Abstract
In order to accomplish its function of transmitting and focusing light, the crystalline lens of the vertebrate eye has evolved a unique cellular structure and protein complement. These distinct adaptations have provided a rich source of scientific discovery ranging from biochemistry and genetics to optics and physics. In addition, because of these adaptations, lens cells persist for the lifetime of an organism, providing an excellent model of the aging process. The chapters dealing with the lens will demonstrate how the different aspects of lens biology and biochemistry combine in this singular refractive organ to accomplish its critical role in the visual system.
1. INTRODUCTION
Like the lens in a camera, the basic function of the eye lens is to transmit and focus light onto the retina. To facilitate this, it contains one of the highest concentrations of proteins of any tissue. The lens has been studied scientifically for over a century, beginning in 1833 when Sir David Brewster deduced the fine structure of the cod lens using only a candle and a finely ruled steel bar.1 In 1894, Mörner first described high concentrations of soluble structural proteins we now call crystallins,2 and Spemann developed the concept of inductive interactions in development by studying the lens in 1901.3 Renwick mapped a cataract locus, one of the first autosomal loci to be localized,4 and chicken lens δ-crystallins were among the first mRNAs to be isolated and cloned.5 Thus, in addition to being important in the study of inherited diseases, the lens has also been a model system invaluable for developmental and structural biology.
2. STRUCTURE AND CELLS OF THE LENS
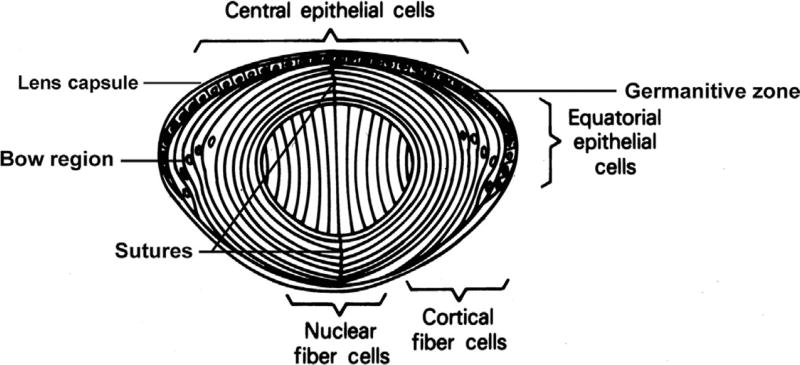
Weighing about 65 mg at birth, the human lens increases in weight to about 160 mg by the age of 10 at which time growth slows substantially so that it weighs about 250 mg by the age of 90.6,7 As much as 60% of the total mass of the lens can be made up of proteins, much higher than almost any other tissue.8 The lens is surrounded by a collagenous capsule, on which the anterior-facing basal poles of the epithelial cells rest, as do the basal poles of the fiber cells facing posteriorly (Fig. 1).9,10 The capsule acts as a barrier to diffusion and contributes to shaping the lens during accommodation.11,12 Its main components are type IV collagen, laminin, entactin, perlecan, type XVIII collagen, heparin sulfate proteoglycan, and fibronectin,13,14 of which the first four are major structural molecules that self-assemble to form a matrix. The capsular filaments, of uniform size and aligned in a parallel fashion, are thinnest at the posterior pole and thicken to a maximum at the equator, where the lens zonules insert.14 Fibrillin and elastin fibers also integrate in the equatorial region, especially in the outer zonular.15 The lens capsule is first detectable at 5–6 weeks of gestation in humans16 and is produced continually throughout life12 anteriorly by the cuboidal epithelium and more slowly posteriorly by the fiber cells.
Figure 1.
Human lens structure. Anterior epithelial cells divide at the 10 and 2 o’clock positions. Cells then move laterally, eventually inverting in the bow region, at which time they elongate and begin degrading their organelles to form cortical fiber cells. Central nuclear fiber cells elongate from the posterior epithelia early in development. The ends of the more peripheral secondary fiber cells abut at the sutures, which are shown here as vertical lines but are seen clinically as the anterior and posterior Y structures.
