Abstract
Introduction
We present a prospective randomized study to compare the efficacy of tamsulosin and silodosin in patients suffering from acute urinary retention caused by benign prostatic hyperplasia, planned for trial without catheter.
Material and methods
Patients with acute urinary retention secondary to benign prostatic hyperplasia (total 160) were catheterized and randomized into two groups: Group A: tamsulosin 0.4 mg (80 patients) and Group B: silodosin 8 mg (80 patients). After three days, the catheter was removed, and patients were put on trial without catheter. Patients with a successful trial without catheter were followed up after two weeks and one month, taking into account the international prostate symptom score (IPSS), post void residual volume (PVR), and peak flow rate (PFR). Statistical analysis of the data was performed.
Results
Both group A (tamsulosin) and group B (silodosin) had similar results of trial without catheter (group A: 67.50%, group: B 60%). In follow up, three patients in group A and four patients in group B had retention of urine, requiring recatheterization. These patients were withdrawn from the study. No significant differences were present between group A and group B patients in regard with IPSS, PVR and PFR measured at the time of successful trial without catheter and during follow up at two weeks and one month.
Conclusions
Efficacy for trial without catheter of tamsulosin was slightly higher than silodosin, but comparable. No statistical difference between tamsulosin & silodosin treated groups were found in regard with IPSS, PVR and PFR.
Keywords: acute urinary retention, benign prostatic hyperplasia, trial without catheter, tamsulosin, silodosin
INTRODUCTION
In aging males, lower urinary tract symptoms (LUTS) are a common problem and the most common cause of LUTS in elderly men over 70 years of age is benign prostatic hyperplasia (BPH). BPH usually starts in men in their 50s and by the age of 60 years, 50% of men have histological evidence of BPH and 28% of men in their 70s suffer from moderate to severe BPH-related LUTS [1].
Acute urinary retention secondary to BPH is a urological emergency which requires urgent catheterization and was followed in the past by prostatic surgery [2]. Any intervention which aims at increasing the rate of a successful trial without a catheter following an acute urinary retention episode would be considered potentially helpful for such patients. Trial Without Catheter (TWOC) is defined as, when a catheter which has been inserted into the bladder via urethra for drainage purposes, is removed for a trial period to determine whether the patient is able to pass urine safely and spontaneously without the need for further catheterization. TWOC has become a standard practice around the world for male patients with BPH and Acute Urinary Retention (AUR). An alpha-blocker is prescribed in most of the cases before starting TWOC which significantly increases the rate of success, provided that the AUR is caused by increased sympathetic activity at the level of the prostatic smooth muscles [3]. Alpha-blockers effectively reduce the symptoms and improve the urodynamic parameters of obstruction associated with BPH [4]. The recommended duration and cost effectiveness of alpha blocker treatment after successful TWOC remains unknown.
Safety of silodosin has been extensively studied regarding old age, but its use in TWOC is sparsely investigated. Our objective in this study was to compare the efficacy of silodosin and tamsulosin in patients suffering from AUR caused by BPH planned for TWOC through a randomized controlled trial (RCT)
MATERIAL AND METHODS
The study was conducted as a parallel group, randomized, controlled trial at our department, during the period July 2014 to December 2016. Institutional Ethical clearance was obtained and the approval number is IEC REF NO., 83/2014-15. Written informed consent was taken from each eligible patient before enrollment. All patients aged >50 years presenting with their first episode of AUR secondary to BPH with a retention volume <1000 ml were recruited in this study. Patient demographics & clinical details were recorded. Clinical details included duration of symptoms, medical history, retention volume, digital rectal examination (DRE) findings, serum creatinine, serum Prostate Specific Antigen, and prostate size on abdomen and pelvis ultrasound. Exclusion criteria includes patients with urinary tract infection, recurrent urinary retention, previous failed trial voiding, more than 1 liter of retention volume, history of prostatic or bladder neck surgery, proven case of prostate carcinoma, urethral stricture, history of neurogenic bladder, renal failure, and liver disease. Total number of patients enrolled in the trial were 160 and randomized in a 1:1 ratio to one of the two study groups, using a computer generated random number list. They took either tamsulosin 0.4 mg controlled-release capsule (group A) or silodosin 8 mg capsule (group B) once daily after dinner for three days and then a TWOC was performed on all patients. All capsules were removed from their commercial blister strip packaging and repackaged in air-tight, screw cap containers and suitably labeled and coded as trial medication.
