of FMS [33]. A meta-analysis in adults reviewed 10 randomized-controlled trials assessing SNRIs against placebo in the treatment of FMS (5 studies examined milnacipran and 5 examined duloxetine) [33]. Milnacipran and duloxetine had a small incremental effect over placebo in reducing pain (standardized mean difference −0.23; 95% CI −0.29 to −0.18; 6.1% relative improvement) but did not substantially reduce fatigue and did not significantly improve quality of life. Additionally, subject dropout rates were statistically significantly higher in the treatment groups with the most frequently reported symptoms including nausea, dry mouth, constipation, headache, somnolence/dizziness and insomnia. Therefore, the minimal benefit of these medications, countered by the potential for side effects must be seriously considered before prescribing these medications to children.
While there are no studies assessing duloxetine in the treatment of JFMS, milnacipran was assessed in a clinical trial program for JFMS in 2015 [34]. The study included children aged 13 to 17 years. Among the exclusion criteria were comorbid psychiatric illness and suicidality. The study was designed such that after receiving open-label milnacipran for 8 weeks, subjects with greater than or equal to 50% improvement in pain underwent double-blind randomization (1:2) to either placebo or continued treatment with milnacipran for an additional 8 weeks. All subjects were eligible to enter an extension study with open-label milnacipran (for a total of 52 weeks). The primary endpoint was loss of therapeutic response during the double-blind period. However, due to low enrollment rates the study was terminated early. The exclusion of children with psychiatric comorbidities and the difficulty in diagnosing JFMS were cited as reasons for a low enrollment rate. Nonetheless, the open-label phase of the study demonstrated improvements in pain and quality of life including function among those treated with milnacipran albeit modest; the pain score decreased from 6.5 (10 the worse) to 5.4 on average. Additionally, the milnacipran was well tolerated with more than two-thirds of patients tolerating the maximum dose of 100 mg per day and a side-effect profile similar to that in adults. These results should be replicated in larger, multi-centered studies that are powered to detect statistically significant differences.
Fluoxetine is a SSRI that has been used in adults with one exploratory, open trial reported in JFMS [35]. In this study, of the 10 recruited subjects, 4 completed all 12 weeks of treatment and these patients demonstrated reduction in pain and global improvement. However, most subjects only tolerated low doses of fluoxetine suggesting increased sensitivity to adverse effects in this pediatric population.
Tricyclic anti-depressants (TCAs) comprise the third class of anti-depressants that are commonly used in the treatment of FMS. Amitriptyline, specifically, is the TCA prescribed for fibromyalgia with sparse data limited to the adult population. In the aforementioned randomized controlled trial study comparing amitriptyline to naproxen, amitriptyline 25 mg nightly was found to be effective in a number of parameters of FMS [22]. Häuser et al, in a meta-analysis, calculated adjusted indirect comparisons of the efficacy of amitriptyline, duloxetine and milnacipran[36]. This study demonstrated that amitriptyline was superior to duloxetine and milnacipran in reducing pain, sleep disturbances and fatigue but the validity of these findings is limited by the low methodological quality. There was no difference in the rate of medication discontinuation between groups. In another systematic review, Nishishinya et al demonstrated that there may be evidence to support the use of short term, low dose (25 mg/day) amitriptyline, with improvements in pain, sleep and fatigue; however, there is no evidence to support the efficacy of long-term (12 weeks or greater) or higher doses (50 mg/day) of amitriptyline[37].
While anti-depressants may have limited evidence to support their use in the treatment of the symptoms of JFMS itself, they do serve a critical role in treating concomitant psychiatric conditions. Previous research demonstrates that co-morbid anxiety and depression are common among children with fibromyalgia, with the prevalence of anxiety ranging as high as 75% and that for depression reported at 25% [18, 38, 39]. Therefore, anti-depressants and mood stabilizers are important in addressing these psychological issues. The black box warning for increased suicidal tendency in young adults with a history of major depressive disorder taking SSRIs should be carefully considered [40]. We recommend that a safety plan be in place in order to monitor for the risk of suicidal ideation and intent and that patients have routine follow-up visits with a psychologist or psychiatrist while prescribed these medications.
3.4 Opioid Analgesics
Opioids are substances that act on opioid receptors to produce morphine-like effects and are most commonly used medically to relieve pain. Opioids include opiates, an older term that refers to drugs derived from opium, as well as semi-synthetic and synthetic drugs such as hydrocodone, oxycodone and fentanyl. The ineffectiveness of opioids in the treatment of FMS is thought to be due to the inability of these medications to target the pathophysiological processes involved in this central sensitization syndrome [41]. In fact, the American Pain Society (APS) guidelines recommend that opioid analgesics be used with caution after all other therapeutic options have been exhausted [42], and current adult FMS treatment guidelines do not recommend opioids. Despite this, approximately 30% of Canadian and American adults with FMS are prescribed opioids [43, 44]. Opioid use for treatment of FMS in adults has been associated with a number of adverse effects, including severe mental illness, substance abuse and suicidal behavior [43]. Opioid use in patients with FMS may also worsen symptoms such as fatigue and cognitive impairment and lead to new-onset depression [45]. Additionally, adult fibromyalgia patients using opioids are more likely to be unemployed and receive disability benefits [46]. Therefore, there is significant harm associated with opioid use for the management of adult FMS.
There is an even greater paucity of data regarding the use of opioids in the treatment of JFMS. However, despite this lack of evidence, opioid prescribing to children has doubled between 1990 and 2010 [47]. This alarming trend has been associated with a number of adverse consequences. Accidental opioid poisoning in young children may lead to disability or death. In one study of U.S. poison center surveillance systems from 2006–2009, more than 9,000 inadvertent exposures to opioids, often necessitating hospitalization, were reported in children younger than 6 years of age and resulted in 8 deaths [48]. Recreational use of opioids by adolescents is also a serious concern, and research shows that teenagers with early onset non-medical use of prescription drugs are more likely to develop prescription drug abuse and dependence in adulthood [49]. Therefore, judicious use of opioids in the management of pediatric chronic pain is critical for the prevention of opioid addiction and inadvertent poisonings and deaths and should not be used for JFMS.
3.5 Opiate Antagonists
Additionally, within the last couple of years there has been active research assessing the potential use of low-dose naltrexone in the treatment of FMS [50, 51]. Naltrexone is an opioid receptor antagonist used in the treatment of alcohol and opioid dependence. In fibromyalgia, this medication, administered in low doses, is hypothesized to cause temporary blockade of opioid receptors with a resultant endorphin rebound and attenuation of pain [51, 52]. Daily pain levels were measured longitudinally. Those taking the low-dose naltrexone had greater reduction in baseline pain (28.8% versus 18.0%; p=0.016), improved satisfaction with life (p=0.045) and improved mood (p=0.039). There were no differences, however, in fatigue and sleep between control and treatment groups. In a pilot study of 10 women with FMS, low dose naltrexone was found to be efficacious in proportion to one’s erythrocyte sedimentation rate and side effects were rare (including insomnia and vivid dreams) and short-lived [53]. These studies need to be repeated in larger trials with replications demonstrating similar findings before low-dose naltrexone can be recommended for the treatment of FMS. There are yet to be published studies assessing the use of low-dose naltrexone in the treatment of JFMS.
