Abstract
Autoimmune disease is typically caused by the activated self-reacted immune cells. The mainstream treatment to autoimmune disorders is composed of different mechanisms of immunosuppression. In recent years, a new subtype of T cells called regulatory T (Treg) cells have been identified to maintain the immune homeostasis in terms of suppressing the activated immune components.
According to this discovery, it is suggested that treating autoimmune patients by supplementing Treg cells would be a good choice. However, due to their natural scarcity, it is difficult to isolate a desired number of Treg for this therapeutical approach. Here, we report that by using stem cells, especially the induced pluripotent stem (iPS) cells, we are able to generate a significant amount of Treg cells for the autoimmune prevention and treatment.
Keywords: iPS cells, Treg cells, Notch signaling, Differentiation, Autoimmune and immunosuppression
1 Introduction
There is a special group of T cells existing in human and mouse immune systems called regulatory T (Treg) cells. The general function of Treg cells is to suppress the activated immune system through different mechanisms such as direct contact or cytokine-mediated immune suppression. Although this Treg-mediated immunosuppression is malicious in timorous condition, on the flip side, it plays an important role in controlling autoimmunity, for example, rheumatoid arthritis and systemic lupus erythematosus. This unique immunosuppressive property of Treg cells renders them good candidates for treating different types of autoimmune diseases. However, due to the nature of the scarcity of Treg cells in humans, it is difficult to collect adequate numbers of active Treg cells in the clinical setting. To find a new approach to generate a large number of Treg cells becomes the hotspot of current research in immunology and stem cell biology. Previous studies have shown that embryonic stem cells are able to differentiate into T cells in a controlled condition and ectopic expression of Treg promoter FoxP3 is able to convert naïve T cells into Treg cells. Therefore, under these circumstances, it is hypothesized that stem cells, especially embryonic stem cells are able to generate Treg cells by introducing both T cell differentiation signals and Treg-inducing signals. For testing this hypothesis, we used induced pluripotent stem (iPS) cells as a model system to bypass the ethic and technique difficulties in using embryonic stem cells. Meanwhile, using iPS cells as a model system could further help to design a stem cell-originated, personalized immunotherapy to autoimmune patients.
In this chapter, the generation and following characterization of Treg cells from iPS cells will be described in a chronological format. After reading this chapter, readers should have an initial impression about how to generate Treg cells from iPS cells.
2 Materials
2.1 Cells and Mice
iPS-MEF-Ng-20D-17 cell line: generated from mouse embryonic fibroblasts by retroviral transduction of Oct3/4 Sox2, Klf4, and c-Myc [1] (see Note 1). iPS cells are routinely maintained on irradiated SNL76/7 feeder cells with conditioned 15 % FBS-supplemented DMEM.
OP9-DL1 cell line (see Note 2) and OP9-DL1 I-Ab cell line: OP9-DL1 I-Ab cell line is generated by introducing MHC-II molecule I-Ab (see Note 3) into OP9-DL1 cells through a retroviral transduction [2]. OP9-DL1 and OP9-DL1 I-Ab cell lines are routinely maintained in 20 % FBS-supplemented α-MEM medium.
SNL76/7 cell line (ATCC): maintained in 10 % FBS-supplemented DMEM. Before using as iPS feeder cells, SNL76/7 cell are irradiated with a dose of 5,000 Rads in the 60Co irradiator, the irradiated SNL76/7 cells are designated as irSNL76/7 cells [3].
4–12 weeks of age C57/BL6, B6.129S7-Rag1tm1Mom (Rag1-deficient), and DBA/1 mice (Jackson Laboratory).
Plat-E packaging cells (see Note 4).
2.2 Cell Culture
6- and 24-well culture plate (BD).
100 mm culture dish (BD).
70 μm cell strainer (BD).
Syringes (1 mL and 10 mL, BD).
Needles (27G1/2 and 18G11/2, BD).
Plastic pipettes (5 mL, 10 mL, 25 mL, 50 mL; BD).
0.22 μM bottle-top filter (Corning).
0.4 μm filter (Millipore).
Dulbecco’s Modified Eagle Medium (DMEM, Invitrogen).
α-Minimal Essential Medium (α-MEM, Invitrogen): one pack of α-MEM powder and 2.2 g NaHCO3 in 1 L ddH2O. Filtration of prepared medium through a 0.22 μM filter is required for sterilization purpose. FBS, antibiotics, and cytokines are added according to different protocols.
