Abstract
In 2007, the clinical and research profile of illusions, hallucinations, delusions and related symptoms in Parkinson disease (PD) was raised with the publication of a consensus definition of PD psychosis. Symptoms that were previously deemed benign and clinically insignificant were incorporated into a continuum of severity, leading to the rapid expansion of literature focusing on clinical aspects, mechanisms and treatment. Here, we review this literature and the evolving view of PD psychosis. Key topics include the prospective risk of dementia in individuals with PD psychosis, and the causal and modifying effects of PD medication. We discuss recent developments, including recognition of an increase in the prevalence of psychosis with disease duration, addition of new visual symptoms to the psychosis continuum, and identification of frontal executive, visual perceptual and memory dysfunction at different disease stages. In addition, we highlight novel risk factors — for example, autonomic dysfunction — that have emerged from prospective studies, structural MRI evidence of frontal, parietal, occipital and hippocampal involvement, and approval of pimavanserin for the treatment of PD psychosis. The accumulating evidence raises novel questions and directions for future research to explore the clinical management and biomarker potential of PD psychosis.
In 2007, consensus recommendations from an international work group redefined the landscape of psychosis in Parkinson disease (PD)1. Symptoms of psychosis, such as illusions, hallucinations and delusions, are collectively referred to as ‘positive’, carrying the implication of an excess of function or brain activity, in contrast with ‘negative’ symptoms of deficit. Though all long recognized as nonmotor manifestations of PD, positive symptoms such as illusions, hallucinations and delusions were traditionally considered to be distinct from one another, with different clinical implications. In particular, hallucinations of a person, animal or indefinite object passing through the peripheral visual field (passage hallucinations), misperception of actual stimuli (illusions) and a distinct class of perceptual experiences without visual content but a ‘feeling’ of someone present (presence hallucinations) were not thought to carry the same clinical significance as formed visual hallucinations of animals, objects or figures. This distinction was reflected in the terminology, whereby illusions and passage and presence hallucinations were described as ‘benign’ or ‘minor’ hallucinations.
After reviewing the available evidence, the combined National Institute of Neurological Disorders and Stroke (NINDS) and National Institute of Mental Health (NIMH) work group concluded that instead of representing distinct symptom classes, the various phenomena formed a continuum progressing over the course of PD. The typical clinical scenario was of minor phenomena evolving to formed visual hallucinations, with insight initially preserved but lost in later stages, and with delusions and non-visual — for example, auditory, tactile and olfactory — hallucinations also developing (BOX 1). This spectrum of positive symptoms was termed ‘PD psychosis’, and the proposed diagnostic criteria included the chronology of symptoms (occurrence after the onset of PD) and symptom duration (recurrent or continuous for 1 month). The NINDS–NIMH recommendations led to a paradigmatic shift in focus from individual positive symptoms to a symptom continuum, which was further characterized in a series of landmark reviews published in the years that followed2–8. These reviews benchmarked knowledge of PD psychosis in terms of its phenomenology, prevalence, clinical implications, mechanisms, treatment and future research directions, and led to a rapidly expanding literature. In this Review, we reflect on the advances that have been made in the 10 years since the publication of the consensus work group criteria, highlighting key research findings and directions in PD psychosis, and their implications for clinical practice and the neuroscience of PD.
Box 1. The psychosis spectrum in Parkinson disease.
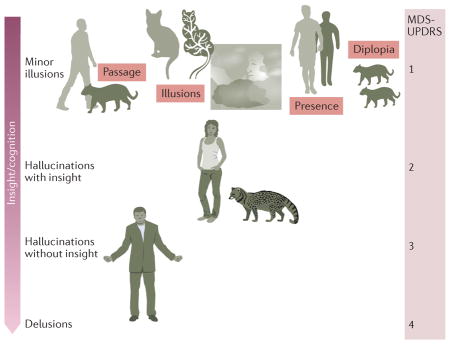
Positive symptoms in Parkinson disease (PD) vary across its course. Early in the disease, symptoms experienced include passage hallucinations (where a person, animal or indefinite object is seen briefly passing in the peripheral visual field), illusions (for example, seeing the branch of a tree as a cat), and presence hallucinations (a feeling that someone is nearby). Pareidolia refers to a specific class of illusion where faces and objects are seen in formless visual stimuli, such as clouds, flames or tree bark, or in geometric visual patterns, such as carpets or wallpaper. This type of illusion can occur as a normal perceptual experience, but is increased in frequency in PD159 and related disorders such as dementia with Lewy bodies.
Later in PD, formed visual hallucinations, typically of animals or people, occur. Insight — that is, recognition that the experiences are hallucinations — is preserved at this stage, but becomes lost as PD progresses, with the onset of false beliefs (delusions) and hallucinations in other sensory modalities (multimodality hallucinations). Cognitive decline and loss of insight parallel symptom progression, as illustrated by the transition in the arrow on the left from dark shading (cognition/insight intact) to light shading (cognition/insight impaired).
Approximate ratings of the Movement Disorder Society United PD Rating Scale (MDS-UPDRS) hallucination/psychosis item are shown on the right of the figure, marking progression through different categories of experience on the continuum. The terms pseudohallucination and hallucinosis are used as synonyms for hallucinations with insight in some psychiatric and neurological traditions, but also have other meanings and were not adopted by the NINDS–NIMH work group1.
Phenomenology and diagnosis
As described by the NINDS–NIMH consensus work group1, symptoms of the psychosis spectrum in early stages of PD include minor experiences, such as passage and presence hallucinations, illusions, and formed hallucinations — most commonly, recurring visual hallucinations of people, animals or inanimate objects — with insight preserved (BOX 1). In later PD stages, delusions and hallucinations occur in other modalities, for example, auditory hallucinations consisting of a voice that may not be comprehensible9, or non-verbal sounds, such as steps or music10. The hallucinations tend to occur in conditions of low ambient stimulation, typically when the individual is alone in a quiet environment, are experienced several times a day, and last for seconds to minutes in the early stages of PD psychosis. Evidence that has emerged since the publication of the consensus work group recommendations has helped to further characterize the phenomenology of different PD psychosis stages, and their relationship with the progression of Lewy body pathology (BOX 2).
Box 2. Psychosis spectrum progression and Braak stage.
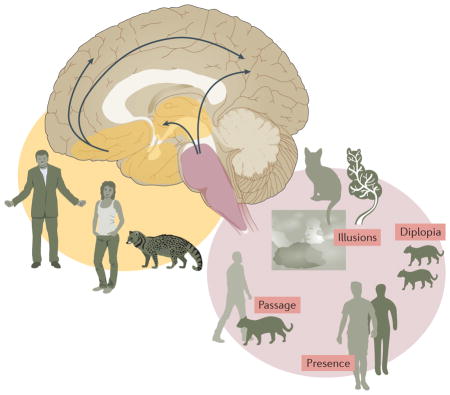
The fact that illusions, passage and presence hallucinations progress to formed visual hallucinations in Parkinson disease (PD) need not imply that they all have the same underlying mechanism. Evidence has emerged that minor hallucinations and formed visual hallucinations are associated with different sets of risk factors. For example, presence hallucinations have been linked to sleep regulation and somnolence scores, illusions are related to somnolence and motor severity scores160, and neurobiological explanations of passage hallucinations implicate dysfunctional brainstem eye movement control mechanisms and subcortical and cortical motion pathways, including dorsal stream areas in the visual parietal lobe105,161. Previous studies of visual hallucinations have linked visual parietal areas to hallucinations in the peripheral visual field162 (as described for passage hallucinations) and palinopsia (helping to account for palinparousia — recently decribed palinopsia-like presence hallucinations11), and subregions of the intraparietal sulcus are linked to eye movement control163. By contrast, formed visual hallucinations are associated with changes in cognition, visual function and affect160, probably reflecting cortical involvement in ventral occipitotemporal lobe regions162. The onset of minor hallucinations might even precede the development of motor symptoms28.
The difference in timing of and risk factors for minor hallucinations and formed visual hallucinations mirrors the Braak progression of Lewy body pathology from brainstem to forebrain systems systems164. This observation suggests that symptom progression along the PD psychosis continuum begins with isolated minor experiences, indicating brainstem pathology (pink shading in figure) and its wider impact on subcortical and cortical motion and eye movement control networks, including the visual parietal lobe (arrows — note that dysfunction within these cortical networks does not necessarily imply cortical Lewy body pathology (BOX 4)). Subsequent emergence of visual hallucinations with insight indicates basal forebrain involvement (orange shading) and its wider impact on cortical cholinergic projections (arrows), in particular, projections to the ventral occipitotemporal cortex. Finally, multimodality hallucinations, delusions and loss of insight indicate widespread cortical Lewy body pathology.
Early PD psychosis symptoms
According to a report on the largest series of PD patients with presence hallucinations to date, the ‘presence’ is seen as an unformed visual shadow or mist in up to one-third of cases, may or may not be recognizable, and can be a persistent or recurrent experience of someone who has just left the room (palinparousia)11. An online questionnaire administered to individuals with PD suggested an overall prevalence of 50% for presence and passage hallucinations (‘extracampine hallucinations’ was used as a collective term for both experiences, although this differs from its classic usage), with individual prevalences of 25% for presence hallucinations and 46% for passage hallucinations12. Studies of formed hallucinations in PD have found an association between the cognitive profile and the type of hallucination experienced13: patients with PD whose typical hallucinations are of unfamiliar content (for example, unidentified figures) have more-profound deficits of inhibitory executive function than do patients with hallucinations that are recognized (for example, family members). Additional visual symptoms that are observed in the early stages of PD and might be considered to belong to the psychosis spectrum are listed in TABLE 1.
Table 1.
Symptoms related to the PD psychosis spectrum
| Symptom | Phenomenology | Association with psychosis spectrum |
|---|---|---|
| Isolated diplopia179 | Image doubling related to an isolated object or figure — as opposed to the whole scene — in the visual environment | May precede the onset of visual hallucinations by 2–3 years |
| Freezing180 | Walking cessation when passing through narrow spaces | Associated with passage hallucinations in PD without cognitive impairment |
| Spatial misjudgement180 | Difficulty estimating distance of objects when walking | Associated with presence and passage hallucinations |
PD, Parkinson disease.
Later PD psychosis symptoms
As PD progresses, hallucinations in non-visual (auditory, tactile and olfactory) modalities occur alongside visual hallucinations. In one cohort of PD patients without hallucinations at baseline, 60% were experiencing hallucinations in multiple sensory modalities by 10 years of follow-up14,15. These non-visual hallucinations are not confined to end-stage PD dementia; they are also found in patients whose cognition is relatively intact (Mini-Mental State Examination (MMSE) score 24 or 25)16,17.
One study has estimated the prevalence of delusions in a PD clinical setting at 16%18, rising to 47% in the subset of patients with PD psychosis18. Of note, an earlier study of patients with PD psychosis taking part in a clinical trial indicated an even higher prevalence of delusions (76%)16. The delusional themes that are identified depend on the assessment instruments used, but they typically encompass sin or guilt, grandiosity, reference, religion, persecution, jealousy, and theft, without prominence of any specific theme16. Delusional mis-identification syndromes, a specific subset of delusions characterized by pathological familiarity, include the Capgras delusion (the belief that someone familiar has been replaced by an imposter), reduplicative paramnesia (the belief that a room or place has been duplicated and is present at two locations simultaneously) and the mirror sign (failure to recognize oneself in the mirror)19,20. In patients who have dementia with Lewy bodies (DLB), the prevalence of misidentification syndromes was found to increase with greater cognitive decline (40% for MMSE score >20 compared with 60% for MMSE score <10)21. A similar trend was observed in individuals with AD, although the prevalence was lower overall (18% for MMSE score >20 and 28% for MMSE score <10). A smaller-scale study of patients with PD dementia found a 16.7% prevalence of misidentification symptoms, and also noted an association between these symptoms and a specific profile of memory and language deficits20.
Assessment scales
Several scales to measure positive symptoms were available in 2007 (REF. 22), but none were specifically designed for use in PD. As a result, many of the studies reviewed here used a definition of psychosis spectrum status that was based on a single question item within the Movement Disorder Society United PD Rating Scale (MDS-UPDRS), with a range of 0–4 (BOX 1). New scales have emerged to better capture the diversity of symptoms and for use in clinical trials, including the North-East Visual Hallucinations Interview (NEVH-I)23 and a shortened version of the Scale for Assessment of Positive Symptoms adapted for PD (SAPS-PD)24. A test to measure susceptibility to pareidolia has been developed for DLB25, and might also have a role in the assessment of early symptoms of PD psychosis.