Mitotic division in the lens occurs in the germinative zone of the anterior epithelium located just anterior to the equator. The anterior epithelial cells of the lens are connected by gap junctions,17 allowing exchange of low-molecular-weight metabolites and ions. They have few or no tight junctions that would make the extracellular spaces impermeable to these molecules.18,19 Anterior cuboidal epithelial cells also are rich in organelles and contain large amounts of cytoskeletal proteins such as microtubules, spectrin, α-actinin, actin, myosin, and vimentin, presumably to help stabilize the cell structures during accommodation.20–22 Both lens epithelial and especially fiber cells contain large amounts of crystallins.
Fiber cells make up the lens nucleus. Layers of nucleated cortical fiber cells form highly ordered concentric shells around the nonnucleated central fiber cells which make up the fetal nucleus, with the ends of the peripheral fiber cells abutting in sutures anteriorly and posteriorly. Both the ordered arrangement of the fiber cells and their sutures as well as their intracellular structure are important for light transmission and lens transparency.23–25 Also contributing to transparency is the presence of only minimal extracellular space between fiber cells, which have many interdigitations.9,26 Junctional complexes between adjacent fiber cells allow for exchange of metabolites.21,22 Lens crystallins, which make up about 90% of the water-soluble protein, are the main soluble components of fiber cells, along with cytoskeletal components, including actin, myosin, vimentin, α-actinin, and microtubules.27
The lens is composed of a single cell type that follows a developmental pattern, beginning as a member of the germinative zone in the single layer of anterior epithelial cells overlaying the fiber cell mass.26 Epithelial cells then migrate laterally toward the equator, where they begin to elongate and invert to form secondary fibers. In order to increase light transmission, organelles such as mitochondria, Golgi bodies, and both rough and smooth ER are degraded in the differentiating lens fiber cells so that they are absent from nuclear fiber cells. The density of their cell membranes increases, approaching that of the cytoplasm, which also decreases light scattering.28 As the cells elongate newer cortical fiber cells are layered over them so that they are moved toward the lens nucleus, stretching anteriorly from the cuboidal epithelial cells posteriorly to the posterior capsule. Transcriptional control plays a significant role in the differential synthesis of lens crystallins (see Ref. 29). The distribution of β-crystallin mRNAs in chickens30 and the β- and γ-crystallin proteins and mRNAs in rats31,32 provides examples of the spatial and temporal control of crystallin gene expression during lens development.
3. TRANSPARENCY
The main optical function of the lens is to transmit light, focusing it on the retina. The cornea contributes about 80% of total refraction, while the lens fine-tunes the focusing of light onto the retina. Although the human lens is colorless at birth, there is a gradual increase in yellowish pigmentation with age33 probably due to the production of 3-hydroxykynurenine and other metabolites of tryptophan that filter UV light.34 The lens transmits light with wavelengths up to 1200 nm efficiently, but transmits very little light below 390 nm. 1200 nm is well above the limit of visual perception, about 720 nm. As discussed previously, the architecture and cellular contents of the lens are critical for its transparency. The transparency and high-refractive index of cells in the lens result from tight packing of their proteins, providing a constant refractive index over distances approximating the wavelength of the transmitted light.24,25 In fact, as lens proteins are diluted to concentrations below that found in the lens, about 450 mg/ml, light scattering actually increases,35,36 because dilution decreases the weak interactions between unlike proteins that occur at high concentrations and help to maintain lens transparency.37,38 Finally, there is a gradual increase in the refractive index of the human lens from 1.38 (73–80% H2O) in the cortex to 1.42 (68%H2O) in the nucleus, in part due to an enrichment of tightly packed γ-crystallins.39
4. AGING
