Introduction
Necrotizing soft tissue infections (NSTI) are rapidly progressive skin and soft tissue infections that cause widespread tissue necrosis and are associated with systemic illness1. The term “NSTI” has been increasing used in lieu of the term “necrotizing fasciitis”, originally coined by BL Wilson in 1952, to encompass cases where necrosis extends beyond the fascia and can involve the muscle, skin and surrounding tissues2. The incidence and prevalence of NSTI varies by season, location and patient population. We know from the active surveillance operations of the CDC that the incidence of NSTI due to invasive group A streptococcal (GAS) infections in the United States is 0.4 per 100,0003. The estimated incidence of all-cause NSTI remains less clear due to wide variability in reporting practices. Despite advances in the care, mortality from NSTI has remained relatively high at 25–30% for the past thirty years, and has only recently seen a decrease to just over 20%4,5,6,7,8. Case fatality rates remain highest when NSTI is accompanied by shock and/or host factors such as advanced age, comorbidities or immunocompromised state1.
Necrotizing soft tissue infections can be classified on the basis of microbiology, location or depth of tissue involvement. Guiliano et al originally described 2 distinct microbiologic profiles in NSTI; however, the classification system has evolved over time with the recognition of additional pathogen classes (Table 1)9. Type 1 is the most common infection seen, and describes polymicrobial infections, often including anerobes, Type 2 infections are monomicrobial and typically involve GAS or less commonly Staphylococcus aureus. Monomicrobial NSTI can also be caused by Clostridium spp., and rarely by Vibrio vulnificans (from exposure to warm coastal seawater or consumption of raw oysters; classified by some as Type III), Aeromonas hydrophila (from exposure to leech therapy or traumatic lesions in fresh water)10 as well as fungi (classified by some as Type IV) such as Apohyphomyces spp. Certain monomicrobial etiologies have presented as local outbreaks (e.g. community-associated MRSA in Los Angeles)11 or exhibited geographic clustering (e.g. Klebsiella pneumoniae among diabetic patients with NSTI in Taiwan)12. Terminology varies by anatomic site as well; Fournier’s gangrene is used to describe NSTIs of the perineum, which is generally polymicrobial. Diabetic foot infections are polymicrobial and associated with an anaerobic milieu and compromised microvasculature and can sometimes progress to a necrotizing pattern. Finally, the depth of necrosis can also help classify NSTI, with necrotizing cellulitis describing an infection involving the dermis and subcutaneous tissue, necrotizing fasciitis involving the fascia, and pyomyositis or myonecrosis describing involvement of the muscle fascicle without necessarily having overlying skin infections.
Table 1.
Microbiologic classification of necrotizing soft tissue infections
| Types of NF | Etiology | Organism(s) | Clinical Progress | Mortality |
|---|---|---|---|---|
| Type I (70–80% of cases) | Polymicrobial/synergistic, often bowel flora-derived | Mixed anaerobes and aerobes | More indolent, better prognosis, easier to recognize | Variable, depends on underlying comorbidities |
| Type II (20–30% of cases) | Often monomicrobial, skin or respiratory-derived | Usually A β-hemolytic streptococcus (GAS), occasionally S. aureus | Aggressive, presentation easily missed | >30%, depends on associated myositis |
| Type III (More common in Asia) | Gram-negative, often marine-related organisms | Vibrio spp. | Seafood ingestion or water contamination in wounds | 30%–40% |
| Type IV (Fungal) | Trauma associated |
Candida spp., immunocompromised patients. Zygomycetes in immunocompetent patients |
Aggressive with rapid extension, especially if immunocompromised | >50%, higher if immunocompromised |
Adapted from: Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect 2010;75:249–57.
Pathophysiology
The vicious cycle of fulminant infection, toxin production, cytokine activation, microthrombosis and ischemia, tissue dysfunction and death, and in turn, greater dissemination of infection is central to the rapidly progressive necrosis seen in NSTI and differentiates it from that of uncomplicated skin and soft tissue infections (Figure 1)13. Inoculation may be related to trauma or surgery; injured skeletal muscle cells have demonstrated greater adherence to bacteria14. The pathogen first spreads in the tissue, releasing a variety of toxins. In the case of GAS and Staphylococcus aureus, these are exotoxins15. Toxins mediate an inflammatory change in the walls of the microvasculature that facilitates microvascular thrombosis. Pyrogenic exotoxins act as superantigens that bind to antigen presenting cells and cause rapid proliferation of T cells, and in turn, production of cytokines that perpetrate shock and multiorgan failure. This is the mechanism for development of toxic shock syndrome (TSS), which is seen with up to half of the NSTI cases due to GAS16 and can also be seen in cases due to Staphylococcus aureus. All the clinical criteria of TSS including macular rash and desquamation of palms and soles are not always present, often making TSS difficult to distinguish from septic shock by the bedside; the latter can be associated with all etiologies of NSTI. Antibiotics penetrate dead and dying tissue poorly and such organism-laden dead tissue represents a perpetual source of infection, underscoring the need for emergent surgical source control in NSTI.
Figure 1.
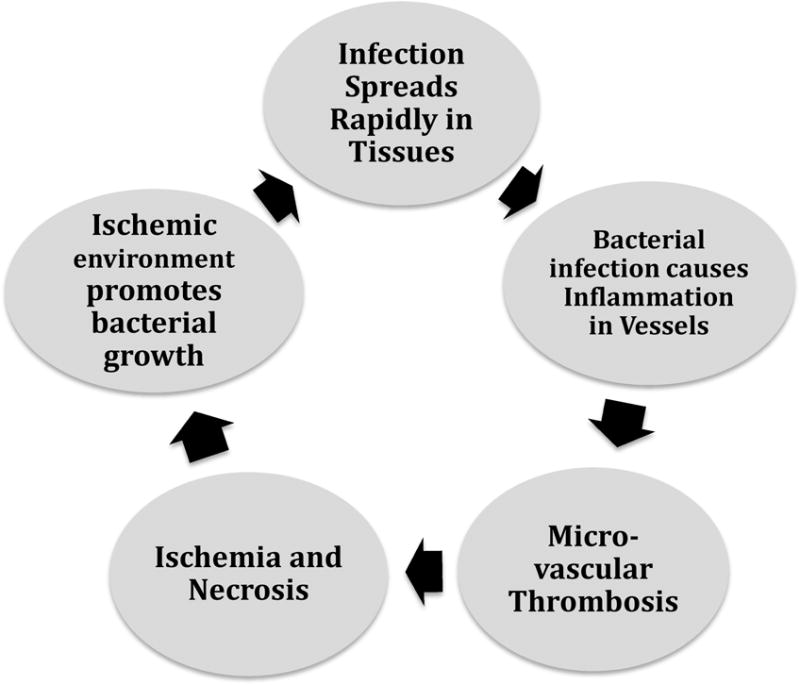
Vicious cycle of necrotizing soft tissue infection
Diagnosis
Clinical assessment
Nothing replaces early recognition and immediate initiation of treatment for NSTI, which are key to a favorable outcome. The majority of cases exhibit swelling and erythema, but the most consistent finding is pain that is out of proportion to exam findings17. However, it can often be difficult to discern a necrotizing process from a simple cellulitis. Patients with NSTI may often present with systemic illness and encephalopathy alone; a thorough exam is valuable when history cannot be easily elicited; suspicion should be very high in patients with a soft tissue infection who rapidly deteriorate with organ system failure18. Additional skin exam findings that should lead to a high index of suspicion include bullae, skin ecchymosis, skin necrosis, and edema outside of the area of erythema, and sometimes, cutaneous anesthesia19.
