Abstract
Purpose of this Review
Over the past decades, osteocytes have emerged as mechano-sensors of bone and master regulators of bone homeostasis. This article summarizes latest research and progress made in understanding osteocyte mechanobiology and critically reviews tools currently available to study these cells.
Recent Findings
Whereas increased mechanical forces promote bone formation, decrease loading is always associated with bone loss and skeletal fragility. Recent studies identified cilia, integrins, calcium channels and G-protein coupled receptors as important sensors of mechanical forces and Ca2+ and cAMP signaling as key effectors. Among transcripts regulated by mechanical forces, sclerostin and RANKL have emerged as potential therapeutic targets for disuse-induced bone loss.
Summary
In this paper we review the mechanisms by which osteocytes perceive and transduce mechanical cues and the models available to study mechano-transduction. Future directions of the field are also discussed.
Keywords: Osteocyte, mechanical forces, sclerostin, bone homeostasis
Introduction
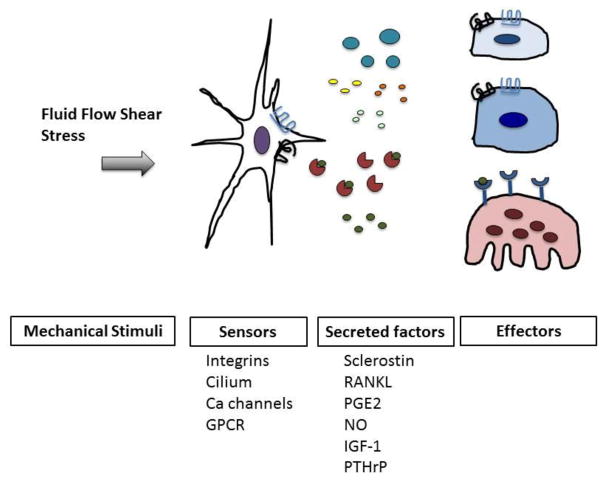
Mechanical forces have long been known to be essential for proper skeletal homeostasis. At the cellular level, bone adaptation to its mechanical environment is orchestrated by osteocytes, the bone cells deeply embedded into the mineralized matrix (1, 2). These cells are capable of sensing mechanical cues applied to the bone and then react to these loads by controlling osteoblast (bone forming cell) and osteoclast (bone resorbing cell) activities through cell-to-cell communication and via secreted factors (Figure 1) (2–5). Mechanical stimuli regulate numerous cellular functions, including gene expression, protein synthesis, cell proliferation and differentiation. Loading is required to preserve bone mass and Galileo was the first one to observe and describe its consequences to the bones. In 1892, Wolff theorized (Wolff’s law on bone transformation) that mechanical stress is the driving force for the architecture of bone (6). His law postulates that the skeleton, through a process known as modeling (i.e. large changes in bone structure driven by independent actions of osteoblasts and osteoclasts), adapts its form to react to mechanical demands. Remodeling, on the other hand, is the continuous and spatially coupled resorption and formation of bone required to preserve its functional integrity. In the late 80’s Harold Frost speculated the existence of a mechanism (named the “mechanostat”) capable of distinguish between bone modeling (changes in shape) and remodeling (continuous replacement) and he identified the osteocyte as the “mechanostat” of bone (7). The scientific evidences supporting his theory came 20 years later when investigators in Japan engineered mice lacking osteocytes, “osteocyte-less mice”, and demonstrated their resistance to unloading induced bone-losses (2). Over the past decade, the identification of osteocyte’s specific transcripts together with new gene editing tools, has pushed forward our understanding of osteocyte functions and biology. In this review we will examine new findings on the mechanisms of mechano-sensation (i.e. signal detection), mechano-transduction (i.e. signal transmission) and downstream biological responses.
Figure 1.
Osteocyte (white stellate cell), sense mechanical forces through cellular sensors (integrins, cilium, calcium channels and GPCR) and then transform these biomechanical stimuli into biological responses that results in the secretion of several factors capable of affecting effector cells such as osteoblasts (dark blue), bone lining cells (light blue) and osteoclasts (pink). Osteocytes, osteoblast and bone lining cells express PTHRs (black) and LRP5/4 (blue) which are involved in mechanobiology
Osteocytes
Osteocytes derive from mature osteoblasts that, during the process of bone formation, assume a more differentiated morphology and become entrapped in the matrix that they are actively synthesizing. Once embedded in the mineralized matrix, the osteocyte maintains its contacts with adjacent cells (including surrounding osteocytes, osteoblasts, endothelial cells and possibly cells in the marrow cavity) and receives nutrients via the dendritic processes that lie within the canaliculi of the bone. Communication between adjacent osteocytes and neighboring cells is mediated by gap junctions. In addition, osteocytes can signal to distant cells or organs via secreted factors released in the bone marrow space or directly into the circulation (8). Their location, deeply into the mineralized bone, and their structural organization of a cellular network, make them ideal to sense mechanical stimuli and to transduce these signals into biochemical cues affecting the surrounding cells.