3.6 Muscle Relaxants
Cyclobenzaprine, which is structurally similar to TCAs, is marketed as a muscle relaxant. Cyclobenzaprine is recommended for the treatment of FMS according to the European United League Against Rheumatism (EULAR) and German and Spanish treatment guidelines [28, 54, 55]. A number of studies have evaluated the efficacy of cyclobenzaprine in the management of adult FMS [56–63]. Three studies compared cyclobenzaprine to placebo and they showed improvement in pain [57, 58, 60] but only two revealed a statistically significant improvement in sleep symptoms [57, 58]. Dosages of 10 mg/day and 30 mg/day have been compared in a crossover design study that showed no additive benefit of a higher dose but rather an increased incidence of adverse events [62]. In a study assessing the synergy of ibuprofen with cyclobenzaprine, combination therapy was not found to be better than cyclobenzaprine alone in reducing pain or sleep disturbances [61]. However, there was additive pain relief in the use of cyclobenzaprine (10 mg/day) combined with fluoxetine (20 mg/day) in 2 studies, one of which was a long-term randomized, placebo-controlled trial [56, 64]. However, when comparing cognitive behavioral therapy (CBT), cyclobenzaprine and combination therapy of the two, it was found that CBT, with or without cyclobenzaprine was responsible for overall improvement in symptoms [63]. Lastly, a meta-analysis of 5 trials of cyclobenzaprine demonstrated that patients treated with this medication were 3 times more likely to have global improvement with moderate reductions in individual symptoms, specifically sleep [65]. The number needed to treat for 1 patient to experience improvement in symptoms was reported to be 4.8 (95% CI: 3.0–11). However, replication of these studies in children and adolescents must be performed before applying any of these findings to the treatment of JFMS. Future studies should consider evaluating the efficacy of a new sublingual formulation of low dose (2.8 mg), slow-release cyclobenzaprine which would provide an ideal route of administration for children by decreasing frequency of administration and providing a non-tablet formulation [25].
4. Non-Pharmacological Management
Given the lack of evidence-based medicine for the pharmacological management of JFMS combined with the risk of adverse effects such as suicidal ideation, drowsiness, weight gain, and nausea, the risks of pharmacologic treatment outweigh any potential benefits. Additionally, the risk of polypharmacy is another deterrent from the prescription of medications for the treatment of JFMS. Therefore, the treatment of JFMS should be conservative with judicial use of medications for symptom management and an emphasis on non-pharmacologic approaches.
4.1 Complementary and Alternative Medicine
A significant number of children with JFMS use complementary therapies. Typically, these are interventions that are sought out and initiated by their parents without receiving specific recommendations from healthcare providers. These modalities are employed with the goal of relieving stress, pain and physical and psychological impairment [66]. Examples of such interventions include herbs, lotions, multivitamins, spiritual healing and dietary manipulation [66].
4.1.1 Dietary Interventions
Regarding dietary manipulation, gluten and hypovitaminosis D are two targets that have been explored in the treatment of FMS. In one study from 2016, vitamin D replacement therapy was assessed in the treatment of chronic nonspecific widespread musculoskeletal pain in adults with FMS [67]. While this study demonstrated improvements in musculoskeletal symptoms, levels of depression and quality of life with vitamin D replacement therapy, there was no control population or placebo treatment for comparison. In a meta-analysis of twelve-studies, hypovitaminosis D was found to be associated with chronic widespread pain, even when adjusting for potential confounders but the causal relationship between low vitamin D levels and chronic widespread pain has yet to be identified [68]. In one pilot study, children with a number of different musculoskeletal and orthopedic conditions and co-morbid hypovitaminosis D were prescribed vitamin D supplementation over the course of 6 months [69]. Measures of pain and physical functioning were found to be improved but again, the validity of these findings has yet to be demonstrated in randomized study designs with a focus on JFMS.
Celiac disease and non-gluten celiac gluten sensitivity (NCGS) both have been found to have a high prevalence in patients with FMS [70, 71]. In a study of 50 children with JFMS, only 1 had celiac disease and her pain level increased when on a gluten free diet [72]. While screening for celiac disease in all subjects with chronic widespread pain may not be feasible, patients may choose to undergo a trial of gluten-free diet in attempt to help alleviate their pain [73]. However, in an open-label randomized clinical trial comparing the effects of a gluten-free diet compared with a hypocaloric diet among adults with FMS, there was no difference between the two diets with regards to reducing the number of gluten sensitivity symptoms or secondary outcomes (sleep, anxiety, depression and overall well-being)[74]. In the authors’ experience, we do not typically recommend dietary changes for the treatment of JFMS. However, if children themselves would like to opt for a gluten-free diet without it taking a toll on their quality of life and overall mood, we would not discourage it. We advise, however, that adhering to a strict gluten free diet is difficult to maintain and may have negative effects on one’s overall quality of life. The same holds true for other dietary changes or supplementation such as the use of omega-3 and probiotics [75].
4.1.2 Mind-Body Interventions
In addition to dietary changes, other complementary medicine interventions include mindfulness-based stress reduction, tai chi and yoga. One study demonstrated improvement in function, pain tolerance as well as reduced catastrophizing among children practicing mindfulness meditation [76]. Additionally, mindfulness training has been shown to be beneficial in adults with FMS [77, 78]. Jastrowski et al performed a randomized controlled pilot study of mindfulness-based stress reduction in the treatment of pediatric chronic pain but due to a small sample size (n=6), the researchers were unable to determine the effect of mindfulness on the outcomes of interest [79]. Yoga and tai-chi, like mindfulness meditation, can improve self-efficacy while promoting physical activity. Similarly, there is limited evidence evaluating the outcomes of yoga but two studies demonstrated improvements in pain and functioning in adolescents experiencing chest pain and gastrointestinal symptoms [80, 81]. A randomized controlled trial of tai chi in adults revealed improvements in both subjective and objective assessments of pain and quality of life, with these improvements sustained at 24 weeks [82]. Further investigations assessing the applicability of these alternative practices to the treatment of JFMS are warranted in order to make formal recommendations; however, the authors see no harm in children with JFMS participating in these practices.
4.1.3 Acupuncture and Transcutaneous Electrical Nerve Stimulation
There are no studies in children but a few in adults examining the effects of acupuncture. Results from these studies varied with some finding no evidence supporting acupuncture [83, 84], two with inconclusive results [85, 86] and three finding positive effects on pain [87–89]. Given the lack of supportive evidence for this invasive modality, the authors do not recommend the use of acupuncture in the management of JFMS and have had children report increased pain during and after a treatment.
Transcranial Electrical Nerve Stimulation (TENS) is the application of electrical current through electrodes placed on the skin for pain control. TENS can be applied with frequencies ranging from low (< 10 Hz) to high (> 50 Hz). Intensity can also be varied from sensory to motor intensities, the latter of which usually involves a motor contraction but is not painful [90]. Determination of the correct settings of TENS and preventing tolerance to repeat application of TENS are factors that make it challenging to determine its overall effectiveness, especially in JMFS.
A recent randomized controlled trial assessed the treatment effects on pain of transcranial direct current stimulation (tDCS) [91]. This modality, similar to TENS, is a non-invasive brain-stimulation technique. While, the results of this study suggest that tDCS reduces pain levels in patients with FMS, the study reported small effect sizes and although statistically significant, the findings do not translate into clinically meaningful findings. While the researchers state that no patients experienced serious adverse effects, the evidence is not sufficiently convincing to apply the treatment to children. The authors have patients who report marked pain after a TENS treatment.
