Abstract
The liver fluke Opisthorchis viverrini (Ov) is endemic in Southeast Asia where more than 10 million people are estimated to be infected. The infection is associated with several hepatobiliary diseases including cholangiocarcinoma (CCA). Northeast Thailand is a hotspot of Ov transmission and despite extensive public health prevention campaigns led by the government, Ov infection prevalence is still high. High infection rates result from cultural and ecological complexities where wet-rice agrarian habitats, centuries old raw food culture and the parasite complex biology combine to create an ideal transmission arena. Here we review the state of our knowledge regarding the social-ecological determinants underlying Ov transmission. We also describe an integrative research rationale for liver fluke control better aligned with sustainable health development.
Keywords: Opisthorchis viverrini, Landscape Epidemiology, Disease ecology, Integrated control, Transdisciplinarity, Global health
Embracing complexity for liver fluke sustainable control
Opisthorchis viverrini (Ov), the Southeast Asian liver fluke, is a fish-borne trematode (Fig. 1) endemic in Thailand, Lao’s People Democratic Republic, Cambodia and southern parts of Vietnam where an under-estimate of 10 million people are infected. The infection is associated with several hepatobiliary diseases including cholangiocarcinoma (CCA), a fatal bile duct cancer. Northeast Thailand, a hotspot of Ov transmission, has been reported as having the world highest incidence (see Glossary) of CCA. Despite nationwide public health prevention campaigns led by the government and private organizations, Ov infection prevalence is still presently high [1].
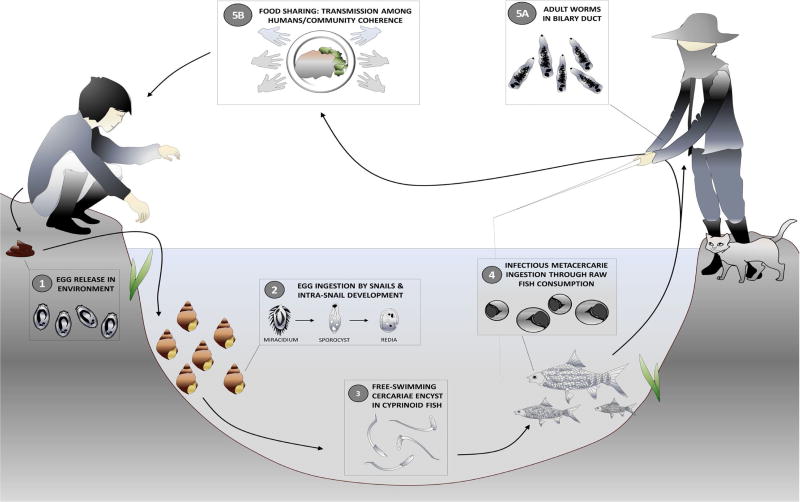
Figure 1. Opisthorchis viverrini transmission cycle.
Human infection occurs when cyprinid fish bearing metacercariae in their tissues and organs are consumed raw or partially cooked (including smoked, pickled and salted). Metacercariae in the fish excyst in the duodenum and enter the bile ducts, where they mature sexually. The adult worms produce eggs which are passed out in faeces to the environment. When freshwater Bithynia snails ingest the eggs, miracidium hatch and develop into sporocysts which undergo asexual multiplication, and develop into rediae and cercariae. Upon release in the environment, free-swimming cercariae actively search for a fish host, most often from the cyprinidae family, to penetrate the tissues and skin and develop into metacercariae infective to humans and other fish-eating mammals.
The persistence of high Ov infection rates in the region is due to (1) cultural behaviors associated with fishing, food preparation, and eating uncooked fish, practices that are deeply embedded as part of the indigenous rice-fish culture of this region; (2) wetland ecosystem-dependent livelihood and an ancient co-evolutionary relationship of Ov, natural hosts and humans; (3) a parasite highly efficient in its transmission within this coupled human-natural system; (4) a complex set of pathological consequences associated with infection and treatment; (5) historically unprecedented environmental change, including those caused by regional and local water resources management and flood control projects that lead to the disruption of natural ecological regulatory mechanisms with consequences on hosts abundance and patterns of exposure; and, (6) possibly the most important, lack of knowledge integration at the policy level, discontinuity in government support including reduction in geographic coverage and lack of community-based control activities.