Osteocytes’ Mechano-sensation
As described above, mechanical forces are required for maintaining the proper functions of living tissues and cells (9, 10). In vertebrates, bone is the tissue best designed to sustain high magnitude of loads (in young human femur the ultimate compressive strength is ~100 MPa) (11, 12). These loads are needed to regulate bone homeostasis, density and strength (13). Example of unloading conditions, such as prolonged bed rest, paralysis or spaceflight, are characterized by rapid bone loss and skeletal fragility (14, 15) whereas increased loads, such as during exercise, increase bone strength. Skeletal adaptation to mechanical stressors is a complex cellular process, which requires the coordinated activity of osteoblastic bone-forming cells and osteoclastic bone-resorbing ones, and entails a biological system capable of sensing and converting applied mechanical cues into biochemical signals. During increased loading, osteoblasts are activated whereas osteoclasts are partially suppressed by mechanisms described below. Conversely, during reduced loading, bone formation is suppressed and bone resorption is increased. Nonetheless, how the external forces are transmitted at the cellular and molecular levels is still unclear. What is now evident is that osteocytes orchestrate both events (2). Several stressors have been proposed as mechanical stimuli, which include fluid flow shear stress (FFSS), hydrostatic pressure, and direct cellular deformation (16). These mechanical stresses are driven by micro-deformation or –strain of bone matrix induced by loading and gravitational forces. Moreover, the specific components of these stressors, such as frequency, amplitude and rate, also influence cellular responses. The theory of loading-induced fluid flow shear stress was first proposed by Cowin et al. (17, 18) in the late 90’s. According to this theory, whereas the calcified matrix is a mechanically rigid material, mechanical loads induces poro-elastic interactions, micro deformation and microstrain of the matrix (maximally on the order of 0.2%). These micro deformations drive the flow of interstitial fluid within the lacuna-canalicular spaces (11, 19). It has been calculated that the magnitude of pressure on an osteocyte in vivo is in the order of 5 Pa. Loading of long bones also increases the intramedullary cavity pressure and generates interstitial fluid flow (IFF) at the endosteal surface as well as within the lacuno-canalicular network (19). Intramedullary pressurization-derived IFF can induce fluid shear stress-related responses not only in osteocytes but also in osteoblasts and osteoclasts on the endosteal surface. In vitro studies demonstrated that FFSS directly promotes migration of MC3T3-E1 osteoblasts, RAW264.7 monocytes and differentiated osteoclasts suggesting that these bone cells are also mechanosensitive (20, 21). Work of several laboratories showed that osteocytes are connected to the canalicular wall via transmembrane proteins, including integrins and Ephrin A (EphrA), and through transverse tethering elements (22–25). These transmembrane molecules provide physical connections between the extracellular matrix, the intracellular protein complexes and cytoskeletal structures.
How are mechanical stimuli sensed by the osteocyte? Several theories have been proposed and experimental studies have identified integrins, cilia, calcium channels and G-protein coupled receptors (GPCRs) as mechano-sensors of bone. Integrins comprise of an α and β dimer and FFSS induces conformational changes in the β-subunit and activation of the cascade signaling (23, 24). Among integrins, αvβ3 is highly expressed in osteocytes and connects the intracellular actin cytoskeleton to the extracellular matrix proteins fibronectin, vitronectin, and osteopontin (22). Another cellular moiety needed to perceive FFSS is the primary cilium, a non-motile structure required for chemo- and mechano-sensation in a variety of tissues, including kidney, liver, cartilage and bone. Initial studies suggested that flow of the canalicular fluid induced bending of the cilium and trigger Ca2+ influx via the transient receptor potential vanilloid 4 (TRPV4), leading to suppression of cAMP signaling (26, 27). This theory raised some skepticisms primarily because physical laws suggest that FFSS occurs around the cell processes and not on the cell body where the primary cilium is located. Later was proposed by Bell et al. (28) that the cilium perceives hydrostatic pressure applied on the cell body and not fluid flow induced shear stress. Ablation of cilia components, such as ITF88, and Kif3a or of the closely related polycystins (Pkd1 and 2)(29–32), in osteoblasts and osteocytes, impairs skeletal homeostasis and responses to mechanical forces (33). The TAZ/YAP pathway has also been identified as an important signaling for mechano-sensation and its deletion is associated with defective mechano-transduction. In osteocytes, and other cells, biophysical stressors are transmitted to the cells by coupling the extracellular matrix to the actin cytoskeleton through focal adhesions. The actin cytoskeleton transmits mechanical forces from one focal adhesion site to another mechano-sensing sites within the cell and to the neighbouring ones. A major constituent of focal adhesions are focal adhesion kinases (FAK) that are required for osteocytes mechano-transduction. Recently, spectrin, another structural cytoskeletal protein required for the differentiation of osteoblasts to osteocytes (34), has been identified as a mechanosensitive element within the osteocyte (35). Disruption of the spectrin network promotes Ca2+ influx and nitric oxide (NO) secretion in response to reduces stiffness (35). Lipid rafts and membrane proteins (caveola) are also connected to F-actin and cholesterol depletion of lipid rafts micro-domains blocks activation of the cadherins and impairs mechano-sensation. Other potential mechano-sensors are ephrins, gap junctions, Connexin 43 (Cx43) hemichannels and ion channels (stretch activated channels). The parathyroid hormone (PTH)-related peptide (PTHrP) and its receptor (PTH1R) have also been shown to be required for skeletal responses to loading and unloading. Trabecular osteoblasts (TO) isolated from PTHrP−/− animals, flown in space for 6 days, were more sensitive to cell death than control TOs and this effects was reversed by treatment with PTHrP (36). Surprisingly, cortical osteoblasts (CO) isolated from same animals, were “insensitive” to microgravity. Furthermore, mice with conditional deletion of PTHR in osteocytes were resistant to bone gain induced by axial ulna loading, demonstrating the need of an intact PTH-PTHrP-PTHR axis for proper skelatal mechano-transduction (37).