4.2 Sleep Hygiene
The role of sleep in improving pain and decreasing symptoms of fatigue in JFMS has been explored and is somewhat controversial. In adults, there is conflicting literature demonstrating whether or not adults with sleep apnea have an increased incidence FMS [92–94]. Previous research demonstrates that children with JFMS have altered alpha-delta sleep but that this sleep abnormality does not correlate with chronic pain or subjective sleep quality [95]. Hoffart et al performed a prospective longitudinal study assessing sleep in adolescents with chronic pain who completed an interdisciplinary rehabilitation program [96]. Similarly, this study revealed improvements in insomnia, sleep satisfaction, daytime fatigue and sleep disturbance; however, these findings were not mirrored in objective sleep data using actigraphy. Therefore, patients’ subjective assessment of sleep quality may affect long-term outcomes more than physiologic changes in actual sleep quality. Therefore, good sleep hygiene is recommended as part of the treatment of JFMS. These recommendations are outlined in Table 5.
Table 5.
Sleep Hygiene Recommendations
|
4.3 Cognitive Behavioral Therapy
Psychological therapies, most prominently cognitive-behavioral therapy (CBT), are the most evidenced-based treatment modalities for JFMS. Prior research in both adults and children has demonstrated the effectiveness of CBT for FMS [97–100]. The treatment is usually delivered by a trained psychologist in a 6–8 weekly session format and parents typically participate in treatment so that they can effectively support their child throughout treatment and maintain these behavioral interventions upon completion of therapy. CBT trains patients to use specific cognitive and behavioral strategies to cope with their pain and reduce disability secondary to their pain. CBT includes educating patients about the body’s pain mechanisms, identifying and changing negative thoughts related to pain and training in behavior management strategies [101]. Thus, CBT replaces maladaptive coping mechanisms with more supportive coping mechanisms that allow the child to continue to function despite his or her pain. CBT, in combination with routine medical care, has been shown to improve daily functioning and mood and a reduction in pain [99, 100, 102]. These improvements have been shown to be sustained up to 6 months after treatment [99]. Sil et al determined that patients with initial high levels of coping efficacy were 1.6 times more likely to have a positive response to CBT[103]; therefore, a patient’s ability to cope should be gauged prior to therapy to help guide the intensity of therapy.
Given the limited number of available psychologists, researchers have sought to leverage technology via internet-based interventions using CBT methods in other pediatric chronic pain conditions, with promising results [104, 105]. Future studies should assess the efficacy of internet-based interventions specifically in a cohort of patients with JFMS.
4.4 Exercise-based Approaches
Physical activity and cardiovascular exercise in addition to physical and occupational therapy are key components in the effective treatment of both adult and pediatric FMS [18, 106–112]. It has been demonstrated via actigraphy that children with chronic pain are less physically active than their peers [113]. Additionally, via biomechanical assessments it has been shown that adolescents with JFMS have functional deficits affecting gait, posture, balance and movement [114]. The APS published treatment recommendations for children and adults with FMS in 2005 that recommended a minimum of 30 minutes per day of cardiovascular exercise for 2–3 days out of the week [115]. In a study assessing the ability of CBT to increase physical activity, it was found via actigraphy monitoring that CBT alone did not result in increased physical activity [116]. Therefore, a multidisciplinary approach, combining CBT and prescribed physical activity, is recommended.
5. Multidisciplinary Approaches
The multidisciplinary treatment regimen for FMS is multifold, including pharmacological, behavioral and exercise-based modalities [117]. This multidisciplinary approach is currently the standard of care for JFMS [19, 118, 119]. Both inpatient and outpatient treatment programs have been found to be effective, with the former producing faster improvements in treatment outcomes [120, 121]. In a study of 64 children with JFMS in a tertiary care inpatient intensive treatment program, consisting of intensive physical and occupational therapy and psychotherapy, children experienced significantly improved function and pain by both subjective and objective measures [18]. The physical and occupational therapy was 1:1, 5 to 6 hours a day for an average of 23 days and each child had a minimum of 4 hours of psychological support. The pain score (10 the worst) was initially 6.6 and fell to 2.5 at the end of the treatment program and 1 year later was 2.0. In a similar manner, function, subjectively and objectively, significantly improved as did the quality of life scores.
Regarding improving the impaired biomechanics seen with JFMS, a combined intervention program of CBT techniques with specialized neuromuscular exercise has been shown to improve the mechanics of walking gait and functional performance [122]. Additionally, this combined therapy program was more successful than an intervention comprised of CBT alone [99]. The study showed high retention rates with improvements in functional disability, depression, fear of movement, energy level and pain catastrophizing [123, 124].
The challenge remains in determining the long-term efficacy of multidisciplinary treatment programs. While children tend to do well in the immediate period, one prospective longitudinal study found that more than 80% of patients with JFMS continue to have symptoms of fibromyalgia in early adulthood and 51.1% of study subjects meet the adult ACR criteria for fibromyalgia [125]. Therefore, more needs to be done to ensure that treatment outcomes persist into adulthood and what treatment factors lead to long-term success.
6. Conclusion
A variety of medications have been prescribed for the treatment of JFMS, including (but not limited to) non-opioid analgesics, opioids, anticonvulsants, antidepressants, and muscle relaxants [126]. Presently, evidence for the efficacy of medications for the treatment of juvenile fibromyalgia is limited. The current mainstay of treatment consists of a multidisciplinary approach including psychological counseling, physical activity and possible pharmacologic interventions, although use of the latter is controversial. We acknowledge that there are a plethora of symptoms that a child with JFMS may experience and therefore children may benefit from symptom-targeted therapeutic approaches (e.g. oral contraceptive for dysmenorrhea) but these are beyond the scope of the review. Examples of other pain manifestations accompanying JFMS include dizziness, postural orthostatic tachycardia syndrome, irritable bowel syndrome, interstitial cystitis, and vulvodynia as well as a host of psychological issues including disordered eating, suicidality, self-inflicted injury such as cutting, and drug addiction. Therefore, patient-centered, individually-tailored treatment programs are ideal.
The current lack of a standardized treatment regimen for JFMS combined with the fact that it is a challenging diagnosis to make, presents potential risks for patient safety as well as increased healthcare expenditures. Currently, given the variety of pharmacologic agents without a clear consensus on the efficacy of these medications, there is a risk of unnecessary polypharmacy. A major concern with polypharmacy is the greater potential for cumulative toxicity and adverse events with drug-drug interactions from multiple medications as well as increasing complexity of a medication regimen. This can lead to drug-drug interactions that may have dangerous consequences for the patient as well as unwarranted excessive medical costs. Additionally, children undergo numerous diagnostic tests and are seen by multiple medical specialists before receiving a diagnosis of JFMS which further magnifies the associated medical costs [127, 128]. There is therefore an increasing need to reduce the over-medicalization of children with JFMS as well as limit polypharmacy.
Future research in JFMS should place an emphasis on promoting lifestyle changes that allow for patients to maintain long-term success. We argue that physicians, mental health providers, and physical and occupational therapists should work towards providing children with the tools to cope with and alleviate their own pain. This will enable them to harness the mechanisms needed to manage their pain should it reoccur, thereby ensuring their long-term success. Additionally, there is a need for long-term outcomes research to include outcomes other than pain and physical functioning such as the presence of mental health disorders (including suicidality and eating disorders) and other non-traditional pain symptoms (e.g. irritable bowel syndrome) or surrogates thereof (e.g. healthcare utilization).
The complexity of the physical and psychosocial manifestations of JFMS demands an equally complex assessment of treatment outcomes. From a pharmacologic standpoint, this means more stringent randomized, controlled trials with longer follow-up periods in order to determine the long-term efficacy and safety of medications. Similarly, improved recognition of JFMS will allow for improved patient recruitment to permit for adequately powered study designs.
