Abstract
Trastuzumab has substantial antitumor activity in metastatic gastric cancer. One such mechanism by which it exerts its antitumor activity is antibody-dependent cell-mediated cytotoxicity, which has been reported to be influenced by FcγRIIA and IIIA polymorphisms. This study is the first to assess their impact on trastuzumab efficacy in patients with metastatic gastric cancer. We retrospectively examined 42 Her-2-positive patients receiving fluorouracil and platinum-based chemotherapy and trastuzumab, and 68 Her-2-negative patients receiving fluorouracil and platinum-based chemotherapy only as the first-line treatment. FcγRIIA and IIIA polymorphisms were assessed, and their associations with efficacy in both settings were analyzed. In patients treated with trastuzumab, the FcγRIIA H/H genotype was associated with significantly superior progression-free survival (PFS) (hazard ratio [HR] [95% CI]: 0.36 [0.16–0.82], adjusted HR [95% CI]: 0.18 [0.07–0.48], P=0.001). When combining FcγRIIA and IIIA polymorphisms, the FcγRIIA H/H or FcγRIIIA V/V genotype was associated with a significantly improved disease control rate (P=0.04) and PFS (HR [95% CI]: 0.29 [0.13–0.67], adjusted HR [95% CI]: 0.17 [0.07–0.45], P<0.001). As expected, no association of FcγRIIA and IIIA polymorphisms with efficacy was found in patients receiving chemotherapy only. We concluded that FcγRIIA and IIIA polymorphisms might predict disease control rate and PFS in metastatic gastric cancer patients receiving trastuzumab treatment.
Keywords: gastric cancer, trastuzumab, antibody-dependent cell-mediated cytotoxicity, FcγRIIA polymorphism, FcγRIIIA polymorphism, human epidermal growth factor receptor-2
Introduction
Gastric cancer (GC) is one of the leading malignancies of the digestive system. There were an estimated 26,370 new cases of GC in the USA in 2016, making it fourth in rank among all cancers of the digestive system.1 The incidence of GC is much higher in China, where it ranks second among all cancers as estimated in 2015.2 The prognosis of GC is relatively poor.3 GC is also the second leading cause of cancer-specific death in China.2 The median overall survival for stage IV patents is about 1 year. The use of targeted therapy prolongs survival for ~2–4 months.4–7 Trastuzumab, the main targeted drug for GC, was approved by the Food and Drug Administration in 2010 for the treatment of epidermal growth factor receptor 2 (ERBb2 or Her-2)-overexpressed metastatic GC or gastroesophageal junction adenocarcinoma.
The major mechanism of the antitumor effect of trastuzumab involves the growth signaling pathways transduced by the transmembrane protein, Her-2, as indicated by in vitro and in vivo laboratory and clinical studies.8–10 However, another Her-2-targeted drug, lapatinib, has not been found to significantly improve survival in advanced or metastatic GC.11 This drug is a small-molecule tyrosine kinase inhibitor and is different from trastuzumab, which is an IgG1 monoclonal antibody that mediates antibody-dependent cell-mediated cytotoxicity (ADCC) and the subsequent lysis of targeted tumor cells.12,13 This efficacy disparity may emphasize the importance of ADCC in the antitumor effect of trastuzumab in GC.
The ADCC effect is mediated by the binding of the fragment C (Fc) portion of the antibody and the Fc γ receptor (FcγR) on immunologic effector cells.13 Genetic polymorphisms of FcγR have been demonstrated to result in differences in binding affinity, with the FcγRIIA and IIIA alleles most frequently reported. For FcγRIIA, a single-nucleotide substitution (A519G, rs1801274) results in the substitution of histidine (H) by arginine (R) at amino acid position 131. Likewise, for FcγRIIIA, a single-nucleotide substitution (A559C, rs396991) leads to the substitution of phenylalanine (F) by valine (V) at amino acid position 158. Both the FcγRIIA 131H/H and FcγRIIIA 158V/V genotypes are identified as having the strongest binding affinity.14–16
An association between patient outcomes and FcγRIIA and/or IIIA genotypes has been discovered in lymphoma treated with rituximab17 and colorectal cancer treated with cetuximab,18,19 and both these drugs are IgG1 monoclonal antibodies. The prognostic value of FcγRIIA and IIIA genotypes in breast cancer treated with trastuzumab was found to be inconsistent in several previous investigations, but these genotypes seemed to be relevant in cases of advanced and metastatic breast cancer.20–23 Only about half of Her-2-positive GC patients respond to trastuzumab,7 and thus, the identification of biomarkers predicting its efficacy has potential clinical application. ADCC may constitute a significant portion of the antitumor mechanism of trastuzumab in GC. To our knowledge, this is the first study to evaluate the clinical and prognostic relevance of FcγRIIA and/or IIIA polymorphisms in trastuzumab-treated metastatic GC.
Materials and methods
Ethics statement
All patients provided written informed consent for their information to be used in our hospital database. Study approval was obtained from independent ethics committees at Sun Yat-sen University Cancer Center. This study was conducted in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Study population
This research was retrospectively conducted at Sun Yat-sen University Cancer Center. We included two sets of patients pathologically diagnosed with metastatic gastric adenocarcinoma in this study. Set A included patients with Her-2-positive GC, who received trastuzumab combined with chemotherapy as the first-line treatment. Set B included patients with Her-2-negative GC, who received only chemotherapy as the first-line treatment. The assessment of Her-2 status followed the criteria of the National Comprehensive Cancer Network guidelines. HER-2 positive was defined as HER-2(+++) by immunohistochemistry or HER-2(++) by immunohistochemistry and HER-2(positive) by fluorescence in situ hybridization. There were 228 patients pathologically diagnosed with metastatic GC from May 1, 2011, to August 30, 2015, in our center, including 58 patients with Her-2-positive cancer and 170 patients with Her-2-negative cancer. We included only those with an efficacy assessment and follow-up information, as well as those who signed informed consent forms and had qualified blood samples. With these eligibility criteria, there were 42 patients enrolled in set A. All of them received trastuzumab combined with fluorouracil and platinum-based chemotherapy as the first-line treatment. As a comparison, enrolled in set B were 68 patients who received only fluorouracil and platinum-based chemotherapy as the first-line treatment. The Consolidated Standards of Reporting Trials (CONSORT) diagram is shown in Figure S1. The clinicopathologic data and follow-up information were retrospectively collected.