Phosphate-buffered saline (PBS): 137 mmol/L NaCl, 2.7 mmol/L KCl, 10 mmol/L Na2HPO4.2H2O, 2 mmol/L KH2PO4, pH 7.4, autoclave before use.
100× Penicillin and Streptomycin Mix (10,000 U/mL, Invitrogen).
Fetal bovine serum (FBS, heat-inactivated, HyClone).
Flt-3ligand (Flt-3L, PeproTech).
Interleukin 7 (IL-7, PeproTech).
Neutral Formalin buffer (Decal Chemical).
Gelatin (Sigma-Aldrich).
Brefeldin A (Invitrogen).
Formaldehyde solution (Sigma-Aldrich).
Fc blocker 24G2 (BD).
Permeabilizing kit (BioLegend).
[3H]-labeled thymidine.
2.3 Retroviral Transduction
GeneJamma transfection reagent (Stratagene).
5 μg/mL polybrene (Sigma-Aldrich).
MoFlo cell sorter.
2.4 Collagen-Induced Arthritis (CIA)
Complete Freund’s Adjuvant (CFA, Chondrex).
Chicken Collagen Type II (Chondrex).
2.5 Immunoblot
RIPA lysis buffer (G-Biosciences).
Bio-Rad DC Protein Assay (Bio-Rad).
Western Blot NuPAGE system (Invitrogen).
ECL immunodetection system (Amersham Pharmacia Biotech).
2.6 Histology and Immunofluorescence
Formical-4 buffer (Decal Chemical).
3 % hydrogen peroxide (VWR).
DAPI reagent (Enzo Life Sciences).
Hematoxylin and eosin (HE) or Safranin O–Fast Green (Sigma-Aldrich).
Xylene (Sigma-Aldrich).
Ethanol (VWR).
Blocking buffer: 3 % BSA in PBS.
Easy-Titer IgG assay kit.
2.7 Antibodies
Antibodies used are listed in Table 1.
Table 1.
Antibodies
| Name | Clone | Company |
|---|---|---|
| CD 3 | 2C11 | BD Pharmingen |
| CD28 | 37.51 | BD Pharmingen |
| CD3 | 17A2 | BioLegend |
| CD4 | GK1.5 | BioLegend |
| CD8 | 6A242 | Santa Cruz |
| CD25 | 3C7 | BioLegend |
| CD44 | 1M7 | BioLegend |
| CD69 | H1.2F3 | BioLegend |
| CD117 | 2B8 | BioLegend |
| CD127 | A7R34 | BioLegend |
| CTLA-4 | UC10-4B9 | BioLegend |
| FoxP3 | 150D | BioLegend |
| TCR-β | H57597 | BioLegend |
| IL-2 | JES6-5H4 | BD Pharmingen |
| IL-10 | JES5-16E3 | BioLegend |
| IFN-γ | XMG1.2 | BD Pharmingen |
| LAP (TGF-β1) | TW7-16B4 | BioLegend |
3 Methods
3.1 Retroviral Transduction: Generation of Retroviral Construct
Construct MSCV-IRES-DsRED (MiDR) vector from MSCV-IRES-GFP vector by substituting the GFP gene by the DsRED gene (see Note 5).
Subclone FoxP3 and Bcl-xl genes into the MiDR vector to make a FoxP3-Bcl-xl-MiDR construct.
3.2 Retroviral Transduction and Cell Sorting (Fig. 1) (See Note 6)
Fig. 1.