The inability of cells to be replaced in the encapsulated lens combined with the inability of lens cell proteins to turn over in the nonnucleated fiber cells makes the lens particularly susceptible to damage with aging and environmental insults such as UV light and other oxidative stresses.40 This results in a decrease in transmission of light and focusing even in the normal aged lens so that the intensity of light reaching the retina is reduced by about 10-fold by 80 years of age.41 It also increases susceptibility to senescent cataract and presbyopia, especially in individuals exposed to environmental insults or having a genetic proclivity.42 With increasing age, vacuoles and multilamellar bodies develop between lens fiber cells, occasionally disrupting the fiber plasma membrane.43 In addition, most of the elaborate cytoskeletal structure found in lens cells disappears with age,44 so that by the fifth decade presbyopia develops with loss of the ability to accommodate.45,46
Enzymatic activity in the lens decreases with age, especially in the central cells of the lens nucleus where the cells are older than those in the cortical nucleus and especially the anterior epithelial cells.47 This compromised intracellular homeostasis might be exacerbated by the decreased metabolic coupling of the active cortex and the inactive nucleus that occurs in older lenses, in part associated with decreased gap junction coupling.48,49 This is particularly relevant for the enzymes that produce a reducing environment by maintaining high levels of reduced glutathione, such as glutathione reductase and glucose-6-phosphate dehydrogenase.50 Decreases in the activity of these and other reducing enzymes decrease defenses against oxidative damage in the lens and exacerbate damage to crystallins and other metabolic support systems.51 Finally, as the lens ages intracellular Na+ and Ca2+ concentrations rise, probably due to an increase in lens permeability or decrease in ion channel pumping efficiency.52
Lens crystallins also show age-related changes that might interfere with lens transparency.37 Between 10 and 50 years of age crystallin modification increases,53 as does the level of high-molecular-weight aggregates and water-insoluble protein.54 Because of their chaperone activity, this is especially notable in the α-crystallins, but is also seen in the β- and γ-crystallins.55,56 Crystallins, membranes, and enzymes are also cleaved and partially degraded, including the nonenzymatic cleavage of αA-crystallin at the bond between Asn101 and Glu102.57 In what might be a positive feedback effect, cleavage products of βA3-crystallin appear to inhibit the chaperone activity of α-crystallin chaperone.58 γ-Crystallins, and particularly γS-crystallin, are often subject to proteolysis, degradation, and modification in age-dependent cataracts, being broken down to low-molecular-weight peptides.59–61
As the lens ages both the amino- and carboxyl-terminal arms of up to half of the intrinsic membrane protein AQP0 (MP26) molecules undergo proteolysis, forming MP22.62 Other posttranslational modifications of AQP0 also occur with aging including C-terminal phosphorylation, possibly involved in intercellular trafficking, and glycation, which influences AQP0 interaction with calmodulin. However, the precise functional significance of these remains unclear.63,64 The lens contains proteasomes, which preferentially degrade oxidized proteins65 tagged with the protease cofactor ubiquitin,66 whose activity is increased by oxidative stress.67 These proteinases are balanced during aging by inhibitors including the chaperones HSP90 and α-crystallin.59
Covalent modifications of crystallins and other lens proteins also increase with aging, with increases in oxidation of methionine, deamidation of asparagine and glutamine residues, disulfide bridges, backbone cleavage, and racemization of aspartic acid residues.59,68,69 Deamidation can destabilize βA3-crystallin, causing it to aggregate,70 while deamidation of glutamines at the interface of γD-crystallin can also destabilize it.71 Asp151 in αA-crystallin is especially susceptible to racemization because it forms a succinimide intermediate easily.72 Racemization at both Asp58 and Asp151 can lead to increased aggregation and decreased chaperone activity and is enhanced by mutations of nearby residues.73 Finally, phosphorylation and nonenzymatic glycosylation (glycation) also occur, especially affecting the ε-amino groups of lysine residues.57,74,75 These can participate in the Maillard reaction, resulting in nondisulfide covalent cross-links, increased pigmentation, and nontryptophan fluorescence.76 Glycation of α-crystallin can also decrease its chaperone function, eventually resulting in aggregation. 77 Lens proteins can also undergo carbamylation with aging or other insult, and this can induce cataract,78 which has been proposed to be the mechanism of cataract associated with chronic diarrhea and its resultant uremia.79 Thus, the development and biology of the lens is directed at establishing transparency and focusing of light, and then defending this highly specialized system against damage by age and environmental insults.