Laboratory values and Scoring Systems
Laboratory values and imaging have little to add to diagnosis when clinical suspicion of NSTI is high enough to warrant treatment. However, clinical features alone might be poorly sensitive for making a diagnosis of NSTI in equivocal cases. Additionally, the disease is rare enough that some practitioners may have limited experience with these severe infections, and supplemental diagnostic assistance may be desirable to those less familiar with NSTI20. Notably, laboratory findings of leukocytosis and hyponatremia have been shown to improve sensitivity from clinical exam alone21. An admission lactate >6 mmol/L and a serum sodium <135 mEq/L have been shown to be independent predictors of in-hospital mortality in those presenting with NSTI22. In 2004, Wong et al developed the Laboratory Risk Indicator for Necrotizing Fasciitis (LRINEC) score. This score uses white blood cell (WBC) count, hemoglobin, sodium, glucose, serum creatinine and serum c-reactive protein to develop a scoring system for the likelihood of necrotizing fasciitis23. In the original publication, a score of ≥6 yielded a positive predictive value of 92% and negative predictive value of 96%, displaying promise for predicting severity of skin and soft tissue infection among patients presenting to emergency care. Although retrospective validation of this scoring system has been attempted in small case-series24,25, a recent multicenter prospective evaluation of the LRINEC score has lessened the excitement around this predictive tool; a cut-off of ≥ or <6 in NSTI patients failed to discriminate between those with and without high cytokine levels, septic shock and death26. Furthermore, the LRINEC score can be artificially elevated in other musculoskeletal infections. The Fournier’s Gangrene Risk Index, although shown to be a predictor of outcome in retrospective studies, has not shown to be any better than the age-adjusted Charlson Co-morbidity Index and remains of research interest alone27. As such, these scoring systems should not be solely relied upon for diagnosing or exluding NSTI.
Imaging
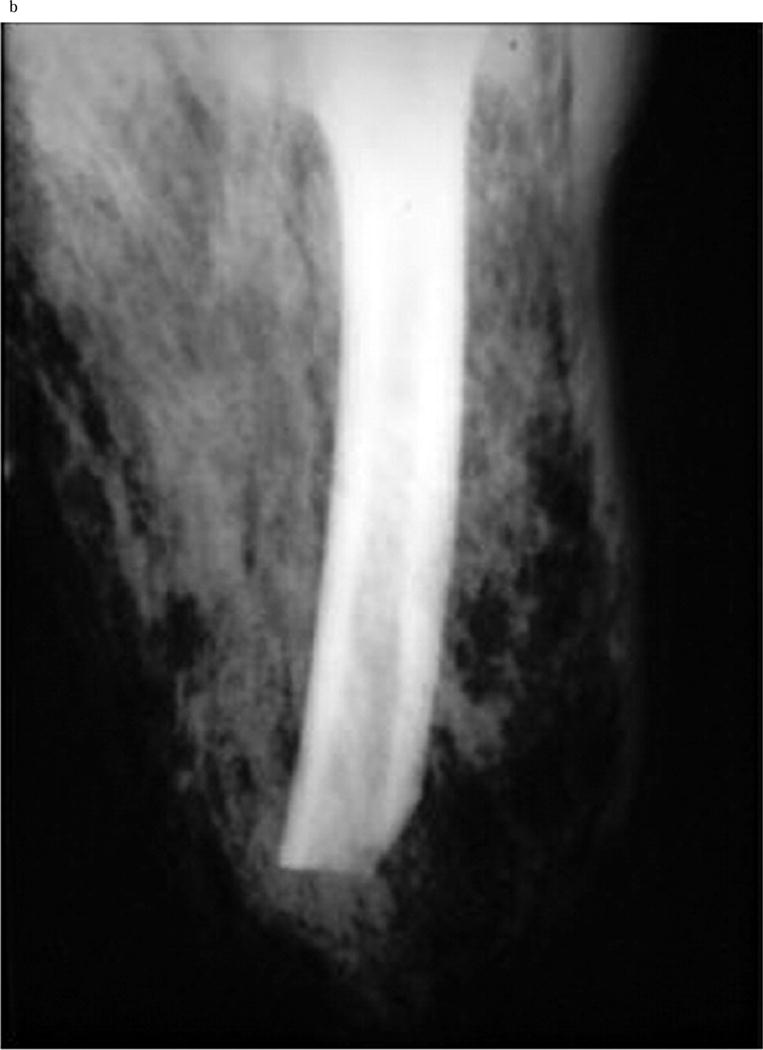
Gas in the soft tissues on plain, portable radiographs, when seen, can aid in the diagnosis of NSTI in patients who are too unstable to travel for more advanced radiographic studies (Figure 2). However, for those patients able to undergo CT scan or MRI, both have been shown to be useful adjuncts for diagnosis when the diagnosis is not certain on clinical evaluation. A CT scan with contrast that demonstrates lack of enhancement of the fascia, along with involvement of the fascia in the infectious process is more specific for NSTI than air or edema alone (Figure 3)28. In the case of MRI, imaging finding consistent with a diagnosis of NSTI include greater thick signal intensity on T2-weighted images and focal nonenhancing areas of abnormal signal intensity in the deep fascia (Figure 4). This is useful in distinguishing a necrotizing infection from a non-necrotizing infection in the case of non-diagnostic CT and plain radiograph findings such as soft tissue swelling29. However, MRI can be overly sensitive as well as time consuming; it can certainly delay necessary surgical management and should be used with caution. Ultrasound can identify soft tissue abscesses in NSTI. The rapidity and portability of point-of-care ultrasound in the emergency room is attractive in principle but evidence is currently limited to sporadic reports and additional data are needed before it can be thought of as a mainstream diagnostic modality for NSTI30.
Figure 2.