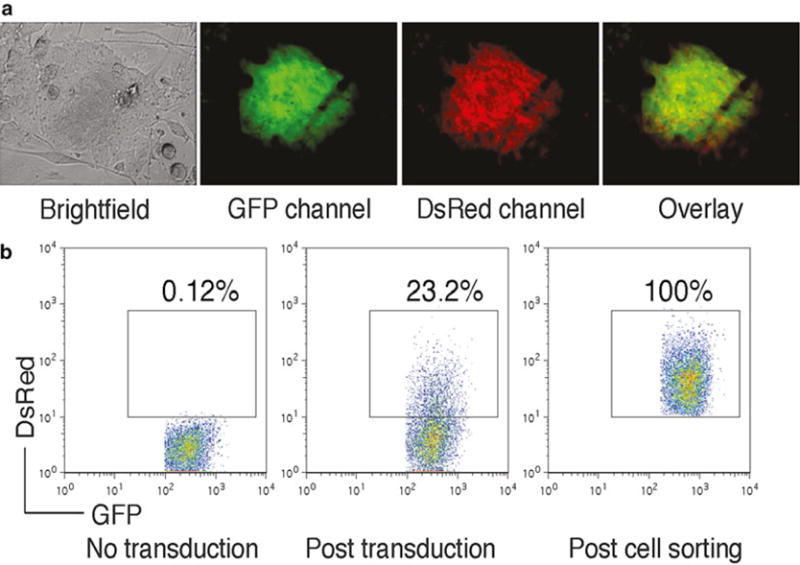
(a) FoxP3-transduced iPS cells were visualized by fluorescence microscopy. (b) GFP+ iPS cells (left) were transduced with the retroviral construct, and GFP+ DsRed+ iPS cells (middle) were analyzed by flow cytometry and sorted by a high-speed cell sorter (right)
Use Plat-E packaging cells to generate a pseudovirus that will be used for the following transduction.
Seed 3 × 106 Plat-E cells on a 100 mm culture dish 1 day prior to transfection with the retroviral construct.
On day 0, transfect Plat-E cells with FoxP3-Bcl-xl-MiDR plasmid by using GeneJamma transfection reagent.
On day 1, seed 1 × 106 iPS cells into one well of a 0.1 % gelatin-pre-coated 24-well plate.
On day 2, collect pseudovirus-containing supernatant from Plat-E culture and pass it through a 0.4 μm filter to exclude potential contaminants.
Perform transduction at 32 °C and centrifugation at 500 × g for 1 h in the presence of 5 μg/mL polybrene.
After centrifuge-based transduction, place cells in 32 °C, 5 % CO2 incubator overnight.
On day 3, repeat the day 2 transduction procedure as described above. Meanwhile, pre-coat a 6-well plate with irSNL76/7 feeder cells for future use.
On day 4, trypsinize transduced iPS cells, centrifuge at 400 × g for 5 min and seed on pre-coated irSNL76/7 feeder cells.
At confluency, trypsinize cells, centrifuge at 400 × g for 5 min and process for cell sorting. GFP and DsRED double positive cells will be sorted by a MoFlo cell sorter. Culture sorted cells on irSNL76/7 feeder cells for future use (see Note 7).
3.3 Coculture with OP9-DL1/I-Ab Cells
3.3.1 In Vitro Coculture System
On day 0, seed 5 × 104 gene-transduced iPS cells (FoxP3/iPS) on a 100 mm culture dish containing a confluent OP9-DL1/I-Ab cell monolayer in 20 % FBS α-MEM medium.
On day 3, change culture medium.
On day 5, trypsinize cells and centrifuge at 400 ×g for 5 min before incubating on a fresh 100 mm culture dish for 30 min in a 37 °C incubator.
Collect and count floating cells, and transfer 5 × 105 cells to a fresh culture dish containing a confluent OP9-DL1/I-Ab cell monolayer in 20 % FBS-supplemented α-MEM medium. Add cytokine mFlt-3L (final concentration: 5 ng/mL) to the culture.
On day 8, gently pipette down loosely attached cells.
Wash the OP9-DL1/I-Ab feeding layer with 10 mL PBS one more time to get the maximal recovery of partially differentiated iPS cells.
After harvesting cells from the coculture, centrifuge cells at 400 ×g for 5 min and resuspend in 20 % FBS-supplemented α-MEM medium supplemented with Flt-3L (5 ng/mL) and IL-7 (1 ng/mL).
At the end, transfer cells into a 6-well culture plate coated with confluent OP9-DL1/I-Ab cells. Usually iPS cells recovered from one 100 mm culture dish will be transferred into one well of the 6-well plate.
From day 10, change culture medium every other day (20 % FBS-supplemented α-MEM medium supplemented with Flt-3L, 5 ng/mL, and IL-7, 1 ng/mL).
Culture plates coated with feeder OP9-DL1/MIAb cells will be changed every 4–6 days depending on the growth of the feeder cells.
3.3.2 In Vitro Differentiation of FoxP3/iPS Cells
At different days of coculture with OP9-DL1/I-Ab cells, take live cell pictures under a conventional light microscope.
Calculate cell recovery rates based on the number of cells harvested from the culture.