Patients were categorized under successful TWOC when there was less than 100 ml post void residual (PVR) volume after voiding at least 100 ml of urine. Patients were assessed with uroflowmetry (UFR), PVR volume & IPSS (International Prostate Symptom Score). TWOC was considered to be failed when patient re-experienced painful acute urinary retention or patients had obstructive LUTS with >100 ml PVR & were re-catheterized and planned for trans urethral resection of prostate (TURP). All patients with successful TWOC were followed up at 2 weeks and one month and were assessed with UFR, IPSS & PVR volume during their follow up.
Power analysis
With Anticipated Mean Difference of success of TWOC between the two study groups as 11% and Anticipated SD as 0.19, the minimum sample size per group is 77 (≈80) with 90% power and 5% level of significance.
Formula used here
where
Z = Z statistic at a level of significance
MD = Anticipated mean difference
SD = Anticipated Standard deviation
Statistical analysis
All characteristics were summarized descriptively. For continuous variables, the summary statistics of N, mean, standard deviation (SD) were used. For categorical data, the numbers and percentages were used in the data summaries. The difference of the means of analysis variables was tested by an unpaired t test. The difference of the proportion was tested by z test of proportion. If the p-value was <0.05, then the results will be considered to be significant. The data was analyzed using IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY: IBM Corp.
RESULTS
Out of 160 patients, 102 (63.75%) patients had a successful TWOC, irrespective of their group and drug given.
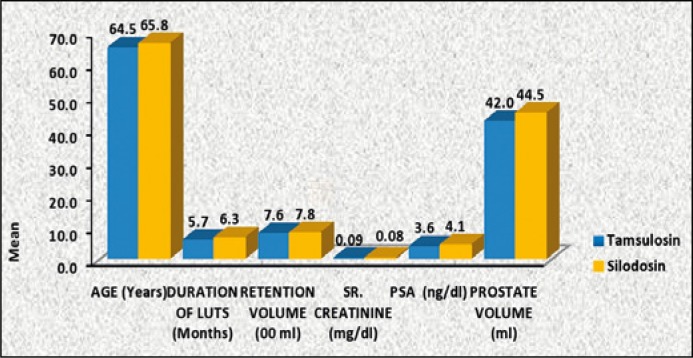
Both the tamsulosin and silodosin group were comparable in regards with age, duration of LUTS (months), retention volume (ml), serum creatinine (mg/dl), PSA (ng/dl), prostate volume (ml) and were statistically insignificant (Table 1, Figure 1).
Table 1.
Comparison of mean value of selected parameters between two study groups
| Parameters (mean+SD) |
Tamsulosin (Group A) |
Silodosin (Group B) |
P value | 95% CI |
|---|---|---|---|---|
| Age (years) | 64.5 + 9.3 | 65.8 + 8.1 | 0.347 | (-4.02, 1.42) |
| Duration of luts (months) | 5.7 + 3.4 | 6.3 + 3.4 | 0.266 | (-1.66, 0.46) |
| Retention volume (ml) | 758 + 154 | 782 + 163 | 0.400 | (-73.52, 25.52) |
| Sr. creatinine (mg/dl) | 0.09 + 0.34 | 0.08 + 0.4 | 0.865 | (-0.11, 0.13) |
| PSA (ng/dl) | 3.6 + 1.4 | 4.1 + 2.8 | 0.156 | (-0.19, 1.19) |
| Prostate volume (ml) | 42.3 + 7.9 | 44.5 + 9.6 | 0.116 | (-4.95, 0.55) |
Figure 1.
Comparison of mean value of selected parameters between two study groups.
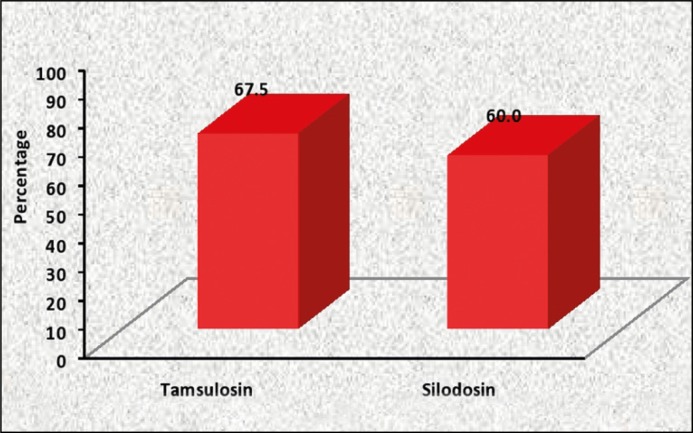
Success rate of TWOC with tamsulosin (Group A) was 67.50.% (58/80), slightly higher but comparable to silodosin (Group B) which was 60% (48/80) (Table 2, Figure 2).