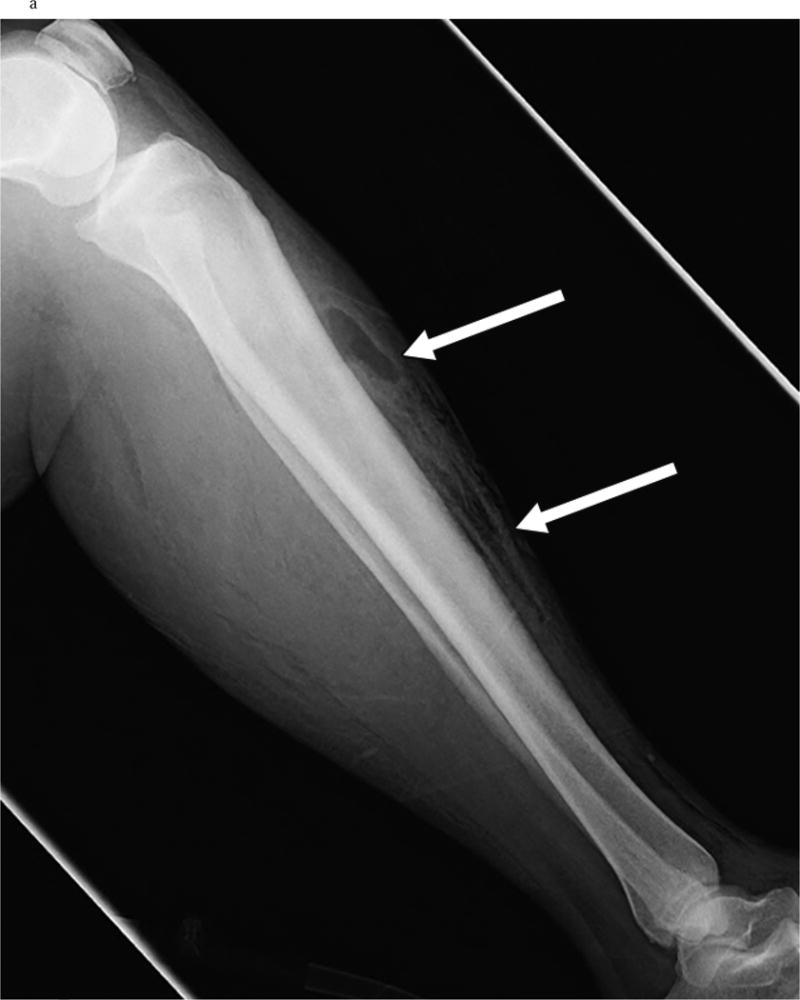
a Evidence of Gas tracking on the fascia on X ray in a patients with NSTI involving the leg. [Used with permission]: Chaudhry A, Baker K, Gould E, Gupta R. Necrotizing Fasciitis and it’s mimics: What Radiologists need to know. AJR 2015; 204: 128–139.
b Subcutaneous emphysema on X ray in a patient with NSTI of the thigh. [Used with permission] from Sarani B, Strong M, Pascual J, Schwab CW. Necrotizing Fasciitis: Current Concepts and Review of the Literature. JACS 2009; 208(2): 279–288.
Figure 3.
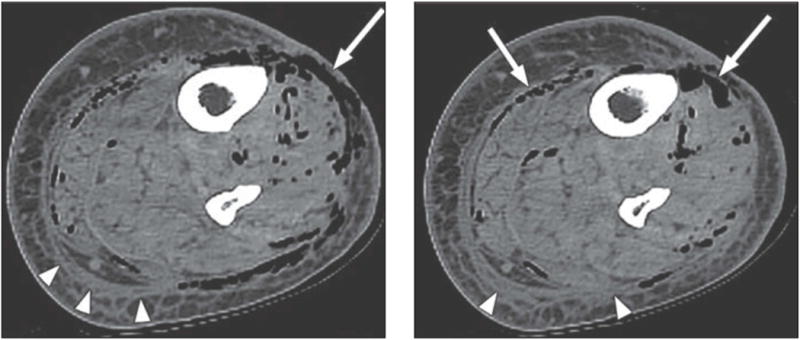
CT scan of a patient with necrotizing soft tissue infection, demonstrating edema in the soft tissues and air tracking along the fascia planes. [Used with permission] : Chaudhry A, Baker K, Gould E, Gupta R. Necrotizing Fasciitis and it’s mimics: What Radiologists need to know. AJR 2015; 204: 128–139.
Figure 4.
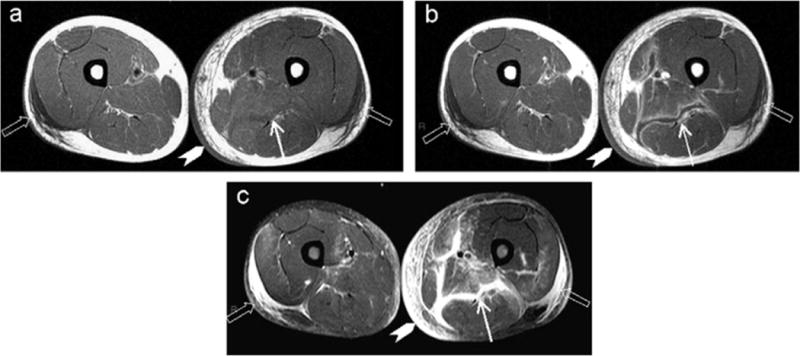
MRI findings for a patient with necrotizing fasciitis and myositis, demonstrating increased T2-weighted soft tissue enhancement and gas in the soft tissues. [Used with permission:]
Bedside exploration and biopsy
The definitive diagnosis of NSTI is made surgically. A large number of equivocal cases exist where additional evidence might be needed before the patient is taken for surgery. In such cases, prior to any formal operation, surgeons can assist in the bedside diagnosis of NSTI by performing a local exploration of the area in question at the bedside under local anesthetic. Alternately, surgeons may proceed to the operating room where additional debridement can be immediately performed if NSTI is diagnosed on local exploration. In this case, a small incision is made over the area of maximal suspicion and the overlying soft tissue is divided. The fascia is examined locally for signs of necrosis, “dishwater” brown fluid or “positive finger sign” in which a finger inserted along the fascial planes easily dissects the overlying tissue without resistance31. Similarly, the underlying muscle tissue can be examined intraoperatively for evidence of necrosis; electrocautery may be used in anesthetized patients without systemic paralytic therapy in place to demonstrate muscle fiber nonreactivity, which indicates tissue death, and high organism density and worse clinics outcome have been suggested; albeit on univariate analyses alone1. Use of biopsy for frozen section analysis might aid in unequivocal cases, however, IDSA guidelines caution against undue reliance on the same due to potential false negatives from sampling error32,33.