Analyze surface marker changes by flow cytometry (Fig. 2).
On different days of coculture, remove cells by trypsinization and wash with cold PBS before proceeding to cell surface staining.
Before staining with different fluorochrome-conjugated antibodies, block cells by Fc blocker 24G2 at 4 °C for 20 min.
Stain cells with fluorochrome-conjugated antibodies after Fc blocking.
After 20 min of staining at 4 °C, wash the cells three times in cold PBS before flow cytometric examination.
Fig. 2.
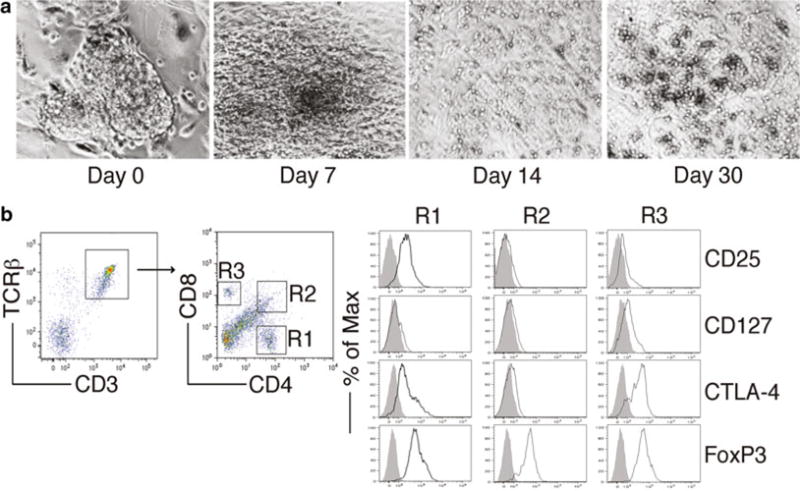
FoxP3-transduced iPS cells were cocultured on OP9-DL1/M I-Ab cells in the presence of murine recombinant Flt3L and IL-7. (a) Morphology of Treg cell differentiation on days 0, 7, 14, and 30. (b) Flow cytometric analysis for the protein expression of iPS cell-derived cells on day 30. CD3+ TCRαβ+ cells were gated as indicated, and analyzed for the expression of CD4 and CD8, with CD25, CD127, CTLA-4, and FoxP3 expression shown for cells gated as CD4+ cells (R1), CD4+CD8+ cells (R2), and CD8+ cells (R3) (dark lines, shaded areas indicate isotype controls)
3.3.3 Functional Analysis of In Vitro Differentiated FoxP3/IPS Cells (Fig. 3)
Fig. 3.
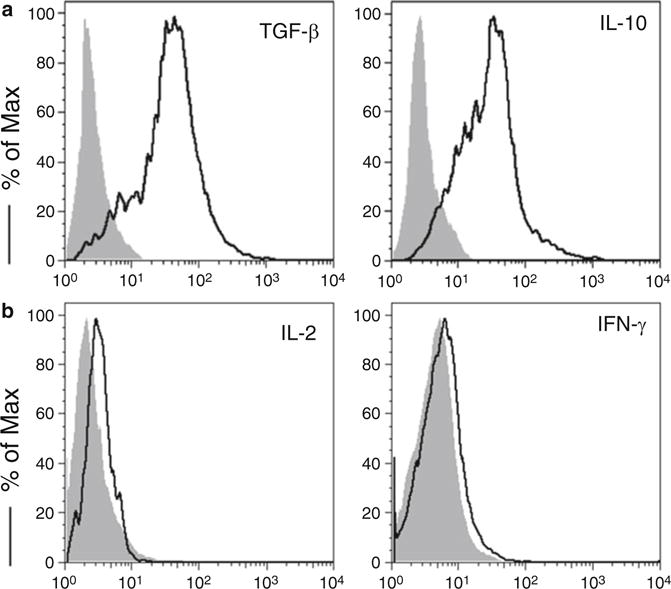
Murine iPS cell-derived Treg cells were stimulated with plate-coated anti-CD3/anti-CD28 mAbs. Intracellular cytokine production was analyzed by flow cytometry after gating on live CD4+ CD25+ cells (dark lines, shaded areas indicate isotype controls). (a) Intracellular TGF-β and IL-10. (b) Intracellular IL-2 and IFN-γ
One day before activation assay, pre-coat a 24-well plate with anti-CD3 (final concentration: 4 μg/mL in PBS) at 4 °C overnight.