References
- 1.Brewster D. On the anatomical and optical structure of the crystalline lens of animals, particularly that of cod. Philos Trans R Soc Lond. 1833;123:323–332. [Google Scholar]
- 2.Morner CT. Untersuchungen der protein-substanzen in den lichtbrechenden Medien des Auges. Hoppe Seylers Z Physiol Chem. 1894;18:61–106. [Google Scholar]
- 3.Spemann H. Uber Korrelationen in der Entwicklung des Auges. Verh Anat Ges. 1901;15:61–79. [Google Scholar]
- 4.Renwick JH, Lawler SD. Probable linkage between a congenital cataract locus and the Duffy blood group locus. Ann Hum Genet. 1963;27:67–84. doi: 10.1111/j.1469-1809.1963.tb00782.x. [DOI] [PubMed] [Google Scholar]
- 5.Zelenka PS, Piatigorsky J. Isolation and in vitro translation of delta-crystallin mRNA from embryonic chick lens fibers. Proc Natl Acad Sci USA. 1974;71:1896–1900. doi: 10.1073/pnas.71.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harding JJ, Rixon KC, Marriott FHC. Men have heavier lenses than women of the same age. Exp Eye Res. 1977;25:651. doi: 10.1016/0014-4835(77)90143-9. [DOI] [PubMed] [Google Scholar]
- 7.Augusteyn RC. Growth of the human eye lens. Mol Vis. 2007;13:252–257. [PMC free article] [PubMed] [Google Scholar]
- 8.Wistow GJ, Piatigorsky J. Lens crystallins: the evolution and expression of proteins for a highly specialized tissue. Annu Rev Biochem. 1988;57:479–504. doi: 10.1146/annurev.bi.57.070188.002403. [DOI] [PubMed] [Google Scholar]
- 9.Kuszak JR. Embryology and anatomy of the lens. In: Tasman W, Jaeger EA, editors. Duane’s Clinical Ophthalmology. Philadelphia: J.B. Lippincott; 1990. pp. 1–9. [Google Scholar]
- 10.Danysh BP, Duncan MK. The lens capsule. Exp Eye Res. 2009;88(2):151–164. doi: 10.1016/j.exer.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koretz JF, Handelman GH. How the human eye focuses. Sci Am. 1988;256:92–99. doi: 10.1038/scientificamerican0788-92. [DOI] [PubMed] [Google Scholar]
- 12.Fisher RF, Pettet BE. The postnatal growth of the capsule of the human crystalline lens. J Anat. 1972;112:207–214. [PMC free article] [PubMed] [Google Scholar]
- 13.Parmigiani C, McAvoy J. Localisation of laminin and fibronectin during rat lens morphogenesis. Differentiation. 1986;28:53–61. doi: 10.1111/j.1432-0436.1984.tb00266.x. [DOI] [PubMed] [Google Scholar]
- 14.Cammarata PR, Cantu-Crouch D, Oakford L, Morrill A. Macromolecular organization of the bovine lens capsule. Tissue Cell. 1986;18:83–97. doi: 10.1016/0040-8166(86)90009-1. [DOI] [PubMed] [Google Scholar]
- 15.Mir S, Wheatley HM, Hussels IE, Whittum-Hudson JA, Traboulsi EI. A comparative histologic study of the fibrillin microfibrillar system in the lens capsule of normal subjects and subjects with Marfan syndrome. Invest Ophthalmol Vis Sci. 1998;39(1):84–93. [PubMed] [Google Scholar]
- 16.Mann I. The Development of the Human Eye. New York: Grune and Stratton; 1964. [Google Scholar]
- 17.Goodenough DA, Dick JSB, Lyons JE. Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J Cell Biol. 1980;86:576–589. doi: 10.1083/jcb.86.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorthy WC, Snavely MR, Berrong ND. Some aspects of transport and digestion in the lens of the normal young adult rat. Exp Eye Res. 1971;12:112–119. doi: 10.1016/0014-4835(71)90135-7. [DOI] [PubMed] [Google Scholar]