Frequency and clinical consequences
As concluded by the NINDS–NIMH psychosis work group and related reviews, psychosis spectrum symptoms are common in PD and have important clinical consequences, in particular, an increased risk of dementia, nursing home placement and mortality. Quality of life is also impaired, both overall and in subdomains of emotional well-being, daily living, cognition and bodily discomfort26. Recent studies have provided revised prevalence estimates for PD psychosis based on longitudinal study data, and have better characterized the link to poor cognitive outcome.
Prevalence
In early cross-sectional studies, the prevalence of the psychosis spectrum varied depending on which symptoms were included, and which PD population was studied. For example, estimates of complex visual hallucinations ranged from 22–38%, compared with 0–22% for auditory hallucinations3. Prevalence estimates from older studies that excluded minor hallucinations required revision after the publication of the consensus guidelines27. According to the latest figures, minor phenomena are reported in 42% of patients with very early disease28, although this high estimate could reflect recall bias29. In a large-scale study (n= 250) that excluded patients with MMSE scores ≤23, the prevalence of PD psychosis was 26%30.
Longitudinal studies conducted in the wake of the consensus guidelines have established that the prevalence of the psychosis spectrum is time dependent, increasing with the duration of PD3,31–33. In a study that used detailed phenomenological interviews31, 82.7% of patients experienced psychosis spectrum symptoms over a period of 36 months. In another study, which excluded minor hallucinations, 60% of patients had experienced psychosis spectrum symptoms by the end of a 12-year follow-up period33. The Parkinson’s Progression Markers Initiative (PPMI) reported an increase in the prevalence of psychosis from 3% around the time of PD diagnosis to 10% at 2 years’ follow-up32.
Risk of cognitive decline
An association between visual hallucinations and the subsequent emergence of dementia was first reported in 2003 (REF. 34). Patients who reported previous visual hallucinations at study entry had an increased risk of dementia at 8 years (OR 3.1, 95% CI 1.6–6.2). This association has been replicated in several recent studies of shorter follow-up duration. One study35 found that visual hallucinations and visual illusions at baseline had separate predictive effects for dementia at ~4 years, and another study36 found that the longitudinal emergence of visual hallucinations — that is, emergence across the duration of the study — increased the risk of dementia at ~6 years. The PRIAMO study of early PD found a decline in cognitive function at 2 years in patients with psychosis spectrum symptoms37. The detailed profile of cognitive decline over 1–2 years has been reported in prospective cohorts defined at baseline by the presence or absence of visual hallucinations38,39. A significant group × time interaction was found, with a steeper decline across a range of cognitive domains and an increased risk of dementia at follow-up in patients with visual hallucinations. A recent metabolic imaging study of PD-associated mild cognitive impairment (PD-MCI) has also found elevated rates of dementia at 30-month follow-up in patients with visual hallucinations40.
Psychosis spectrum symptoms are not the sole predictors of future cognitive decline in PD: a range of factors have been identified, the interrelationship of which has yet to be clarified. According to multivariate analyses34,36, visual hallucinations are independent of other factors, but their predicitive statistical significance is reduced when cognitive measures are included36, suggesting that cognition and visual hallucinations are closely related.
Association with motor subtypes
Exploratory and confirmatory cluster analysis of a large PD data set revealed associations between non-tremor-dominant PD and psychopathology, including hallucinations and cognitive impairment41. The association between clinically defined non-tremor subtypes and dementia, but not the association with hallucinations, was replicated in a subsequent study42. This discrepancy between studies might be attributable to differences in methodology.
Mechanisms and risk factors
A range of possible mechanisms to explain the psychosis spectrum, including changes in visual function, sleep, medication effects and cognition, were identified by the NINDS–NIMH work group and in related reviews, with unifying models being presented to summarize the proposed interrelationship between these mechanisms1–3.
The eye and low-level vision
The predominantly visual nature of PD psychosis spectrum symptoms points to dysfunction in the visual system, and early studies focused on low-level deficits in colour vision, contrast sensitivity and acuity (reviewed elsewhere2). More recently, evidence has emerged of retinal changes in PD, as measured by techniques such as ocular coherence tomography (OCT) and electroretinography43; however, to date, only one study using such techniques has investigated the retinal associations of the PD psychosis spectrum. In patients with visual hallucinations, OCT revealed thinning of the retinal ganglion cell layer in the dominant eye at both nasal and temporal retinal locations44. Such thinning could be the result of a primary process within the eye, or could be secondary to retrograde trans-synaptic degeneration caused by changes in the brain. Thinning was more pronounced in PD psychosis patients without dementia (as measured by MMSE score), pointing to a primary process within the eye.
Visual perception and cognition
Early studies of the psychosis spectrum focused on visual hallucinations, and included patients with a range of severity of what might now be termed PD-MCI. More-recent studies have concentrated on differences in cognitive profiles for specific symptom subtypes, and the cognitive profile of the psychosis spectrum at different stages of global cognitive impairment. TABLE 2 shows a range of cognitive tests that have been repeated in different studies, although the same version of a given test is rarely used by different groups. Most studies investigating visual perception, irrespective of the selection criteria (for example, unselected PD-MCI cohorts versus participants selected for higher cognitive function) have shown deficits in the perception of objects, including faces. Similarly, most studies investigating executive function, as measured by the Stroop task, response inhibition and verbal fluency, have found deficits in patients with visual hallucinations.
Table 2.
Visual and cognitive deficits in the PD psychosis spectrum
| Domain | Subdomain or task | Significant deficit associated with PD psychosis spectrum | Minor hallucinations | |
|---|---|---|---|---|
|
| ||||
| Unselected PD-MCI | MMSE score >25 | |||
| Visual perception | Object | Yes51,55,181 | Yes47,55 | – |
| Face | Yes45 | – | – | |
| Spatial | Yes47,51 | – | – | |
|
| ||||
| Executive function | Stroop | Yes46,49,182,183 | Yes13 | – |
| Go–no-go | Yes46,182 | Yes13 | – | |
| Verbal fluency | Yes45,49,182–184; No50 | Yes13 | No52 | |
|
| ||||
| Attention | – | Yes51,181 | – | No52 |
|
| ||||
| Memory | Visual | Yes45,46,49 | Yes47 | – |
| Verbal | Yes45,49,184; No50 | – | No52 | |
‘Yes’ and ‘No’ refer to presence and absence, respectively, of significant deficit reported in studies cited; ‘–’ indicates test not performed. In most studies, PD psychosis spectrum was defined by the presence of visual hallucinations. Studies that pooled z-transformed scores from a range of tests into a single domain summary (for example, Goldman et al.101) are not included, as individual test scores were lost. Studies using evidence from items within global assessment scales such as MOCA (for example, Chung et al.185) or SCOPA-COG (for example, Gallagher et al.55) are also excluded. MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; PD, Parkinson disease.
Deficits in memory and related hippocampal function tests are found in PD patients with visual hallucinations, in both unselected PD-MCI and higher-function groups. These deficits withstand correction for MMSE score, depression and PD stage45. Some studies have reported deficits that are specific to visual — as opposed to verbal — memory46. Greater memory deficits have also been found for black-and-white visual stimuli than for coloured visual stimuli, perhaps reflecting more-limited encoding of visually impoverished stimuli in patients with visual hallucinations47. A transitive inference test thought to measure hippocampal function has uncovered impairments in patients with PD psychosis48.
TABLE 2 helps to illustrate agreement between studies regarding cognitive functions that are deficient, but it does not reveal whether some cognitive functions show relative sparing. Tests purporting to measure visuospatial function, such as the Benton judgement of line orientation and the Rey complex figure test, are often included in test batteries for PD psychosis, but tend not to show significant differences46,49 (although see Katzen et al.50 for evidence of deficits on the Benton test). The lack of consistent findings could be a test sensitivity issue, as other measures of visuospatial function — for example, the Visual Object and Space Perception (VOSP) number location subtest47,51 — have been shown to differ between patients with and without visual hallucinations. Tests examining motion, spatiotemporal aspects of visual perception, and eye movements in the PD psychosis spectrum have not been reported to date, but are of particular relevance given the hypothesized role of dysfunctional motion perception and eye movement control in passage and presence hallucinations.
Minor hallucinations
Only one study has focused on the cognitive profile associated with minor hallucinations in PD52. Compared with patients without psychosis spectrum symptoms, patients with minor hallucinations had no significant differences in verbal memory, verbal fluency or sustained attention. Progressive decreases in cognitive performance were seen across the spectrum from no hallucinations, through minor hallucinations and hallucinations with insight, to hallucinations without insight. The lack of cognitive deficits in patients with minor hallucinations raises the issue of whether the onset of psychosis spectrum symptoms predates measurable cognitive decline.
Multimodal hallucinations
One might expect patients with non-visual hallucinations in the later stages of PD to exhibit greater cognitive deficits or a different cognitive profile compared with PD patients with visual hallucinations alone. However, one study that compared patients with visual only and visual plus non-visual hallucinations50 found no evidence of significant additional decline in the latter group for any of the cognitive domains tested (including verbal memory and verbal fluency). The number of patients with hallucinations in multiple modalities was small (n = 12), so the study might have been underpowered to detect such changes.
Delusions
Only one study has compared cognitive profiles between PD psychosis spectrum patients with hallucinations but not delusions and those with delusions but not hallucinations18. Cognitive profiles did not differ significantly between the two groups. Cognitive scores in a range of domains were found to correlate with the severity and number of hallucination modalities (for example, auditory, olfactory somatic and/or visual), but not with the severity and number of delusion modalities (for example, jealousy, grandiosity and/or religious delusions). These findings suggest that cognitive risk factors for hallucinations and delusions might differ. This view is supported by evidence from a study investigating risk factors for a specific delusion (delusional jealousy), where an association was found with dopamine agonist treatment but not dementia, in contrast to hallucinations, where an association was found with dementia but not dopamine agonist treatment53.
Neuropathology
Neuropathological studies provide important insights into the distribution of Lewy body pathology at different stages of the PD psychosis spectrum (BOX 2). The first study to report on this issue found significantly higher Lewy body load in the amygdala and parahippocampal gyrus in patients with visual hallucinations, half of whom met the clinicopathological criteria for PD and half of whom met the criteria for DLB54. Subsequent studies have replicated the finding of limbic pathology associated with visual hallucinations55,56, with increased pathology also found in other regions, including the superior and lateral frontal cortex (Brodmann area 8/9), inferior and lateral temporal cortex (Brodmann areas 20 and 21), inferior parietal cortex (Brodmann areas 39 and 40), and cingulate cortex (Brodmann area 24). Visual hallucinations are also linked to higher levels of amyloid and tau pathology in frontal, parietal and hippocampal areas57. Only one study has investigated the occipital lobe in patients with visual hallucinations: Lewy body and tau pathologies were found to be absent, and amyloid burden was rated as mild58.
Unlike patients with PD psychosis who have dementia, those without dementia do not have cortical Lewy body involvement. An early study of PD patients with visual hallucinations and MMSE scores >25 found increased Lewy body load in the basolateral nucleus of the amygdala, but only sparse Lewy bodies in the cortex and hippocampus59. Consistent with the lack of cortical involvement, a recent study has found no association between Lewy body, tau or amyloid pathology in frontal, parietal and temporal regions and visual hallucinations after controlling for dementia, but found strong associations with dementia after controlling for visual hallucinations58. Such findings suggest that the neuropathological changes underlying visual hallucinations are distinct from those underlying dementia.
Involvement of the cholinergic system in visual hallucinations is suggested by brainstem atrophy in the pedunculopontine nucleus60 and atrophy of the substantia innominata, which contains the nucleus basalis of Meynert46. The cause of the atrophy is unclear, as no association has been found between visual hallucinations and Lewy body, tau or amyloid load in the nucleus basalis of Meynert58.
Non-cognitive risk factors
Patients with visual hallucinations exhibit abnormalities on a range of autonomic tests, including the tilt-table test, the Valsalva manouevre and cardiac 123I-metaiodobenzylguanidine imaging, indicating sympathetic dysfunction61. In addition, autonomic dysfunction was found to be an independent risk factor for hallucinations (all modalities included) in PROPARK, a large-scale 5-year prospective study62. The same study also reported female sex as an independent risk factor62. REM sleep behaviour disorder (RBD) is also associated with visual hallucinations (recently reviewed elsewhere63), although the association is weak64, and the proportions of patients with visual hallucinations who do and do not have RBD are similar. Vivid dreams are associated with visual hallucinations after controlling for factors including PD duration, depression, anxiety and UPDRS scores65. Depression has been associated with hallucinations in PD2. The urinary concentration of the oxidative stress marker 8-hydroxydeoxyguanosine was found to correlate with the hallucination score (modality unspecified) after controlling for MMSE score, age, duration and UPDRS part 3 score66.