On day 45 of coculture, harvest FoxP3/iPS cell-derived T cells from the culture and wash with cold PBS before stimulating with plate-coated anti-CD3 and soluble anti-CD28 antibodies (final concentration: 4 μg/mL).
Incubate plate in 37 °C, 5 % CO2 incubator for 40 h and then add Brefeldin A to the culture for another 4 h.
At the end of coculture, harvest cells, wash, and block by Fc blocker as described above. Stain blocked cells for surface markers such as CD3, CD4, CD8, and TCRβ chain by using fluorochrome-conjugated antibodies.
After cell surface staining, fix cells by using 4 % formaldehyde, and permeabilize by using BioLegend’s Permeabilizing kit.
After permeabilization, stain intracellular molecules like TGF-β, IL-10, IL-2, and IFN-γ by using fluorochrome-conjugated antibodies.
Before final flow cytometric examination, wash the cells three times in cold PBS to exclude excessive antibodies.
3.3.4 Immunosuppression Analysis of In Vitro Differentiated FoxP3/ iPS Cells (Fig. 4)
Fig. 4.
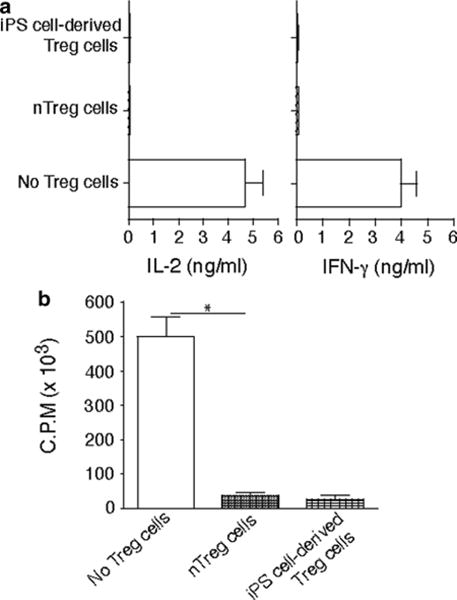
Murine iPS cell-derived Treg cells were cocultured with naive CD4+ T cells from C57BL/6 mice (Tregs/T cells = 1:1) in the presence of anti-CD3/anti-CD28 mAbs for 2 days. A group of CD4+ T cells stimulated with CD4+ CD25+ Treg cells from C57BL/6 mice as the positive control and a group of CD4+ T cells stimulated without Treg cells as the negative control. Cytokine production was analyzed by ELISA, and proliferation was determined by [3H] thymidine incorporation. (a) IL-2 and IFN-γ. (b) Thymidine incorporation during the last 12 h. *P< 0.05, one-way AN OVA tests
One day before activation assay, pre-coat a 24-well plate with anti-CD3 (final concentration: 4 μg/mL in PBS) at 4 °C overnight.
On day 45 of coculture, harvest FoxP3/iPS cell-derived T cells from culture and wash with cold PBS before stimulating with plate-coated anti-CD3 and soluble anti-CD28 antibodies (final concentration: 4 μg/mL).
Harvest naïve CD4 T cells from C57BL/6 mice and mix with in vitro differentiated FoxP3/iPS cells in a 1:1 ratio before the co-stimulatory activation assay.
Incubate the mixed population in 37 °C, 5 % CO2 incubator for 48 h. Add [3H]-labeled thymidine 12 h after the incubation.
At the end of coculture, harvest culture supernatant to determine IL-2 and IFN-γ concentrations by ELISA.
Later on, harvest cells to check the thymidine incorporation rate.
3.4 Adoptive Transfer
FoxP3-Bcl-xl-MiDR-transduced and in vitro-induced iPS (FoxP3/iPS) cells are prepared as described above.
After 7 days of in vitro coculture, FoxP3/iPS cells are trypsinized, centrifuged at 400 × g for 5 min and resuspended in fresh medium.
30 min incubation on a fresh culture dish in a 37 °C incubator is required to eliminate differentiated cells and remnant feeder cells.
At the end of incubation, collect floating cells and centrifuge at 400×g for 5 min.
Wash cell pellet in cold PBS three times, and pass cells through a 70 μm nylon strainer in between of two washes to exclude cell clumps (2 × filtration).
After wash, count and resuspend cells in cold PBS at a concentration of 1.5 × 107 cells/mL (see Note 8).