- 19.Rae JL, Stacey T. Lanthanum and procion yellow as extracellular markers in the crystalline lens of the rat. Exp Eye Res. 1979;28:1–21. doi: 10.1016/0014-4835(79)90101-5. [DOI] [PubMed] [Google Scholar]
- 20.Ramaekers FCS, Bloemendal H. Cytoskeletal and contractile structures in lens cell differentiation. In: Bloemendal H, editor. Molecular and Cellular Biology of the Eye Lens. New York: John Wiley & Sons; 1981. pp. 85–136. [Google Scholar]
- 21.Benedetti L, Dunia I, Ramaekers FCS, Kibbelaar MA. Lenticular plasma membranes and cytoskeleton. In: Bloemendal H, editor. Molecular and Cellular Biology of the Eye Lens. New York: John Wiley & Sons; 1981. pp. 137–188. [Google Scholar]
- 22.Alcala H, Maisel H. Biochemistry of lens plasma membranes and cytoskeleton. In: Maisel H, editor. The Ocular Lens. New York: Marcel Dekker Inc.; 1985. pp. 169–222. [Google Scholar]
- 23.Kuszak JR, Zoltoski RK, Sivertson C. Fibre cell organization in crystalline lenses. Exp Eye Res. 2004;78(3):673–687. doi: 10.1016/j.exer.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Benedek GB. Theory of transparency of the eye. Appl Opt. 1971;10:459–473. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 25.Delaye M, Tardieu A. Short-range order of crystallin proteins accounts for eye lens transparency. Nature. 1983;302:415–417. doi: 10.1038/302415a0. [DOI] [PubMed] [Google Scholar]
- 26.Rafferty NS. Lens morphology. In: Maisel H, editor. The Ocular Lens. New York: Marcel Dekker Inc.; 1985. pp. 1–60. [Google Scholar]
- 27.Ireland M, Maisel H. A family of lens fiber cell specific proteins. Lens Eye Toxic Res. 1989;6:623–638. [PubMed] [Google Scholar]
- 28.Michael R, van Marle J, Vrensen GF, van den Berg TJ. Changes in the refractive index of lens fibre membranes during maturation—impact on lens transparency. Exp Eye Res. 2003;77(1):93–99. doi: 10.1016/s0014-4835(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 29.Piatigorsky J. Gene expression and genetic engineering in the lens. Friedenwald lecture. Invest Ophthalmol Vis Sci. 1987;28:9–28. [PubMed] [Google Scholar]
- 30.Hejtmancik JF, Beebe DC, Ostrer H, Piatigorsky J. delta- and beta-Crystallin mRNA levels in the embryonic and posthatched chicken lens: temporal and spatial changes during development. Dev Biol. 1985;109:72–81. doi: 10.1016/0012-1606(85)90347-1. [DOI] [PubMed] [Google Scholar]
- 31.van Leen RW, van Roozendaal KEP, Lubsen NH, Schoenmakers JG. Differential expression of crystallin genes during development of the rat eye lens. Dev Biol. 1987;120:457–464. doi: 10.1016/0012-1606(87)90249-1. [DOI] [PubMed] [Google Scholar]
- 32.Aarts HJ, Lubsen NH, Schoenmakers JG. Crystallin gene expression during rat lens development. Eur J Biochem. 1989;183:31–36. doi: 10.1111/j.1432-1033.1989.tb14892.x. [DOI] [PubMed] [Google Scholar]
- 33.Lerman S. Radiant Energy and the Eye. New York: MacMillan; 1980. [Google Scholar]
- 34.Korlimbinis A, Truscott RJ. Identification of 3-hydroxykynurenine bound to proteins in the human lens. A possible role in age-related nuclear cataract. Biochemistry. 2006;45(6):1950–1960. doi: 10.1021/bi051744y. [DOI] [PubMed] [Google Scholar]