Dopamine agonists
The role of medication in PD psychosis was noted to be “the most controversial aspect of these criteria” in the 2007 consensus recommendations1. Clinical experience suggested that the onset of PD psychosis could be linked to medication onset and improved with dose reduction, but there was no evidence of a direct causal relationship between pharmacological treatments or medication dose and PD psychosis (for example, levodopa infusion did not induce hallucinations67), and PD psychosis was recognized to occur in unmedicated patients.
In the intervening years, the role of medication has received relatively little research attention. The lack of a direct causal role for medication is supported by a high prevalence of minor symptoms28 or visual hallucinations68 in drug-naive patients, and the finding that levodopa dose equivalence did not increase the risk of psychosis in some prospective studies14,31, including the PPMI cohort32. However, though failing to reach statistical significance, a link to medication is implied in the threefold increase in psychosis onset for patients starting dopamine replacement therapy in the PPMI study. Similarly, a large-scale prospective study identified elevated baseline levodopa dose as a risk factor for PD psychosis, with an odds ratio of 1.26 per 100 mg of dose equivalence increase33, and a prospective study of early PD (PRIAMO) found increased rates of dopamine agonist treatment in patients who went on to develop PD psychosis37. The PROPARK study also identified dyskinesia and levodopa equivalence measures as independent risk factors for PD psychosis62.
The NINDS–NIMH work group1 suggested “anti-parkinsons medication as a modifier rather than a necessary feature for the diagnosis of PD psychosis” to help reconcile the medication-related and medication-independent viewpoints. Evidence that has since emerged supports this ‘modifier’ account, but highlights the need for further work in this area to clarify the relationship between dopamine replacement therapy and PD psychosis.
Genetics
A recent review of the genetics of psychotic symptoms in PD identified 26 published articles on this topic69. The most commonly studied genetic factor is the apolipoprotein E ε4 (APOE*ε4) allele, although dopaminergic pathway genes have been the focus of a number of studies.
With regard to APOE*ε4, little evidence is available to suggest an association with psychotic symptoms in PD (only one of seven studies reported an association with visual hallucinations)70–76, a finding that is mirrored in the AD literature77. The extended microtubule-associated protein tau (MAPT) haplotype is less well studied in PD psychosis: an initial study reporting an association between visual hallucinations and the H1 haplotype was not replicated by a second larger study70,71. By contrast, an increasing body of evidence suggests that glucosylceramidase (GBA) mutations are associated with psychosis in PD78–80. Of note, the influence of GBA is not limited to psychosis: GBA, like APOE and MAPT, has been associated with cognition in PD, as well as faster progression to dementia and more-severe motor stages79,81. Any associations between psychosis and these three genes are perhaps most likely to be driven by fundamental disease processes, such as tau or Lewy body accumulation. Therefore, considering any associations with a particular symptom in isolation would be too narrow an approach. Specifically, it will be important to establish whether any subtypes of PD have a pattern of cognitive impairment that is more similar to AD and carries a relatively low risk of psychosis (which might be predicted by APOE), compared with an early dysexecutive syndrome and greater risk of hallucinations (as indicated by the pattern of association with GBA).
Consistent evidence links GBA, but not APOE, with psychosis in PD; however, findings related to other genes have been equivocal. Polymorphisms in dopaminergic pathway genes have been examined in 15 studies (BOX 3). Although there is some evidence of a relationship between polymorphisms in dopamine transporter and cholecystokinin genes and psychosis, the overall number of studies examining these associations is small, making it difficult to draw reliable conclusions. Research in serotonin (5-hydroxytryptamine, or 5-HT) pathway genes is limited to two studies82,83, but with the recent demonstration of efficacy of pimavanserin for the treatment of PD psychosis (see below), serotonergic genes or other genes in serotonergic signalling pathways may provide promising avenues for future research.
Box 3. Genetic risk factors for psychosis in Parkinson disease.
GBA
Associated with an increased risk of psychosis in three studies78–80. One study including only two cases with hallucinations found no association165.
MAPT
Mixed findings: one positive association and one negative71,72.
APOE
No association (in six of seven studies). The APOE*ε4 allele was associated with hallucinations within 5 years of diagnosis in one study70–76.
COMT
SLC6A3 (also known as DAT1)
Mixed findings: two of three studies reported an association, one with the variable number tandem repeat and one with the 839C>T polymorphism167–169.
DRD2
Mixed findings: one of four studies reported an association73,167,168,170.
DRD3
DRD4
CCK
Mixed findings: two of four studies show evidence of an association with CCK 45C>T, and one reported a combined effect with the CCKAR CC genotype171–174.
HTR2A
No association83.
SLC6A4
One study reported an increased risk associated with the LL genotype in a mixed Lewy body dementia sample82.
ACE
Mixed findings: one study reported a significant association with levodopa-induced psychosis, whereas another reported no association175,176.
HOMER1
Mixed findings: one study reported an association with the A allele, and a second reported an association with the G allele and a reduced risk of adverse effects of levodopa treatment177,178.
The genetic aetiology of psychosis in PD will be complex, and is likely to involve many genes each with a small effect size. As such, the most promising genetic approaches will probably be those that leverage genome-wide data, which has the advantage of full genome coverage and is not constrained by biases resulting from a priori hypotheses. In the broader literature, recent advances in the analysis of genome-wide data sets have uncovered genetic pleiotropy between clinically distinct diseases84,85. Moreover, analyses whereby biological pathways (for example, molecular, cellular, organ/system or disease) are constructed on the basis of genomic data and tested for enrichment of single nucleotide polymorphisms in cases versus controls have shown promise in late-life depression86. These types of emerging techniques have clear applications in heterogeneous conditions such as PD, where questions surrounding the overlapping aetiology between psychosis across neurodegenerative diseases and psychiatric disorders remain unanswered. The increasing availability of larger, better-characterized data sets will provide greater potential to conduct these more-sophisticated genetic analyses.
Explanatory models
Explanatory models of PD psychosis attempt to integrate risk factors into unifying accounts (reviewed elsewhere87). The Activation–Input–Modulation (AIM) model88 takes a dimensional approach to PD psychosis, with contributions from an activation factor (the level of arousal), an input factor (the spectrum of externally and internally driven visual percepts), and a modulation factor (neurotransmitter and medication effects). The activation factor may help to explain why hallucinations typically occur with low ambient stimulation. The Perception and Attention Deficit (PAD) model89 argues that the core mechanism of complex hallucinations is dysfunctional integration of perceptual and attentional processing, leading to the intrusion of generic representations (proto-objects) into perceptual consciousness. In the attentional control model90, the core pathology of PD psychosis is dysfunctional integration between three networks (dorsal attention network, ventral attention network and default mode network), with a failure to engage the dorsal attention network in the context of ambiguous visual input and the intrusion of default mode contents into perceptual consciousness90–92. This account is supported by evidence from PD patients with visual hallucinations or illusions of elevated strength of mental imagery93, attentional set-shifting deficits94, altered resting state and task activation networks95, and increased functional connectivity in the default mode network96. A dopamine-based model of PD psychosis97 argues for abnormalities of biasing and gating access to a global workspace that is thought, according to some accounts, to underlie conscious experience.
These unifying models are informed by clinical associations — for example, the combination of visual perceptual and frontal executive deficits — that were recognized at the time that the models were formulated. As the range of risk factors increases, the question arises as to whether all risk factors are linked to the mechanisms underlying PD psychosis. Distinctions may need to be drawn between associations that are integral to the mechanisms of PD psychosis or its individual symptoms, and syndromic associations caused by the spread of PD pathology to neighbouring brain regions or to separate brain regions at a similar disease stage. Which associations and risk factors are integral to PD psychosis and which are syndromic associations is unclear at present, and such information will be pivotal to the development of novel treatment approaches.
Imaging and electrophysiology
The raised profile of PD psychosis, combined with developments in imaging methodology, has resulted in a rapidly advancing evidence base that helps to inform the risk factor and mechanistic accounts of the symptom spectrum.
Structural MRI
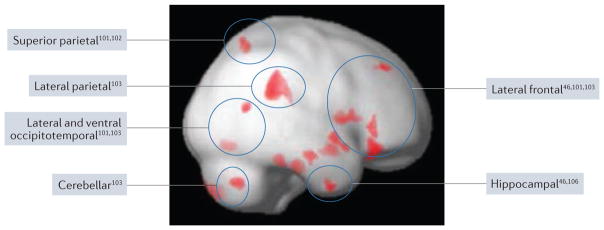
Despite methodological differences, structural imaging studies of visual hallucinations have yielded a number of consistent findings98–100. Several studies have reported atrophy in the visual cortex101–103 — broadly defined as extending into lateral and ventral occipitotemporal regions including the fusiform gyrus and visual parietal cortex (corresponding to dorsal and ventral visual streams) — although this finding is not univeral104. One study of PD patients with and without visual hallucinations (some patients had hallucinations in both visual and other modalities), matched for cognitive status, only detected atrophy in posterior cortical regions101, whereas another study of visual hallucinations, in which global cognition (MMSE score) was included as a covariate, found atrophy in frontal, hippocampal and thalamic regions46. A study focusing on minor hallucinations found atrophy in the midbrain, cerebellar vermis and visual parietal cortex, as well as areas of increased cortical volume in the limbic cortex and the posterior lobe of the cerebellum, which might be linked to compensatory mechanisms105. Studies that did not control specifically for cognition found atrophy of the hippocampal head106, frontal regions (particularly the lateral frontal cortex)103,107,108, cerebellum103 and lateral parietal cortex103 in patients with visual hallucinations.
The distribution of atrophy described in the various studies is consistent with the profile of cognitive deficits found in PD psychosis (FIG. 1). At 30-month follow-up, patients who report visual hallucinations at baseline have greater progression of cortical atrophy within limbic, frontal and thalamic regions, and are more likely to have developed dementia38. The greater rate of atrophy and hypothesized progression from hippocampal head involvement to diffuse hippocampal atrophy in patients with visual hallucinations106 might account for the association between visual hallucinations and poor cognitive outcome.
Figure 1. Regions of cortical atrophy in PD psychosis.
The figure summarizes studies that have reported regions of increased atrophy in patients with Parkinson disease (PD) psychosis (defined by visual hallucinations in most studies) compared with PD controls. The results are superimposed on a lateral view of the right hemisphere. The approximate locations of regions of atrophy identified in previous work are indicated by circles annotated by the relevant references. The red regions indicate atrophy in our own study (ffytche, D. et al., unpublished work), in which PD patients with visual hallucinations (n = 8; mean Mini-Mental State Examination (MMSE) score 27.3) were compared with PD controls (n = 9; mean MMSE score 29.6). A lenient statistical threshold (P < 0.01; 100 contiguous voxels) is used for illustrative purposes. Lighter red shading indicates regions deep to the rendered cortical surface. These summary findings indicate that visual hallucinations in PD are associated with widely distributed but specific regions of cortical atrophy.
Few studies have investigated white matter changes in PD psychosis. An early study found no differences with regard to a range of clinically defined white matter lesion indices in patients with and without visual hallucinations109. A more recent volumetric study described decreased white matter volume in occipital and parahippocampal regions103. Another study showed that the microstructural integrity of deep white matter, as measured by fractional anisotropy, was affected in PD psychosis (defined as the presence of perceptual errors, hallucinations in any modality, or delusions). When fractional anisotropy was compared across groups as an absolute measure or normalized to globus pallidus or substantia nigra values, reduced fractional anisotropy was detected within regions of interest in the occipital lobe, frontal lobe and cingulate gyrus, and deep to the left hippocampal head110.
Neurotransmitter imaging
Reduced dopamine transporter binding in the striatum in early PD, as measured by the 123I-β-CIT tracer, is associated with an increased prospective risk of PD psychosis at 5 years (assessed using the UPDRS thought disorder item)111. It is unclear whether this binding reduction represents the underlying mechanism of the psychosis spectrum or is an indirect association, for example, reflecting more-extensive neurodegenerative involvement in PD psychosis.