How much strain or shear stress does an osteocyte feel? Jacobs et al. (38) suggested that habitual strains in bone tissue may not be constant between subjects or anatomical sites, and proposed an upper limit for bone tissue strain of roughly 3%. The application of 2000 microstrain macroscopically to a piece of bone resulted in a much greater microscopic strain surrounding the osteocyte lacunae of over 30,000 microstrain. Thi et al. (39) demonstrated that osteocyte processes are extremely responsive to piconewton-level mechanical loading, whereas the osteocyte cell body and processes with no local attachments are not. Inhibition of αVβ3 integrin attachment sites compromises the response to probe stimulation (40).
Osteocytes’ Mechano-transduction
Once the signal is sensed by the osteocyte, via the mechanisms described above, it needs to be transduced into biological cues. The most studied and best described pathways induced by mechanical forces are intracellular Ca2+, ATP, nitrogen oxide (NO), Prostaglandins (PGE2) and Wnts. Whereas some of these signals acts exclusively intracellularly (i.e. Ca2+), others are also secreted and affect osteoblasts and osteoclasts (i.e. NO and PGE2). Opening of stretch-activated calcium channel or TRPV6 induces a rapid increase in intracellular Ca2+ with subsequent cellular responses. Pharmacological inhibition of Ca channels impairs osteocyte’s ability of respond to mechanical cues and in vivo treatment with Ca channel inhibitors reduces skeletal responses to mechanical forces. ATP quickly increases upon mechanical stimulation and several in vitro studies demonstrated that intracellular Ca2+ in required for ATP response. The exact mechanism by which Ca2+ controls ATP release is still not completely understood. Osteocytes synthesize and release PGE2 in response to mechanical forces. FFSS stimulates gap junction-mediated intercellular communication, increases Cx43 expression which in turn forms hemichannels allowing the release of prostaglandins (41). PGE2 then functions in an autocrine fashion to activate EP2-EP4 receptors expressed on osteocytes and in a paracrine fashion to modulate osteoblast and osteoclast activities. Upon EP2-EP4 activation there is an increase in intracellular cAMP and activation of protein kinase A (PKA). In osteocytes, this signaling pathway regulates the expression of several downstream effectors, including Sost, Dmp1, RANKL and others. Indeed the release of PGE2 by bone in response to mechanical loading has been known to be one of the earliest responses to loading (3, 42–44).
The canonical Wnt-signaling plays an important role in the development and maintenance of many organs, including bone and several rare genetic disorders that affect the Wnt signaling pathways have provided strong evidences for its role in skeletal homeostasis. Among bone cells, osteocytes have emerged as major targets of this signaling pathway. Mice lacking βcatenin in osteocytes have severe osteopenia, skeletal fragility and die prematurely whereas mice heterozygous for βcatenin deletion have impaired responses to mechanical loading (1). Wnt activity increases by mechanical loading and decreases by unloading and most of these effects are mediated by sclerostin, as described below. Interestingly, Wang et al. (45) reported that, in vascular endothelial cells, FFSS stimulates HDAC5 phosphorylation and nuclear export through calcium/calmodulin-dependent signal pathway. HDAC5 has been shown as a potent regulator of Sost expression (46) so it is plausible to speculate that HDAC5 might also be mechanically regulated in osteocytes.
Sex hormones regulation of bone remodeling attracted some attention in recent decades and several studies investigated the link between sex hormones and mechanical transduction. In mice lacking estrogen receptor-α, periosteal and endosteal bone formation rate, following ulna loading were significantly reduced (47). Ciani et al. used ovariectomy-induced estrogen-deficient rats to explore the transport of solute around osteocyte. They injected FITC-labeled bovine serum albumin tracer into rats’ tibia during loading and demonstrated that the load-induced solute transport around osteocyte was enhanced in ovariectomized animals (48). Another study reported that androgens inhibits osteogenic responses to mechanical loading in adult male mice (49) demonstrating a close relationship between sex hormones and skeletal mechanobiology.