- 35.Bettelheim FA, Siew EL. Effect of changes in concentration upon lens turbidity as predicted by the random fluctuation theory. Biophys J. 1983;41:29–33. doi: 10.1016/S0006-3495(83)84402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Delaye M, Gromiec A. Mutual diffusion of crystallin proteins at finite concentrations: a light scattering study. Biopolymers. 1983;22:1203–1221. doi: 10.1002/bip.360220413. [DOI] [PubMed] [Google Scholar]
- 37.Takemoto L, Sorensen CM. Protein-protein interactions and lens transparency. Exp Eye Res. 2008;87(6):496–501. doi: 10.1016/j.exer.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stradner A, Foffi G, Dorsaz N, Thurston G, Schurtenberger P. New insight into cataract formation: enhanced stability through mutual attraction. Phys Rev Lett. 2007;99(19):198103. doi: 10.1103/PhysRevLett.99.198103. [DOI] [PubMed] [Google Scholar]
- 39.Uhlhorn SR, Borja D, Manns F, Parel JM. Refractive index measurement of the isolated crystalline lens using optical coherence tomography. Vision Res. 2008;48(27):2732–2738. doi: 10.1016/j.visres.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lynnerup N, Kjeldsen H, Heegaard S, Jacobsen C, Heinemeier J. Radiocarbon dating of the human eye lens crystallines reveal proteins without carbon turnover throughout life. PLoS One. 2008;3(1):e1529. doi: 10.1371/journal.pone.0001529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sample PA, Esterson FD, Weinreb RN, Boynton RM. The aging lens: in vivo assessment of light absorption in 84 human eyes. Invest Ophthalmol Vis Sci. 1988;8:1306–1311. [PubMed] [Google Scholar]
- 42.Bron AJ, Vrensen GF, Koretz J, Maraini G, Harding JJ. The ageing lens. Ophthalmologica. 2000;214(1):86–104. doi: 10.1159/000027475. [DOI] [PubMed] [Google Scholar]
- 43.Vrensen G, Kappelhof J, Willikens B. Aging of the human lens. Lens Eye Toxic Res. 1990;7:1–30. [PubMed] [Google Scholar]
- 44.Kuszak JR, Deutsch TA, Brown HG. Anatomy of aged and senile cataractous lenses. In: Albert D, Jacobiec F, editors. Principles and Practice of Ophthalmology: Basic Sciences. Philadelphia: W.B. Saunders; 1994. pp. 82–97. [Google Scholar]
- 45.Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye—aging of the anterior segment. Vision Res. 1989;29:1685–1692. doi: 10.1016/0042-6989(89)90150-8. [DOI] [PubMed] [Google Scholar]
- 46.Davson H. Physiology of the Eye. New York: Pergamon Press; 1990. [Google Scholar]
- 47.Hockwin O, Ohrloff C. The eye in the elderly: lens. In: Platt D, editor. Geriatrics. Berlin: Springer-Verlag; 1984. pp. 373–424. [Google Scholar]
- 48.Truscott RJ. Age-related nuclear cataract: oxidation is the key. Exp Eye Res. 2005;80(5):709–725. doi: 10.1016/j.exer.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Gao J, Wang H, Sun X, et al. The effects of age on lens transport. Invest Ophthalmol Vis Sci. 2013;54(12):7174–7187. doi: 10.1167/iovs.13-12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ganea E, Harding JJ. Glutathione-related enzymes and the eye. Curr Eye Res. 2006;31(1):1–11. doi: 10.1080/02713680500477347. [DOI] [PubMed] [Google Scholar]
- 51.Wei M, Xing KY, Fan YC, Libondi T, Lou MF. Loss of thiol repair systems in human cataractous lenses. Invest Ophthalmol Vis Sci. 2015;56(1):598–605. doi: 10.1167/iovs.14-15452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Duncan G, Hightower KR, Gandolfi SA, Tomlinson J, Maraini G. Human lens membrane cation permeability increases with age. Invest Ophthalmol Vis Sci. 1989;30(8):1855–1859. [PubMed] [Google Scholar]