In a serotonergic imaging study that used the 5-HT2A receptor ligand 18F-setoperone, increased binding in ventral occipitotemporal regions and bilateral frontal cortex was identified in patients with visual hallucinations112. By contrast, a 5-HT1A receptor binding study in postmortem tissue found no association with visual hallucinations, although 5-HT1A binding was elevated in sublayers of the orbitofrontal, ventral temporal and motor cortex in patients with PD, irrespective of visual hallucinations113.
Metabolic and blood flow imaging
Occipital hypometabolism and reduced regional cerebral blood flow (rCBF) are characteristics of DLB114,115, and have been linked to visual hallucination severity116. Whether the same regions exhibit reduced metabolism and rCBF in PD psychosis is less clear. Metabolic imaging studies of PD patients with and without visual hallucinations have identified reductions in posterior metabolism that spare the occipital pole117 or primary visual cortex (data from table in Nagano-Saito et al.118). In addition to posterior metabolic deficits, one study found increased frontal metabolism in PD psychosis118. Another study found that posterior metabolic deficits were more pronounced in patients with visual hallucinations and cognitive impairment (defined by the K-MMSE cut-off score, the Korean version of the MMSE), but were also present in patients with K-MMSE scores above the threshold value119.
In contrast to spared occipital metabolism in the studies described above, a recent study of PD-MCI has found evidence of occipital hypometabolism in PD patients with visual hallucinations40. The hypometabolism did not differ between patients who did and did not subsequently develop dementia40. Evidence also exists of decreased posterior perfusion in ‘medication-induced’ hallucinations (mostly visual) that would now be termed PD psychosis, with significant reductions of rCBF in the posterior temporo-occipital cortex but not in the occipital lobe120. Similarly, another study of visual hallucinations in PD found trend-level posterior perfusion deficits that spared occipital cortices121. A region of decreased perfusion outside the occipital lobe in the ventral temporal cortex (fusiform gyrus) became significant after controlling for confound covariates, as did a region of increased perfusion in the superior and middle temporal gyrus121.
In summary, consistent evidence points towards posterior changes in metabolism and perfusion in PD patients with visual hallucinations, but the presence or absence of involvement of the occipital lobe remains unclear.
Functional MRI
There is a paucity of data on brain changes occurring in PD at the time of a hallucination (capture studies). One functional MRI (fMRI) case study found a decrease in activity in visual areas coincident with visual hallucinations122. This decrease in occipital activity contrasts with evidence from eye disease, schizophrenia and induced visual hallucinations, which indicates that activity in visual areas increases at the time of visual hallucinations123. The PD case study also reported increased activity in the frontal lobe during the hallucinations122. Frontal activations are not found in eye disease and schizophrenia during hallucinations; however, another PD case report, in which a different technique was used (single-photon emission CT with technetium-99m ethyl cysteinate dimer), documented frontal and anterior temporal increases in rCBF during visual hallucinations124. Contrasting findings of occipital increases or decreases and the presence or absence of frontal activations across clinical contexts may suggest differences in the underlying mechanisms of hallucinations.
Studies in which visual stimulation was used to probe cerebral activity have reported decreased activation in the parietal lobe, lateral occipitotemporal cortex125 and occipital cortex126 in PD patients with a history of visual hallucinations. An early fMRI study reported decreased activity in the primary visual cortex but increased activation in the visual association cortex127. In the frontal lobe, both activation increases125 and activation decreases126,128 have been reported in response to visual stimulation. The contrasting findings might relate to differences in the stimuli and tasks used.
Reduced occipital low frequency fluctuations have been found in the resting state in PD patients with a history of visual hallucinations129. These fluctuations are thought to be an indirect measure of cerebral glucose metabolism and local field potentials, supporting the idea of occipital hypometabolism in patients with PD who are susceptible to visual hallucinations. Increased connectivity between occipital and frontal regions, the striatum and the thalamus129 and greater co-activation of default mode network nodes in the posterior cingulate/precuneus and lateral frontal cortex are associated with susceptibility to visual hallucinations in PD96.
Electrophysiology
In PD patients with a history of visual hallucinations, the P100 component of visual evoked potentials in response to reversing checkerboard stimulation was found to be delayed130, as was the P300 component of a visual odd-ball task131. A delay in evoked potential latency has also been found in association with illusions and visual hallucinations in an immersive virtual reality environment following short-term PD medication deprivation132. The evoked potential delays might fall within the distribution of normal variation, however, as rates of retinal and cortical evoked measures categorized as normal or abnormal on the basis of normal population standard deviation cut-off values do not differ between patients with and without hallucinations132,133. Changes in cholinergic-related electrophysiological measures (short-latency afferent inhibition) associated with susceptibility to visual hallucinations in PD are consistent with a loss of cortical cholinergic input134.
Treatment
As outlined in previous reviews, treatment of PD psychosis includes psychological therapies, dose reduction of PD medication, and medication to treat psychosis symptoms1,2,135. TABLE 3 summarizes double-blind, placebo controlled trials in patients with PD psychosis, including trials of pimavanserin (a 5-HT2A inverse agonist) and memantine that have been published since this topic was last reviewed. For patients without prominent cognitive impairment, clozapine and pimavanserin have the best evidence of efficacy in PD psychosis. In patients with cognitive impairment and visual hallucinations, rivastigmine was found to reduce the Neuropsychiatric Inventory score (predominantly the agitation component) in a secondary analysis, and unpublished evidence suggests efficacy of pimavanserin in PD psychosis with cognitive impairment. The clozapine and pimavanserin trials have indicated a therapeutic effect specific to hallucinations as a whole but, to date, no studies have reported a specific effect on visual hallucinations. No treatment trials targeting minor symptoms have yet been reported.
Table 3.
Double-blind studies of psychosis spectrum treatments
| Reference | Drug | Number of participants | Mean MMSE score | Psychosis (improvement over placebo) | Effect size | Visual hallucinations (improvement over placebo) |
|---|---|---|---|---|---|---|
| Atypical antipsychotics | ||||||
| Pollak et al. (2014)136 | Clozapine (4 weeks) |
|
|
CGI – 1.2* (P= 0.001) | 0.90* | PANNS – 4.8 (P<0.0001) |
| The Parkinson Study Group137 | Clozapine (4 weeks) |
|
|
CGI – 1.1* (P<0.001) | 4.40* | SAPS – 8* (P = 0.01) |
| Kurlan et al. (2007)141 | Quetiapine (10 weeks) |
|
|
BPRS – 2.2 (not significant) | – | – |
| Ondo et al. (2005)139 | Quetiapine (12 weeks) |
|
|
No significant change on Baylor Hallucination Questionnaire or BPRS | – | – |
| Shotbolt et al. (2009)140 | Quetiapine (12 weeks) |
|
|
Secondary outcome: no significant change on BPRS | – | No significant change on Baylor Hallucination Questionnnaire or NPI |
| Breier et al. (2002)186 | Olanzapine (4 weeks) |
|
|
No significant change on BPRS positive symptom cluster subscore | – | No significant change on NPI hallucinations score |
| Breier et al. (2002)186 | Olanzapine (4 weeks) |
|
|
No significant change on BPRS positive symptom cluster subscore | – | No significant change on NPI hallucinations score |
| Ondo et al. (2002)142 | Olanzapine (9 weeks) |
|
26.8‡ | No significant change on UPDRS thought disorder item | – | – |
| Cholinesterase inhibitor | ||||||
| Burn et al. (2006)187 | Rivastigmine(24 weeks): visual hallucination subgroup |
|
|
Secondary outcome: NPI 10 – 4.19 (P= 0.01) | 0.40* | No significant change on NPI-10 visual hallucination subscore |
| NMDA receptor antagonist | ||||||
| Aarsland et al. (2009)188 | Memantine (24 weeks) |
|
|
Secondary outcome: NPI – 0.1 (not significant) | – | – |
| Serotonin 2A receptor inverse agonist | ||||||
| Cummings et al. (2014)147 | Pimavanserin (6 weeks) |
|
|
SAPS PD – 3.06 (P = 0.001) | 0.50 | SAPS hallucinations domain score – 2.08 (P= 0.003) |
| Ballard, C et al., unpublished work | Pimavanserin (6 weeks) |
|
|
SAPS PD – 6.64 | 3.50* | – |
| Meltzer et al. (2010)146 | Pimavanserin (4 weeks) |
|
Individuals with dementia excluded | SAPS total domain score – 4.6 (trend: P= 0.09) | 0.56 | No significant change on SAPS visual hallucinations score |
Calculated from tabulated data: drug versus placebo difference divided by mean standard deviation of drug versus placebo change.
MMSE scores for drug and placebo groups combined. BPRS, Brief Psychiatric Rating Scale; CGI, Clinical Global Impression; MMSE, Mini-Mental State Examination; NPI, Neuropsychiatric Inventory; PANNS, Positive and Negative Symptom Scale; SAPS, Scale for Assessment of Positive Symptoms; PD, Parkinson disease; UPDRS, Unified PD Rating Scale (thought disorder item relates to illusions, hallucinations and delusions).
Atypical antipsychotics
Evidence for the efficacy of clozapine in PD psychosis136–138 sparked interest in the potential efficacy of quetiapine, a structurally related compound. Although early studies suggested a beneficial effect of quetiapine, several subsequent studies failed to replicate the findings in PD139,140 and across the spectrum of dementia with parkinsonian symptoms141. Similarly, open-label studies suggested that olanzapine was efficacious for PD psychosis, but placebo-controlled142 and comparative143 trials found a worsening of motor symptoms without significant improvement of hallucinations. The 2011 Movement Disorder Society evidence-based medicine review of treatments for nonmotor symptoms concluded that there was insufficient evidence on the efficacy of quetiapine, and that olanzapine carried unacceptable risk135.
Despite the lack of an evidence base, atypical and typical antipsychotics continue to be used in clinical practice144, but are associated with increased mortality in the context of clinical trials145 and a retrospective database analysis, which ranked haloperidol as carrying the highest risk, followed by olanzapine, risperidone and quetiapine144. Such evidence cautions against the use of antipsychotics in PD, and highlights the need for further studies in this area, as well as the development of new therapeutic approaches for PD psychosis.
Pimavanserin
The therapeutic effect of clozapine in PD psychosis is thought to relate, in part, to blockade at the 5-HT2A receptor146. A large double-blind, placebo-controlled study of pimavanserin found a significant improvement on the SAPS-PD147, with greater improvement in a subgroup with more-pronounced cognitive impairment (Ballard, C. et al., unpublished work, see TABLE 3). In an earlier study, a global measure of hallucinations assessed as a secondary end point improved in patients treated with pimavanserin, but the reduction in visual hallucinations was not significant when considered separately146. The relative lack of efficacy of pimavanserin for visual hallucinations suggests that the wider improvement in psychotic symptoms is mediated by a different subset of 5-HT receptors than those stimulated by lysergic acid diethylamide (LSD), the use of which is associated with predominantly visual phenomena. Pimavanserin received FDA approval in April 2016 for the treatment of PD psychosis. Its adverse event profile to date suggests advantages over other anti-psychotics in terms of risk of stroke, falls, fatigue, blood dyscrasia, neuroleptic malignant syndrome, orthostatic hypotension, and worsening of motor symptoms.
Apomorphine
The chemical structure of apomorphine includes a piperidine moiety and, unlike other dopamine agonists, this drug exerts intrinsic antagonist effects at the 5-HT2A receptor as well as agonist affects at both D1-like and D2-like receptors148. This unique profile might account for case report and open-label study evidence that apomorphine does not exacerbate psychosis symptoms and, in some studies, may ameliorate these symptoms149 or improve visual contrast sensitivity150. The mode of apomorphine administration may influence its impact on psychotic symptoms151. The open-label EUROINF study found improvements in perceptual and hallucination symptoms in patients treated with either apomorphine or intrajejunal levodopa152. The effect size for apomorphine (0.32) was larger than for intrajejunal levodopa (0.29), although the difference was not significant152. Apomophine might have a place in the treatment of PD psychosis; however, a better evidence base is needed to guide future recommendations148,152,153.
Electroconvulsive therapy
No sham-controlled studies of electroconvulsive therapy (ECT) for PD psychosis have been undertaken, but two case series have reported improvements in PD psychosis spectrum symptoms. In one study (n = 5), Brief Psychiatric Rating Scale scores improved after five to 12 ECT sessions, and the effects persisted for 5–30 weeks154. In another study (n = 8), SAPS scores improved at 1 week (longer-term data not reported) after a mean of six ECT sessions155.