Treatment
Any patient with evidence of septic shock should be treated in the critical care setting. The intensivist should maintain a high clinical suspicion and heighten the level of urgency among members of the care team at the point of initial patient contact so that all aspect of work up and treatment are expedited wherever possible. Once the diagnosis of NSTI is suspected, early consultation with a surgeon is warranted. Even in institutions with immediate surgical capabilities, however, a period of time will be spent evaluating the patient and preparing transport to the operating room, during which delivery of antibiotic therapy and supportive critical care must be expedited. In the case of patients with systemic illness and shock, resuscitation is performed in a similar manner as is done for septic shock and initial management occurs simultaneous to the search for pathogen and source.
Antibiotic therapy
Early and aggressive use of antibiotic therapy is essential and should be performed concomitant to the patient undergoing surgical evaluation and treatment. Blood cultures, and where possible, deep tissue, abscess and/or operative cultures must be obtained promptly as these will help tailor antibiotic therapy. Antibiotic therapy for necrotizing infections in particular has not been studied in randomized controlled trials. Data for antibiotic treatment is extrapolated from proposed therapy for non-necrotizing complicated skin and soft tissue infections.
Initial empiric therapy should encompass broad-spectrum coverage of polymicrobial infections, as about half of these infections will be polymicrobial in nature33. This should include a MRSA-active agent such as vancomycin, daptomycin, linezolid or ceftaroline as well as a broad-spectrum agent against gram-negative pathogens such as piperacillin–tazobactam, ampicillin–sulbactam, ticarcilin-clavulanate, extended-spectrum cephalosporins or carbapenems. If the selected regimen lacks anerobic activity, an agent such as metronidazole or clindamycin must be added. More recently, a German study has suggested tigecycline as possible single agent therapy in patients previously colonized with resistant bacteria, such as patients who have been recently hospitalized or institutionalized34; however, such practice must be guided by local epidemiologic patterns. Similarly, empiric use of fluoroquinolones and ceftriaxone in areas with high prevalence of resistance to these agents among gram-negative bacteria must be avoided. Empiric antifungal therapy is not essential, but an appropriate antifungal agent may be added upon visual evidence on stains or growth in blood or operative cultures of fungal elements such as Candida or Mucorales spp.
Animal models have demonstrated greater efficacy with the clindamycin–a lincosamide antibiotic that works by inhibition of ribosomal tranlocation–compared to β-lactams in GAS infection; these findings were corroborated in two small retrospective cohort studies35,36. Notably, clindamycin may have multiple advantages over β-lactams including an effect that is independent of inoculum size or infection stage as well as potential antitoxin properties. In toxic shock syndrome (TSS), clindamycin is thought to mitigate the severity of shock by decreasing toxin production37. Although macrolide resistance in GAS remains low in the US, it tends to be relatively higher among invasive strains of GAS; consequently, penicillins being universally active against GAS could offer coverage in clindamycin resistant infections. Hence, the Surgical Infection Society and Infectious Disease Society of America (IDSA) guidelines both strongly recommend combination therapy with penicillin and clindamycin in NSTI (with or without TSS) due to GAS38,39, 33. Since the causative pathogen is not usually known upfront, it is reasonable to add clindamycin to the empiric regimen for suspected NSTI. Like clindamycin, Linezolid is also a protein synthesis inhibitor with potential toxin inhibiting properties (particularly in the case of S. aureus infection); however, no clinical studies to date have evaluated the clinical impact of this property of Linezolid in NSTI.
After the organism(s) have been identified in the microbiology lab, therapy can usually be tailored further. The absence of growth of MRSA in cultures demonstrates a high negative predictive value and can facilitate discontinuation of the MRSA-active agent. For known or suspected Vibrio spp. NSTIs, doxycycline plus a third generation cephalosporin is recommended, and combination therapy is key when a cell wall inhibiting agent is used40. For known or suspected Aeromonas infections, doxycycline is recommended in combination with ciprofloxacin for community-acquired infections or cefepime for leech therapy acquired infections, which have been reported to be resistant to ciprofloxacin41.
No clinical trials have evaluated duration of therapy in NSTI; guidelines suggest continuation of appropriate antibiotics for a minimum of 48–72 h after resolution of fever and other systemic signs of infection as well as hemodynamic stabilization. Please refer to IDSA practice guidelines for skin and soft tissue infections for additional details on antibiotic therapy for NSTI33.
Surgical intervention
While antibiotic therapy, resuscitation and critical care evaluation are necessary in the treatment of patients presenting with NSTIs, the mainstay of therapy remains surgical treatment (refer to Figure 5 for a proposed management pathway in NSTI). Multiple large studies cite the need for early and aggressive debridement in NSTIs, and claim it as the single most important treatment intervention for this disease process, although no randomized controlled trial has studied the timing or extent of surgical therapy or clearly defined an “adequate” debridement42,43,44,45. Delay in the identification or early surgical management of these infections clearly increases mortality46. In addition, recent data suggests that delay not only increases mortality, but in survivors, also increases the number of subsequent operations needed to control the infection47. Increased number of operations may increase the total tissue loss from the disease process, as more tissue is removed with each operation, and therefore limit functional recovery as more muscle and possibly critical structures such as nerves, are sacrificed. There is also associated increased cost with each subsequent operation. Early identification and aggressive treatment, therefore, remains critical in the treatment of these infections48. Time to surgical debridement has been demonstrated as an independent predictor of improved outcome in large studies49,50. In another study, the presence of a 24-hour in-house emergency general surgical team provided both the expertise and expeditious treatment needed to reduce time to operation and improve mortality51. While this was an isolated single center experience, it underscores the importance of early surgical evaluation and advocates for widespread emergency surgical capability or early transfer to a facility with these capabilities.
Figure 5.