FcγRIIA and IIIA polymorphism genotyping
Blood samples were collected before treatment. Archived peripheral blood mononuclear cells were used for DNA extraction with the QIAamp DNA blood mini kit (Qiagen). Nested polymerase chain reaction (PCR) was conducted to detect single-nucleotide polymorphisms in FcγRIIA and/or IIIA using the same primers as in a previous study.21 PCR was carried out using the HiFi HotStart ReadyMix (KAPA Biosystems) and optimized protocols. The PCR products were purified using the PCR clean kit (Qiagen) and then sequenced on an ABI3730XL (Applied Biosystems) with the BigDye Terminator v3.1 Cycle Sequencing Kit. The PCR products of 110 samples were also analyzed on MassARRAY analyzer (Sequenom) with the iPLEX Gold assay (Sequenom) using primers, as in a previous study,21 to identify the A559C polymorphism in FcγRIIIA and the A519G polymorphism in FcγRIIA.
Statistical analysis
All statistical analyses were performed using the SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA). A two-tailed P-value of <0.05 was considered statistically significant. The associations between FcγRIIA and/or IIIA genotypes and clinicopathologic characteristics were analyzed with a chi-square test or Kruskal–Wallis H-test, based on the type of the data. Comparisons of tumor responses between FcγRIIA and/or IIIA genotypes were also conducted with chi-square test or Kruskal–Wallis H-test. Survival curves were plotted by the Kaplan–Meier method and compared using the log-rank test. The prognostic value of FcγRIIA and/or IIIA genotypes was evaluated using univariate and multivariate Cox regression analyses.
Results
Patient characteristics
Patient characteristics according to treatment settings are shown in Table S1. All 42 patients in set A were Her-2 positive; by contrast, all 68 patients in set B were Her-2 negative. Accordingly, differences in some clinicopathologic features existed between the two sets. For example, tumors in set A presented with more intestinal types compared with those in set B (33.3% vs 2.9%). The correlations between FcγRIIA and/or IIIA genotypes and clinicopathologic features are shown in Table 1. The frequency of FcγRIIA and/or IIIA genotypes did not differ between the two study sets, as demonstrated by the lack of associations between FcγRIIA and/or IIIA genotypes and Her-2 status (negative/positive). In addition, we did not find any correlations between other clinicopathologic features including age (<52/≥52 years), gender (male/female), Eastern Cooperative Oncology Group score (0/1), gastrectomy (yes/no), location of tumor (proximal/middle/distal/others), degree of differentiation (well-differentiated adenocarcinoma/moderately differentiated adenocarcinoma/poorly differentiated/signet ring cell carcinoma/mucinous adenocarcinoma), Lauren classification (intestinal type/diffuse type/mixed type), smoking status (present or previous/never), drinking status (present or previous/never), and metastasis (single/multiple).
Table 1.
Patient characteristics by genotypes in metastatic gastric cancer
| Features |
FcγRIIA
|
FcγRIIIA
|
FcγRIIA and IIIA
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H/H (%), n=56 | R/R (%), n=14 | H/R (%), n=40 | P-value | V/V (%), n=19 | F/F (%), n=40 | V/F (%), n=51 | P-value | H/H or V/V, n=61 | Others, n=49 | P-value | |
| Her-2 status | 0.52 | 0.38 | 0.37 | ||||||||
| Positive | 19 (45.2) | 7 (16.7) | 16 (38.1) | 5 (11.9) | 18 (42.9) | 19 (45.2) | 21 (50.0) | 21 (50.0) | |||
| Negative | 37 (54.4) | 7 (10.3) | 24 (35.3) | 14 (20.6) | 22 (32.4) | 32 (47.1) | 40 (58.8) | 28 (41.2) | |||
| Age (years, median 52) | 0.52 | 0.97 | 0.38 | ||||||||
| <52 | 23 (45.1) | 7 (13.7) | 21 (41.2) | 9 (17.6) | 19 (37.3) | 23 (45.1) | 26 (51.0) | 25 (49.0) | |||
| ≥52 | 33 (55.9) | 7 (11.9) | 19 (32.2) | 10 (16.9) | 21 (35.6) | 28 (47.5) | 35 (59.3) | 24 (40.7) | |||
| Gender | 0.08 | 0.08 | 0.56 | ||||||||
| Male | 33 (50.0) | 5 (7.6) | 28 (42.4) | 7 (10.6) | 26 (39.4) | 33 (50.0) | 35 (53.0) | 31 (47.0) | |||
| Female | 23 (52.3) | 9 (20.5) | 12 (27.3) | 12 (27.3) | 14 (31.8) | 18 (40.9) | 26 (59.1) | 18 (40.9) | |||
| ECOG score | 0.25 | 0.08 | |||||||||