Table 2.
Comparison of Success proportion of TWOC between two study groups
| Study Groups | Total no of patient | Successful TWOC | Failed TWOC | Percentage of success | p value | (95%CI) |
|---|---|---|---|---|---|---|
| Tamsulosin (Group A) | 80 | 54 | 26 | 67.5% | 0.324 | (-0.07, 0.22) |
| Silodosin (Group B) | 80 | 48 | 32 | 60.0% |
Figure 2.
Comparison of Success proportion of TWOC between two study groups.
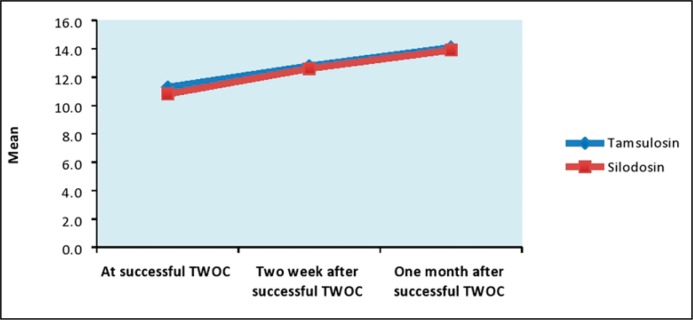
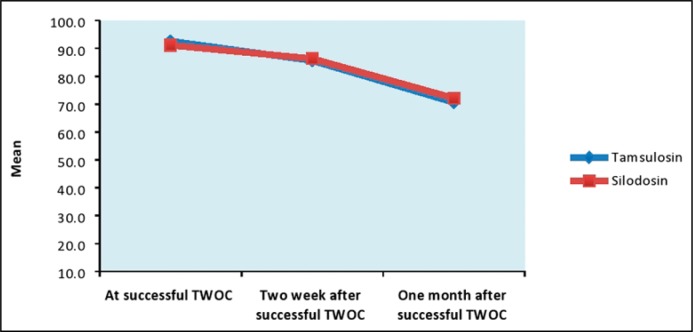
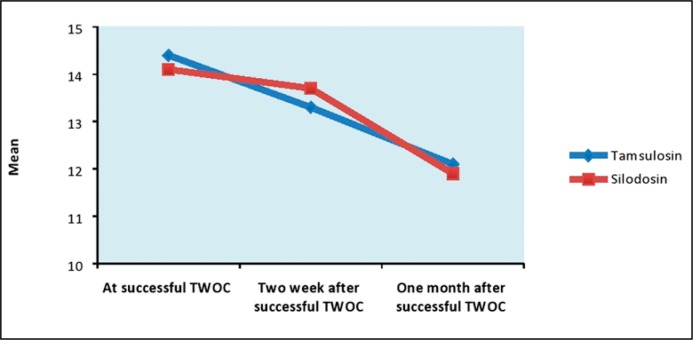
No statistically significant differences between silodosin and tamsulosin treated groups were found for IPSS score, PVR volume and UFR measured at the time of successful TWOC and during follow up after 2 weeks and 1 month of successful TWOC (Table 3, Figures 3, 4 & 5).
Table 3.
Comparison of Qmax, PVR & IPSS Score
| Parameters (mean+SD) | Follow-up points | Tamsulosin | Silodosin | p value | 95% CI |
|---|---|---|---|---|---|
| (Group A) | (Group B) | ||||
| Qmax (ml/sec) | At successful TWOC | 11.3 + 1.9 | 10.8 + 1.7 | 0.081 | (-0.06, 1.06) |
| Two week after successful TWOC | 12.8 + 1.2 | 12.6 + 1.3 | 0.313 | (-0.19, 0.59) | |
| One month after successful TWOC | 14.1 + 1.7 | 13.9 + 1.6 | 0.445 | (-0.32, 0.72) | |
| PVR urine volume (ml) | At successful TWOC | 92.6 + 16.3 | 91.1 + 17.2 | 0.572 | (-3.73, 6.73) |
| Two week after successful TWOC | 85.6 + 17.3 | 86.3 + 16.2 | 0.792 | (-5.93, 4.53) | |
| One month after successful TWOC | 70.6 + 18.3 | 72.1 + 17.2 | 0.594 | (-7.05, 4.05) | |
| IPSS | At successful TWOC | 14.4 + 2.8 | 14.1 + 2.1 | 0.445 | (-0.47, 1.07) |
| Two week after successful TWOC | 13.3 + 2.1 | 13.7 + 1.7 | 0.187 | (-0.99, 0.20) | |
| One month after successful TWOC | 12.1 + 2.8 | 11.9 + 2.1 | 0.610 | (-0.57, 0.97) |
Figure 3.