Table 3.
| 1. Widespread pain index* (WPI) ≥7 and symptom severity scale** (SSS) score ≥ 5 or WPI 3-6 and SSS score ≥ 9. |
| 2. Symptoms have been present at a similar level for at least 3 months. |
| 3. The patient does not have a disorder that would otherwise explain the pain. |
Widespread pain index calculated by adding number of body regions in pain within the last week including: jaw, shoulder, upper arm, lower arm, buttocks, upper leg, lower leg (2 points if bilateral) and single points for neck, chest, abdomen, upper back and lower back for a total possible score of 19.
Symptom severity scale score may be as high as 12 and is calculated by adding the sum of the self-reported severity scores for 3 core symptoms: fatigue, waking unrefreshed and thinking difficulty (0 = no problem, 1 = mild problem, 2 = moderate problem, 3 = severe problem for a total possible score of 9 and a symptom checklist score, 0 = no symptoms, 1 = 1-10 symptoms, 2 = 11-24 symptoms, 3 = 25+ symptoms for an additional possible 3 points.
The symptoms may include: muscle pain, headaches, tiredness, muscle weakness, abdominal pain, nausea, nervousness, dizziness, thinking problems, irritable bowel symptoms (bloating, gas, intermittent diarrhea/constipation), chest pain, insomnia – getting or staying asleep, easy bruising, depression, ringing in ears, numbness, dry mouth, loss of appetite, heartburn, blurry vision, shortness of breath, frequent urination, dry eyes, itching, Raynaud – hands or feet with color changes , ongoing constipation, ongoing diarrhea, oral ulcers, hair loss, loss of taste or change of taste, hearing difficulties, wheezing, vomiting, bladder spasms, painful urination, hives/welts, seizures, sun sensitivity, light sensitivity other than sun sensitivity, noise sensitivity, sensitivity to strong smells, seeing spots, partial or total blindness, double vision, difficulty reading, lightheadedness or feeling woozy , balance problems, fainting or near fainting, heart racing/palpitations, uncontrollable shaking of muscles, twitching of muscles or tics, inability to move part of your body/paralysis, part of your body so stiff it cannot bend or move, reversal of day/night sleep wake cycle, severe menstrual cramps
Key Points.
While a variety of medications have been prescribed for the treatment of juvenile fibromyalgia syndrome, the efficacy of these medications is limited.
The most evidence-based treatment modalities for juvenile fibromyalgia syndrome are psychological therapies, prominently cognitive-behavioral therapy.
A multidisciplinary approach, combining pharmacological, behavioral and exercise-based modalities is currently the standard of care for juvenile fibromyalgia syndrome.
Acknowledgments
Funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number T32-AR007442 (Gmuca) and by the Snider Family (Dr. Sherry). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest: SG and DDS declare they have no conflicts of interest to disclose.
Compliance with Ethical Standards:
Ethical approval & informed consent: This study does not qualify as human subjects research therefore ethical approval and informed consent were not required.
References
- 1.Buskila D. Pediatric fibromyalgia. Rheum Dis Clin North Am. 2009;35:253–261. doi: 10.1016/j.rdc.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Kashikar-Zuck S, King C, Ting TV, Arnold LM. Juvenile Fibromyalgia: Different from the Adult Chronic Pain Syndrome? Curr Rheumatol Rep. 2016;18:19. doi: 10.1007/s11926-016-0569-9. [DOI] [PubMed] [Google Scholar]
- 3.Anthony KK, Schanberg LE. Assessment and management of pain syndromes and arthritis pain in children and adolescents. Rheum Dis Clin North Am. 2007;33:625–660. doi: 10.1016/j.rdc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Schikler KN. Is it juvenile rheumatoid arthritis or fibromyalgia? Med Clin North Am. 2000;84:967–982. doi: 10.1016/s0025-7125(05)70269-8. [DOI] [PubMed] [Google Scholar]
- 5.Buskila D, Press J, Gedalia A, Klein M, Neumann L, Boehm R, Sukenik S. Assessment of nonarticular tenderness and prevalence of fibromyalgia in children. J Rheumatol. 1993;20:368–370. [PubMed] [Google Scholar]
- 6.Clark P, Burgos-Vargas R, Medina-Palma C, Lavielle P, Marina FF. Prevalence of fibromyalgia in children: a clinical study of Mexican children. J Rheumatol. 1998;25:2009–2014. [PubMed] [Google Scholar]
- 7.Gerloni VGM, Fantini F. Assessment of nonarticular tenderness and prevalence of primary fibromyalgia syndrome in healthy Italian schoolchildren. Arthritis Rheum. 1998;41:1405. [Google Scholar]
- 8.Sherry DD. An overview of amplified musculoskeletal pain syndromes. J Rheumatol Suppl. 2000;58:44–48. [PubMed] [Google Scholar]
- 9.Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Semin Arthritis Rheum. 1981;11:151–171. doi: 10.1016/0049-0172(81)90096-2. [DOI] [PubMed] [Google Scholar]
- 10.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985;28:138–145. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research. 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 13.Wolfe F, Hauser W. Fibromyalgia diagnosis and diagnostic criteria. Ann Med. 2011;43:495–502. doi: 10.3109/07853890.2011.595734. [DOI] [PubMed] [Google Scholar]
- 14.Ting TV, Barnett K, Lynch-Jordan A, Whitacre C, Henrickson M, Kashikar-Zuck S. 2010 American College of Rheumatology Adult Fibromyalgia Criteria for Use in an Adolescent Female Population with Juvenile Fibromyalgia. J Pediatr. 2016;169:181–187.e181. doi: 10.1016/j.jpeds.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Hauser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46:319–329. doi: 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Malleson PN, al-Matar M, Petty RE. Idiopathic musculoskeletal pain syndromes in children. J Rheumatol. 1992;19:1786–1789. [PubMed] [Google Scholar]
- 17.Sherry DD, McGuire T, Mellins E, Salmonson K, Wallace CA, Nepom B. Psychosomatic musculoskeletal pain in childhood: clinical and psychological analyses of 100 children. Pediatrics. 1991;88:1093–1099. [PubMed] [Google Scholar]
- 18.Sherry DD, Brake L, Tress JL, Sherker J, Fash K, Ferry K, Weiss PF. The Treatment of Juvenile Fibromyalgia with an Intensive Physical and Psychosocial Program. J Pediatr. 2015;167:731–737. doi: 10.1016/j.jpeds.2015.06.036. [DOI] [PubMed] [Google Scholar]
- 19.Hoffart CM, Wallace DP. Amplified pain syndromes in children: treatment and new insights into disease pathogenesis. Curr Opin Rheumatol. 2014;26:592–603. doi: 10.1097/BOR.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 20.Buskila D, Ablin JN. Treating juvenile fibromyalgia: cognitive-behavioral therapy, exercise and pharmacotherapy. Pain Manag. 2013;3:323–324. doi: 10.2217/pmt.13.37. [DOI] [PubMed] [Google Scholar]