Systemic illness
Psychosis is part of a range of acute events in PD, and is often seen in hospitalized patients with systemic infection, dopamine agonist withdrawal syndrome or parkinsonism– hyperpyrexia syndrome, as well as in postsurgical states156. Management of the underlying precipitant, along with rehydration, cautious use of antipsychotic agents and — in suitable cases — gentle introduction of low-dose dopamine agonists, may be required.
Conclusions
The paradigmatic shift in perspective that followed the 2007 consensus definition of PD psychosis revitalized research interest and led to a rapidly expanding literature. The impact of these studies is still evolving, but there have already been important clinical consequences, such as a change in our understanding of the prevalence of PD psychosis, given that it increases with PD duration; expansion of the range of symptoms considered part of the psychosis spectrum; and recognition of new prospective risk factors for PD psychosis. The largest expansion of literature has been in the mechanism domain, particularly in studies of cognitive profile, structural brain imaging and genetics.
Despite substantial progress over the past decade, our current state of knowledge leaves key questions unanswered (BOX 4). For example, the relationship between PD psychosis, PD-MCI and PD dementia is unclear. Most patients with PD dementia will have psychosis, but only a small proportion of those with PD-MCI have such symptoms. Does this observation imply a difference in PD-MCI cognitive profiles for patients with and without psychosis? If so, an understanding of these profile differences might help to explain the link between early PD psychosis symptoms and the subsequent development of dementia. Another unresolved issue is the relationship between individual symptoms in the PD psychosis spectrum in terms of underlying pathophysiological mechanisms. Evidence from studies of minor hallucinations, formed hallucinations and delusions, for example, suggest that the mechanisms are not the same for all symptoms, with important implications for the assessment of PD psychosis in research studies. A continuum assessment scale might be helpful in epidemiological and risk factor studies, but may be less useful for studying specific symptoms and their underlying mechanism.
Box 4. Relationship between brain structure, function and neuropathology in PD psychosis.
The relationship between different imaging biomarkers of Parkinson disease (PD) psychosis remains unclear. Areas of reduced posterior cortical metabolism40 overlap with areas of reduced activation in functional MRI (fMRI) studies125, so might reflect similar underlying functional alterations or a causal link (for example, an area of reduced metabolism might result in a reduced blood oxygen level-dependent (BOLD) signal). Likewise, regions of increased metabolic activity in the frontal cortex118, as identified in some studies, may relate to regions of increased frontal fMRI activation125.
One question that arises is why specific subregions of frontal, parietal, limbic and occipital lobes are altered in PD psychosis. Some regions with atrophic or metabolic change have shown increased Lewy body and/or amyloid and tau pathology in postmortem studies55–57. However, other regions, in particular the occipital cortex, have limited neuropathological load even in end-stage disease58, contrasting with atrophy and possible hypometabolism in imaging studies. Therefore, in vivo imaging changes may not simply reflect localized neuropathology, but have other causes, for example, loss of cholinergic or serotonergic cortical projections, leading to a specific distribution of receptor upregulation, functional alterations and, in the longer term, atrophy.
Other areas identified as requiring further research include the role of PD medication as a modifier or unmasker of psychosis symptoms, motion perception and eye movement control deficits associated with passage and presence hallucinations, and the transition from hallucinations with insight to those without insight and its impact on quality of life and the move to supervised care. These negative outcomes are arguably the most clinically relevant aspects of PD psychosis; however, the brain changes that underlie them are unclear.
Finally, visual hallucinations are not unique to synucleinopathies, and patients with eye disease, serotonergic dysfunction or AD also have visual symptoms that overlap with those of PD but are associated with different cognitive profiles157,158. The progressive improvement of visual hallucinations in eye disease contrasts with the progressive deterioration in PD and AD psychosis157,158, and comparative studies of brain changes associated with visual hallucination susceptibility across clinical contexts might shed light on why these differences exist.
Overall, two major themes can be recognized in the literature that has emerged over the past decade. One theme highlights the clinical challenges of PD psychosis, with an urgent need for better characterization of its symptoms and their impact, and for evidence on how to assess and treat these symptoms. The second theme highlights what the psychosis spectrum reveals about PD in terms of disease stage and distribution, and its role as a clinical biomarker for PD outcomes. Further research in this area will have implications that extend beyond the symptoms themselves to the wider issues of cognitive decline in PD, patient and carer experience, and the development of novel treatments for positive symptoms in PD and other clinical contexts.
Key points.
Parkinson disease (PD) psychosis refers to a spectrum of illusions, hallucinations and delusions that occur throughout the disease course
Evolving literature highlights the importance of recognizing and treating PD psychosis, and understanding its role as a clinical biomarker of disease stage, distribution and future progression
Current evidence points to PD psychosis as a set of symptoms with distinct pathophysiological mechanisms, as opposed to a single pathophysiological symptom with a spectrum of severity
The relationship between neuropathology in PD psychosis and in vivo measures of reduced metabolism, functional MRI alterations and cortical volume loss remains unclear
Further studies are needed to explore the role of PD medication in unmasking psychosis symptoms, why psychosis symptoms predict worse cognitive outcome, comparisons of psychosis symptoms and mechanisms in different clinical conditions, and development of novel treatments
Acknowledgments
The authors thank Dr Michael Haworth for help in preparing the final manuscript, Dr Rowena Carter for help preparing Figure 1, and the National Institute for Health Research (NIHR) Maudsley Biomedical Research Centre and Dementia Unit and NIHR Programme Grants for Applied Research (SHAPED RP-PG-0610-10100) for supporting their involvement in this work. K.R.C. acknowledges support from the European Union, Parkinson’s UK, the NIHR and the Parkinson’s Disease Non Motor group. D.A. is a Royal Society Wolfson Research Merit Award Holder and thanks the Wolfson Foundation and the Royal Society for their support. The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health.
Footnotes
Competing interests statement
K.R.C. has consulted and served on advisory boards for Britannia, AbbVie, Neuronova, Mundipharma and UCB, and has also served on advisory boards for Synapsus and Medtronic. He has received honoraria from Boehringer Ingelheim, GlaxoSmithKline, AbbVie, Britannia, UCB, Mundipharma, Otsuka and Zambon, and grants from Boehringer Ingelheim, GlaxoSmithKline, Britannia, AbbVie, UCB and Neuronova. He holds intellectual property rights for the KPP scale and the PDSS, and receives royalties for the books Non-Motor Symptoms of Parkinson’s Disease and Fastfacts: Parkinson’s Disease. C.B. declares grants and personal fees from Lundbeck and Acadia, and personal fees from Roche, Orion, GlaxoSmithKline, Otusaka, Heptares and Lilly. D.A. has received research support and/or honoraria from Astra-Zeneca, H. Lundbeck, Novartis Pharmaceuticals and GE Health, and serves as a paid consultant for H. Lundbeck and Axovant. The other authors declare no competing interests.
References
- 1.Ravina B, et al. Diagnostic criteria for psychosis in Parkinson’s disease: report of an NINDS, NIMH work group. Mov Disord. 2007;22:1061–1068. doi: 10.1002/mds.21382. [DOI] [PubMed] [Google Scholar]
- 2.Diederich NJ, Fénelon G, Stebbins G, Goetz CG. Hallucinations in Parkinson disease. Nat Rev Neurol. 2009;5:331–342. doi: 10.1038/nrneurol.2009.62. [DOI] [PubMed] [Google Scholar]
- 3.Fénelon G, Alves G. Epidemiology of psychosis in Parkinson’s disease. J Neurol Sci. 2010;289:12–17. doi: 10.1016/j.jns.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 4.Rabey JM. Hallucinations and psychosis in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15(Suppl 4):S105–S110. doi: 10.1016/S1353-8020(09)70846-6. [DOI] [PubMed] [Google Scholar]
- 5.Friedman JH. Parkinson’s disease psychosis 2010: a review article. Parkinsonism Relat Disord. 2010;16:553–560. doi: 10.1016/j.parkreldis.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Friedman JH. Parkinson disease psychosis: update. Behav Neurol. 2013;27:469–477. doi: 10.3233/BEN-129016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goetz CG. New developments in depression, anxiety, compulsiveness, and hallucinations in Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S104–S109. doi: 10.1002/mds.22636. [DOI] [PubMed] [Google Scholar]
- 8.Fénelon G. Psychosis in Parkinson’s disease: phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr. 2008;13:18–25. doi: 10.1017/s1092852900017284. [DOI] [PubMed] [Google Scholar]
- 9.Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:533–535. doi: 10.1136/jnnp.64.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fénelon G, Mahieux F, Huon R, Ziegler M. Hallucinations in Parkinson’s disease: prevalence, phenomenology and risk factors. Brain. 2000;123:733–745. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 11.Fénelon G, Soulas T, Cleret de Langavant L, Trinkler I, Bachoud-Levi AC. Feeling of presence in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2011;82:1219–1224. doi: 10.1136/jnnp.2010.234799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood RA, Hopkins SA, Moodley KK, Chan D. Fifty percent prevalence of extracampine hallucinations in Parkinson’s disease patients. Front Neurol. 2015;6:263. doi: 10.3389/fneur.2015.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boubert L, Barnes J. Phenomenology of visual hallucinations and their relationship to cognitive profile in Parkinson’s disease patients: preliminary observations. SAGE Open. 2015 http://dx.doi.org/10.1177/2158244015585827.