Conditional ablation of insulin-like growth factor 1 (IGF-1) in osteocytes abolished loading-induces skeletal responses, indicating that this signaling pathways is also required for proper mechano-transduction (50). At the molecular level, IGF-1 −/− failed to suppress Sost expression which is required for appropriate anabolic response to mechanical loading.
Mechano-sensitive Genes and Secreted Factors
Once the osteocyte perceives the mechanical stimulus, it activates a cascade of events which culminates in gene regulations. Over the past decades, the number of mechano-sensitive genes has expanded quite dramatically. Here we discuss the function and effects of main mechanosensitive genes.
Sclerostin
Sclerostin, the product of the Sost gene, an osteocyte-specific protein, has recently emerged as an important therapeutic target for bone diseases such as osteoporosis and osteopenia. This protein inhibits bone formation, both in vitro and in vivo, by directly reducing proliferation and differentiation of osteoblasts via inhibition of the canonical Wnt signaling pathway. Sclerostin was first identified as the disease causing protein for sclerosteosis and Van Buchem disease, two rare bone diseases. Sclerostin, a cysteine rich protein with homology to gremlin, acts by binding and inactivating the canonical Wnt signaling pathway. Patients with sclerosteosis carry a point mutation in the Sost gene whereas van Buchem disease is characterized by a 52kb deletion downstream of the Sost gene (51). Osteoclasts were initially identified as the main source and target of sclerostin (52) but soon it became clear that this protein was secreted predominantly, if not exclusively by osteocytes. Sclerostin binds to low-density lipoprotein (LDL)-related protein 5, 6 and 4 (LRP5,6 and 4) receptors and inhibiting the Wnt-βcatenin signaling pathway. Human gain-of-function mutations of LRP5 which decrease its affinity to sclerostin are characterized by high bone mass (HBM). Inhibition of Wnt-βcatenin in osteocytes, in turn, suppresses osteoblast proliferation and function by mechanisms not fully understood. Sclerostin is exquisitely regulated by mechanical forces; serum levels increases in humans after immobilization (53, 54) and in animals subjected to tail suspension whereas the protein is suppressed by increased mechanical stimuli (55). These increases in Sost/sclerostin likely contribute to the reduced bone formation seen in microgravity. Similarly, mice lacking Sost gene have high bone mass and are resistant to unload-induced bone loss (56) and treatment of tail-suspended mice with sclerostin antibodies prevent unload-induced bone loss (57). Importantly, however, the regulation of Sost expression is poorly understood and how exactly mechanical cues regulate Sost/sclerostin expression is still unclear.
RANKL
Recent findings indicate that osteocytes are a major source of the pro-osteoclastic cytokine RANKL, and that osteocyte-derived RANKL is a key contributor to disuse-induced bone loss in rodent models of unloading (5). RANKL is required for osteoclasts differentiation and function; in its absence, mice develop severe osteopetrosis whereas its over-expression induces osteopenia. Osteoprotegerin (OPG) is also expressed and secreted by osteocytes and acts as a decoy receptor for RANKL preventing its binding to osteoclast progenitors. In vitro studies using mechanically loaded osteocytic cells, demonstrated that upon FFSS, RANKL is suppressed whereas when cells are subjected to simulated microgravity, this cytokine is increased (58). Osteocytes also produce matrix extracellular phospho-glycoprotein (Mepe) which upregulate OPG and decrease RANKL/OPG ratio leading to osteoclast inhibition (59).
Fibroblast growth factor 23
Fibroblast growth factor 23 (FGF23) is another secreted factor produced mainly by osteocytes (60). FGF23, together with PTH, controls phosphate homeostasis by binding to its receptor FGFR1 and the co-receptor Klotho, both in kidney and parathyroid gland. FGF23 prevents phosphate reabsorption and induces hypophosphatemia. The role of FGF23 in osteocyte mechanobiology is controversial and whereas initial studies suggested that this protein was regulated by mechanical forces, subsequent studies failed to corroborate these initial findings. Whether FGF-23 is indeed a mechanosensitive gene and whether its regulation has physiological implications is still unknown.
Besides the factors listed above, a plethora of other genes and molecules are regulated by mechanical forces. Dmp1, Phex, Mepe and osteopontin (and others) have all been shown to be mechanosensitive genes although the exact function of these factors in osteocytes’ mechanobiology is still unclear.