| 0 | 42 (48.3) | 10 (11.5) | 35 (40.2) | 15 (17.2) | 36 (41.4) | 36 (41.4) | 47 (54.0) | 40 (46.0) | 0.56 | ||
| 1 | 14 (60.9) | 4 (17.4) | 5 (21.7) | 4 (17.4) | 4 (17.4) | 15 (65.2) | 14 (60.9) | 9 (39.1) | |||
| Gastrectomy | 0.47 | 0.27 | 0.99 | ||||||||
| Yes | 43 (51.8) | 12 (14.5) | 28 (33.7) | 12 (14.5) | 33 (39.8) | 38 (45.8) | 46 (55.4) | 37 (44.6) | |||
| No | 13 (48.1) | 2 (7.4) | 12 (44.4) | 7 (25.9) | 7 (25.9) | 13 (48.1) | 15 (55.6) | 12 (44.4) | |||
| Location of tumor | 0.87 | 0.36 | 0.81 | ||||||||
| Proximal | 14 (60.9) | 3 (13.0) | 6 (26.1) | 3 (13.0) | 9 (39.1) | 11 (47.8) | 14 (60.9) | 9 (39.1) | |||
| Middle | 16 (50.0) | 4 (12.5) | 12 (37.5) | 8 (25.0) | 10 (31.3) | 14 (43.8) | 18 (56.3) | 14 (43.8) | |||
| Distal | 23 (46.9) | 7 (14.3) | 19 (38.8) | 6 (12.2) | 21 (42.9) | 22 (44.9) | 25 (51.0) | 24 (49.0) | |||
| Others | 3 (50.0) | 0 (0.0) | 3 (50.0) | 2 (33.3) | 0 (0.0) | 4 (66.7) | 4 (66.7) | 2 (33.3) | |||
| Degree of differentiation | 0.53 | 0.16 | 0.50 | ||||||||
| Well-differentiated adenocarcinoma | 1 (33.3) | 0 (0.0) | 2 (66.71) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 1 (33.3) | 2 (66.7) | |||
| Moderately differentiated adenocarcinoma | 13 (65.0) | 2 (10.0) | 5 (25.0) | 2 (10.0) | 7 (35.0) | 11 (55.0) | 13 (65.0) | 7 (35.0) | |||
| Poorly differentiated/signet ring cell carcinoma/mucinous adenocarcinoma | 42 (48.3) | 12 (13.8) | 33 (37.9) | 17 (19.5) | 30 (34.5) | 51 (46.0) | 47 (54.0) | 40 (46.0) | |||
| Lauren classification | 0.48 | 0.81 | 0.83 | ||||||||
| Intestinal type | 10 (62.5) | 0 (0.0) | 6 (37.5) | 2 (12.5) | 6 (37.5) | 8 (50.0) | 10 (62.5) | 6 (37.5) | |||
| Diffuse type | 34 (50.0) | 9 (13.2) | 25 (36.8) | 14 (20.6) | 23 (33.8) | 31 (45.6) | 37 (54.4) | 31 (45.6) | |||
| Mixed type | 12 (46.2) | 5 (19.2) | 9 (34.6) | 3 (11.5) | 11 (42.3) | 12 (46.2) | 14 (53.8) | 12 (46.2) | |||
| Smoke | 0.53 | 0.69 | 0.98 | ||||||||
| Present or previous | 20 (52.6) | 3 (7.9) | 15 (39.5) | 5 (13.2) | 15 (39.5) | 18 (47.4) | 21 (55.3) | 17 (44.7) | |||
| Never | 36 (50.0) | 11 (15.3) | 25 (34.7) | 14 (19.4) | 25 (34.7) | 33 (45.8) | 40 (55.6) | 32 (44.4) | |||
| Drink | 0.69 | 0.51 | 0.94 | ||||||||
| Present or previous | 15 (48.4) | 3 (9.7) | 13 (41.9) | 4 (12.9) | 10 (32.3) | 17 (54.8) | 17 (54.8) | 14 (45.2) | |||
| Never | 41 (51.9) | 11 (13.9) | 27 (34.2) | 15 (19.0) | 30 (38.0) | 34 (43.0) | 44 (55.7) | 35 (44.3) | |||
| Metastasis | 0.95 | 0.86 | 0.38 | ||||||||
| Single | 26 (51.0) | 7 (13.7) | 18 (35.3) | 8 (15.7) | 18 (35.3) | 25 (49.0) | 26 (51.0) | 25 (49.0) | |||
| Multiple | 30 (50.8) | 7 (11.9) | 22 (37.3) | 11 (18.6) | 22 (37.3) | 26 (44.1) | 35 (59.3) | 24 (40.7) | |||
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
FcγR genotypes and tumor responses
The frequencies of the FcγRIIA H/H, H/R, and R/R genotypes were 45.2%, 38.1%, and 16.7%, respectively, in set A (patients receiving trastuzumab and chemotherapy) and 54.4%, 35.3%, and 10.3%, respectively, in set B (patients receiving chemotherapy only). The frequencies of the FcγRIIIA V/V, V/F, and F/F genotypes were 11.9%, 45.2%, and 42.9%, respectively, in set A and 20.6%, 47.1%, and 32.4%, respectively, in set B. The objective response rate (ORR) and the disease control rate (DCR) were 59.5% and 83.3%, respectively, in set A, but were lower in set B (32.4% and 72.1%, respectively). We did not find any significant associations between the FcγRIIA H/H, H/R, and R/R genotypes and the FcγRIIIA V/V, V/F, and F/F genotypes with tumor response in set B. However, we observed a trend of higher DCR in the FcγRIIA H/H genotype compared with the H/R or R/R genotypes (94.7% vs 73.9%, P=0.07) in set A. We further classified patients based on their combination of FcγRIIA and IIIA into two groups: the H/H or V/V group, which represented the group with higher affinity, and all the others, which represented the group with lower affinity. Still, no association between FcγRIIA and IIIA genotypes and tumor response was found in set B, but the H/H or V/V genotype was associated with a significantly higher DCR in set A (95.2% vs 71.4%, P=0.04). The detailed information is presented in Table 2.
Table 2.