- 14.Goetz CG, Stebbins GT, Ouyang B. Visual plus nonvisual hallucinations in Parkinson’s disease: development and evolution over 10 years. Mov Disord. 2011;26:2196–2200. doi: 10.1002/mds.23835. [DOI] [PubMed] [Google Scholar]
- 15.Goetz CG, Fan W, Leurgans S, Bernard B, Stebbins GT. The malignant course of “benign hallucinations” in Parkinson disease. Arch Neurol. 2006;63:713–716. doi: 10.1001/archneur.63.5.713. [DOI] [PubMed] [Google Scholar]
- 16.Chou KL, et al. Drug-induced psychosis in Parkinson disease: phenomenology and correlations among psychosis rating instruments. Clin Neuropharmacol. 2005;28:215–219. doi: 10.1097/01.wnf.0000180228.77802.32. [DOI] [PubMed] [Google Scholar]
- 17.Papapetropoulos S, et al. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson’s disease. BMC Neurol. 2008;8:21. doi: 10.1186/1471-2377-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Factor SA, et al. Cognitive correlates of hallucinations and delusions in Parkinson’s disease. J Neurol Sci. 2014;347:316–321. doi: 10.1016/j.jns.2014.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moro A, Munhoz RP, Moscovich M, Arruda WO, Teive HA. Delusional misidentification syndrome and other unusual delusions in advanced Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:751–754. doi: 10.1016/j.parkreldis.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Pagonabarraga J, et al. A prospective study of delusional misidentification syndromes in Parkinson’s disease with dementia. Mov Disord. 2008;23:443–448. doi: 10.1002/mds.21864. [DOI] [PubMed] [Google Scholar]
- 21.Ballard C, et al. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer’s disease. Am J Psychiatry. 1999;156:1039–1045. doi: 10.1176/ajp.156.7.1039. [DOI] [PubMed] [Google Scholar]
- 22.Fernandez HH, et al. Scales to assess psychosis in Parkinson’s disease: critique and recommendations. Mov Disord. 2008;23:484–500. doi: 10.1002/mds.21875. [DOI] [PubMed] [Google Scholar]
- 23.Mosimann UP, et al. A semi-structured interview to assess visual hallucinations in older people. Int J Geriatr Psychiatry. 2008;23:712–718. doi: 10.1002/gps.1965. [DOI] [PubMed] [Google Scholar]
- 24.Voss T, et al. Performance of a shortened Scale for Assessment of Positive Symptoms for Parkinson’s disease psychosis. Parkinsonism Relat Disord. 2013;19:295–299. doi: 10.1016/j.parkreldis.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 25.Yokoi K, et al. Hallucinators find meaning in noises: pareidolic illusions in dementia with Lewy bodies. Neuropsychologia. 2014;56:245–254. doi: 10.1016/j.neuropsychologia.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 26.McKinlay A, et al. A profile of neuropsychiatric problems and their relationship to quality of life for Parkinson’s disease patients without dementia. Parkinsonism Relat Disord. 2008;14:37–42. doi: 10.1016/j.parkreldis.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Fénelon G, Soulas T, Zenasni F, Cleret de Langavant L. The changing face of Parkinson’s disease-associated psychosis: a cross-sectional study based on the new NINDS–NIMH criteria. Mov Disord. 2010;25:763–766. doi: 10.1002/mds.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagonabarraga J, et al. Minor hallucinations occur in drug-naive Parkinson’s disease patients, even from the premotor phase. Mov Disord. 2016;31:45–52. doi: 10.1002/mds.26432. [DOI] [PubMed] [Google Scholar]
- 29.Friedman JH. Editorial on: Pagonabarraga, J et al Minor hallucinations occur in drug-naive Parkinson’s disease patients even from the premotor phase Movement Disorders 2015; available from: 10.1002/mds.26432. Mov Disord. 2016;31:9–10. doi: 10.1002/mds.26472. [DOI] [PubMed] [Google Scholar]
- 30.Mack J, et al. Prevalence of psychotic symptoms in a community-based Parkinson disease sample. Am J Geriatr Psychiatry. 2012;20:123–132. doi: 10.1097/JGP.0b013e31821f1b41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibson G, et al. Frequency, prevalence, incidence and risk factors associated with visual hallucinations in a sample of patients with Parkinson’s disease: a longitudinal 4-year study. Int J Geriatr Psychiatry. 2013;28:626–631. doi: 10.1002/gps.3869. [DOI] [PubMed] [Google Scholar]
- 32.de la Riva P, Smith K, Xie SX, Weintraub D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology. 2014;83:1096–1103. doi: 10.1212/WNL.0000000000000801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forsaa EB, et al. A 12-year population-based study of psychosis in Parkinson disease. Arch Neurol. 2010;67:996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- 34.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 35.Anang JB, et al. Predictors of dementia in Parkinson disease: a prospective cohort study. Neurology. 2014;83:1253–1260. doi: 10.1212/WNL.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uc EY, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009;73:1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgante L, et al. Psychosis associated to Parkinson’s disease in the early stages: relevance of cognitive decline and depression. J Neurol Neurosurg Psychiatry. 2012;83:76–82. doi: 10.1136/jnnp-2011-300043. [DOI] [PubMed] [Google Scholar]
- 38.Ibarretxe-Bilbao N, et al. Differential progression of brain atrophy in Parkinson’s disease with and without visual hallucinations. J Neurol Neurosurg Psychiatry. 2010;81:650–657. doi: 10.1136/jnnp.2009.179655. [DOI] [PubMed] [Google Scholar]
- 39.Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Tolosa E. Cognitive changes in Parkinson’s disease patients with visual hallucinations. Dement Geriatr Cogn Disord. 2007;23:281–288. doi: 10.1159/000100850. [DOI] [PubMed] [Google Scholar]
- 40.Gasca-Salas C, Clavero P, Garcia-Garcia D, Obeso JA, Rodriguez-Oroz MC. Significance of visual hallucinations and cerebral hypometabolism in the risk of dementia in Parkinson’s disease patients with mild cognitive impairment. Hum Brain Mapp. 2016;37:968–977. doi: 10.1002/hbm.23080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reijnders JS, Ehrt U, Lousberg R, Aarsland D, Leentjens AF. The association between motor subtypes and psychopathology in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:379–382. doi: 10.1016/j.parkreldis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Rana AQ, Vaid HM, Edun A, Dogu O, Rana MA. Relationship of dementia and visual hallucinations in tremor and non-tremor dominant Parkinson’s disease. J Neurol Sci. 2012;323:158–161. doi: 10.1016/j.jns.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 43.Bodis-Wollner I. Foveal vision is impaired in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:1–14. doi: 10.1016/j.parkreldis.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Lee JY, et al. Retinal nerve fiber layer thickness and visual hallucinations in Parkinson’s disease. Mov Disord. 2014;29:61–67. doi: 10.1002/mds.25543. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez-Ruiz B, Junque C, Marti MJ, Valldeoriola F, Tolosa E. Neuropsychological deficits in Parkinson’s disease patients with visual hallucinations. Mov Disord. 2006;21:1483–1487. doi: 10.1002/mds.20965. [DOI] [PubMed] [Google Scholar]
- 46.Shin S, et al. Neuroanatomical substrates of visual hallucinations in patients with non-demented Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2012;83:1155–1161. doi: 10.1136/jnnp-2012-303391. [DOI] [PubMed] [Google Scholar]
- 47.Barnes J, Boubert L. Visual memory errors in Parkinson’s disease patient with visual hallucinations. Int J Neurosci. 2011;121:159–164. doi: 10.3109/00207454.2010.539308. [DOI] [PubMed] [Google Scholar]
- 48.Moustafa AA, Krishna R, Frank MJ, Eissa AM, Hewedi DH. Cognitive correlates of psychosis in patients with Parkinson’s disease. Cogn Neuropsychiatry. 2014;19:381–398. doi: 10.1080/13546805.2013.877385. [DOI] [PubMed] [Google Scholar]
- 49.Ozer F, et al. Cognitive impairment patterns in Parkinson’s disease with visual hallucinations. J Clin Neurosci. 2007;14:742–746. doi: 10.1016/j.jocn.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 50.Katzen H, et al. Multi-modal hallucinations and cognitive function in Parkinson’s disease. Dement Geriatr Cogn Disord. 2010;30:51–56. doi: 10.1159/000314875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koerts J, et al. Attentional and perceptual impairments in Parkinson’s disease with visual hallucinations. Parkinsonism Relat Disord. 2010;16:270–274. doi: 10.1016/j.parkreldis.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 52.Llebaria G, et al. Neuropsychological correlates of mild to severe hallucinations in Parkinson’s disease. Mov Disord. 2010;25:2785–2791. doi: 10.1002/mds.23411. [DOI] [PubMed] [Google Scholar]
- 53.Poletti M, et al. Dopamine agonists and delusional jealousy in Parkinson’s disease: a cross-sectional prevalence study. Mov Disord. 2012;27:1679–1682. doi: 10.1002/mds.25129. [DOI] [PubMed] [Google Scholar]
- 54.Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125:391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- 55.Gallagher DA, et al. Testing an aetiological model of visual hallucinations in Parkinson’s disease. Brain. 2011;134:3299–3309. doi: 10.1093/brain/awr225. [DOI] [PubMed] [Google Scholar]
- 56.Papapetropoulos S, McCorquodale DS, Gonzalez J, Jean-Gilles L, Mash DC. Cortical and amygdalar Lewy body burden in Parkinson’s disease patients with visual hallucinations. Parkinsonism Relat Disord. 2006;12:253–256. doi: 10.1016/j.parkreldis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 57.Jacobson SA, et al. Plaques and tangles as well as Lewy-type alpha synucleinopathy are associated with formed visual hallucinations. Parkinsonism Relat Disord. 2014;20:1009–1014. doi: 10.1016/j.parkreldis.2014.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalaitzakis ME, et al. Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:196–204. doi: 10.1016/j.parkreldis.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 59.Harding AJ, Stimson E, Henderson JM, Halliday GM. Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain. 2002;125:2431–2445. doi: 10.1093/brain/awf251. [DOI] [PubMed] [Google Scholar]
- 60.Janzen J, et al. The pedunculopontine nucleus is related to visual hallucinations in Parkinson’s disease: preliminary results of a voxel-based morphometry study. J Neurol. 2012;259:147–154. doi: 10.1007/s00415-011-6149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oka H, et al. Impaired cardiovascular autonomic function in Parkinson’s disease with visual hallucinations. Mov Disord. 2007;22:1510–1514. doi: 10.1002/mds.21581. [DOI] [PubMed] [Google Scholar]
- 62.Zhu K, van Hilten JJ, Putter H, Marinus J. Risk factors for hallucinations in Parkinson’s disease: results from a large prospective cohort study. Mov Disord. 2013;28:755–762. doi: 10.1002/mds.25389. [DOI] [PubMed] [Google Scholar]
- 63.Lenka A, Hegde S, Jhunjhunwala KR, Pal PK. Interactions of visual hallucinations, rapid eye movement sleep behavior disorder and cognitive impairment in Parkinson’s disease: a review. Parkinsonism Relat Disord. 2016;22:1–8. doi: 10.1016/j.parkreldis.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 64.Meral H, et al. Relationship between visual hallucinations and REM sleep behavior disorder in patients with Parkinson’s disease. Clin Neurol Neurosurg. 2007;109:862–867. doi: 10.1016/j.clineuro.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 65.Gama RL, et al. Risk factors for visual hallucinations in patients with Parkinson’s disease. Neurol Res. 2015;37:112–116. doi: 10.1179/1743132814Y.0000000418. [DOI] [PubMed] [Google Scholar]
- 66.Hirayama M, et al. Urinary 8-hydroxydeoxyguanosine correlate with hallucinations rather than motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2011;17:46–49. doi: 10.1016/j.parkreldis.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Goetz CG, et al. Intravenous levodopa in hallucinating Parkinson’s disease patients: high-dose challenge does not precipitate hallucinations. Neurology. 1998;50:515–517. doi: 10.1212/wnl.50.2.515. [DOI] [PubMed] [Google Scholar]
- 68.Dotchin CL, Jusabani A, Walker RW. Non-motor symptoms in a prevalent population with Parkinson’s disease in Tanzania. Parkinsonism Relat Disord. 2009;15:457–460. doi: 10.1016/j.parkreldis.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 69.Lenka A, Arumugham SS, Christopher R, Pal PK. Genetic substrates of psychosis in patients with Parkinson’s disease: a critical review. J Neurol Sci. 2016;364:33–41. doi: 10.1016/j.jns.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Papapetropoulos S, et al. Phenotypic associations of tau and ApoE in Parkinson’s disease. Neurosci Lett. 2007;414:141–144. doi: 10.1016/j.neulet.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 71.Factor SA, et al. Disease-related and genetic correlates of psychotic symptoms in Parkinson’s disease. Mov Disord. 2011;26:2190–2195. doi: 10.1002/mds.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.de la Fuente-Fernandez R, Nunez MA, Lopez E. The apolipoprotein E epsilon 4 allele increases the risk of drug-induced hallucinations in Parkinson’s disease. Clin Neuropharmacol. 1999;22:226–230. [PubMed] [Google Scholar]