Despite the long acknowledged complexity underlying Ov transmission dynamics and the myriad of disease drivers, understanding of which requires expertise from outside medicine and public health [2], most control interventions, until recently, have been designed based on a reductionist biomedical frame of understanding. Although valuable in many regards and responsible of great achievements, this frame of understanding is inherently limited in its capability to identify and evaluate the synergies and cross-scale influences among disease drivers which are responsible for disease incidence and severity variation across spatial and temporal scales [3], making sustainable control difficult.
For instance, most interventions targeting liver fluke until now have only partially met success in their capacity to reduce liver fluke prevalence and to contribute to health development, as they neglected to account for the broader development context and its inherent epidemiological and livelihood transitions [4]. These transitions and their associated changes in ecological character, communities social capital, resilience and vulnerability in turn influence transmission and condition susceptibility to disease [5].
What has become the greatest challenge in liver fluke infection and related disease prevention, is therefore operating a shift in our understanding and conception of Ov from a host-parasite biomedical problem to an ecological problem [6] that embraces the social-ecological complexity underlying transmission and disease manifestation. Such a conceptual and procedural shift requires systems thinking and a ‘real life problem’ (rather than a ‘disciplinary science problem’) focused approach that bridges disciplinary gaps, enhances collaborative research and education, and addresses previously unresolved questions related to epidemiological data and disease risk patterns related to Ov. This, in turn, should foster more sustainable disease control interventions that are simultaneously compatible with rural communities’ health promotion We review here the state of our knowledge regarding the social-ecological determinants of Ov transmission and we highlight the role of neglected social, ecological and complex system sciences in improving our understanding of the systems transmission dynamic. We then describe an integrative research rationale based on transdisciplinarity pointing to the needs to enhance collaborative research and education as well as provide a broader human health and sustainable development framework in the context of the rapidly changing Southeast Asia waterscape.
Liver fluke landscape epidemiology: environmental changes and their impact on transmission dynamics
Deagrarianization in Southeast Asia [7] involves numerous socio-economic transitions, including the diversification of occupations and livelihoods in the countryside, a shift in the balance between farm and non-farm household incomes, increased mobility and delocalized employments, cultural and social changes implicated in livelihood modifications [8]. These profound changes that orchestrate an overall detachment from agricultural livelihoods and increased penetration of commercial activities in rural areas [7–9], are also associated with severe landscape restructuration and environmental degradation, as the remaining agricultural sector has shifted towards an intensified form of agriculture [10] (Box 1).
Box 1.
Agriculture intensification, health and disease in Southeast Asia
It is estimated that irrigation for agriculture accounts for around 90% of all water diversions in Southeast Asia (Cambodia: 94%; Laos: 82%; Vietnam: 86%; and Thailand: 91 %). Global irrigated areas increased from 168 million hectares in 1970 to about 300 million hectares at the end of the 1990s, with Thailand and Vietnam in particular having extensively increased their irrigated areas in more recent time [52]. In addition to irrigated areas, more than 85 dams are now proposed to be built on the main channel and tributaries of the Mekong River in Southeast Asia [13,53] to support agriculture, energy demands and drinking necessities.
In the case of livestock production, in 2001, three countries—China, Thailand, and Vietnam— accounted for more than half the hogs and one-third the chickens produced worldwide and these figures have continued to grow until now (http://www.fao.org/3/a-a0261e.pdf). Animals in industrial concentrated feeding operations are fed hormones and antibiotics for growth improvement and disease control [54,55]. Estimated antimicrobial consumption of cattle, chickens, and pigs in members of the Association of Southeast Asian Nations in 2010, suggest that Indonesia, Vietnam, and Myanmar are the three leading consumers of antimicrobials for farm use on a total per-country basis. China, which is the largest animal feeding country in the world is also experiencing the expansion and intensification of animal feeding operations in many areas. Due to the absence of relevant regulation, the residues of a variety of antibiotics have been consistently reported at high levels in animal waste [56,57].