- 21.Crofford LJ. Pain management in fibromyalgia. Curr Opin Rheumatol. 2008;20:246–250. doi: 10.1097/BOR.0b013e3282fb0268. [DOI] [PubMed] [Google Scholar]
- 22.Goldenberg DL, Felson DT, Dinerman H. A randomized, controlled trial of amitriptyline and naproxen in the treatment of patients with fibromyalgia. Arthritis Rheum. 1986;29:1371–1377. doi: 10.1002/art.1780291110. [DOI] [PubMed] [Google Scholar]
- 23.Romano TJ. Fibromyalgia in children; diagnosis and treatment. W V Med J. 1991;87:112–114. [PubMed] [Google Scholar]
- 24.Clark S, Tindall E, Bennett RM. A double blind crossover trial of prednisone versus placebo in the treatment of fibrositis. J Rheumatol. 1985;12:980–983. [PubMed] [Google Scholar]
- 25.Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother. 2015;16:1347–1368. doi: 10.1517/14656566.2015.1047343. [DOI] [PubMed] [Google Scholar]
- 26.Ablin J, Fitzcharles MA, Buskila D, Shir Y, Sommer C, Hauser W. Treatment of Fibromyalgia Syndrome: Recommendations of Recent Evidence-Based Interdisciplinary Guidelines with Special Emphasis on Complementary and Alternative Therapies. Evid Based Complement Alternat Med. 2013;2013:485272. doi: 10.1155/2013/485272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Hauser W, Fluss E, Choy E, Kosek E, Amris K, Branco J, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76:318–328. doi: 10.1136/annrheumdis-2016-209724. [DOI] [PubMed] [Google Scholar]
- 28.Hauser W, Arnold B, Eich W, Felde E, Flugge C, Henningsen P, Herrmann M, Kollner V, Kuhn E, Nutzinger D, et al. Management of fibromyalgia syndrome--an interdisciplinary evidence-based guideline. Ger Med Sci. 2008;6 Doc14. [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzcharles M-A, Ste-Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinière M, Ko G, Moulin DE, Panopalis P, Proulx J, et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: Executive summary. Pain Research & Management J Can Pain Soc. 2013;18:119–126. doi: 10.1155/2013/918216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnold LM, Schikler KN, Bateman L, Khan T, Pauer L, Bhadra-Brown P, Clair A, Chew ML, Scavone J. Safety and efficacy of pregabalin in adolescents with fibromyalgia: a randomized, double-blind, placebo-controlled trial and a 6-month open-label extension study. Pediatr Rheumatol Online J. 2016;14:46. doi: 10.1186/s12969-016-0106-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnold LM, Goldenberg DL, Stanford SB, Lalonde JK, Sandhu HS, Keck PE, Jr, Welge JA, Bishop F, Stanford KE, Hess EV, et al. Gabapentin in the treatment of fibromyalgia: a randomized, double-blind, placebo-controlled, multicenter trial. Arthritis Rheum. 2007;56:1336–1344. doi: 10.1002/art.22457. [DOI] [PubMed] [Google Scholar]
- 32.North JM, Hong KS, Rauck RL. The Effect of a Novel form of Extended-Release Gabapentin on Pain and Sleep in Fibromyalgia Subjects: An Open-Label Pilot Study. Pain Pract. 2016;16:720–729. doi: 10.1111/papr.12319. [DOI] [PubMed] [Google Scholar]
- 33.Hauser W, Urrutia G, Tort S, Uceyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database Syst Rev. 2013;1 doi: 10.1002/14651858.CD010292. Cd010292. [DOI] [PubMed] [Google Scholar]
- 34.Arnold LM, Bateman L, Palmer RH, Lin Y. Preliminary experience using milnacipran in patients with juvenile fibromyalgia: lessons from a clinical trial program. Pediatr Rheumatol Online J. 2015;13:27. doi: 10.1186/s12969-015-0025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mariutto EN, Stanford SB, Kashikar-Zuck S, Welge JA, Arnold LM. An exploratory, open trial of fluoxetine treatment of juvenile fibromyalgia. J Clin Psychopharmacol. 2012;32:293–295. doi: 10.1097/JCP.0b013e31824858dc. [DOI] [PubMed] [Google Scholar]
- 36.Hauser W, Petzke F, Uceyler N, Sommer C. Comparative efficacy and acceptability of amitriptyline, duloxetine and milnacipran in fibromyalgia syndrome: a systematic review with meta-analysis. Rheumatology. 2011;50:532–543. doi: 10.1093/rheumatology/keq354. [DOI] [PubMed] [Google Scholar]
- 37.Nishishinya B, Urrutia G, Walitt B, Rodriguez A, Bonfill X, Alegre C, Darko G. Amitriptyline in the treatment of fibromyalgia: a systematic review of its efficacy. Rheumatology. 2008;47:1741–1746. doi: 10.1093/rheumatology/ken317. [DOI] [PubMed] [Google Scholar]
- 38.Cunningham NR, Tran ST, Lynch-Jordan AM, Ting TV, Sil S, Strotman D, Noll JG, Powers SW, Arnold LM, Kashikar-Zuck S. Psychiatric Disorders in Young Adults Diagnosed with Juvenile Fibromyalgia in Adolescence. J Rheumatol. 2015;42:2427–2433. doi: 10.3899/jrheum.141369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kashikar-Zuck S, Parkins IS, Graham TB, Lynch AM, Passo M, Johnston M, Schikler KN, Hashkes PJ, Banez G, Richards MM. Anxiety, mood, and behavioral disorders among pediatric patients with juvenile fibromyalgia syndrome. Clin J Pain. 2008;24:620–626. doi: 10.1097/AJP.0b013e31816d7d23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walitt B, Urrutia G, Nishishinya MB, Cantrell SE, Hauser W. Selective serotonin reuptake inhibitors for fibromyalgia syndrome. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD011735. Cd011735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ngian GS, Guymer EK, Littlejohn GO. The use of opioids in fibromyalgia. Int J Rheum Dis. 2011;14:6–11. doi: 10.1111/j.1756-185X.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- 42.Peng X, Robinson RL, Mease P, Kroenke K, Williams DA, Chen Y, Faries D, Wohlreich M, McCarberg B, Hann D. Long-term evaluation of opioid treatment in fibromyalgia. Clin J Pain. 2015;31:7–13. doi: 10.1097/AJP.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 43.Fitzcharles MA, Ste-Marie PA, Gamsa A, Ware MA, Shir Y. Opioid use, misuse, and abuse in patients labeled as fibromyalgia. Am J Med. 2011;124:955–960. doi: 10.1016/j.amjmed.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 44.Robinson RL, Kroenke K, Mease P, Williams DA, Chen Y, D’Souza D, Wohlreich M, McCarberg B. Burden of illness and treatment patterns for patients with fibromyalgia. Pain Med. 2012;13:1366–1376. doi: 10.1111/j.1526-4637.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- 45.Scherrer JF, Svrakic DM, Freedland KE, Chrusciel T, Balasubramanian S, Bucholz KK, Lawler EV, Lustman PJ. Prescription Opioid Analgesics Increase the Risk of Depression. J Gen Intern Med. 2014;29:491–499. doi: 10.1007/s11606-013-2648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aguglia A, Salvi V, Maina G, Rossetto I, Aguglia E. Fibromyalgia syndrome and depressive symptoms: comorbidity and clinical correlates. J Affect Disord. 2011;128:262–266. doi: 10.1016/j.jad.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 47.Schechter NL. Pediatric pain management and opioids: the baby and the bathwater. JAMA Pediatr. 2014;168:987–988. doi: 10.1001/jamapediatrics.2014.1441. [DOI] [PubMed] [Google Scholar]