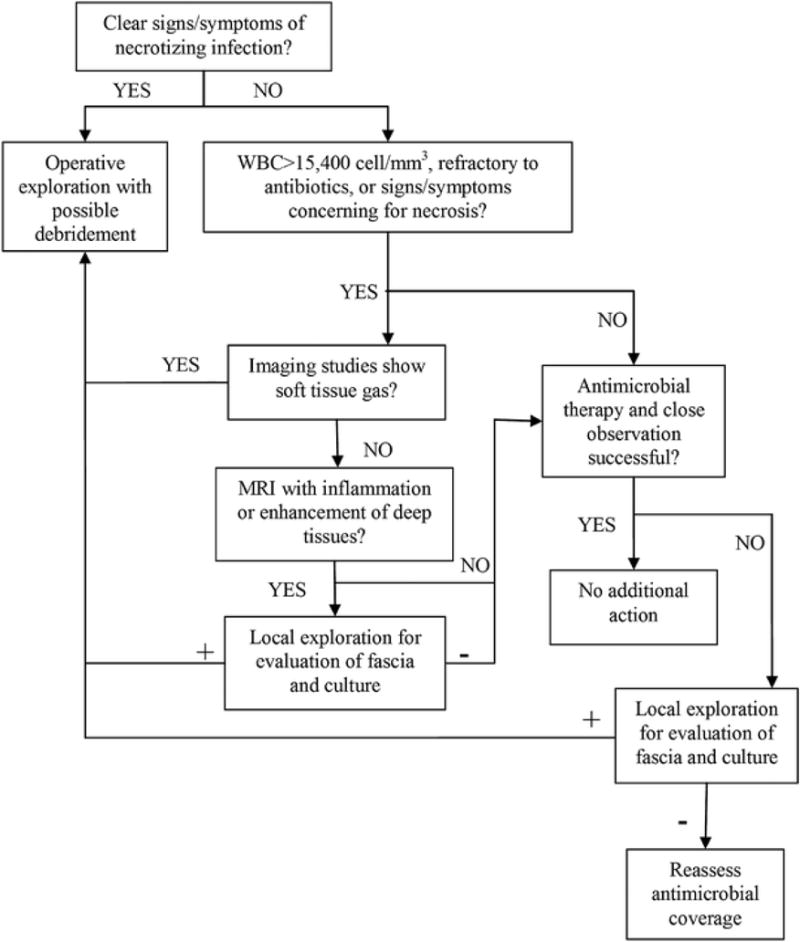
Management pathway in NSTI. [Used with permission]. Original source: Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect 2010;75:249–57.
Despite being a mainstay of therapy for this infection, no study has defined what an “adequate” debridement is, although typical training dictates that all necrotic tissue should be removed. Debridement, therefore, remains at the discretion of the operating surgeon43. Many studies refer to “aggressive” debridement without objectively quantifying the term. It is, however, well demonstrated that wounds should be frequently reevaluated, typically with re-exploration in the operating room within 24–48 hours of the initial debridement procedure. The return to the operating room is intended for re-evaluation of the wound, debridement of any further necrotic tissue, confirming the absence of progression, and to facilitate dressing changes. The average number of debridement procedures is 3–4 before further dressing changes are performed at the bedside47,49,50.
In the case of wide or disfiguring debridements, involvement of additional teams, including urology for perineal wounds or wounds involving the penis or scrotum, plastic surgery for complex reconstruction or muscle flap reconstruction, or orthopedics for bony involvement may be necessary. In the case of perineal wounds, it may be necessary to divert the fecal stream away from the area of contamination with a loop colostomy52. Amputations may be necessary in the case of diabetic foot infections or larger scale debridements of entire muscle compartments, resulting in a nonfunctioning limb. Reconstruction with rotational flaps or skin graft techniques may be necessary and warrant early intervention and co-management with plastic surgery. Widespread use of vacuum-assisted closure devices is generally used to provide consistent and easy nursing care, suctioning of soft tissue edema, and promoting granulation tissue53. Newer vacuum assisted closure products are accompanied by continuous wound irrigation, which may be beneficial in wounds from debridement of NSTI54. Negative pressure dressings can provide dermotraction to limit the wound size and facilitate closure55. In the case of complex and repeated reconstructive surgeries, rehabilitation, physical therapy or occupational therapy may be necessary and treatment courses can be significantly life-altering and prolonged.
The impact of early transfer vs on-site initial debridement in NSTI has not been systematically investigated in a clinical trial, and as such, is difficult to decipher retrospectively. Initial resuscitation, initial debridement (when available) and control of the infectious process must be prioritized at the presenting hospital. Often, however, the decion of when to operate and when to transfer is complex and must be made carefully taking into account clinical severity and institutional capabilities. Institutional factors that might prompt transfer include the lack of an intensive care unit, lack of availability of advanced services such as continuous renal replacement therapy, large volume blood transfusion or on-call surgical staff and need for complex reconstruction techniques that may not be available at certain hospitals.
Adjuvant therapies
The two most common adjunctive medical treatments discussed for NSTIs include intravenous immune globulin and hyperbaric oxygen. Intravenous immunoglobulin has been suggested as a treatment for superantigen-mediated toxic shock syndrome due to streptococcal56 or staphylococcal NF57. The proposed mechanism of action is that IVIG binds and inactivates circulating superantigens, thereby blunting the superantigen-mediated cytokine cascade. Initial retrospective studies demonstrated some promise, but a randomized control trial on the subject was terminated early and lacked sufficient power to detect a survival benefit58. A subsequent pediatric study also demonstrated no benefit of IVIG therapy59. Additionally, the cost associated with the treatment is high. In 2016, Kadri et al reported findings of a propensity-matched analysis of administrative data from 130 US hospitals evaluating the role of adjunctive IVIG in NSTI and validated administrative data algorithms against clinical data from 4 hospitals; there was no clinical benefit to IVIG therapy observed, regardless of timing of treatment60. Also, not surprisingly, IVIG was found to be used rather sporadically at 4%. In 2017, the INSTINCT study, a Danish multicenter randomized controlled trial also evaluating the adjunctive potential of 3 days of IVIG therapy in NSTI found no benefit of the same on physical functioning or survival at 6-months61. Plasmapheresis, in principle, could remove circulating inflammatory mediators and potentially decrease the host’s intrinsic inflammatory response, lessening the severity of vasodilatory shock. However, the data supporting this strategy remains anecdotal62.
Hyperbaric oxygen has been proposed as an adjunctive therapy after surgical debridement for NSTI63. The fascia is known to be a relatively hypoxic environment owing to its tenuous blood supply when compared to surrounding muscle or skin. By increasing plasma dissolved oxygen concentration, hyperbaric oxygen is believed to potentially enhance oxygen delivery to hypoxic tissues surrounding areas of necrosis, directly killing anaerobic bacteria and improving leukocyte activity64. This proposed mechanism has led to a series of retrospective studies, with some showing decreased mortality and others showing no effect65,66. These studies are not compelling to recommend hyperbaric therapy. Furthermore, the greatest barrier to practical use of this modality in NSTI is the limited number of centers nationwide with hyperbaric chambers where critically ill patients can be adequately monitored. In summary, despite theoretical benefits, no prospective literature exists to support its use as adjuvant therapy and society guidelines to do not recommend it’s routine use in these infections17.