FcγR polymorphisms and tumor responses in metastatic GC
| Set A (n=42)
|
Set B (n= 68)
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No (%) | Response
|
No (%) | Response
|
|||||||||||||||
| CR no (%) | PR no (%) | SD no (%) | PD no (%) | ORR no (%) | DCR no (%) | P-value for ORR | P-value for DCR | CR no (%) | PR no (%) | SD no (%) | PD no (%) | ORR no (%) | DCR no (%) | P-value for ORR | P-value for DCR | |||
| FcγRIIA | ||||||||||||||||||
| H/H | 19 (45.2) | 1 (5.3) | 11 (57.9) | 6 (31.6) | 1 (5.3) | 12 (63.2) | 18 (94.7) | 37 (54.4) | 0 (0.0) | 14 (37.8) | 13 (35.1) | 10 (27.0) | 14 (37.8) | 27 (73.0) | ||||
| H/R | 16 (38.1) | 0 (0.0) | 8 (50.0) | 2 (12.5) | 6 (37.5) | 8 (50.0) | 10 (62.5) | 24 (35.3) | 0 (0.0) | 6 (25.0) | 13 (54.2) | 5 (20.8) | 6 (25.0) | 19 (79.1) | ||||
| R/R | 7 (16.7) | 0 (0.0) | 5 (71.0) | 2 (28.6) | 0 (0.0) | 5 (71.0) | 7 (100.0) | 7 (10.3) | 0 (0.0) | 2 (28.6) | 1 (14.3) | 4 (57.1) | 2 (28.6) | 3 (42.9) | ||||
| H/R or R/R | 23 (54.8) | 0 (0.0) | 13 (56.5) | 4 (17.4) | 6 (26.1) | 13 (56.5) | 17 (73.9) | 31 (45.6) | 0 (0.0) | 8 (25.8) | 14 (45.2) | 9 (29.0) | 8 (25.8) | 22 (71.0) | ||||
| H/H vs H/R vs R/R | 0.58 | 0.02 | 0.57 | 0.18 | ||||||||||||||
| H/H vs H/R or R/R | 0.66 | 0.07 | 0.29 | 0.85 | ||||||||||||||
| FcγRIIIA | ||||||||||||||||||
| V/V | 5 (11.9) | 0 (0.0) | 4 (80.0) | 1 (20.0) | 0 (0.0) | 4 (80.0) | 5 (100.0) | 14 (20.6) | 0 (0.0) | 7 (50.0) | 1 (7.1) | 6 (42.9) | 7 (50.0) | 8 (57.0) | ||||
| V/F | 19 (45.2) | 1 (5.3) | 10 (52.6) | 3 (15.8) | 5 (26.3) | 11 (57.9) | 14 (73.7) | 32 (47.1) | 0 (0.0) | 9 (28.1) | 15 (46.9) | 8 (25.0) | 9 (28.1) | 24 (75.0) | ||||
| F/F | 18 (42.9) | 0 (0.0) | 10 (55.6) | 6 (33.3) | 2 (11.1) | 10 (55.6) | 16 (88.9) | 22 (32.4) | 0 (0.0) | 6 (27.3) | 11 (50.0) | 5 (22.7) | 6 (27.3) | 17 (77.3) | ||||
| V/F or F/F | 37 (88.1) | 0 (0.0) | 21 (56.8) | 9 (24.3) | 7 (18.9) | 21 (56.8) | 30 (81.0) | 54 (79.4) | 0 (0.0) | 15 (27.8) | 26 (48.1) | 13 (24.1) | 15 (27.8) | 41 (75.9) | ||||
| V/V vs V/F vs F/F | 0.61 | 0.27 | 0.29 | 0.38 | ||||||||||||||
| V/V vs V/F or F/F | 0.32 | 0.29 | 0.11 | 0.16 | ||||||||||||||
| FcγRIIA and IIIA | 0.35 | 0.04 | 0.28 | 0.65 | ||||||||||||||
| H/H or V/V | 21 (50.0) | 1 (61.9) | 13 (66.7) | 6 (28.6) | 1 (4.8) | 14 (66.7) | 20 (95.2) | 40 (58.8) | 0 (0.0) | 15 (37.5) | 13 (32.50) | 12 (30.0) | 15 (37.5) | 28 (70.0) | ||||
| Others | 21 (50.0) | 0 (0.0) | 11 (52.4) | 4 (19.0) | 6 (28.6) | 11 (52.4) | 15 (71.4) | 28 (41.2) | 0 (0.0) | 7 (25.0) | 14 (50.0) | 7 (25.0) | 7 (25.0) | 21 (75.0) | ||||
Notes: Set A: patients with Her-2–positive GC who received trastuzumab combined with chemotherapy as the first-line treatment. Set B: patients with Her-2–negative GC who received chemotherapy only as the first-line treatment.
Abbreviations: CR, complete response; DCR, disease control rate; FcγR, Fc γ receptor; GC, gastric cancer; ORR, objective response rate; PD, progressed disease; PR, partial response; SD, stable disease.
FcγR genotypes and progression-free survival
The median follow-up time was 13.15 (range: 3.5–73.63) and 7.30 (range: 1.3–63.1) months in set A and set B, respectively. The median progression-free survival (PFS) was estimated to be 7.23 (95% CI: 4.93–9.53) and 5.27 (95% CI: 3.66–6.87) months in set A and set B, respectively.