- 48.Bailey JE, Campagna E, Dart RC. The underrecognized toll of prescription opioid abuse on young children. Ann Emerg Med. 2009;53:419–424. doi: 10.1016/j.annemergmed.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 49.McCabe SE, West BT, Morales M, Cranford JA, Boyd CJ. Does early onset of non-medical use of prescription drugs predict subsequent prescription drug abuse and dependence? Results from a national study. Addiction. 2007;102:1920–1930. doi: 10.1111/j.1360-0443.2007.02015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Younger J, Parkitny L, McLain D. The use of low-dose naltrexone (LDN) as a novel anti-inflammatory treatment for chronic pain. Clin Rheumatol. 2014;33:451–459. doi: 10.1007/s10067-014-2517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Younger J, Noor N, McCue R, Mackey S. Low-dose naltrexone for the treatment of fibromyalgia: findings of a small, randomized, double-blind, placebo-controlled, counterbalanced, crossover trial assessing daily pain levels. Arthritis Rheum. 2013;65:529–538. doi: 10.1002/art.37734. [DOI] [PubMed] [Google Scholar]
- 52.Metyas SK, Yeter K, Solyman J, Arkfeld D. Low Dose Naltrexone in the Treatment of Fibromyalgia. Curr Rheumatol Rev. 2017 doi: 10.2174/1573397113666170321120329. [DOI] [PubMed] [Google Scholar]
- 53.Younger J, Mackey S. Fibromyalgia symptoms are reduced by low-dose naltrexone: a pilot study. Pain Med. 2009;10:663–672. doi: 10.1111/j.1526-4637.2009.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carville SF, Arendt-Nielsen L, Bliddal H, Blotman F, Branco JC, Buskila D, Da Silva JA, Danneskiold-Samsoe B, Dincer F, Henriksson C, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536–541. doi: 10.1136/ard.2007.071522. [DOI] [PubMed] [Google Scholar]
- 55.de Miquel CA, Campayo J, Florez MT, Arguelles JM, Tarrio EB, Montoya MG, Martin A, Salio AM, Fuentes JV, Alberch EA, de la Camara AG. Interdisciplinary consensus document for the treatment of fibromyalgia. Actas Esp Psiquiatr. 2010;38:108–120. [PubMed] [Google Scholar]
- 56.Cantini F, Bellandi F, Niccoli L, Di Munno O. [Fluoxetin combined with cyclobenzaprine in the treatment of fibromyalgia] Minerva Med. 1994;85:97–100. [PubMed] [Google Scholar]
- 57.Bennett RM, Gatter RA, Campbell SM, Andrews RP, Clark SR, Scarola JA. A comparison of cyclobenzaprine and placebo in the management of fibrositis. A double-blind controlled study. Arthritis Rheum. 1988;31:1535–1542. doi: 10.1002/art.1780311210. [DOI] [PubMed] [Google Scholar]
- 58.Quimby LG, Gratwick GM, Whitney CD, Block SR. A randomized trial of cyclobenzaprine for the treatment of fibromyalgia. J Rheumatol Suppl. 1989;19:140–143. [PubMed] [Google Scholar]
- 59.Reynolds WJ, Moldofsky H, Saskin P, Lue FA. The effects of cyclobenzaprine on sleep physiology and symptoms in patients with fibromyalgia. J Rheumatol. 1991;18:452–454. [PubMed] [Google Scholar]
- 60.Moldofsky H, Harris HW, Archambault WT, Kwong T, Lederman S. Effects of bedtime very low dose cyclobenzaprine on symptoms and sleep physiology in patients with fibromyalgia syndrome: a double-blind randomized placebo-controlled study. J Rheumatol. 2011;38:2653–2663. doi: 10.3899/jrheum.110194. [DOI] [PubMed] [Google Scholar]
- 61.Fossaluzza V, De Vita S. Combined therapy with cyclobenzaprine and ibuprofen in primary fibromyalgia syndrome. Int J Clin Pharmacol Res. 1992;12:99–102. [PubMed] [Google Scholar]
- 62.Santandrea S, Montrone F, Sarzi-Puttini P, Boccassini L, Caruso I. A double-blind crossover study of two cyclobenzaprine regimens in primary fibromyalgia syndrome. J Int Med Res. 1993;21:74–80. doi: 10.1177/030006059302100202. [DOI] [PubMed] [Google Scholar]
- 63.Garcia J, Simon MA, Duran M, Canceller J, Aneiros FJ. Differential efficacy of a cognitive-behavioral intervention versus pharmacological treatment in the management of fibromyalgic syndrome. Psychol Health Med. 2006;11:498–506. doi: 10.1080/13548500600745286. [DOI] [PubMed] [Google Scholar]
- 64.Cantini F, Niccoli L, Bellandi F. Effectiveness of fluoxetine associated with cyclobenzaprine in the treatment of fibromyalgia: Twelve-months-follow up results. Reumatismo. 1995;47:103–108. [Google Scholar]
- 65.Tofferi JK, Jackson JL, O’Malley PG. Treatment of fibromyalgia with cyclobenzaprine: A meta-analysis. Arthritis Rheum. 2004;51:9–13. doi: 10.1002/art.20076. [DOI] [PubMed] [Google Scholar]
- 66.Patkar AA, Bilal L, Masand PS. Management of fibromyalgia. Curr Psychiatry Rep. 2003;5:218–224. doi: 10.1007/s11920-003-0046-9. [DOI] [PubMed] [Google Scholar]
- 67.Yilmaz R, Salli A, Cingoz HT, Kucuksen S, Ugurlu H. Efficacy of vitamin D replacement therapy on patients with chronic nonspecific widespread musculoskeletal pain with vitamin D deficiency. Int J Rheum Dis. 2016 doi: 10.1111/1756-185X.12960. [DOI] [PubMed] [Google Scholar]
- 68.Hsiao MY, Hung CY, Chang KV, Han DS, Wang TG. Is Serum Hypovitaminosis D Associated with Chronic Widespread Pain Including Fibromyalgia? A Meta-analysis of Observational Studies. Pain Physician. 2015;18:E877–887. [PubMed] [Google Scholar]
- 69.Blagojevic Z, Nikolic V, Kisic-Tepavcevic D, Terzic Supic Z, Kovacevic R, Zivkovic Z, Stevanovic D. Musculoskeletal Pain and Vitamin D Deficiency in Children: A Pilot Follow-up Study of Vitamin D Therapy in Musculoskeletal/Orthopedic Conditions. Acta Chir Orthop Traumatol Cech. 2016;83:21–26. [PubMed] [Google Scholar]
- 70.Rodrigo L, Blanco I, Bobes J, de Serres FJ. Clinical impact of a gluten-free diet on health-related quality of life in seven fibromyalgia syndrome patients with associated celiac disease. BMC Gastroenterol. 2013;13:157. doi: 10.1186/1471-230X-13-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rodrigo L, Blanco I, Bobes J, de Serres FJ. Remarkable prevalence of celiac disease in patients with irritable bowel syndrome plus fibromyalgia in comparison with those with isolated irritable bowel syndrome: a case-finding study. Arthritis Res Ther. 2013;15:R201. doi: 10.1186/ar4391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taubman B, Mamula P, Sherry DD. Prevalence of asymptomatic celiac disease in children with fibromyalgia: a pilot study. Pediatr Rheumatol Online J. 2011;9:11. doi: 10.1186/1546-0096-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nisihara R, Marques AP, Mei A, Skare T. Celiac disease and fibromyalgia: Is there an association? Rev Esp Enferm Dig. 2016;108:107–108. doi: 10.17235/reed.2015.3992/2015. [DOI] [PubMed] [Google Scholar]
- 74.Slim M, Calandre EP, Garcia-Leiva JM, Rico-Villademoros F, Molina-Barea R, Rodriguez-Lopez CM, Morillas-Arques P. The Effects of a Gluten-free Diet Versus a Hypocaloric Diet Among Patients With Fibromyalgia Experiencing Gluten Sensitivity-like Symptoms: A Pilot, Open-Label Randomized Clinical Trial. J Clin Gastroenterol. 2016 doi: 10.1097/MCG.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 75.Young L, Kemper KJ. Integrative care for pediatric patients with pain. J Altern Complement Med. 2013;19:627–632. doi: 10.1089/acm.2012.0368. [DOI] [PubMed] [Google Scholar]