Summary/Discussion
In summary, NSTI remains a disease with high morbidity and mortality, despite improvements in care. In the past several decades, our understanding of this disease process has improved such that we know that early diagnosis, along with rapid aggressive treatment with broad spectrum antimicrobial treatment and wide surgical debridement are necessary to effectively treat this disease process. Adjunctive therapies have been explored and found to be largely ineffective and are not routinely recommended. More recently, differences in the timing of antibiotic therapy administration has been observed between high and low volume centers for the treatment of necrotizing fasciitis, and suggests that differences in care may exist between centers with high volume care of this disease67. If indeed, patients must be identified early, treated expeditiously, and supported with the best available critical care, then perhaps further advances in the care of this disease will be less about finding a better treatment modality. It may be that improving the systems that bring patients to the attention and care of appropriate clinicians will be the intervention that moves the needle on the burden of this disease.
Key Points.
Necrotizing soft tissue infections (NSTI) are generally severe and rapidly progressive and accompanied by sepsis, multisystem organ failure and often death.
Rapid recognition and early surgical intervention form the mainstay of management of NSTIs. Most cases require more than one debridement. Imaging can facilitate diagnosis and the decision to operate but should not delay treatment in unequivocal cases; direct exploration remains the gold standard for diagnosis.
Initial surgical debridement should be promptly performed preferably at the presenting hospital when adequate surgical infrastructure and personnel exist. Transfer of the patient to a referral center may be necessary for definitive surgical and complex wound care.
Broad-spectrum empiric antibiotics directed at the likely organisms is essential early in the treatment course, but do not substitute surgical management. Antibiotic therapy should be subsequently tailored to the etiologic agent. In cases of documented NSTI due to group A Streptococcus, clindamycin should be administered in addition to penicillin.
There is insufficient data to warrant routine use of adjuvant hyperbaric oxygen. Adjuvant intravenous immunoglobulin is an expensive intervention that is not likely to improve survival or quality of life and is best reserved for use on a case-by-case basis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Stephanie Bonne, Assistant Professor of Surgery, Rutgers New Jersey Medical School, Newark, NJ.
Sameer S. Kadri, Head, Clinical Epidemiology Section, Critical Care Medicine Department, National Institutes of Health Clinical Center, Bethesda, MD.
References
- 1.Faraklas I, Yang D, Eggerstedt M, Zhai Y, et al. A Multi-Center Review of Care Patterns and Outcomes in Necrotizing Soft Tissue Infections. Surg Infect (Larchmt) 2016;17(6):73–778. doi: 10.1089/sur.2015.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson B. Necrotizing fasciitis. Am Surg. 1952;18:416–31. [PubMed] [Google Scholar]
- 3.Nelson GE, Pondo T, Toews KA, et al. Epidemiology of Invasive Group A Streptococcal Infections in the United States, 2005–2012. Clin Infect Dis. 2016 Aug 15;63(4):478–86. doi: 10.1093/cid/ciw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yilmazlar T, Ozturk E, Alsoy A, et al. Necrotizing soft tissue infections: APACHE II score, dissemination, and survival. World J Surg. 2007;31:1858–1862. doi: 10.1007/s00268-007-9132-1. [DOI] [PubMed] [Google Scholar]
- 5.Miller AT, Saadai P, Greenstein A, et al. Postprocedural necrotizing fasciitis: A 10-year retrospective review. Am Surg. 2008;74:405–409. [PubMed] [Google Scholar]
- 6.Lee TC, Carrick MM, Scott BG, et al. Incidence and clinical characteristics of methicillin-resistant Staphylococcus aureus necrotizing fasciitis in a large urban hospital. Am J Surg. 2007;194:809–812. doi: 10.1016/j.amjsurg.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 7.Hefny AF, Eid HO, Al Hussona M, et al. Necrotizing fasciitis: A challenging diagnosis. Eur J Emerg Med. 2007;14:50–52. doi: 10.1097/01.mej.0000228447.48276.7b. [DOI] [PubMed] [Google Scholar]
- 8.Childers BJ, Potyondy LD, Nachreiner R, et al. Necrotizing fasciitis: A fourteen-year retrospective study of 163 consecutive patients. Am Surg. 2002;68:109–116. [PubMed] [Google Scholar]
- 9.Giuliano A, Lewis F, Hadley K, et al. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134:52–57. doi: 10.1016/0002-9610(77)90283-5. [DOI] [PubMed] [Google Scholar]
- 10.Sartor C, Limouzin-Perotti F, Legré R, Casanova D, Bongrand MC, Sambuc R, Drancourt M. Nosocomial Infections with Aeromonas hydrophila from Leeches. Clin Infect Dis. 2002 Jul 1;35(1):E1–5. doi: 10.1086/340711. [DOI] [PubMed] [Google Scholar]
- 11.Miller LG, Perdreau-Remington F, Rieg G, Mehdi S, Perlroth J, Bayer AS, Tang AW, Phung TO, Spellberg B. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445–53. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- 12.Cheng NC, Yu YC, Tai HC, Hsueh PR, Chang SC, Lai SY, Yi WC, Fang CT. Recent trend of necrotizing fasciitis in Taiwan: focus on monomicrobial Klebsiella pneumonia necrotizing fasciitis. Clin Infect Dis. 2012;55(7):930–9. doi: 10.1093/cid/cis565. [DOI] [PubMed] [Google Scholar]
- 13.Anaya DA, McMahon K, Nathens AB, Sullivan SR, Foy H, Bulger E. Predictors of mortality and limb loss in necrotizing soft tissue infections. Arch Surg. 2005;140(2):151–7. doi: 10.1001/archsurg.140.2.151. [DOI] [PubMed] [Google Scholar]
- 14.Bryant AE, Bayer CR, Huntinton JD, Stevens DL. Group A streptococcal myonecrosis: Increased vimentin expression after skeletal-muscle injury mediates the binding of Streptococcus pyogenes. J Infect Dis. 2006;193(12):1685–92. doi: 10.1086/504261. [DOI] [PubMed] [Google Scholar]
- 15.Bohach GA, Fast DJ, Nelson RD, Schlievert PM. Staphylococcal and streptococcal pyrogenic toxins involved in toxic shock syndrome and related illnesses. Crit Rev Microbiol. 1990;17:251–72. doi: 10.3109/10408419009105728. [DOI] [PubMed] [Google Scholar]