Agrochemical use, and large scale irrigation project have been associated with increased soil erosion and salinization, lower soil fertility, reduced biodiversity, pollution of ground water and eutrophication of rivers and lakes [58–62]. Nitrogen and phosphorous run-offs resulting from both crop fertilization and animal production have become a major source of aquatic dead zones, noxious odors, and ecological change, including the loss of biodiversity-based regulatory potential of agro-ecosystems in face of infectious disease transmission [63–67]. Industrial livestock production systematically utilizing anabolic steroids and antibiotics as growth accelerators, contribute to the increasing prevalence of antibiotic resistant bacteria (ARB) and antibiotic resistance genes (ARGs) in the environment [68,69], a main contributor to the growing number of emerging infectious diseases in the region and worldwide [65,70].
These transitions and their inherently associated socio-ecological modifications exhibit specific and transient epidemiological characteristics, different from the more stable epidemiological states that precede and follow them [11], which from a research and intervention standpoint should deserve particular attention and accrued vigilance, responsiveness and adaptive management capacity. Dam building, irrigation expansion and associated water movement, in combination to soil and water nutrient enrichment associated with the intensification of agricultural practices, are anticipated to strongly influence liver fluke transmission dynamics [12,13]. For instance, in Lao PDR, road infrastructure improvement and flood reduction have facilitated the excavation of small aquaculture ponds within villages, which now provide anthropogenic microhabitats in which the full life cycle of Ov can occur [12]. Similarly, in intensive irrigation areas, in Kalasin Province, Thailand, Sri-Aroon et al. [14] found a total of 15 snail species, including 7 species with suspected involvement in human infections of angiostrongyliasis, cercarial dermatitis, echinostomiasis, opisthorchiasis and paragonimiasis. Snails of the species Bithynia siamensis goniomphalos (Bsg), which are intermediate hosts of the liver fluke Ov, accounted for 89 % of all collected samples, and exhibited a high infection rate compared to studies from endemic areas in Khon Kaen province, Thailand [14].
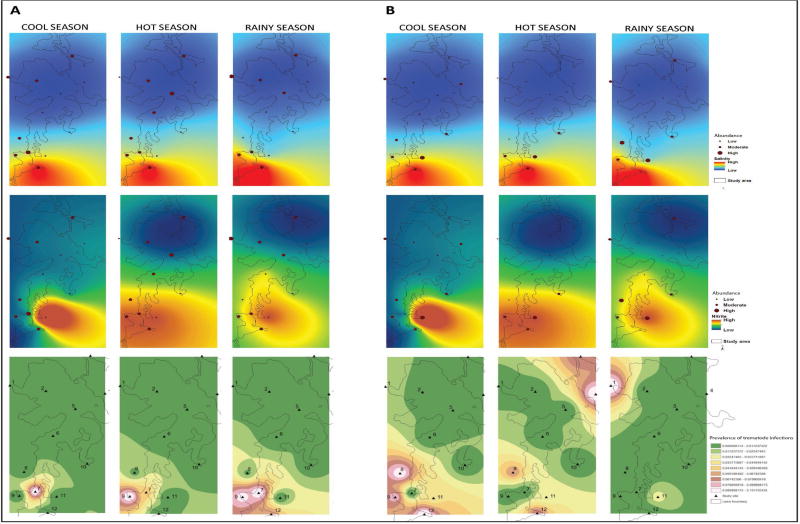
Following a ‘landscape epidemiology’ rationale [3,15,16], a recent extensive study conducted in northeastern Thailand, describes an ecological gradient of Ov intermediate hosts abundance and documents a strong influence of environmental changes on Ov transmission dynamics [17]. In the Lawa lake region of Khon Kaen province, the authors observed high relative abundance of Bsg snails and cyprinid fish (the two first intermediate host for Ov) over their counterpart species in Lawa Lake. The patterns of abundance described varied both spatially and temporally, with sites located near the shore and in the southern region of the lake, particularly during the rainy season (August – October in the region), being more prone to Bsg and cyprinid fish presence, respectively. These patterns of distribution and abundance were significantly related to nutrient enrichment and high levels of salinity and nitrite-nitrogen, suggesting that water contaminants may modulate local freshwater snail community structure (i.e. species richness and abundance) through environment-mediated and species-specific physiological tolerance processes. In other words, different freshwater snail species do not handle disturbance (i.e. water contamination) equally well, with species such as Lymnaea rubiginosa, being particularly sensitive while Bsg snails appeared to be relatively immune to these physiological challenges. As a consequence, Bsg are able to maintain higher relative population abundance in the areas of the lake exhibiting higher degree of contamination [17] (Fig. 2). Similar conclusions were drawn by Wang et al. [18] who showed that the freshwater habitats of ponds, streams and rice paddies possessed significantly different abiotic water qualities with different freshwater snail diversity and species evenness, with high Bsg snail abundance in certain habitats (rice paddies). In particular, the relative abundance of Bsg snails was found to be negatively correlated with that of Filopaludina martensi martensi snails, further underscoring the possible influence of species interaction on Bsg snail population and the potential effect of anthropogenic irrigation practices on Bsg snail ecology [18]. The influence of agricultural practices on water quality parameters resulting in changes in the ecology of intermediate hosts has also been shown for several other helminthes of public health significance, including schistosomes [19].