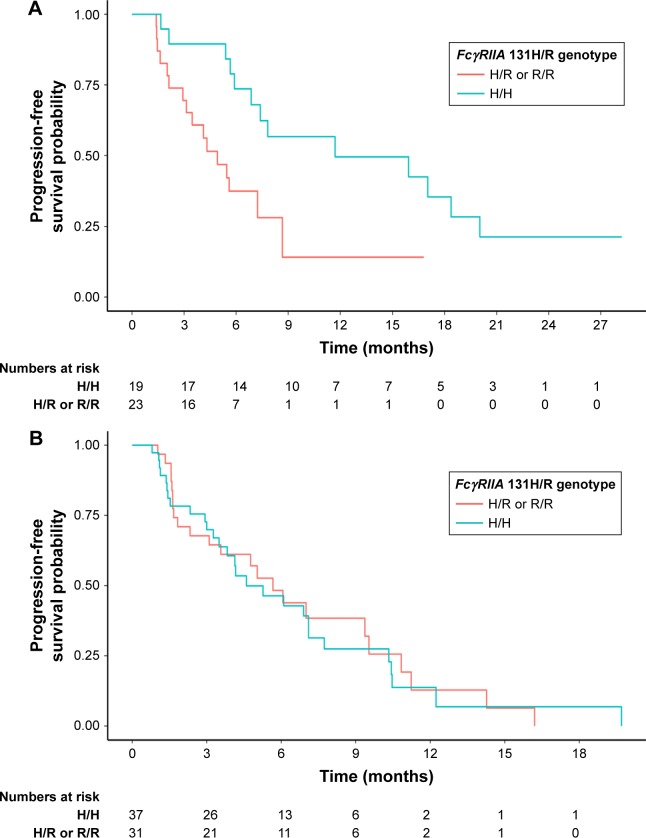
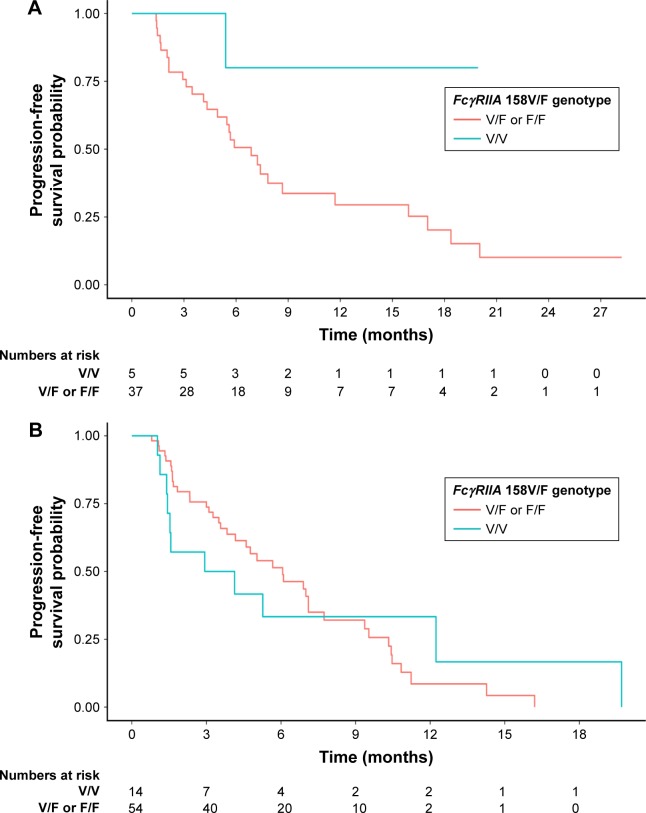
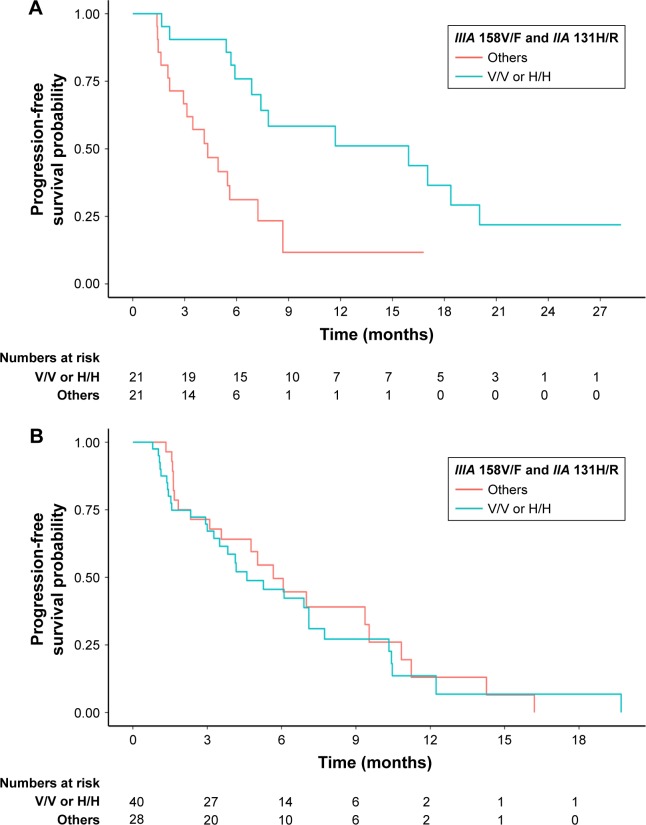
In set A, univariate analysis showed that FcγRIIA polymorphism with the H/H genotype was associated with significantly superior PFS compared with the H/R or R/R genotype (hazard ratio [HR] [95% CI]: 0.36 [0.16–0.82], P=0.02). At the same time, FcγRIIIA polymorphism had no impact on PFS (HR [95% CI]: 0.20 [0.03–1.49], P=0.12). When considering the prognostic value of the combination of FcγRIIA and IIIA polymorphisms, we found that the H/H or V/V genotype was also related to significantly better PFS compared with other genotypes (HR [95% CI]: 0.29 [0.13–0.67], P=0.004). The prognostic value of FcγRIIA and/or IIIA was further adjusted for the following factors in the multivariate analysis: age (<52/≥52 years), gender (male/female), Eastern Cooperative Oncology Group score (0/1), degree of differentiation (well-differentiated adenocarcinoma/moderately differentiated adenocarcinoma/poorly differentiated/signet ring cell carcinoma/mucinous adenocarcinoma), and Lauren classification (intestinal type/diffuse type/mixed type). FcγRIIA polymorphism and the combination of FcγRIIA and IIIA polymorphisms remained independent and significant prognostic factors for PFS in set A (adjusted HR [95% CI]: 0.18 [0.07–0.48] and 0.17 [0.07–0.45], P=0.001 and P<0.001, respectively). FcγRIIIA polymorphism also showed a tendency to be prognostic in the multivariate analysis in set A (adjusted HR [95% CI]: 0.15 [0.02–1.31], P=0.09). However, in set B, no associations between FcγRIIA and/or IIIA polymorphisms and PFS were identified in either univariate or multivariate analysis. Detailed information on the univariate and multivariate survival analyses is given in Table 3. The prognostic differences in PFS between FcγRIIA and/or IIIA polymorphisms in set A and set B were analyzed by the Kaplan–Meier method, as shown in Figure 1 (FcγRIIA), Figure 2 (FcγRIIIA), and Figure 3 (the combination of FcγRIIA and IIIA).