- 16.Darenberg J, Luca-Harari B, Jasir A, Sandgren A, Pettersson H, Schalén C, Norgren M, Romanus V, Norrby-Teglund A, Normark BH. Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin Infect Dis. 2007 Aug 15;45(4):450–8. doi: 10.1086/519936. [DOI] [PubMed] [Google Scholar]
- 17.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft-tissue infections. Clin Infect Dis. 2005;41:1373–1406. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 18.Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections: Risk factors for mortality and strategies for management. Ann Surg. 1996;224:672–683. doi: 10.1097/00000658-199611000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wall DB, de Virgilio C, Black S, et al. Objective criteria may assist in distinguishing necrotizing fasciitis from nonnecrotizing soft tissue infection. Am J Surg. 2000;179:17–21. doi: 10.1016/s0002-9610(99)00259-7. [DOI] [PubMed] [Google Scholar]
- 20.Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705–10. doi: 10.1086/511638. [DOI] [PubMed] [Google Scholar]
- 21.Chan T1, Yaghoubian A, Rosing D, Kaji A, de Virgilio C. Low sensitivity of physical examination findings in necrotizing soft tissue infection is improved with laboratory values: a prospective study. Am J Surg. 2008 Dec;196(6):926–30. doi: 10.1016/j.amjsurg.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 22.Yaghoubian A1, de Virgilio C, Dauphine C, Lewis RJ, Lin M. Use of admission serum lactate and sodium levels to predict mortality in necrotizing soft-tissue infections. Arch Surg. 2007 Sep;142(9):840–6. doi: 10.1001/archsurg.142.9.840. [DOI] [PubMed] [Google Scholar]
- 23.Wong CH1, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004 Jul;32(7):1535–41. doi: 10.1097/01.ccm.0000129486.35458.7d. [DOI] [PubMed] [Google Scholar]
- 24.Kincius M, Telksnys T, Trumbeckas D, Jievaltas M, Milonas D. Evaluation of LRINEC Scale Feasibility for Predicting Outcomes of Fournier Gangrene. Surg Infect (Larchmt) 2016 Aug;17(4):448–53. doi: 10.1089/sur.2015.076. [DOI] [PubMed] [Google Scholar]
- 25.Chao WN, Tsai SJ, Tsai CF, et al. The Laboratory Risk Indicator for Necrotizing Fasciitis score for discernment of necrotizing fasciitis originated from Vibrio vulnificus infections. J Trauma Acute Care Surg. 2012 Dec;73(6):1576–82. doi: 10.1097/TA.0b013e318270d761. [DOI] [PubMed] [Google Scholar]
- 26.Hansen MB, Rasmussen LS, Svensson M, Chakrakodi B, Bruun T, Madsen MB, Perner A, Garred P, Hyldegaard I, onrrby-Teglund A. Association between cytokine response, the LRINEC score and outcome in patients with necrotizing soft tissue infection: a multicetre, prospective study. Sci Rep. 2017;7:42179. doi: 10.1038/srep42179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roghmann F, von Bodman C, Loppenberg B, Hinkel A, Palisaar J, Noldus J. Is there a need for the Fournier’s gangrene severity index? Comparison of scoring systems for outcome prediction in patients with Fournier’s gangrene. BJU Int. 2012;110(9):1359–65. doi: 10.1111/j.1464-410X.2012.11082.x. [DOI] [PubMed] [Google Scholar]
- 28.Carbonetti F, Cremona A, Carusi V, et al. The role of contrast enhanced computed tomography in the diagnosis of necrotizing fasciitis and comparison with the laboratory risk indicator for necrotizing fasciitis (LRINEC) Radiol Med. 2016 Feb;121(2):106–21. doi: 10.1007/s11547-015-0575-4. [DOI] [PubMed] [Google Scholar]
- 29.Kim KT, Kim YJ, Won Lee J, Kim YJ, Park SW, Lim MK, Suh CH. Can necrotizing infectious fasciitis be differentiated from nonnecrotizing infectious fasciitis with MR imaging? Radiology. 2011 Jun;259(3):816–24. doi: 10.1148/radiol.11101164. [DOI] [PubMed] [Google Scholar]
- 30.Kehrl T. Point-of-care ultrasound diagnosis of necrotizing fasciitis missed by computed tomography and magnetic resonance imaging. J Emerg Med. 2014 Aug;47(2):172–5. doi: 10.1016/j.jemermed.2013.11.087. [DOI] [PubMed] [Google Scholar]
- 31.Green RJ, Dafoe DC, Raffin TA. Necrotizing fasciitis. Chest. 1996;110:219–229. doi: 10.1378/chest.110.1.219. [DOI] [PubMed] [Google Scholar]
- 32.Hietbrink F, Bode LG, Riddez L, Leenen LP, van Dijk MR. Triple diagnostics for early detection of ambivalent necrotizing fasciitis. World J Emerg Surg. 2016 Oct 11;11:51. doi: 10.1186/s13017-016-0108-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevens DL, Bisno AL, Chambers HF, et al. Practice guidelines for the diagnosis and management of skin and soft tissue infections: 2014 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2014;59:147–159. doi: 10.1093/cid/ciu296. [DOI] [PubMed] [Google Scholar]
- 34.Eckmann C, Heizmann W, Bodmann KF, et al. Tigecycline in the treatment of necrotizing soft tissue infections due to multiresistant bacteria. Surg Infec. 2015;16:618–625. doi: 10.1089/sur.2014.089. [DOI] [PubMed] [Google Scholar]
- 35.Zimbelman J, Palmer A, Todd J. Improved outcome of clindamycin compared with beta-lactam antibiotic treatment for invasive Streptococcus pyogenes infection. Pedatr Infect Dis J. 1999;18(12):1096–100. doi: 10.1097/00006454-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Mulla ZD, Leaverton PE, Wiersma ST. Invasive group A Streptococcal infections in Florida. South Med J. 2003;96(10):968–73. doi: 10.1097/01.SMJ.0000051060.95210.9A. [DOI] [PubMed] [Google Scholar]
- 37.Bernardo K, Pakulat N, Fleer S, et al. Subinhibitory concentrations of linezolid reduce Staphylococcus aureus virulence factor expression. Antimicrob Agents Chemother. 2004;48:546–444. doi: 10.1128/AAC.48.2.546-555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gemmell CG, Ford CW. Virulence factor expression by gram-positive cocci exposed to subinhibitory concentrations of linezolid. J Antimicrob Chemother. 2002;50:665–672. doi: 10.1093/jac/dkf192. [DOI] [PubMed] [Google Scholar]
- 39.May AK, Stafford RE, Bulger EM, Heffernan D, Guillamondegui O, Bochicchio G, Eachempati SR, Surgical Infection Society Surg Infect (Larchmt) 2009 Oct;10(5):467–99. doi: 10.1089/sur.2009.012. [DOI] [PubMed] [Google Scholar]
- 40.Zanetti S, Spanu T, Deriu A, et al. In vitro susceptibility of Vibrio spp isolated from the environment. Int J Antimicrob Agents. 2001;17:407–409. doi: 10.1016/s0924-8579(01)00307-7. [DOI] [PubMed] [Google Scholar]