Tools to study mechano-transduction
One of the limitations in elucidating osteocyte mechano-biology is the paucity of available tools and cell lines suitable for studing the intricated and complex mechanisms of mechano-transduction. Moreover, cells in culture might not faithfull represent an osteocyte in vivo since they lack both the native extracellular milieu and the tethering elements required for strain amplification. Currently there are several in vitro and in vivo models routinely used to study osteocyte mechanobiology and cellular responses to changes in mechanical forces. Several osteocytic cell lines are now available to examine, at the cellular and molecular level, mechanisms of mechano-transduction, and each cell line differs slightly in terms of basal gene expression and skeletal origin. The most studied and characterized one is MLOY-4, (61) a conditionally immortalized cell line derived from long bone of mice in which the SV40 antigen was driven by the osteocalcin promoter. Although these cells possess most of the characteristic of an osteocyte, they do not express high level of Sost/sclerostin or express other osteocyte specific gene, namely FGF23. Other osteocytic cell lines currently available are Oc14 (62), derived from PTHR−/− calvarial bones and two new ones isolated from long bones of conditionally immortalized animals expressing GFP under the Dmp-1 promoter, IDG-SW and Ocy454 (58, 63). These cells can be subjected, in vitro, to load, as achieved by laminar continuous unidirectional flow or by pulsatile fluid flow. Commercially available systems or investigator custom-made devices have been used to impose FFSS on 2D cultured cells. Alternatively, cells can be grown on flexible-bottom tissue culture plates and exposed to tensile forces or subjected to hypotonic conditions. These systems have been widely used to study the effects of loads on osteocytes and their limitation is that cells are grown in 2D monolayers, not fully recapitulating the physiological relationship and forces present in bone cells in vivo. The use of 3D structures, or scaffolds, should be preferred when studying osteocytes (or other bone cells) under altered mechanical conditions (loading or microgravity). A multitude of scaffolds or inert support are currently available for bone research and they include collagen-based sponges, hydroxyapatite substrates, and synthetic materials, such as polypropylene. The choice of scaffold is often guided by both the experimental end-point (compatibility of the substrate with the end applications) and the culture conditions (geometry of the scaffolds). In vivo studies also provided important insights into osteocytes mechano-transduction. Cyclical loads of long bones (tibia or ulna) have been used, in vivo, to analyze skeletal responses to increased forces. Animals undergo daily repetitive loading of the tibia or femur utilizing non-invasive loading devices. Recently, vibration platforms have also been used to study bone adaptation. Similarly, several experimental settings have been developed to study cells, or animals, under reduced mechanical cues. Cells can be subjected to simulated microgravity using NASA developed slow-rotating wall vessels (SRWV) or using random positioning machine (RPM) or 3D clinostat. The NASA SRWV bioreactor analog for simulating microgravity operates on the principle of subjecting cells to a rotating fluid environment that randomizes the gravity vector over one revolution. Similar principle of “gravity vector averaging” applies to the RPM. Thus, using the rotating wall analog model alone is not sufficient to fully validate the observed morphological, gene, and hormonal changes of the osteocyte network solely due to unloading conditions. Earth based cell culture unloading analogs (for the study of in vitro osteocyte cellular network) cannot separate effects of fluid flow shear stress from the effects of simulated mechanical unloading. Thus, utilizing only earth analogs for osteocyte network mechano-sensing investigations is insufficient to characterize the osteocyte network changes arising from mechanical unloading alone. Real microgravity environment and minimal fluid shear culture conditions available onboard of the International Space Station (ISS) are therefore the gold standard for analyzing osteocytes’s responses to unloading. In vivo studies, using both animals and humans are also used to gain insights into mechanisms regulating the skeletal response to reduced mechanical loading. In mice and rats, disuse-induced bone loss is achieved by suspending the animal by the tail, so that a coronal rotation of 30° (head-down) is produced, weight bearing by the hindquarters is eliminated, and a cephalad fluid shift occurs. This technique has become one of the most frequently employed for studies of disuse bone loss, with a plethora of data produced. Botox injections, used to paralyze the animal hindlimb, are an alternative approach to hind-limb unloading. Humans studies are far more complex than animal ones and have involved astronauts, spinal cord injured patients or healthy volunteers subjected to prolonged bed-rest (up to 90 days) with a 6 ° head-down tilt.
Conclusions and Future Directions