Figure 2. Environmental influences on Opisthorchis viverrini natural hosts and infection prevalence.
Kriging geographic analysis of the abundance of A) cyprinid fish and B) Bithynia siamensis goniomphalos in relation to salinity (top panel) and nitrite (middle panel). Bottom panel shows the prevalence of Opisthorchis viverrini infection. Data for the rainy season, cool season and hot season is shown. Adapted from [37].
More long-term ecological studies are needed to confirm and dissect the mechanisms underlying the influence of nutrient-enrichment and eutrophication on freshwater snail community structure seasonal variation and Ov epidemiology. However, large amounts of evidence suggest the highly dynamic nature of Ov transmission, both spatially and temporally, and strongly identify it as an ecological process modulated by agriculture intensification. As such, Ov transmission needs to be understood in its social-ecological context, including not only natural wetland ecology, but also with an appreciation for the need to connect local landscape management strategies and cultural/agrarian practices/policies with regional and global economic decisions [20]. This is particularly important considering the current context of agriculture intensification, waterscape fragmentation [21] and livelihood shifts in northeastern Thailand where unregulated agrochemical use [22] and large-scale irrigation systems disturb local wetlands ecological dynamics, modify patterns of local communities’ environmental risk exposure, wealth, poverty and vulnerability while seemingly improving regional and national capital.
Climate change, liver fluke transmission dynamics and control
Recent experimental research indicate that Ov transmission potential and infection success in snails is highly temperature-dependent [23,24]. Subsequent simulations incorporating these experimental data, as well as regional temperature and precipitation records, snail abundance and egg availability in the environment, provided a useful modeling framework to assess and predict seasonal transmission risk [24]. The model outputs suggested that transmission risk varies greatly over the year (see also [25,26]), with very low transmission risk during the dry season and increasing transmission risk at the onset of the rainy season (i.e. June–July) with a peak in September. This outcome indicates a strong seasonal pattern of transmission in snails and the crucial, although indirect, role of environmental and climatic parameter variation in modulating the transmission process. Of note, human activity in the environment is usually high during the rainy season, as rain-fed rice farming peaks and coincides with increased fecal bacterial contamination and potentially Ov egg presence in natural water reservoirs [18,27,28]. These findings are supported by a recent Thailand-wide climate modeling investigation of Ov transmission [29] and strengthen the case that control can and should be practiced seasonally during the year rather than year-round in order to maximize resource utility [24,30]. These seasonal patterns of transmission risk and the insights they provide regarding control initiatives may be subject to change as temperature and precipitation regimes in the central Mekong Basin are forecasted to change [31]. The predictive model for Khon Kaen Province referred to above [24] suggests that the transmission risk seasonal peak will shift to later in the year, in October rather than September, in response to precipitation and seasonal timing changes in a context of climate change. However, the overall transmission risk throughout the year may decrease as increasing temperatures become less favorable to snails and less conducive to successful Ov egg hatching based on this model. The modification of precipitation and temperature regimes in Southeast Asia may also induce changes in rain-dependent rice farming schedules, growing season duration and irrigation schemes that will influence patterns of snail distribution, human activity in rice fields and, indirectly, egg prevalence in the environment. Climate change will also modify infection risk for cyprinid fish and the direct risk for infection in human populations. Although the preliminary model described above does not incorporate data from fish population, human socio-economic, or agriculture intensification trends, these are likely to modulate snail-egg encounter rates and will need to be accounted for in future modeling investigations [24].