Comparison of Qmax (ml/sec).
Figure 4.
Comparison of PVR urine volume (ml).
Figure 5.
Comparison of IPSS score.
In subsequent follow ups after one month, three patients in group A, and four patients in group B had retention of urine, requiring re-catheterization. They were withdrawn from the study and subsequently taken up for definitive treatment i.e. TURP.
Safety
Both silodosin and tamsulosin were well tolerated with minimal side effects. Dizziness and vertigo were present in 7 patients of tamsulosin group and 2 patients in silodosin group.
Complaints of headaches were present in 4 patients from the tamsulosin group and none from the silodosin group. Retrograde ejaculation was reported in two patients treated with tamsulosin. No side effects during the trial required cessation of medication.
DISCUSSION
BPH is a progressive disease which is characterized by deterioration of symptoms over time and leads to serious outcomes such as acute retention of urine which in turn requires BPH related surgery in some patients [5]. AUR is defined as a sudden and painful inability to pass urine and it is one of the most common urological emergencies. The incidence of this complication varies widely from 0.4 to 25% in men with benign prostatic hyperplasia [6].
Bladder decompression by a urethral catheter is the immediate treatment provided to the patient followed almost exclusively by prostatic surgery after a first episode of AUR.
The greater morbidity and mortality associated with prostatic surgery and morbidity associated with prolonged catheterization, has led to the increased use of a trial without catheter. The urethral catheter is removed after one to three days which allows the patient to void in 23–40% of cases. In patients who fail the TWOC, surgery can be planned at a later stage [7].
The sympathetic nervous system plays an important role in controlling the myogenic tone of the bladder outlet, hence its activity is partly responsible for urinary outflow resistance.
The alpha (1)-adrenoreceptor antagonists i.e. doxazosin, terazosin, alfuzosin or tamsulosin, are able to decrease bladder outflow resistance resulting in significant relief of LUTS (20–65%) and improvement of voiding flow (1–4.3 ml/s) in men with symptomatic BPH [8].
Many randomized, controlled trials have provided evidence supporting the efficacy as well as tolerability of alpha (1)-adrenoreceptor antagonists in BPH, and for this cause of LUTS, they are the most frequently used as the initial treatment option [9].
Newer alpha-blocking agents such as silodosin and tamsulosin tend to demonstrate improved selectivity for the prostate and bladder. Tamsulosin (in 0.4 mg once daily dose) is better tolerated than terazosin and doxazosin due to the low risk of postural hypotension, obviating the need for dose titration and having better gastro-intestinal tolerance [10].
There have been several prospective randomized trials on the role of either silodosin or tamsulosin in comparison to placebo for TWOC in patients of acute urinary retention due to BPH but the aim of the present study was to evaluate and compare the efficacy of silodosin with tamsulosin in these patients. The success rate of TWOC of the tamsulosin group in our study was 67.5%, which is higher than previous observations by Lucas et al. and Hua et al., who had reported success rates of 48% and 61% respectively when compared with placebo [11, 12].
Success rate of TWOC of silodosin group in our study was 60%, which is lower than previous trails by Kumar et al. who had reported success rates of 76.7% in the silodosin group when compared to placebo [13].
Ginka et al. observed slightly higher success rate for TWOC with Silodosin when compared to tamsulosin i.e. 72.33% and 66.7% respectively [14]. In contrary to this, we have observed in our study that the efficacy and success of TWOC in the tamsulosin group is slightly higher than the silodosin group but they are comparable i.e. 67.50% and 60% respectively (Table 4). Both silodosin and tamsulosin were well tolerated. To the best of our knowledge, the present study is one of only a few prospective randomized trials, with head-to-head comparison between these two drugs for TWOC in AUR. Most studies available have promoted silodosin as the drug of choice for BPH with AUR. Contrary to this popular perception that silodosin has an advantage, it seems that both drugs have almost similar efficacy and profile, while tamsulosin is cost effective and cheaper as compared to silodosin.