- 53.Lampi KJ, Ma Z, Hanson SR, et al. Age-related changes in human lens crystallins identified by two-dimensional electrophoresis and mass spectrometry. Exp Eye Res. 1998;67(1):31–43. doi: 10.1006/exer.1998.0481. [DOI] [PubMed] [Google Scholar]
- 54.Datiles MB, III, Ansari RR, Suh KI, et al. Clinical detection of precataractous lens protein changes using dynamic light scattering. Arch Ophthalmol. 2008;126(12):1687–1693. doi: 10.1001/archophthalmol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roy D, Spector A. Absence of low-molecular weight alpha-crystallin in nuclear region of old human lens. Proc Natl Acad Sci USA. 1976;73:3484–3487. doi: 10.1073/pnas.73.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McFall-Ngai MJ, Ding LL, Takemoto LJ, Horwitz J. Spatial and temporal mapping of the age-related changes in human lens crystallins. Exp Eye Res. 1985;41:745–758. doi: 10.1016/0014-4835(85)90183-6. [DOI] [PubMed] [Google Scholar]
- 57.Voorter CE, De Haard-Hoekman WA, Roersma ES, Meyer HE, Bloemendal H, de Jong WW. The in vivo phosphorylation sites of bovine alpha B-crystallin. FEBS Lett. 1989;259:50–52. doi: 10.1016/0014-5793(89)81491-7. [DOI] [PubMed] [Google Scholar]
- 58.Rao G, Santhoshkumar P, Sharma KK. Anti-chaperone betaA3/A1(102–117) peptide interacting sites in human alphaB-crystallin. Mol Vis. 2008;14:666–674. [PMC free article] [PubMed] [Google Scholar]
- 59.Harding JJ, Crabbe MJC. In: The lens: development, proteins, metabolism and cataract. 3. Davson H, editor. Orlando: Academic Press; 1984. pp. 207–492. The Eye; vol IB. [Google Scholar]
- 60.Straatsma BR, Horwitz J, Takemoto LJ, Lightfoot DO, Ding LL. Clinicobiochemical correlations in aging-related human cataract. Am J Ophthalmol. 1984;97:457–469. doi: 10.1016/s0002-9394(14)76129-x. [DOI] [PubMed] [Google Scholar]
- 61.David LL, Shearer TR. Role of proteolysis in lenses: a review. Lens Eye Toxic Res. 1989;6:725–747. [PubMed] [Google Scholar]
- 62.Horwitz J, Wong MM. Peptide mapping by limited proteolysis in sodium dodecyl sulfate of the main intrinsic polypeptides isolated from human and bovine lens plasma membranes. Biochim Biophys Acta. 1980;622:134–143. doi: 10.1016/0005-2795(80)90165-8. [DOI] [PubMed] [Google Scholar]
- 63.Ball LE, Garland DL, Crouch RK, Schey KL. Post-translational modifications of aquaporin 0 (AQP0) in the normal human lens: spatial and temporal occurrence. Biochemistry. 2004;43(30):9856–9865. doi: 10.1021/bi0496034. [DOI] [PubMed] [Google Scholar]
- 64.Swamy-Mruthinti S. Glycation decreases calmodulin binding to lens transmembrane protein, MIP. Biochim Biophys Acta. 2001;1536(1):64–72. doi: 10.1016/s0925-4439(01)00031-x. [DOI] [PubMed] [Google Scholar]
- 65.Wagner BJ, Margolis JW, Garland D, Roseman JE. Bovine lens neutral proteinase preferentially hydrolyses oxidatively modified glutamine synthetase. Exp Eye Res. 1986;43:1141–1143. doi: 10.1016/0014-4835(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 66.Jahngen JH, Lipman RD, Eisenhauer DA, Jahngen EG, Jr, Taylor A. Aging and cellular maturation cause changes in ubiquitin-eye lens protein conjugates. Arch Biochem Biophys. 1990;276:32–37. doi: 10.1016/0003-9861(90)90006-k. [DOI] [PubMed] [Google Scholar]