Table 3.
The prognostic value of FcγRIIA and FcγRIIIA polymorphisms for PFS in patients with metastatic GC: univariate and multivariate analyses
| Set A (n=42)
|
Set B (n=68)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median PFS (months) | Univariate
|
Multivariate
|
Median PFS (months) | Univariate
|
Multivariate
|
|||||
| Hazard ratio (95% CI) | P-value | Adjusted hazard ratio (95% CI) | P-value | Hazard ratio (95% CI) | P-value | Adjusted hazard ratio (95% CI) | P-value | |||
| General | 7.23 | 5.27 | ||||||||
| FcγRIIA polymorphism | 0.02 | 0.001 | 0.83 | 0.78 | ||||||
| H/R or R/R | 4.93 | 1 (reference) | 1 (reference) | 5.67 | 1 (reference) | 1 (reference) | ||||
| H/H | 11.7 | 0.36 (0.16–0.82) | 0.18 (0.07–0.48) | 4.60 | 1.06 (0.61–1.86) | 0.91 (0.50–1.69) | ||||
| FcγRIIIA polymorphism | 0.12 | 0.09 | 0.96 | 0.94 | ||||||
| F/V or F/F | 6.87 | 1 (reference) | 1 (reference) | 6.07 | 1 (reference) | 1 (reference) | ||||
| V/V | 0.20 (0.03–1.49) | 0.15 (0.02–1.31) | 2.93 | 1.02 (0.50–2.06) | 0.97 (0.45–2.09) | |||||
| FcγRIIA and IIIA | − | 0.004 | <0.001 | 0.62 | 0.94 | |||||
| Others | 4.33 | 1 (reference) | 1 (reference) | 5.67 | 1 (reference) | 1 (reference) | ||||
| H/H or V/V | 15.93 | 0.29 (0.13–0.67) | 0.17 (0.07–0.45) | 4.60 | 1.15 (0.66–2.03) | 1.03 (0.56–1.90) | ||||
Notes: Set A: patients with Her-2-positive GC who received trastuzumab combined with chemotherapy as the first-line treatment. Set B: patients with Her-2–negative GC who received chemotherapy only as the first-line treatment. Multivariate analysis for the prognostic value of FcγRIIA and FcγRIIIA polymorphisms was adjusted by age (<02/≥52 years), gender (male/female), ECOG score (0/1), degree of differentiation (well-differentiated adenocarcinoma/moderately differentiated adenocarcinoma/poorly differentiated/signet ring cell carcinoma/mucinous adenocarcinoma), and Lauren classification (intestinal type/diffuse type/mixed type).
Abbreviations: ECOG, Eastern Cooperative Oncology Group; FcγR, Fc γ receptor; GC, gastric cancer; ORR, objective response ratio; PFS, progression-free survival.
Figure 1.
Progression-free survival for patients with metastatic gastric cancer receiving chemotherapy and trastuzumab (A, P=0.02) or chemotherapy only (B, P=0.83) as the first-line treatment, according to FcγRIIA polymorphisms (H/R or R/R vs H/H).
Abbreviation: FcγR, Fc γ receptor.
Figure 2.
Progression-free survival for patients with metastatic gastric cancer receiving chemotherapy and trastuzumab (A, P=0.12) or chemotherapy only (B, P=0.96) as the first-line treatment, according to FcγRIIIA polymorphisms (F/V or F/F vs V/V).
Abbreviation: FcγR, Fc γ receptor.
Figure 3.
Progression-free survival for patients with metastatic gastric cancer receiving chemotherapy and trastuzumab (A, P=0.004) or chemotherapy only (B, P=0.62) as the first-line treatment, according to FcγRIIA and IIIA polymorphisms (others vs H/H or V/V).
Abbreviation: FcγR, Fc γ receptor.
Discussion
To our knowledge, this is the first study to assess the impact of FcγRIIA and/or IIIA polymorphisms on tumor response and PFS in metastatic GC patients receiving chemotherapy and trastuzumab or chemotherapy alone. We found that the FcγRIIA H/H genotype was associated with a trend of higher DCR and significantly superior PFS, compared with the H/R or R/R genotype. In addition, when combining FcγRIIA and IIIA polymorphisms together, the H/H or V/V genotype was related to significantly higher DCR and better PFS compared with other genotypes.
Her-2 overexpression is identified to be a negative prognostic factor, and anti-Her-2–targeted therapy with trastuzumab could remedy the survival gap between Her-2-positive GC and Her-2-negative GC, as suggested by a cohort study.10 In ToGA trial, the addition of trastuzumab to cisplatin-based chemotherapy significantly prolonged PFS from 5.5 to 6.7 months and improved ORR from 35% to 47% in Her-2-positive GC.7 However, primary resistance to trastuzumab was also observed in a small portion of patients. The identification of predictive markers for screening of patients for the efficacy of trastuzumab is important to avoid unnecessary costs.