Table 4.
Various studies performed to look for efficacy of tamsulosin and silodosin for TWOC
| Study | Drugs | Results and efficacy |
|---|---|---|
| Hua LX, et al. | Tamsulosin vs. placebo | Tamsulosin = 61% Placebo = 28% |
| Kumar, et al. | Silodosin vs. placebo | Silodosin = 76.7% Placebo = 36.7% |
| Ginka, et al. | Tamsulosin vs. silodosin | Tamsulosin = 66.7% Silodosin = 72.33% |
| Our study | Tamsulosin vs. silodosin | Tamsulosin = 67.50% Silodosin = 60% |
CONCLUSIONS
In our study, the efficacy for TWOC of tamsulosin was slightly higher than silodosin, but comparable. No statistical difference between tamsulosin& silodosin treated groups were found in regard with IPSS score, PVR volume and peak flow rate.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: A population-based survey of urinary symptoms. J Urol. 1993;150:85–89. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 2.Emberton M, Fitzpatrick JM. The Reten-World survey of the management of acute urinary retention: preliminary results. BJU Int. 2008;101(Suppl 3):27–32. doi: 10.1111/j.1464-410X.2008.07491.x. [DOI] [PubMed] [Google Scholar]
- 3.Altarac S. Alpha-adrenergic blockers as a support in the treatment of acute urinary retention. Lijec Vjesn. 2006;128:233–237. [PubMed] [Google Scholar]
- 4.McNeill SA. The role of alpha-blockers in the management of acute urinary retention caused by benign prostatic obstruction. Eur Urol. 2004;45:325–332. doi: 10.1016/j.eururo.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Emberton M. Definition of at-risk patients: dynamic variables. BJU Int. 2006;97(Suppl 2):12–15. doi: 10.1111/j.1464-410X.2006.06099.x. [DOI] [PubMed] [Google Scholar]
- 6.Hartung R. Do alpha-blockers prevent the occurrence of acute urinary retention? EurUrol. 2001;39(Suppl 6):13–18. doi: 10.1159/000052594. [DOI] [PubMed] [Google Scholar]
- 7.Fitzpatrick JM, Kirby RS. Management of acute urinary retention. BJU Int. 2006;97(Suppl 2):16–20. doi: 10.1111/j.1464-410X.2006.06100.x. [DOI] [PubMed] [Google Scholar]
- 8.Oelke M, Höfner K, Berges RR, Jonas U. Drug therapy of benign prostatichyperplasia syndrome with alpha 1-receptor blockers. Basic principles and clinical results. Urologe A. 2002;41:425–441. doi: 10.1007/s00120-002-0236-9. [DOI] [PubMed] [Google Scholar]
- 9.Nickel JC. The use of alpha1-adrenoceptor antagonists in lower urinary tract symptoms: beyond benign prostatic hyperplasia. Urology. 2003;62(Suppl 1):34–41. doi: 10.1016/s0090-4295(03)00472-2. [DOI] [PubMed] [Google Scholar]
- 10.Milani S, Djavan B. Lower urinary tract symptoms suggestive of benignprostatic hyperplasia: latest update on alpha-adrenoceptor antagonists. BJU Int. 2005;95(Suppl 4):29–36. doi: 10.1111/j.1464-410X.2005.05485.x. [DOI] [PubMed] [Google Scholar]
- 11.Lucas MG, Stephenson TP, Nargund V. Tamsulosin in the management ofpatients in acute urinary retention from benign prostatic hyperplasia. BJU Int. 2005;95:354–357. doi: 10.1111/j.1464-410X.2005.05299.x. [DOI] [PubMed] [Google Scholar]
- 12.Hua LX, Wu HF, Sui YG, et al. Tamsulosinin the treatment of benign prostatic hyperplasia patients with acuteurinary retention. Zhonghua Nan KeXue. 2003;9:510–511. [PubMed] [Google Scholar]
- 13.Kumar S, Tiwari DP, Ganesamoni R, et al. Prospective randomized placebo-controlled study to assess the safety and efficacy of silodosin in the management of acute urinary retention. Urology. 2013;82:171–175. doi: 10.1016/j.urology.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 14.Ginka B. A prospective study of silodosin in comparision with tamsulosin in the management of patients in acute urinary retention due to benign hyperplasia of prostate. J Urol. 2015;(193 Suppl):e330–331. [Google Scholar]