- 41.Aravena-Román M, Inglis TJ, Henderson B, Riley TV, Chang BJ. Antimicrobial susceptibilities of Aeromonas strains isolated from clinical and environmental sources to 26 antimicrobial agents. Antimicrob Agents Chemother. 2012 Feb;56(2):1110–2. doi: 10.1128/AAC.05387-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McHenry CR, Piotrowski JJ, Petrinic D, et al. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–563. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudarsky LA, Laschinger JC, Coppa GF, et al. Improved results from a standardized approach in treating patients with necrotizing fasciitis. Ann Surg. 1987;206:661–665. doi: 10.1097/00000658-198711000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tillou A, St Hill CR, Brown C, et al. Necrotizing soft tissue infections: Improved outcomes with modern care. Am Surg. 2004;70:841–844. [PubMed] [Google Scholar]
- 45.Bilton BD, Zibari GB, McMillan RW, et al. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: A retrospective study. Am Surg. 1998;64:397–400. [PubMed] [Google Scholar]
- 46.Bilton BD, Zibari GB, McMillan RW, et al. Aggressive surgical management of necrotizing fasciitis serves to decrease mortality: A retrospective study. Am Surg. 1998;64:397–400. [PubMed] [Google Scholar]
- 47.Kobayashi L, Konstantinidis A, Shackelford S, Chan LS, Talving P, Inaba K, Demetriades D. J Necrotizing soft tissue infections: delayed surgical treatment is associated with increased number of surgical debridements and morbidity. Trauma. 2011 Nov;71(5):1400–5. doi: 10.1097/TA.0b013e31820db8fd. [DOI] [PubMed] [Google Scholar]
- 48.Sartelli M, Malangoni MA, May AK, et al. World Society of Emergency Surgery (WSES) guidelines for the management of skin and soft tissue infections. World J Emerg Surg. 2014;9:57. doi: 10.1186/1749-7922-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elliott DC, Kufera JA, Myers RA. Necrotizing soft tissue infections: Risk factors for mortality and strategies for management. Ann Surg. 1996;224:672–683. doi: 10.1097/00000658-199611000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McHenry CR, Piotrowski JJ, Petrinic D, et al. Determinants of mortality for necrotizing soft-tissue infections. Ann Surg. 1995;221:558–563. doi: 10.1097/00000658-199505000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gunter OL, Guillamondegui OD, May AK, et al. Outcome of necrotizing skin and soft tissue infections. Surg Infect. 2008;9:443–450. doi: 10.1089/sur.2007.053. [DOI] [PubMed] [Google Scholar]
- 52.Kilic A, Aksoy Y, Kilic L. Fournier’s gangrene: Etiology, treatment, and complications. Ann Plast Surg. 2001;47:523–527. doi: 10.1097/00000637-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 53.Czymek R, Schmidt A, Eckmann C, et al. Fournier’s gangrene: vacuum- assisted closure versus conventional dressings. Am J Surg. 2009;197:168–176. doi: 10.1016/j.amjsurg.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 54.Kiyokawa K, Takahashi N, Rikimaru H, Yamauchi T, Inoue Y. New Continuous negative-pressure and irrigation treatment for infected wounds and intractable ulcers. Plast Reconstr Surg. 2007 Oct;120(5):1257–65. doi: 10.1097/01.prs.0000279332.27374.69. [DOI] [PubMed] [Google Scholar]
- 55.Lee JY, Jung H, Kwon H, Jung SN. Extended negative pressure wound & therapy-assisted dermatotraction for the closure of large open fasciotomy wounds in necrotizing faciitis patients. World J Emergency Surg. 2014;9:29–39. doi: 10.1186/1749-7922-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linnér A, Darenberg J, Sjölin J, Henriques-Normark B, Norrby-Teglund A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxicshock syndrome: a comparative observational study. Clin Infect Dis. 2014 Sep;59(6):15. 851–7. doi: 10.1093/cid/ciu449. [DOI] [PubMed] [Google Scholar]
- 57.Darenberg J1, Söderquist B, Normark BH, Norrby-Teglund A. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigens: implica tions for therapy of toxic shock syndrome. Clin Infect Dis. 2004 Mar;38(6):15. 836–42. doi: 10.1086/381979. [DOI] [PubMed] [Google Scholar]
- 58.Darenberg J, Ihendyane N, Sjolin J, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: A European randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2003;37:333–340. doi: 10.1086/376630. [DOI] [PubMed] [Google Scholar]
- 59.Shah SS, Hall M, Srivastava R, Subramony A, Levin JE. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis. 2009;49:1369–76. doi: 10.1086/606048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kadri SS, Swihart BJ, Bonne SL, et al. Impact of Intravenous Immunoglobulin on Survival in Necrotizing Fasciitis with Vasopressor-dependent Shock: A Propensity-Score Matched Analysis from 130 US Hospitals. Clin Infect Dis. 2016 Dec;:29. doi: 10.1093/cid/ciw871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madsen MB, Hjortrup PB, Hansen MB, Lange T, Norrby-Teglund, Hydegaard O, Perner A. Immunoglobulinith necrotizing soft tissue infection (INSTINCT): a randomized, blinded, placebo-controlled trial. Intensive Care Med. 2017 Apr 18; doi: 10.1007/s00134-017-4786-0. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 62.Kyles DM, Baltimore J. Adjunctive use of plasmapheresis and intravenous immunoglobulin therapy in sepsis: A case report. Am J Crit Care. 2005;14:109–112. [PubMed] [Google Scholar]
- 63.Shupak A, Shoshani O, Goldenberg I, et al. Necrotizing fasciitis: An indication for hyperbaric oxygenation therapy? Surgery. 1995;118:873–878. doi: 10.1016/s0039-6060(05)80278-8. [DOI] [PubMed] [Google Scholar]
- 64.Jallali N, Withey S, Butler PE. Hyperbaric oxygen as adjuvant therapy in the management of necrotizing fasciitis. Am J Surg. 2005;189:462–466. doi: 10.1016/j.amjsurg.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 65.Riseman JA, Zamboni WA, Curtis A, et al. Hyperbaric oxygen therapy for necrotizing fasciitis reduces mortality and the need for debridements. Surgery. 1990;108:847–850. [PubMed] [Google Scholar]
- 66.Shupak A, Shoshani O, Goldenberg I, et al. Necrotizing fasciitis: An indication for hyperbaric oxygenation therapy? Surgery. 1995;118:873–878. doi: 10.1016/s0039-6060(05)80278-8. [DOI] [PubMed] [Google Scholar]
- 67.Faraklas Iris, Yang Derek, Eggerstedt Michael, Zhai Yan, Liebel Patrick, Graves Gareth, Dissanaike Sharmila, Mosier Michael, Cochran Amalia. Surgical Infections. 2016 Dec;17(6):773–778. doi: 10.1089/sur.2015.238. [DOI] [PMC free article] [PubMed] [Google Scholar]