ADCC has been suggested as another antitumor mechanism in addition to blocking the signal transduction pathway.24,25 FcγRIIA and IIIA polymorphisms have a substantial impact on the binding affinity in ADCC.15,16 Consistent with some previous findings, we identified that the FcγRIIA H/H or FcγRIIIA V/V genotype benefits more from trastuzumab than other genotypes. Although it had no impact on ORR, the H/H or V/V genotype was associated with significantly improved DCR and PFS. This impact did not exist in patients receiving chemotherapy-only treatment. This finding is consistent with the mechanism of ADCC. Lymphocyte infiltration of tumors might not lead to obvious shrinkage of tumor size, and sometimes even results in enlargement of the tumor size, as observed in immune checkpoint blockage therapy. However, once this treatment is effective, patients might gain a durable benefit for a long time.26
In this study, we suggested that the FcγRIIA H/H genotype, but not the FcγRIIIA V/V genotype, predicts a benefit in PFS from trastuzumab treatment in Her-2-positive metastatic GC. This is in accordance with the findings on metastatic breast cancer. In addition, we demonstrated that the combination of FcγRIIA and IIIA polymorphisms in the FcγRIIA H/H or FcγRIIIA V/V genotype definitely results in benefits from trastuzumab treatment, with a significant improvement in both DCR and ORR. Thus, the FcγRIIIA polymorphism might also have a predictive role in trastuzumab treatment in metastatic GC. Actually, as shown in Table 2, FcγR IIIA V/V seemed to demonstrate higher ORR and DCR, but a statistically significant difference was not obtained. However, in the combined analysis, positive results were demonstrated when the sample size was enlarged. Thus, our study is limited by a small sample size, and verification by further studies is warranted.
The major limitation of our study was that it was a retrospective, single-center study. Trastuzumab was approved for Her-2-positive metastatic GC in late 2012, and the frequency of Her-2 overexpression was relatively low (~20%) in GC. In addition, trastuzumab was not covered by health insurance in most areas in China. Thus, the sample size was relatively small. Our conclusions need to be verified by larger studies. Nevertheless, to our knowledge, this study demonstrated the predictive value of FcγRIIA and/or IIIA polymorphisms for benefits from trastuzumab treatment among patients with metastatic GC for the first time.
Supplementary materials
The CONSORT diagram.
Table S1.
Patient characteristics by treatment settings
| Characteristics | Set A, no (%) | Set B, no (%) |
|---|---|---|
| Her-2 status | ||
| Positive | 42 (100.0) | 0 |
| Negative | 0 | 68 (100.0) |
| Age (years) | ||
| Median (range) | 56 (25–72) | 51 (22–73) |
| Gender | ||
| Male | 32 (76.2) | 34 (50.0) |
| Female | 10 (23.8) | 34 (50.0) |
| Gastrectomy | ||
| Yes | 33 (78.6) | 50 (73.5) |
| No | 9 (21.4) | 18 (26.5) |
| Location of tumor | ||
| Proximal | 12 (28.6) | 11 (16.2) |
| Middle | 15 (35.7) | 17 (25.0) |
| Distal | 14 (33.3) | 35 (51.5) |
| Others | 1 (2.4) | 5 (7.4) |
| Degree of differentiation | ||
| Well-differentiated adenocarcinoma | 2 (4.8) | 1 (1.5) |
| Moderate differentiated adenocarcinoma | 13 (31.0) | 7 (10.3) |
| Poorly differentiated/signet ring cell carcinoma/mucinous adenocarcinoma | 27 (64.3) | 60 (88.2) |
| Lauren classification | ||
| Intestinal type | 14 (33.3) | 2 (2.9) |
| Diffuse type | 23 (54.8) | 45 (66.2) |
| Mixed type | 5 (11.9) | 21 (30.9) |
Notes: Setting A: patients with Her-2-positive GC who received trastuzumab combined with chemotherapy as the first-line treatment. Setting B: patients with Her-2-negative GC who received chemotherapy only as the first-line treatment.
Abbreviation: GC, gastric cancer.
Acknowledgments
We gratefully thank Dr Wei-xiong Xia and Hu Liang in Sun Yat-sen University Cancer Center for their statistical suggestions and assistance. Shanghai Roche Pharmaceuticals Limited provided scientific advice, especially Ying-meng Zhang and Zhi-heng Peng. We gratefully thank Huai-qiang Ju, Dong-liang Chen, Ren Chao, Hai-yu Mo, Ying Jin, Feng Wang, Zhi-wei Zhou, Zhao-lei Zeng, and Dong-sheng Zhang in Sun Yat-sen University Cancer Center for their help in this project. We acknowledge the invaluable contributions of the patients who participated in this study, their families, and the Sun Yat-sen University Cancer Center GI Oncology group. This work is supported by the following funders for Rui-hua Xu, Yu-hong Li, and De-shen Wang:
National High Technology Research and Development Program of China (863 Program), China (No 2015AA020103)
National Natural Science Foundation of China (No 81602070)
Major Special Project from Guangzhou Health and Medical Collaborative Innovation (No 15570006)
There is no financial support for other authors in this article. This study has been presented in part: De-shen Wang, Rui-hua Xu: FcγR IIA and IIIA polymorphisms predict clinical outcome of trastuzumab treated metastatic gastric cancer. Poster presentation at the European Society for Medical Oncology (ESMO) 2016 Congress, October 7–11, Copenhagen, Denmark.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Chen YS, Chen JG, Zhu J, Zhang YH, Ding LL. Long-term survival trends of gastric cancer patients between 1972 and 2011 in Qidong. Chin J Cancer. 2015;34(12):602–607. doi: 10.1186/s40880-015-0058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu MZ, Xu RH. The progress of targeted therapy in advanced gastric cancer. Biomark Res. 2013;1(1):32. doi: 10.1186/2050-7771-1-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs CS, Tomasek J, Yong CJ, et al. REGARD Trial Investigators Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383(9911):31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 6.Wilke H, Muro K, Van Cutsem E, et al. RAINBOW Study Group Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15(11):1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 7.Bang YJ, Van Cutsem E, Feyereislova A, et al. ToGA Trial Investigators Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 8.Goren D, Horowitz AT, Zalipsky S, Woodle MC, Yarden Y, Gabizon A. Targeting of stealth liposomes to erbB-2 (Her/2) receptor: in vitro and in vivo studies. Br J Cancer. 1996;74(11):1749–1756. doi: 10.1038/bjc.1996.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yamashita-Kashima Y, Iijima S, Yorozu K, et al. Pertuzumab in combination with trastuzumab shows significantly enhanced antitumor activity in HER2-positive human gastric cancer xenograft models. Clin Cancer Res. 2011;17(5):5060–5070. doi: 10.1158/1078-0432.CCR-10-2927. [DOI] [PubMed] [Google Scholar]