Although the past decade has seen an exponential increase of current knowledge on osteocytes mechanobiology, the precise mechanisms by which these cells perceive and transduce mechanical cues are still unclear. What have emerged is the multiplicity and complexity of the signaling systems activated by the mechanical inputs. The unique environment of an osteocyte in vivo, make it difficult to establish in vitro model that faithfully recapitulate it. Recent technological advances have demonstrated an impressive progress in understanding osteocyte biology and functions and further elucidation on the mechanisms of osteocyte mechanobiology holds promises of biological and medical implications.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Yuhei Uda, Chao Shi, Ehab Azab, and Ningyuan Sun declare no conflict of interest. Divieti Pajevic reports grants from NIH/NIAMS during the conduct of the study.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
•Of importance
••Of major importance
- 1.Bonewald LF, Johnson ML. Osteocytes, mechanosensing and Wnt signaling. Bone. 2008;42(4):606–15. doi: 10.1016/j.bone.2007.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2**.Tatsumi S, Ishii K, Amizuka N, Li M, Kobayashi T, Kohno K, Ito M, Takeshita S, Ikeda K. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–75. doi: 10.1016/j.cmet.2007.05.001. This study provide the fisrt scientific evidence that osteocytes are mechano-sensors. [DOI] [PubMed] [Google Scholar]
- 3.Bonewald L. Osteocytes as multifunctional cells. J Musculoskelet Neuronal Interact. 2006;6(4):331–3. [PMC free article] [PubMed] [Google Scholar]
- 4.Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med. 17(10):1231–4. doi: 10.1038/nm.2452. [DOI] [PubMed] [Google Scholar]
- 5.Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA. Matrix-embedded cells control osteoclast formation. Nat Med. 17(10):1235–41. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff J. Das Gesetz der Transformation der Knochen Kirschwald. 1892. [Google Scholar]
- 7.Frost HM. Bone “mass” and the “mechanostat”: a proposal. Anat rec. 1987;219:1–9. doi: 10.1002/ar.1092190104. [DOI] [PubMed] [Google Scholar]
- 8.Fulzele K, Lai F, Dedic C, Saini V, Uda Y, Shi C, Tuck P, Aronson JL, Liu X, Spatz JM, et al. Osteocyte-Secreted Wnt Signaling Inhibitor Sclerostin Contributes to Beige Adipogenesis in Peripheral Fat Depots. J Bone Miner Res. 2017;32(2):373–84. doi: 10.1002/jbmr.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annual review of physiology. 1997;59:575–99. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 10.Vogel V, Sheetz M. Local force and geometry sensing regulate cell functions. Nature reviews Molecular cell biology. 2006;7(4):265–75. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 11.Burger EH, Klein-Nulend J. Mechanotransduction in bone--role of the lacuno-canalicular network. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 1999;13(Suppl):S101–12. [PubMed] [Google Scholar]
- 12.Pal S. Mechanical Properties of Biological Materials. 2014:23–40. [Google Scholar]
- 13.Ozcivici E, Luu YK, Adler B, Qin YX, Rubin J, Judex S, Rubin CT. Mechanical signals as anabolic agents in bone. Nature reviews Rheumatology. 2010;6(1):50–9. doi: 10.1038/nrrheum.2009.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SM, Zwart SR, Heer M, Hudson EK, Shackelford L, Morgan JL. Men and women in space: bone loss and kidney stone risk after long-duration spaceflight. J Bone Miner Res. 2014;29(7):1639–45. doi: 10.1002/jbmr.2185. [DOI] [PubMed] [Google Scholar]
- 15.Ohshima H. Bone loss and bone metabolism in astronauts during long-duration space flight. Clinical calcium. 2006;16(1):81–5. [PubMed] [Google Scholar]
- 16.Takano-Yamamoto T. Osteocyte function under compressive mechanical force. Japanese Dental Science Review. 2014;50(2):29–39. [Google Scholar]
- 17.Cowin SC, Moss-Salentijn L, Moss ML. Candidates for the mechanosensory system in bone. Journal of biomechanical engineering. 1991;113(2):191–7. doi: 10.1115/1.2891234. [DOI] [PubMed] [Google Scholar]
- 18.Weinbaum S, Cowin SC, Zeng Y. A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. Journal of biomechanics. 1994;27(3):339–60. doi: 10.1016/0021-9290(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 19.Kwon RY, Meays DR, Meilan AS, Jones J, Miramontes R, Kardos N, Yeh JC, Frangos JA. Skeletal adaptation to intramedullary pressure-induced interstitial fluid flow is enhanced in mice subjected to targeted osteocyte ablation. PLoS One. 2012;7(3):e33336. doi: 10.1371/journal.pone.0033336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riehl BD, Lee JS, Ha L, Kwon IK, Lim JY. Flowtaxis of osteoblast migration under fluid shear and the effect of RhoA kinase silencing. PLoS One. 2017;12(2):e0171857. doi: 10.1371/journal.pone.0171857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu CL, Li SN, Ji BH, Huo B. Flow-Induced Migration of Osteoclasts and Regulations of Calcium Signaling Pathways. Cell Mol Bioeng. 2015;8(1):213–23. [Google Scholar]
- 22.Miyauchi A, Gotoh M, Kamioka H, Notoya K, Sekiya H, Takagi Y, Yoshimoto Y, Ishikawa H, Chihara K, Takano-Yamamoto T, et al. AlphaVbeta3 integrin ligands enhance volume-sensitive calcium influx in mechanically stretched osteocytes. Journal of bone and mineral metabolism. 2006;24(6):498–504. doi: 10.1007/s00774-006-0716-x. [DOI] [PubMed] [Google Scholar]