- 76.Petter M, Chambers CT, McGrath PJ, Dick BD. The role of trait mindfulness in the pain experience of adolescents. J Pain. 2013;14:1709–1718. doi: 10.1016/j.jpain.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 77.Grossman P, Tiefenthaler-Gilmer U, Raysz A, Kesper U. Mindfulness Training as an Intervention for Fibromyalgia: Evidence of Postintervention and 3-Year Follow-Up Benefits in Well-Being. Psychotherapy and Psychosomatics. 2007;76:226–233. doi: 10.1159/000101501. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan KH, Goldenberg DL, Galvin-Nadeau M. The impact of a meditation-based stress reduction program on fibromyalgia. Gen Hosp Psychiatry. 1993;15:284–289. doi: 10.1016/0163-8343(93)90020-o. [DOI] [PubMed] [Google Scholar]
- 79.Jastrowski Mano KE, Salamon KS, Hainsworth KR, Anderson Khan KJ, Ladwig RJ, Davies WH, Weisman SJ. A randomized, controlled pilot study of mindfulness-based stress reduction for pediatric chronic pain. Altern Ther Health Med. 2013;19:8–14. [PubMed] [Google Scholar]
- 80.Kuttner L, Chambers CT, Hardial J, Israel DM, Jacobson K, Evans K. A randomized trial of yoga for adolescents with irritable bowel syndrome. Pain Res Manag. 2006;11:217–223. doi: 10.1155/2006/731628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evans S, Lung KC, Seidman LC, Sternlieb B, Zeltzer LK, Tsao JC. Iyengar yoga for adolescents and young adults with irritable bowel syndrome. J Pediatr Gastroenterol Nutr. 2014;59:244–253. doi: 10.1097/MPG.0000000000000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang C, Schmid CH, Rones R, Kalish R, Yinh J, Goldenberg DL, Lee Y, McAlindon T. A randomized trial of tai chi for fibromyalgia. N Engl J Med. 2010;363:743–754. doi: 10.1056/NEJMoa0912611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Terhorst L, Schneider MJ, Kim KH, Goozdich LM, Stilley CS. Complementary and alternative medicine in the treatment of pain in fibromyalgia: a systematic review of randomized controlled trials. J Manipulative Physiol Ther. 2011;34:483–496. doi: 10.1016/j.jmpt.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 84.Martin-Sanchez E, Torralba E, Diaz-Dominguez E, Barriga A, Martin JL. Efficacy of acupuncture for the treatment of fibromyalgia: systematic review and meta-analysis of randomized trials. Open Rheumatol J. 2009;3:25–29. doi: 10.2174/1874312900903010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Baranowsky J, Klose P, Musial F, Hauser W, Dobos G, Langhorst J. Qualitative systemic review of randomized controlled trials on complementary and alternative medicine treatments in fibromyalgia. Rheumatol Int. 2009;30:1–21. doi: 10.1007/s00296-009-0977-5. [DOI] [PubMed] [Google Scholar]
- 86.Mayhew E, Ernst E. Acupuncture for fibromyalgia--a systematic review of randomized clinical trials. Rheumatology (Oxford) 2007;46:801–804. doi: 10.1093/rheumatology/kel406. [DOI] [PubMed] [Google Scholar]
- 87.Holdcraft LC, Assefi N, Buchwald D. Complementary and alternative medicine in fibromyalgia and related syndromes. Best Pract Res Clin Rheumatol. 2003;17:667–683. doi: 10.1016/s1521-6942(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 88.Deare JC, Zheng Z, Xue CC, Liu JP, Shang J, Scott SW, Littlejohn G. Acupuncture for treating fibromyalgia. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD007070.pub2. Cd007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Langhorst J, Klose P, Musial F, Irnich D, Hauser W. Efficacy of acupuncture in fibromyalgia syndrome--a systematic review with a meta-analysis of controlled clinical trials. Rheumatology. 2010;49:778–788. doi: 10.1093/rheumatology/kep439. [DOI] [PubMed] [Google Scholar]
- 90.DeSantana JM, Walsh DM, Vance C, Rakel BA, Sluka KA. Effectiveness of Transcutaneous Electrical Nerve Stimulation for Treatment of Hyperalgesia and Pain. Curr Rheumatol Rep. 2008;10:492–499. doi: 10.1007/s11926-008-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain. 2015;156:62–71. doi: 10.1016/j.pain.0000000000000006. [DOI] [PubMed] [Google Scholar]
- 92.Donald F, Esdaile JM, Kimoff JR, Fitzcharles MA. Musculoskeletal complaints and fibromyalgia in patients attending a respiratory sleep disorders clinic. J Rheumatol. 1996;23:1612–1616. [PubMed] [Google Scholar]
- 93.Marvisi M, Balzarini L, Mancini C, Ramponi S, Marvisi C. Fibromyalgia is frequent in obstructive sleep apnea and responds to CPAP therapy. Eur J Intern Med. 2015;26:e49–50. doi: 10.1016/j.ejim.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 94.Germanowicz D, Lumertz MS, Martinez D, Margarites AF. Sleep disordered breathing concomitant with fibromyalgia syndrome. J Bras Pneumol. 2006;32:333–338. [PubMed] [Google Scholar]
- 95.Olsen MN, Sherry DD, Boyne K, McCue R, Gallagher PR, Brooks LJ. Relationship between sleep and pain in adolescents with juvenile primary fibromyalgia syndrome. Sleep. 2013;36:509–516. doi: 10.5665/sleep.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoffart C, Fortney S, Wallace D. Sleep Disruption in Children with Chronic Pain [abstract] Arthritis Rheumatol. 2016;68(suppl 10) [Google Scholar]
- 97.Bernardy K, Fuber N, Kollner V, Hauser W. Efficacy of cognitive-behavioral therapies in fibromyalgia syndrome - a systematic review and metaanalysis of randomized controlled trials. J Rheumatol. 2010;37:1991–2005. doi: 10.3899/jrheum.100104. [DOI] [PubMed] [Google Scholar]
- 98.Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: a meta-analysis. Pain. 2010;151:280–295. doi: 10.1016/j.pain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 99.Kashikar-Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, Passo MH, Schikler KN, Hashkes PJ, Spalding S, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multisite, single-blind, randomized, controlled clinical trial. Arthritis Rheum. 2012;64:297–305. doi: 10.1002/art.30644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kashikar-Zuck S, Swain NF, Jones BA, Graham TB. Efficacy of cognitive-behavioral intervention for juvenile primary fibromyalgia syndrome. J Rheumatol. 2005;32:1594–1602. [PubMed] [Google Scholar]
- 101.Sil S, Kashikar-Zuck S. Understanding why cognitive-behavioral therapy is an effective treatment for adolescents with juvenile fibromyalgia. Int J Clin Rheumtol. 2013;8 doi: 10.2217/IJR.13.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Degotardi PJ, Klass ES, Rosenberg BS, Fox DG, Gallelli KA, Gottlieb BS. Development and evaluation of a cognitive-behavioral intervention for juvenile fibromyalgia. J Pediatr Psychol. 2006;31:714–723. doi: 10.1093/jpepsy/jsj064. [DOI] [PubMed] [Google Scholar]