- 10.Qiu MZ, Li Q, Wang ZQ, et al. HER2-positive patients receiving trastuzumab treatment have a comparable prognosis with HER2-negative advanced gastric cancer patients: a prospective cohort observation. Int J Cancer. 2014;134(10):2468–2477. doi: 10.1002/ijc.28559. [DOI] [PubMed] [Google Scholar]
- 11.Satoh T, Xu RH, Chung HC, et al. Lapatinib plus paclitaxel versus paclitaxel alone in the second-line treatment of HER2-amplified advanced gastric cancer in Asian populations: TyTAN a randomized, phase III study. J Clin Oncol. 2014;32(19):2039–2049. doi: 10.1200/JCO.2013.53.6136. [DOI] [PubMed] [Google Scholar]
- 12.Voskens CJ, Watanabe R, Rollins S, Campana D, Hasumi K, Mann DL. Ex-vivo expanded human NK cells express activating receptors that mediate cytotoxicity of allogeneic and autologous cancer cell lines by direct recognition and antibody directed cellular cytotoxicity. J Exp Clin Cancer Res. 2010;29:134. doi: 10.1186/1756-9966-29-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 14.Warmerdam PA, van de Winkel JG, Gosselin EJ, Capel PJ. Molecular basis for a polymorphism of human Fc gamma receptor II (CD32) J Exp Med. 1990;172(1):19–25. doi: 10.1084/jem.172.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stavenhagen JB, Gorlatov S, Tuaillon N, et al. Fc optimization of therapeutic antibodies enhances their ability to kill tumor cells in vitro and controls tumor expansion in vivo via low-affinity activating Fcgamma receptors. Cancer Res. 2007;67(18):8882–8890. doi: 10.1158/0008-5472.CAN-07-0696. [DOI] [PubMed] [Google Scholar]
- 16.Koene HR, Kleijer M, Algra J, Roos D, von Dem BA, de Haas M. Fc gammaRIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gammaRIIIa, independently of the Fc gammaRIIIa-48L/R/H phenotype. Blood. 1997;90(3):1109–1114. [PubMed] [Google Scholar]
- 17.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Gordon M, Schultheis AM, et al. FCGR2A and FCGR3A polymorphisms associated with clinical outcome of epidermal growth factor receptor expressing metastatic colorectal cancer patients treated with single-agent cetuximab. J Clin Oncol. 2007;25(24):3712–3718. doi: 10.1200/JCO.2006.08.8021. [DOI] [PubMed] [Google Scholar]
- 19.Bibeau F, Lopez-Crapez E, Di Fiore F, et al. Impact of Fc{gamma}RIIa-Fc{gamma}RIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27(7):1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 20.Tamura K, Shimizu C, Hojo T, et al. FcgammaR2A and 3A polymorphisms predict clinical outcome of trastuzumab in both neoadjuvant and metastatic settings in patients with HER2-positive breast cancer. Ann Oncol. 2011;22(6):1302–1307. doi: 10.1093/annonc/mdq585. [DOI] [PubMed] [Google Scholar]
- 21.Hurvitz SA, Betting DJ, Stern HM, et al. Analysis of Fcgamma receptor IIIa and IIa polymorphisms: lack of correlation with outcome in trastuzumab-treated breast cancer patients. Clin Cancer Res. 2012;18(12):3478–3486. doi: 10.1158/1078-0432.CCR-11-2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 23.Norton N, Olson RM, Pegram M, et al. Association studies of Fcgamma receptor polymorphisms with outcome in HER2+ breast cancer patients treated with trastuzumab in NCCTG (Alliance) Trial N9831. Cancer Immunol Res. 2014;2(10):962–969. doi: 10.1158/2326-6066.CIR-14-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boero S, Morabito A, Banelli B, et al. Analysis of in vitro ADCC and clinical response to trastuzumab: possible relevance of FcgammaRIIIA/FcgammaRIIA gene polymorphisms and HER-2 expression levels on breast cancer cell lines. J Transl Med. 2015;13:324. doi: 10.1186/s12967-015-0680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petricevic B, Laengle J, Singer J, et al. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. 2013;11:307. doi: 10.1186/1479-5876-11-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber JS, D’Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The CONSORT diagram.
Table S1.
Patient characteristics by treatment settings
| Characteristics | Set A, no (%) | Set B, no (%) |
|---|---|---|
| Her-2 status | ||
| Positive | 42 (100.0) | 0 |
| Negative | 0 | 68 (100.0) |
| Age (years) | ||
| Median (range) | 56 (25–72) | 51 (22–73) |
| Gender | ||
| Male | 32 (76.2) | 34 (50.0) |
| Female | 10 (23.8) | 34 (50.0) |
| Gastrectomy | ||
| Yes | 33 (78.6) | 50 (73.5) |
| No | 9 (21.4) | 18 (26.5) |
| Location of tumor | ||
| Proximal | 12 (28.6) | 11 (16.2) |
| Middle | 15 (35.7) | 17 (25.0) |
| Distal | 14 (33.3) | 35 (51.5) |
| Others | 1 (2.4) | 5 (7.4) |
| Degree of differentiation | ||
| Well-differentiated adenocarcinoma | 2 (4.8) | 1 (1.5) |
| Moderate differentiated adenocarcinoma | 13 (31.0) | 7 (10.3) |
| Poorly differentiated/signet ring cell carcinoma/mucinous adenocarcinoma | 27 (64.3) | 60 (88.2) |
| Lauren classification | ||
| Intestinal type | 14 (33.3) | 2 (2.9) |
| Diffuse type | 23 (54.8) | 45 (66.2) |
| Mixed type | 5 (11.9) | 21 (30.9) |
Notes: Setting A: patients with Her-2-positive GC who received trastuzumab combined with chemotherapy as the first-line treatment. Setting B: patients with Her-2-negative GC who received chemotherapy only as the first-line treatment.
Abbreviation: GC, gastric cancer.