- 73.Goetz CG, et al. Genetic variation analysis in parkinson disease patients with and without hallucinations: case–control study. Arch Neurol. 2001;58:209–213. doi: 10.1001/archneur.58.2.209. [DOI] [PubMed] [Google Scholar]
- 74.Camicioli R, et al. Apolipoprotein E ε4 and catechol-O-methyltransferase alleles in autopsy-proven Parkinson’s disease: relationship to dementia and hallucinations. Mov Disord. 2005;20:989–994. doi: 10.1002/mds.20481. [DOI] [PubMed] [Google Scholar]
- 75.Feldman B, Chapman J, Korczyn AD. Apolipoprotein ε4 advances appearance of psychosis in patients with Parkinson’s disease. Acta Neurol Scand. 2006;113:14–17. doi: 10.1111/j.1600-0404.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 76.Monsell SE, et al. Clinical and pathologic presentation in Parkinson’s disease by apolipoprotein e4 allele status. Parkinsonism Relat Disord. 2014;20:503–507. doi: 10.1016/j.parkreldis.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Murray PS, Kumar S, Demichele-Sweet MA, Sweet RA. Psychosis in Alzheimer’s disease. Biol Psychiatry. 2014;75:542–552. doi: 10.1016/j.biopsych.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neumann J, et al. Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain. 2009;132:1783–1794. doi: 10.1093/brain/awp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Oeda T, et al. Impact of glucocerebrosidase mutations on motor and nonmotor complications in Parkinson’s disease. Neurobiol Aging. 2015;36:3306–3313. doi: 10.1016/j.neurobiolaging.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, et al. Clinicogenetic study of GBA mutations in patients with familial Parkinson’s disease. Neurobiol Aging. 2014;35:935.e3–935.e8. doi: 10.1016/j.neurobiolaging.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 81.Winder-Rhodes SE, et al. Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain. 2013;136:392–399. doi: 10.1093/brain/aws318. [DOI] [PubMed] [Google Scholar]
- 82.Creese B, et al. Determining the association of the 5HTTLPR polymorphism with delusions and hallucinations in Lewy body dementias. Am J Geriatr Psychiatry. 2014;22:580–586. doi: 10.1016/j.jagp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 83.Kiferle L, et al. Visual hallucinations in Parkinson’s disease are not influenced by polymorphisms of serotonin 5-HT2A receptor and transporter genes. Neurosci Lett. 2007;422:228–231. doi: 10.1016/j.neulet.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 84.Andreassen OA, et al. Genetic pleiotropy between multiple sclerosis and schizophrenia but not bipolar disorder: differential involvement of immune-related gene loci. Mol Psychiatry. 2015;20:207–214. doi: 10.1038/mp.2013.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Desikan RS, et al. Genetic overlap between Alzheimer’s disease and Parkinson’s disease at the MAPT locus. Mol Psychiatry. 2015;20:1588–1595. doi: 10.1038/mp.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nho K, et al. Comprehensive gene- and pathway-based analysis of depressive symptoms in older adults. J Alzheimers Dis. 2015;45:1197–1206. doi: 10.3233/JAD-148009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Onofrj M, et al. Visual hallucinations in PD and Lewy body dementias: old and new hypotheses. Behav Neurol. 2013;27:479–493. doi: 10.3233/BEN-129022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson’s disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord. 2005;20:130–140. doi: 10.1002/mds.20308. [DOI] [PubMed] [Google Scholar]
- 89.Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci. 2005;28:737–757. doi: 10.1017/S0140525X05000130. [DOI] [PubMed] [Google Scholar]
- 90.Shine JM, Halliday GM, Naismith SL, Lewis SJ. Visual misperceptions and hallucinations in Parkinson’s disease: dysfunction of attentional control networks? Mov Disord. 2011;26:2154–2159. doi: 10.1002/mds.23896. [DOI] [PubMed] [Google Scholar]
- 91.Shine JM, O’Callaghan C, Halliday GM, Lewis SJ. Tricks of the mind: visual hallucinations as disorders of attention. Prog Neurobiol. 2014;116:58–65. doi: 10.1016/j.pneurobio.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 92.Muller AJ, Shine JM, Halliday GM, Lewis SJ. Visual hallucinations in Parkinson’s disease: theoretical models. Mov Disord. 2014;29:1591–1598. doi: 10.1002/mds.26004. [DOI] [PubMed] [Google Scholar]
- 93.Shine JM, et al. Imagine that: elevated sensory strength of mental imagery in individuals with Parkinson’s disease and visual hallucinations. Proc Biol Sci. 2015;282:20142047. doi: 10.1098/rspb.2014.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shine JM, Halliday GH, Carlos M, Naismith SL, Lewis SJ. Investigating visual misperceptions in Parkinson’s disease: a novel behavioral paradigm. Mov Disord. 2012;27:500–505. doi: 10.1002/mds.24900. [DOI] [PubMed] [Google Scholar]
- 95.Shine JM, et al. The role of dysfunctional attentional control networks in visual misperceptions in Parkinson’s disease. Hum Brain Mapp. 2014;35:2206–2219. doi: 10.1002/hbm.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yao N, et al. The default mode network is disrupted in Parkinson’s disease with visual hallucinations. Hum Brain Mapp. 2014;35:5658–5666. doi: 10.1002/hbm.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Botha H, Carr J. Attention and visual dysfunction in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:742–747. doi: 10.1016/j.parkreldis.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 98.Carter R, ffytche DH. On visual hallucinations and cortical networks: a trans-diagnostic review. J Neurol. 2015;262:1780–1790. doi: 10.1007/s00415-015-7687-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lenka A, Jhunjhunwala KR, Saini J, Pal PK. Structural and functional neuroimaging in patients with Parkinson’s disease and visual hallucinations: a critical review. Parkinsonism Relat Disord. 2015;21:683–691. doi: 10.1016/j.parkreldis.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 100.Ibarretxe-Bilbao N, Junque C, Marti MJ, Tolosa E. Cerebral basis of visual hallucinations in Parkinson’s disease: structural and functional MRI studies. J Neurol Sci. 2011;310:79–81. doi: 10.1016/j.jns.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 101.Goldman JG, et al. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson’s disease with hallucinations. Brain. 2014;137:849–859. doi: 10.1093/brain/awt360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramirez-Ruiz B, et al. Cerebral atrophy in Parkinson’s disease patients with visual hallucinations. Eur J Neurol. 2007;14:750–756. doi: 10.1111/j.1468-1331.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- 103.Watanabe H, et al. Cortical and subcortical brain atrophy in Parkinson’s disease with visual hallucination. Mov Disord. 2013;28:1732–1736. doi: 10.1002/mds.25641. [DOI] [PubMed] [Google Scholar]
- 104.Meppelink AM, de Jong BM, Teune LK, van Laar T. Regional cortical grey matter loss in Parkinson’s disease without dementia is independent from visual hallucinations. Mov Disord. 2011;26:142–147. doi: 10.1002/mds.23375. [DOI] [PubMed] [Google Scholar]
- 105.Pagonabarraga J, et al. Neural correlates of minor hallucinations in non-demented patients with Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:290–296. doi: 10.1016/j.parkreldis.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 106.Ibarretxe-Bilbao N, et al. Hippocampal head atrophy predominance in Parkinson’s disease with hallucinations and with dementia. J Neurol. 2008;255:1324–1331. doi: 10.1007/s00415-008-0885-8. [DOI] [PubMed] [Google Scholar]
- 107.Sanchez-Castaneda C, et al. Frontal and associative visual areas related to visual hallucinations in dementia with Lewy bodies and Parkinson’s disease with dementia. Mov Disord. 2010;25:615–622. doi: 10.1002/mds.22873. [DOI] [PubMed] [Google Scholar]
- 108.Gama RL, et al. Structural brain abnormalities in patients with Parkinson’s disease with visual hallucinations: a comparative voxel-based analysis. Brain Cogn. 2014;87:97–103. doi: 10.1016/j.bandc.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 109.Kraft E, Winkelmann J, Trenkwalder C, Auer DP. Visual hallucinations, white matter lesions and disease severity in Parkinson’s disease. Acta Neurol Scand. 1999;99:362–367. doi: 10.1111/j.1600-0404.1999.tb07365.x. [DOI] [PubMed] [Google Scholar]
- 110.Zhong J, et al. Why psychosis is frequently associated with Parkinson’s disease? Neural Regen Res. 2013;8:2548–2556. doi: 10.3969/j.issn.1673-5374.2013.27.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ravina B, et al. Dopamine transporter imaging is associated with long-term outcomes in Parkinson’s disease. Mov Disord. 2012;27:1392–1397. doi: 10.1002/mds.25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ballanger B, et al. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67:416–421. doi: 10.1001/archneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 113.Huot P, et al. Increased levels of 5-HT1A receptor binding in ventral visual pathways in Parkinson’s disease. Mov Disord. 2012;27:735–742. doi: 10.1002/mds.24964. [DOI] [PubMed] [Google Scholar]
- 114.Minoshima S, et al. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50:358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- 115.Lobotesis K, et al. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology. 2001;56:643–649. doi: 10.1212/wnl.56.5.643. [DOI] [PubMed] [Google Scholar]
- 116.Firbank MJ, Lloyd J, O’Brien JT. The relationship between hallucinations and FDG-PET in dementia with Lewy bodies. Brain Imaging Behav. 2015;10:636–639. doi: 10.1007/s11682-015-9434-0. [DOI] [PubMed] [Google Scholar]
- 117.Boecker H, et al. Metabolic alterations in patients with Parkinson disease and visual hallucinations. Arch Neurol. 2007;64:984–988. doi: 10.1001/archneur.64.7.984. [DOI] [PubMed] [Google Scholar]
- 118.Nagano-Saito A, et al. Visual hallucination in Parkinson’s disease with FDG PET. Mov Disord. 2004;19:801–806. doi: 10.1002/mds.20129. [DOI] [PubMed] [Google Scholar]
- 119.Park HK, et al. Visual hallucinations and cognitive impairment in Parkinson’s disease. Can J Neurol Sci. 2013;40:657–662. doi: 10.1017/s0317167100014888. [DOI] [PubMed] [Google Scholar]
- 120.Okada K, Suyama N, Oguro H, Yamaguchi S, Kobayashi S. Medication-induced hallucination and cerebral blood flow in Parkinson’s disease. J Neurol. 1999;246:365–368. doi: 10.1007/s004150050364. [DOI] [PubMed] [Google Scholar]
- 121.Oishi N, et al. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65:1708–1715. doi: 10.1212/01.wnl.0000187116.13370.e0. [DOI] [PubMed] [Google Scholar]
- 122.Goetz CG, Vaughan CL, Goldman JG, Stebbins GT. I finally see what you see: Parkinson’s disease visual hallucinations captured with functional neuroimaging. Mov Disord. 2014;29:115–117. doi: 10.1002/mds.25554. [DOI] [PubMed] [Google Scholar]
- 123.ffytche DH. The hodology of hallucinations. Cortex. 2008;44:1067–1083. doi: 10.1016/j.cortex.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 124.Kataoka H, Furiya Y, Morikawa M, Ueno S, Inoue M. Increased temporal blood flow associated with visual hallucinations in Parkinson’s disease with dementia. Mov Disord. 2008;23:464–465. doi: 10.1002/mds.21923. [DOI] [PubMed] [Google Scholar]
- 125.Stebbins GT, et al. Altered cortical visual processing in PD with hallucinations: an fMRI study. Neurology. 2004;63:1409–1416. doi: 10.1212/01.wnl.0000141853.27081.bd. [DOI] [PubMed] [Google Scholar]
- 126.Meppelink AM, et al. Impaired visual processing preceding image recognition in Parkinson’s disease patients with visual hallucinations. Brain. 2009;132:2980–2993. doi: 10.1093/brain/awp223. [DOI] [PubMed] [Google Scholar]
- 127.Holroyd S, Wooten GF. Preliminary FMRI evidence of visual system dysfunction in Parkinson’s disease patients with visual hallucinations. J Neuropsychiatry Clin Neurosci. 2006;18:402–404. doi: 10.1176/jnp.2006.18.3.402. [DOI] [PubMed] [Google Scholar]
- 128.Ramírez-Ruiz B, et al. Brain response to complex visual stimuli in Parkinson’s patients with hallucinations: a functional magnetic resonance imaging study. Mov Disord. 2008;23:2335–2343. doi: 10.1002/mds.22258. [DOI] [PubMed] [Google Scholar]
- 129.Yao N, et al. Resting activity in visual and corticostriatal pathways in Parkinson’s disease with hallucinations. Parkinsonism Relat Disord. 2015;21:131–137. doi: 10.1016/j.parkreldis.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 130.Matsui H, et al. The relation between visual hallucinations and visual evoked potential in Parkinson disease. Clin Neuropharmacol. 2005;28:79–82. doi: 10.1097/01.wnf.0000157066.50948.65. [DOI] [PubMed] [Google Scholar]
- 131.Kurita A, Murakami M, Takagi S, Matsushima M, Suzuki M. Visual hallucinations and altered visual information processing in Parkinson disease and dementia with Lewy bodies. Mov Disord. 2010;25:167–171. doi: 10.1002/mds.22919. [DOI] [PubMed] [Google Scholar]