- 103.Sil S, Arnold LM, Lynch-Jordan A, Ting TV, Peugh J, Cunningham N, Powers SW, Lovell DJ, Hashkes PJ, Passo M, et al. Identifying Treatment Responders and Predictors of Improvement after Cognitive-Behavioral Therapy for Juvenile Fibromyalgia. Pain. 2014;155:1206–1212. doi: 10.1016/j.pain.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an Internet-delivered family cognitive-behavioral therapy intervention for children and adolescents with chronic pain. Pain. 2009;146:205–213. doi: 10.1016/j.pain.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stinson JN, McGrath PJ, Hodnett ED, Feldman BM, Duffy CM, Huber AM, Tucker LB, Hetherington CR, Tse SM, Spiegel LR, et al. An internet-based self-management program with telephone support for adolescents with arthritis: a pilot randomized controlled trial. J Rheumatol. 2010;37:1944–1952. doi: 10.3899/jrheum.091327. [DOI] [PubMed] [Google Scholar]
- 106.Hauser W, Klose P, Langhorst J, Moradi B, Steinbach M, Schiltenwolf M, Busch A. Efficacy of different types of aerobic exercise in fibromyalgia syndrome: a systematic review and meta-analysis of randomised controlled trials. Arthritis Res Ther. 2010;12:R79. doi: 10.1186/ar3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hooten WM, Qu W, Townsend CO, Judd JW. Effects of strength vs aerobic exercise on pain severity in adults with fibromyalgia: a randomized equivalence trial. Pain. 2012;153:915–923. doi: 10.1016/j.pain.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 108.van Koulil S, Effting M, Kraaimaat FW, van Lankveld W, van Helmond T, Cats H, van Riel PL, de Jong AJ, Haverman JF, Evers AW. Cognitive-behavioural therapies and exercise programmes for patients with fibromyalgia: state of the art and future directions. Ann Rheum Dis. 2007;66:571–581. doi: 10.1136/ard.2006.054692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jones KD, Adams D, Winters-Stone K, Burckhardt CS. A comprehensive review of 46 exercise treatment studies in fibromyalgia (1988-2005) Health Qual Life Outcomes. 2006;4:67. doi: 10.1186/1477-7525-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sherry DD. Amplified musculoskeletal pain: treatment approach and outcomes. J Pediatr Gastroenterol Nutr. 2008;47:693–694. doi: 10.1097/01.mpg.0000338962.17185.18. [DOI] [PubMed] [Google Scholar]
- 111.Sherry DD. Physical activity levels in the treatment of juvenile fibromyalgia. Nat Rev Rheumatol. 2013;9:8–9. doi: 10.1038/nrrheum.2012.193. [DOI] [PubMed] [Google Scholar]
- 112.Stephens S, Feldman BM, Bradley N, Schneiderman J, Wright V, Singh-Grewal D, Lefebvre A, Benseler SM, Cameron B, Laxer R, et al. Feasibility and effectiveness of an aerobic exercise program in children with fibromyalgia: results of a randomized controlled pilot trial. Arthritis Rheum. 2008;59:1399–1406. doi: 10.1002/art.24115. [DOI] [PubMed] [Google Scholar]
- 113.Wilson AC, Palermo TM. Physical activity and function in adolescents with chronic pain: a controlled study using actigraphy. J Pain. 2012;13:121–130. doi: 10.1016/j.jpain.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sil S, Thomas S, DiCesare C, Strotman D, Ting TV, Myer G, Kashikar-Zuck S. Preliminary evidence of altered biomechanics in adolescents with juvenile fibromyalgia. Arthritis Care Res. 2015;67:102–111. doi: 10.1002/acr.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.American Pain Society. Guideline for the management of fibromyalgia syndrome pain in adults and children. American Pain Society. 2005 [online] [Google Scholar]
- 116.Kashikar-Zuck S, Flowers SR, Strotman D, Sil S, Ting TV, Schikler KN. Physical activity monitoring in adolescents with juvenile fibromyalgia: findings from a clinical trial of cognitive-behavioral therapy. Arthritis Care Res. 2013;65:398–405. doi: 10.1002/acr.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kashikar-Zuck S, Ting TV. Juvenile fibromyalgia: current status of research and future developments. Nat Rev Rheumatol. 2014;10:89–96. doi: 10.1038/nrrheum.2013.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.American Pain Society. Assessment and management of children with chronic pain: a position statement from the American Pain Society. American Pain Society. 2012 [Google Scholar]
- 119.Tesher MS. Juvenile Fibromyalgia: A Multidisciplinary Approach to Treatment. Pediatr Ann. 2015;44:e136–141. doi: 10.3928/00904481-20150611-08. [DOI] [PubMed] [Google Scholar]
- 120.Simons LE, Sieberg CB, Pielech M, Conroy C, Logan DE. What does it take? Comparing intensive rehabilitation to outpatient treatment for children with significant pain-related disability. J Pediatr Psychol. 2013;38:213–223. doi: 10.1093/jpepsy/jss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sherry DD, Wallace CA. Resolution of fibromyalgia with an intensive exercise program. Clin Exp Rheumatol. 1992;10:196. [Google Scholar]
- 122.Tran ST, Thomas S, DiCesare C, Pfeiffer M, Sil S, Ting TV, Williams SE, Myer GD, Kashikar-Zuck S. A pilot study of biomechanical assessment before and after an integrative training program for adolescents with juvenile fibromyalgia. Pediatr Rheumatol Online J. 2016;14:43. doi: 10.1186/s12969-016-0103-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tran ST, Guite JW, Pantaleao A, Pfeiffer M, Myer GD, Sil S, Thomas SM, Ting TV, Williams SE, Edelheit B, et al. Preliminary outcomes of a cross-site cognitive-behavioral and neuromuscular integrative training intervention for juvenile fibromyalgia. Arthritis Care Res. 2016 doi: 10.1002/acr.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kashikar-Zuck S, Tran ST, Barnett K, Bromberg MH, Strotman D, Sil S, Thomas SM, Joffe N, Ting TV, Williams SE, Myer GD. A Qualitative Examination of a New Combined Cognitive-Behavioral and Neuromuscular Training Intervention for Juvenile Fibromyalgia. Clin J Pain. 2016;32:70–81. doi: 10.1097/AJP.0000000000000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kashikar-Zuck S, Cunningham N, Sil S, Bromberg MH, Lynch-Jordan AM, Strotman D, Peugh J, Noll J, Ting TV, Powers SW, et al. Long-Term Outcomes of Adolescents With Juvenile-Onset Fibromyalgia in Early Adulthood. Pediatrics. 2014;133:e592–e600. doi: 10.1542/peds.2013-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gregoire MC, Finley GA. Drugs for chronic pain in children: a commentary on clinical practice and the absence of evidence. Pain Res Manag. 2013;18:47–50. doi: 10.1155/2013/402863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaufman EL, Tress J, Sherry DD. Trends in Medicalization of Children with Amplified Musculoskeletal Pain Syndrome. Pain Med. 2016 [Google Scholar]
- 128.Tian F, Guittar P, Bout-Tabaku S. Chronic Pain in Children Seen at a Rheumatology Clinic: Healthcare Utilization Patterns [abstract] Arthritis Rheumatol. 2016;68(suppl 10) [Google Scholar]