Maintain cells on ice before injection.
For adoptive transfer, 4–6 weeks old male C57BL/6J mice are used. Before i.v. injection through the tail vein, place mice under an infrared light to dilate their tail veins.
3.5 Collagen-Induced Arthritis (CIA)
Fifteen days prior to the adoptive transfer of FoxP3/iPS cells, collagen-induced arthritis (CIA) shall be induced in the recipient male C57BL/6 J mice.
Animal handlings and experimental protocols have to be in accordance with the national regulations and guidelines.
Prepare Chicken type-II collagen (CII) according to the manufacturer’s protocol. Before CIA, all reagents shall be maintained on ice (see Note 9).
On day 0, CIA is induced in male C57BL/6J mice by one intradermal immunization at two sides in the base and slightly above the tail with chicken CII in CFA.
CIA development is monitored by visual inspection as joint swelling and immobility.
3.6 Immunoblot
For checking the protein expression level in FoxP3/iPS cells, lyse live cells in RIPA buffer for 30 min to break cell structure and release intracellular proteins.
Protein concentration is determined by using Bio-Rad protein assay kit, and equal amounts of protein (30–50 μg) are used for subsequent immunoblotting.
All immunoblots are conducted by using a commercial immunoblot kit (Invitrogen’s Nu-PAGE system) and all immunoblots are developed with the ECL immunodetection system.
3.7 Histology and Immunofluorescence (Fig. 5)
Fig. 5.
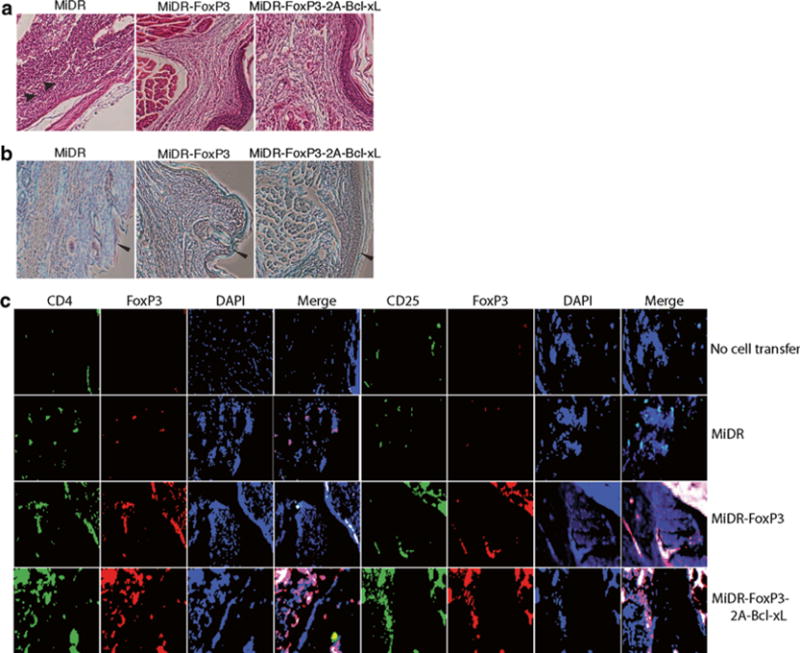
Murine iPS cells were transduced with retroviral constructs: vector (MiDR), FoxP3 (MiDR-FoxP3), or FoxP3 with Bcl-xL (MiDR-Bcl-xL-2A-FoxP3), and cocultured on OP9-DL1/MIAb cells. On day 7, DsRed-positive (DsRed+) T cells were sorted and prepared for adoptive cell transfer. Collagen-induced arthritis (CIA) was induced in male C57BL/6 mice by one (day 0) intradermal immunization at two sites in the base and slightly above of the tail with chicken type II collagen in complete Freund’s adjuvant. On day 15 after the immunization, mice received transduced DsRed+ cells (2.5 × 106/mouse). On day 60 of immunization, hind foot paws were amputated, fixed, and decalcified. The tissues were embedded in paraffin, sectioned, and stained. (a) Hematoxylin and eosin (HE) staining. (b) Safranin O–Fast Green staining. Infiltrations of polymorphonuclear (PMN) cells (arrow heads) in HE staining and massive destruction of cartilage (leftward arrow heads) in Safranin O–Fast Green staining are indicated. (c) Immunofluorescent staining. There were iPS cell-derived Treg cells (red) infiltrating in joints from mice receiving iPS cell-derived Treg cells, but not in tissues from mice receiving vector control-transduced iPS cells or without cell transfer (green: CD4 or CD25, red. FoxP3, blue: DAPI).