- 23.Weyts FA, Li YS, van Leeuwen J, Weinans H, Chien S. ERK activation and alpha v beta 3 integrin signaling through Shc recruitment in response to mechanical stimulation in human osteoblasts. J Cell Biochem. 2002;87(1):85–92. doi: 10.1002/jcb.10278. [DOI] [PubMed] [Google Scholar]
- 24.Yavropoulou MP, Yovos JG. The molecular basis of bone mechanotransduction. J Musculoskelet Neuronal Interact. 2016;16(3):221–36. [PMC free article] [PubMed] [Google Scholar]
- 25.Weinbaum S, Duan Y, Thi MM, You L. An Integrative Review of Mechanotransduction in Endothelial, Epithelial (Renal) and Dendritic Cells (Osteocytes) Cell Mol Bioeng. 2011;4(4):510–37. doi: 10.1007/s12195-011-0179-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoey DA, Chen JC, Jacobs CR. The primary cilium as a novel extracellular sensor in bone. Frontiers in endocrinology. 2012;3(75) doi: 10.3389/fendo.2012.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee KL, Guevarra MD, Nguyen AM, Chua MC, Wang Y, Jacobs CR. The primary cilium functions as a mechanical and calcium signaling nexus. Cilia. 2015;4(7) doi: 10.1186/s13630-015-0016-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell A. The pipe and the pinwheel: is pressure an effective stimulus for the 9+0 primary cilium? Cell Biol Int. 2008;32(4):462–8. doi: 10.1016/j.cellbi.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Qiu N, Xiao Z, Cao L, Buechel MM, David V, Roan E, Quarles LD. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J Cell Sci. 2012;125(Pt 8):1945–57. doi: 10.1242/jcs.095893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao Z, Dallas M, Qiu N, Nicolella D, Cao L, Johnson M, Bonewald L, Quarles LD. Conditional deletion of Pkd1 in osteocytes disrupts skeletal mechanosensing in mice. FASEB J. 25(7):2418–32. doi: 10.1096/fj.10-180299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Z, Zhang S, Mahlios J, Zhou G, Magenheimer BS, Guo D, Dallas SL, Maser R, Calvet JP, Bonewald L, et al. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281(41):30884–95. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temiyasathit S, Tang WJ, Leucht P, Anderson CT, Monica SD, Castillo AB, Helms JA, Stearns T, Jacobs CR. Mechanosensing by the primary cilium: deletion of Kif3A reduces bone formation due to loading. PLoS One. 2012;7(3):e33368. doi: 10.1371/journal.pone.0033368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Z, Quarles LD. Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev Endocr Metab Disord. 2015;16(2):115–29. doi: 10.1007/s11154-015-9313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonewald LF. The amazing osteocyte. J Bone Miner Res. 2011;26(2):229–38. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu XT, Sun LW, Yang X, Ding D, Han D, Fan YB. The potential role of spectrin network in the mechanotransduction of MLO-Y4 osteocytes. Scientific reports. 2017;7(40940) doi: 10.1038/srep40940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camirand A, Goltzman D, Gupta A, Kaouass M, Panda D, Karaplis A. The Role of Parathyroid Hormone-Related Protein (PTHrP) in Osteoblast Response to Microgravity: Mechanistic Implications for Osteoporosis Development. PLoS One. 2016;11(7):e0160034. doi: 10.1371/journal.pone.0160034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delgado-Calle J, Tu X, Pacheco-Costa R, McAndrews K, Edwards R, Pellegrini GG, Kuhlenschmidt K, Olivos N, Robling A, Peacock M, et al. Control of Bone Anabolism in Response to Mechanical Loading and PTH by Distinct Mechanisms Downstream of the PTH Receptor. J Bone Miner Res. 2017;32(3):522–35. doi: 10.1002/jbmr.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jacobs CR, Temiyasathit S, Castillo AB. Osteocyte mechanobiology and pericellular mechanics. Annu Rev Biomed Eng. 12:369–400. doi: 10.1146/annurev-bioeng-070909-105302. [DOI] [PubMed] [Google Scholar]
- 39**.Thi MM, Suadicani SO, Schaffler MB, Weinbaum S, Spray DC. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require alphaVbeta3 integrin. Proc Natl Acad Sci U S A. 2013;110(52):21012–7. doi: 10.1073/pnas.1321210110. This study emonstrated the polarity of mechanosensing and mechanotransduction in osteocytes and its dependence on the αvβ3 integrin attachment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Na S, Collin O, Chowdhury F, Tay B, Ouyang M, Wang Y, Wang N. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci U S A. 2008;105(18):6626–31. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical strain opens connexin 43 hemichannels in osteocytes: a novel mechanism for the release of prostaglandin. Mol Biol Cell. 2005;16(7):3100–6. doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng B, Kato Y, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. PGE(2) is essential for gap junction-mediated intercellular communication between osteocyte-like MLO-Y4 cells in response to mechanical strain. Endocrinology. 2001;142(8):3464–73. doi: 10.1210/endo.142.8.8338. [DOI] [PubMed] [Google Scholar]