- 67.Shang F, Gong X, Taylor A. Activity of ubiquitin-dependent pathway in response to oxidative stress. Ubiquitin-activating enzyme is transiently up-regulated. J Biol Chem. 1997;272(37):23086–23093. doi: 10.1074/jbc.272.37.23086. [DOI] [PubMed] [Google Scholar]
- 68.Masters PM, Bada JL, Zigler JS., Jr Aspartic acid racemisation in the human lens during ageing and in cataract formation. Nature. 1977;268:71–73. doi: 10.1038/268071a0. [DOI] [PubMed] [Google Scholar]
- 69.Hanson SR, Hasan A, Smith DL, Smith JB. The major in vivo modifications of the human water-insoluble lens crystallins are disulfide bonds, deamidation, methionine oxidation and backbone cleavage. Exp Eye Res. 2000;71(2):195–207. doi: 10.1006/exer.2000.0868. [DOI] [PubMed] [Google Scholar]
- 70.Takata T, Oxford JT, Demeler B, Lampi KJ. Deamidation destabilizes and triggers aggregation of a lens protein, betaA3-crystallin. Protein Sci. 2008;17(9):1565–1575. doi: 10.1110/ps.035410.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flaugh SL, Mills IA, King J. Glutamine deamidation destabilizes human gammaD-crystallin and lowers the kinetic barrier to unfolding. J Biol Chem. 2006;281(41):30782–30793. doi: 10.1074/jbc.M603882200. [DOI] [PubMed] [Google Scholar]
- 72.Groenen PJ, van den Ijssel PR, Voorter CE, Bloemendal H, de Jong WW. Site-specific racemization in aging alpha A-crystallin. FEBS Lett. 1990;269:109–112. doi: 10.1016/0014-5793(90)81131-7. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura T, Sakai M, Sadakane Y, et al. Differential rate constants of racemization of aspartyl and asparaginyl residues in human alpha A-crystallin mutants. Biochim Biophys Acta. 2008;1784(9):1192–1199. doi: 10.1016/j.bbapap.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 74.Spector A, Chiesa R, Sredy J, Garner W. cAMP-dependent phosphorylation of bovine lens alpha-crystallin. Proc Natl Acad Sci USA. 1985;82:4712–4716. doi: 10.1073/pnas.82.14.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garlick RL, Mazer JS, Chylack LT, Jr, Tung WH, Bunn HF. Nonenzymatic glycation of human lens crystallin. Effect of aging and diabetes mellitus. J Clin Investig. 1984;74:1742–1749. doi: 10.1172/JCI111592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Augusteyn RC. Distribution of fluorescence in the human cataractous lens. Ophthalmic Res. 1975;7:217–224. [Google Scholar]
- 77.Kumar PA, Kumar MS, Reddy GB. Effect of glycation on alpha-crystallin structure and chaperone-like function. Biochem J. 2007;408(2):251–258. doi: 10.1042/BJ20070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harding JJ. Possible causes of the unfolding of proteins in cataract and a new hypothesis to explain the high prevalence of cataract in some countries. In: Regnault F, Hockwin O, Courtois Y, editors. Aging of the Lens. Amsterdam: Elsevier; 1980. pp. 71–80. [Google Scholar]
- 79.Harding JJ, Rixon KC. Carbamylation of lens proteins: a possible factor in cataractogenesis in some tropical countries. Exp Eye Res. 1980;31:567–571. doi: 10.1016/s0014-4835(80)80015-7. [DOI] [PubMed] [Google Scholar]