At day 60 of the CIA experiment, mice will be sacrificed for checking the arthritis development.
Amputate hind feet, fix them in Neutral Formalin buffer, and further decalcify in Formical-4 solution.
Decalcified samples are prepared as removal of excessive tissue and embedded in paraffin. Make 4 μm sections and stain with HE or Safranin O–Fast Green as described before [4].
Perform immunofluorescent staining on the sections after deparaffinization and rehydration by using xylene and ethanol.
Block endogenous peroxidase activity by 3 % hydrogen peroxide for 30 min after antibody retrieval.
Block nonspecific binding in blocking buffer at room temperature in a moist chamber for 60 min.
Add fluorochrome-labeled antibodies in blocking buffer and apply prepared solution onto the slides.
Incubate the slides at room temperature in a dark moist chamber for 60 min.
At the end of incubation, wash the slides in PBS five times before applying DAPI-containing antifade reagent.
Evaluate immunofluorescence under fluorescent microscope later.
3.8 Antibody Detection
On day 60 of CIA development, sacrifice mice and collect blood from different groups of mice by heart puncture.
Collect serum from whole blood from different groups of mice by centrifugation at 1,000 × g for 10 min.
Total IgG levels are determined by the Easy-Titer IgG assay kit in accordance with the recommendations of the manufacturer.
The levels of anti-CII IgG in different blood samples are measured by ELISA as described previously [4].
Acknowledgments
This project was funded, in part, under grants with the Grant Number K18CA151798 from the National Institutes of Health and the Barsumian Trust to J.S.
Footnotes
iPS-MEF-Ng-20D-17 cell line is a kind gift from Dr. Shinya Yamanaka at the Kyoto University through the RIKEN cell bank via a completed material transfer agreement (MTA).
OP9-DL1 cell line is a kind gift from Dr. J.C. Zuniga-Pflucker [5] at the University of Toronto via a completed material transfer agreement (MTA).
MHC-II molecule I-Ab construct is obtained from Dr. Pin Wang at the University of Southern California via a completed material transfer agreement (MTA).
Plat-E cells are constructed based on the 293T cell line to permanently express retroviral packaging signals env and gag-pol, proteins [6].
The detailed instruction about retroviral vector, gene fragment manipulation, and construct assembly belongs to the scope of molecular biology. Mastering those molecular biology skills is required to perform this section of work.
Usually the retroviral transduction efficiency to induced pluripotent stem cells is low. Adding VSV-G glycoprotein-mediated pseudotyping can increase the transduction efficiency.
For cell sorting, cells need to be passed through a cell strainer several times to prevent cell clogging.
For adoptive transfer, cells need to be serum-free and clump-free to prevent lung embolism and allergic response in recipient mice. To remove serum, multiple PBS wash is mandatory, and to remove cell clump, pass cells multiple times through cell strainers.
When preparing CII in CFA, make sure complete emulsification is achieved otherwise the arthritis induction could be significantly compromised.
References
- 1.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–317. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 2.Dai B, Wang P. In vitro differentiation of adult bone marrow progenitors into antigen-specific CD4 helper T cells using engineered stromal cells expressing a notch ligand and a major histocompatibility complex class II protein. Stem Cells Dev. 2009;18:235–245. doi: 10.1089/scd.2008.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lei F, Zhao B, Haque R, Xiong X, Budgeon L, Christensen ND, Wu Y, Song J. In vivo programming of tumor antigen-specific T lymphocytes from pluripotent stem cells to promote cancer immunosurveillance. Cancer Res. 2011;71:4742–4747. doi: 10.1158/0008-5472.CAN-11-0359. [DOI] [PubMed] [Google Scholar]
- 4.Haque R, Lei F, Xiong X, Wu Y, Song J. FoxP3 and Bcl-xL cooperatively promote regulatory T cell persistence and prevention of arthritis development. Arthritis Res Ther. 2010;12:R66. doi: 10.1186/ar2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 6.Morita S, Kojima T, Kitamura T. Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther. 2000;7:1063–1066. doi: 10.1038/sj.gt.3301206. [DOI] [PubMed] [Google Scholar]