- 43.Cheng B, Zhao S, Luo J, Sprague E, Bonewald LF, Jiang JX. Expression of functional gap junctions and regulation by fluid flow in osteocyte-like MLO-Y4 cells. J Bone Miner Res. 2001;16(2):249–59. doi: 10.1359/jbmr.2001.16.2.249. [DOI] [PubMed] [Google Scholar]
- 44.Cherian PP, Siller-Jackson AJ, Gu S, Wang X, Bonewald LF, Sprague E, Jiang JX. Mechanical Strain Opens Connexin 43 Hemichannels in Osteocytes: A Novel Mechanism for the Release of Prostaglandin. Mol Biol Cell. 2005 doi: 10.1091/mbc.E04-10-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang W, Ha CH, Jhun BS, Wong C, Jain MK, Jin ZG. Fluid shear stress stimulates phosphorylation-dependent nuclear export of HDAC5 and mediates expression of KLF2 and eNOS. Blood. 2010;115(14):2971–9. doi: 10.1182/blood-2009-05-224824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wein MN, Liang Y, Goransson O, Sundberg TB, Wang J, Williams EA, O’Meara MJ, Govea N, Beqo B, Nishimori S. SIKs control osteocyte responses to parathyroid hormone. Nature communications. 2016;7 doi: 10.1038/ncomms13176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L. Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature. 2003;424(6947):389. doi: 10.1038/424389a. [DOI] [PubMed] [Google Scholar]
- 48.Ciani C, Sharma D, Doty SB, Fritton SP. Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone. Bone. 2014;59:229–34. doi: 10.1016/j.bone.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sinnesael M, Laurent MR, Jardi F, Dubois V, Deboel L, Delisser P, Behets GJ, D’Haese PC, Carmeliet G, Claessens F. Androgens inhibit the osteogenic response to mechanical loading in adult male mice. Endocrinology. 2015;156(4):1343–53. doi: 10.1210/en.2014-1673. [DOI] [PubMed] [Google Scholar]
- 50.Lau KH, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li Z, Ruffoni D, Muller R, Kesavan C, Sheng MH. Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab. 2013;305(2):E271–81. doi: 10.1152/ajpendo.00092.2013. [DOI] [PubMed] [Google Scholar]
- 51.Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–7. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kusu N, Laurikkala J, Imanishi M, Usui H, Konishi M, Miyake A, Thesleff I, Itoh N. Sclerostin is a novel secreted osteoclast-derived bone morphogenetic protein antagonist with unique ligand specificity. J Biol Chem. 2003;278(26):24113–7. doi: 10.1074/jbc.M301716200. [DOI] [PubMed] [Google Scholar]
- 53*.Gaudio A, Pennisi P, Bratengeier C, Torrisi V, Lindner B, Mangiafico RA, Pulvirenti I, Hawa G, Tringali G, Fiore CE. Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab. 95(5):2248–53. doi: 10.1210/jc.2010-0067. This study demosntrated an increase in serum sclerostin in immobilized patients. [DOI] [PubMed] [Google Scholar]
- 54*.Spatz JM, Fields EE, Yu EW, Divieti Pajevic P, Bouxsein ML, Sibonga JD, Zwart SR, Smith SM. Serum sclerostin increases in healthy adult men during bed rest. J Clin Endocrinol Metab. 97(9):E1736–40. doi: 10.1210/jc.2012-1579. This study demosntrated an increase in serum sclerostin in helathy volunteer under bed-rest. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55**.Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283(9):5866–75. doi: 10.1074/jbc.M705092200. Fisrt study demosntrating thst sclerostin is regulated by mechanical forces. [DOI] [PubMed] [Google Scholar]
- 56.Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L. Sclerostin Mediates Bone Response to Mechanical Unloading via Antagonizing Wnt/beta-Catenin Signaling. J Bone Miner Res. 2009;24:1651–61. doi: 10.1359/jbmr.090411. [DOI] [PubMed] [Google Scholar]
- 57.Spatz JM, Ellman R, Cloutier AM, Louis L, van Vliet M, Suva LJ, Dwyer D, Stolina M, Ke HZ, Bouxsein ML. Sclerostin antibody inhibits skeletal deterioration due to reduced mechanical loading. J Bone Miner Res. 28(4):865–74. doi: 10.1002/jbmr.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spatz JM, Wein MN, Gooi JH, Qu Y, Garr JL, Liu S, Barry KJ, Uda Y, Lai F, Dedic C, et al. The Wnt Inhibitor Sclerostin Is Up-regulated by Mechanical Unloading in Osteocytes in Vitro. J Biol Chem. 2015;290(27):16744–58. doi: 10.1074/jbc.M114.628313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J. Inhibition of osteoclastogenesis by mechanically loaded osteocytes: involvement of MEPE. Calcif Tissue Int. 2010;87(5):461–8. doi: 10.1007/s00223-010-9407-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hu MC, Shiizaki K, Kuro-o M, Moe OW. Fibroblast growth factor 23 and Klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol. 2013;75:503–33. doi: 10.1146/annurev-physiol-030212-183727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bonewald LF. Establishment and characterization of an osteocyte-like cell line, MLO-Y4. J Bone Miner Metab. 1999;17(1):61–5. doi: 10.1007/s007740050066. [DOI] [PubMed] [Google Scholar]
- 62.Divieti P, Inomata N, Chapin K, Singh R, Juppner H, Bringhurst FR. Receptors for the carboxyl-terminal region of pth(1–84) are highly expressed in osteocytic cells. Endocrinology. 2001;142(2):916–25. doi: 10.1210/endo.142.2.7955. [DOI] [PubMed] [Google Scholar]
- 63.Woo SM, Rosser J, Dusevich V, Kalajzic I, Bonewald LF. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. J Bone Miner Res. 2011;26(11):2634–46. doi: 10.1002/jbmr.465. [DOI] [PMC free article] [PubMed] [Google Scholar]