- 132.Onofrj M, et al. Visual hallucinations in Parkinson’s disease: clues to separate origins. J Neurol Sci. 2006;248:143–150. doi: 10.1016/j.jns.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 133.Onofrj M, et al. Incidence of RBD and hallucination in patients affected by Parkinson’s disease: 8-year follow-up. Neurol Sci. 2002;23(Suppl 2):S91–S94. [Google Scholar]
- 134.Manganelli F, et al. Functional involvement of central cholinergic circuits and visual hallucinations in Parkinson’s disease. Brain. 2009;132:2350–2355. doi: 10.1093/brain/awp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seppi K, et al. The Movement Disorder Society Evidence-Based Medicine Review Update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord. 2011;26(Suppl 3):S42–S80. doi: 10.1002/mds.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pollak P, et al. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75:689–695. doi: 10.1136/jnnp.2003.029868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.The Parkinson Study Group. Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N Engl J Med. 1999;340:757–763. doi: 10.1056/NEJM199903113401003. [DOI] [PubMed] [Google Scholar]
- 138.Wolters EC, et al. Clozapine in the treatment of parkinsonian patients with dopaminomimetic psychosis. Neurology. 1990;40:832–834. doi: 10.1212/wnl.40.5.832. [DOI] [PubMed] [Google Scholar]
- 139.Ondo WG, Tintner R, Voung KD, Lai D, Ringholz G. Double-blind, placebo-controlled, unforced titration parallel trial of quetiapine for dopaminergic-induced hallucinations in Parkinson’s disease. Mov Disord. 2005;20:958–963. doi: 10.1002/mds.20474. [DOI] [PubMed] [Google Scholar]
- 140.Shotbolt P, Samuel M, Fox C, David AS. A randomized controlled trial of quetiapine for psychosis in Parkinson’s disease. Neuropsychiatr Dis Treat. 2009;5:327–332. doi: 10.2147/ndt.s5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kurlan R, Cummings J, Raman R, Thal L Alzheimer’s Disease Cooperative Study Group. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology. 2007;68:1356–1363. doi: 10.1212/01.wnl.0000260060.60870.89. [DOI] [PubMed] [Google Scholar]
- 142.Ondo WG, Levy JK, Vuong KD, Hunter C, Jankovic J. Olanzapine treatment for dopaminergic-induced hallucinations. Mov Disord. 2002;17:1031–1035. doi: 10.1002/mds.10217. [DOI] [PubMed] [Google Scholar]
- 143.Goetz CG, Blasucci LM, Leurgans S, Pappert EJ. Olanzapine and clozapine: comparative effects on motor function in hallucinating PD patients. Neurology. 2000;55:789–794. doi: 10.1212/wnl.55.6.789. [DOI] [PubMed] [Google Scholar]
- 144.Weintraub D, et al. Association of antipsychotic use with mortality risk in patients with Parkinson disease. JAMA Neurol. 2016;73:535–541. doi: 10.1001/jamaneurol.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ballard C, et al. Impact of current antipsychotic medications on comparative mortality and adverse events in people with Parkinson disease psychosis. J Am Med Dir Assoc. 2015;16:898.e1–898.e7. doi: 10.1016/j.jamda.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 146.Meltzer HY, et al. Pimavanserin, a serotonin2A receptor inverse agonist, for the treatment of Parkinson’s disease psychosis. Neuropsychopharmacology. 2010;35:881–892. doi: 10.1038/npp.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cummings J, et al. Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533–540. doi: 10.1016/S0140-6736(13)62106-6. [DOI] [PubMed] [Google Scholar]
- 148.Borgemeester RW, Lees AJ, van Laar T. Parkinson’s disease, visual hallucinations and apomorphine: a review of the available evidence. Parkinsonism Relat Disord. 2016;27:35–40. doi: 10.1016/j.parkreldis.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 149.van Laar T, Postma AG, Drent M. Continuous subcutaneous infusion of apomorphine can be used safely in patients with Parkinson’s disease and pre-existing visual hallucinations. Parkinsonism Relat Disord. 2010;16:71–72. doi: 10.1016/j.parkreldis.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 150.Geerligs L, Meppelink AM, Brouwer WH, van Laar T. The effects of apomorphine on visual perception in patients with Parkinson disease and visual hallucinations: a pilot study. Clin Neuropharmacol. 2009;32:266–268. doi: 10.1097/WNF.0b013e3181a6a92b. [DOI] [PubMed] [Google Scholar]
- 151.Pietz K, Hagell P, Odin P. Subcutaneous apomorphine in late stage Parkinson’s disease: a long term follow up. J Neurol Neurosurg Psychiatry. 1998;65:709–716. doi: 10.1136/jnnp.65.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Martinez-Martin P, et al. EuroInf: a multicenter comparative observational study of apomorphine and levodopa infusion in Parkinson’s disease. Mov Disord. 2015;30:510–516. doi: 10.1002/mds.26067. [DOI] [PubMed] [Google Scholar]
- 153.Todorova A, Ray Chaudhuri K. Subcutaneous apomorphine and non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord. 2013;19:1073–1078. doi: 10.1016/j.parkreldis.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 154.Ueda S, Koyama K, Okubo Y. Marked improvement of psychotic symptoms after electroconvulsive therapy in Parkinson disease. J ECT. 2010;26:111–115. doi: 10.1097/YCT.0b013e3181c18a3d. [DOI] [PubMed] [Google Scholar]
- 155.Usui C, et al. Improvements in both psychosis and motor signs in Parkinson’s disease, and changes in regional cerebral blood flow after electroconvulsive therapy. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1704–1708. doi: 10.1016/j.pnpbp.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 156.Chaudhuri KR, Fung VS. Fast Facts: Parkinson’s Disease. 4. Health Press Limited; 2016. [Google Scholar]
- 157.ffytche DH. Visual hallucinatory syndromes: past, present, and future. Dialogues Clin Neurosci. 2007;9:173–189. doi: 10.31887/DCNS.2007.9.2/dffytche. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.ffytche DH. Visual hallucinations in eye disease. Curr Opin Neurol. 2009;22:28–35. doi: 10.1097/wco.0b013e32831f1b3f. [DOI] [PubMed] [Google Scholar]
- 159.Ebersbach G. An artist’s view of drug-induced hallucinosis. Mov Disord. 2003;18:833–834. doi: 10.1002/mds.10437. [DOI] [PubMed] [Google Scholar]
- 160.Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Visual symptoms in Parkinson’s disease and Parkinson’s disease dementia. Mov Disord. 2011;26:2387–2395. doi: 10.1002/mds.23891. [DOI] [PubMed] [Google Scholar]
- 161.Diederich NJ, Stebbins G, Schiltz C, Goetz CG. Are patients with Parkinson’s disease blind to blindsight? Brain. 2014;137:1838–1849. doi: 10.1093/brain/awu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Santhouse AM, Howard RJ, ffytche DH. Visual hallucinatory syndromes and the anatomy of the visual brain. Brain. 2000;123:2055–2064. doi: 10.1093/brain/123.10.2055. [DOI] [PubMed] [Google Scholar]
- 163.Ettinger U, et al. Decomposing the neural correlates of antisaccade eye movements using event-related FMRI. Cereb Cortex. 2008;18:1148–1159. doi: 10.1093/cercor/bhm147. [DOI] [PubMed] [Google Scholar]
- 164.Braak H, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 165.De Marco EV, et al. Glucocerebrosidase gene mutations are associated with Parkinson’s disease in southern Italy. Mov Disord. 2008;23:460–463. doi: 10.1002/mds.21892. [DOI] [PubMed] [Google Scholar]
- 166.Creese B, et al. No association of COMT val158met polymorphism and psychotic symptoms in Lewy body dementias. Neurosci Lett. 2012;531:1–4. doi: 10.1016/j.neulet.2012.09.062. [DOI] [PubMed] [Google Scholar]
- 167.Kaiser R, et al. L-Dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology. 2003;60:1750–1755. doi: 10.1212/01.wnl.0000068009.32067.a1. [DOI] [PubMed] [Google Scholar]
- 168.Wang J, Zhao C, Chen B, Liu ZL. Polymorphisms of dopamine receptor and transporter genes and hallucinations in Parkinson’s disease. Neurosci Lett. 2004;355:193–196. doi: 10.1016/j.neulet.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 169.Schumacher-Schuh AF, et al. Polymorphisms in the dopamine transporter gene are associated with visual hallucinations and levodopa equivalent dose in Brazilians with Parkinson’s disease. Int J Neuropsychopharmacol. 2013;16:1251–1258. doi: 10.1017/S1461145712001666. [DOI] [PubMed] [Google Scholar]
- 170.Makoff AJ, et al. Association study of dopamine receptor gene polymorphisms with drug-induced hallucinations in patients with idiopathic Parkinson’s disease. Pharmacogenetics. 2000;10:43–48. doi: 10.1097/00008571-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 171.Fujii C, et al. Association between polymorphism of the cholecystokinin gene and idiopathic Parkinson’s disease. Clin Genet. 1999;56:394–399. doi: 10.1034/j.1399-0004.1999.560508.x. [DOI] [PubMed] [Google Scholar]
- 172.Wang J, Si YM, Liu ZL, Yu L. Cholecystokinin, cholecystokinin-A receptor and cholecystokinin-B receptor gene polymorphisms in Parkinson’s disease. Pharmacogenetics. 2003;13:365–369. doi: 10.1097/00008571-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 173.Goldman JG, Goetz CG, Berry-Kravis E, Leurgans S, Zhou L. Genetic polymorphisms in Parkinson disease subjects with and without hallucinations: an analysis of the cholecystokinin system. Arch Neurol. 2004;61:1280–1284. doi: 10.1001/archneur.61.8.1280. [DOI] [PubMed] [Google Scholar]
- 174.Goldman JG, et al. Racial differences may influence the role of cholecystokinin polymorphisms in Parkinson’s disease hallucinations. Mov Disord. 2011;26:1781–1782. doi: 10.1002/mds.23655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin JJ, Yueh KC, Lin SZ, Harn HJ, Liu JT. Genetic polymorphism of the angiotensin converting enzyme and L-dopa-induced adverse effects in Parkinson’s disease. J Neurol Sci. 2007;252:130–134. doi: 10.1016/j.jns.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 176.Pascale E, et al. Genetic polymorphism of angiotensin-converting enzyme is not associated with the development of Parkinson’s disease and of L-dopa-induced adverse effects. J Neurol Sci. 2009;276:18–21. doi: 10.1016/j.jns.2008.08.017. [DOI] [PubMed] [Google Scholar]
- 177.Schumacher-Schuh AF, et al. Association of common genetic variants of HOMER1 gene with levodopa adverse effects in Parkinson’s disease patients. Pharmacogenomics J. 2014;14:289–294. doi: 10.1038/tpj.2013.37. [DOI] [PubMed] [Google Scholar]
- 178.De Luca V, et al. HOMER1 promoter analysis in Parkinson’s disease: association study with psychotic symptoms. Neuropsychobiology. 2009;59:239–245. doi: 10.1159/000230689. [DOI] [PubMed] [Google Scholar]
- 179.Nebe A, Ebersbach G. Selective diplopia in Parkinson’s disease: a special subtype of visual hallucination? Mov Disord. 2007;22:1175–1178. doi: 10.1002/mds.21298. [DOI] [PubMed] [Google Scholar]
- 180.Urwyler P, et al. Visual complaints and visual hallucinations in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:318–322. doi: 10.1016/j.parkreldis.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 181.Meppelink AM, Koerts J, Borg M, Leenders KL, van Laar T. Visual object recognition and attention in Parkinson’s disease patients with visual hallucinations. Mov Disord. 2008;23:1906–1912. doi: 10.1002/mds.22270. [DOI] [PubMed] [Google Scholar]
- 182.Barnes J, Boubert L. Executive functions are impaired in patients with Parkinson’s disease with visual hallucinations. J Neurol Neurosurg Psychiatry. 2008;79:190–192. doi: 10.1136/jnnp.2007.116202. [DOI] [PubMed] [Google Scholar]
- 183.Imamura K, Wada-Isoe K, Kitayama M, Nakashima K. Executive dysfunction in non-demented Parkinson’s disease patients with hallucinations. Acta Neurol Scand. 2008;117:255–259. doi: 10.1111/j.1600-0404.2007.00933.x. [DOI] [PubMed] [Google Scholar]
- 184.Grossi D, et al. Frontal dysfunction contributes to the genesis of hallucinations in non-demented Parkinsonian patients. Int J Geriatr Psychiatry. 2005;20:668–673. doi: 10.1002/gps.1339. [DOI] [PubMed] [Google Scholar]
- 185.Chung EJ, Seok K, Kim SJ. A comparison of montreal cognitive assessment between patients with visual hallucinations and without visual hallucinations in Parkinson’s disease. Clin Neurol Neurosurg. 2015;130:98–100. doi: 10.1016/j.clineuro.2014.12.022. [DOI] [PubMed] [Google Scholar]
- 186.Breier A, et al. Olanzapine in the treatment of dopamimetic-induced psychosis in patients with Parkinson’s disease. Biol Psychiatry. 2002;52:438–445. doi: 10.1016/s0006-3223(02)01392-6. [DOI] [PubMed] [Google Scholar]
- 187.Burn D, et al. Effects of rivastigmine in patients with and without visual hallucinations in dementia associated with Parkinson’s disease. Mov Disord. 2006;21:1899–1907. doi: 10.1002/mds.21077. [DOI] [PubMed] [Google Scholar]
- 188.Aarsland D, et al. Memantine in patients with Parkinson’s disease dementia or dementia with Lewy bodies: a double-blind, placebo-controlled, multicentre trial. Lancet Neurol. 2009;8:613–618. doi: 10.1016/S1474-4422(09)70146-2. [DOI] [PubMed] [Google Scholar]