Although the above-discussion focuses on Ov, it is worthy noting that the transmission dynamics of most helminthes is expected to be altered (i.e. in context specific ways) following sustained climatic anomalies. Evidence indicates that the effects of climate change on helminthes are more significant in northern latitudes as well as in high altitude areas, where modifications of climate variables appear to be more pronounced. Temperature and precipitation regimes are the meteorological factors that have been more frequently linked to the impact of climate change on helminthes in general [32–34].
Human social-ecology, livelihood transitions and liver fluke transmission
Past public health interventions related to liver fluke control have targeted two transmission interfaces where liver fluke stages and hosts encounter each other. First, in rural endemic areas, the sustenance of parasite egg input into the environment is fostered by labor-related defecation practices where rice farmers and fishermen, spending most of their day time out in the fields or in wetlands, cannot reasonably afford the time to go back home or find latrines to defecate [35]. Interventions targeting defecation practices have used education, improved sanitation and hygiene to reduce infection [36] but the complexity of the problem, and its only partially addressed underlying behavioral nature, has prevented sustainable transmission interuption at this interface. Secondly, the human-fish interface is even more complex due to the dietary significance of fish in local diets, livelihood connection with the ecosystem, a relatively high localized dependence on fisheries, and cultural food practices based on a variety of raw and fermented fish dishes [37]. Thus multiple complex socio-cultural and ecological factors associated with the “wetland livelihoods” of the local communities underline the apparent preference for raw cyprinid fish consumption in Isaan and Laos inadvertently contributing to high Ov infection rates in the region.
Recent medical anthropology studies confirm that, despite the transition from traditional eating practices to more westernized food consumption patterns in younger generations, current Isaan iconic raw fish dishes are the expressions of traditional knowledge and adapted practices selected through centuries, thus deeply engrained in the local culture [38]. Until relatively recently (50 years ago), Isaan people had to rely largely on sourcing foods in their local environment for survival. Northeastern Thailand’s dry climate is characterized by high variability in both rainfall amounts and intensities, and also prolonged periods of drought. This harsh environment imposed serious challenges for food security. Fish, mostly cyprinids in this region, are the most easily accessible protein food source representing up to 70% of the people’s diet [39]. During periods of abundance (rainy season) certain fish would be consumed upon capture while others, mostly the smallest ones (cyprinids) would be prepared for long-term storage in anticipation of periods of limited food supply. The most adapted storage procedures are fermentation or salt-preservation, which do not cook the fish but prevent them from wasting.
Furthermore, the distribution network of fish and infection among communities and to the cities is strongly influenced by local needs and market economy [38]. Middlemen on-sell some fish to locals at the piers but mainly distribute the fish to the market in near-by urban centers [38]. This pattern changes depending on the type of fish as larger, more economically attractive (and non-infected) fish are preferably transported away from local village communities to district town markets. Smaller and less economic, potentially infected pla khao noi (Thai for small cyprinid fish) typically stay in the local villages for cheap sale, or free distribution, to family and friends of the fishermen. Thus, “fish economics” becomes a further variable for concentrating Ov-infection endemicity in these villages [38].
Raw food sharing among households in Northeastern Thailand rural villages is also an important, although overlooked, factor contributing to Ov infection and transmission [40]. Human eating behavior is known to be influenced by a variety of factors including ethnicity, culture, religion, age and gender [41]. In subsistence farming and hunting and gathering societies, both of which historically characterize the Isaan livelihood [42], what is eaten and how, is fundamentally a response to the local environment and its available resources [43]. Who eats together and how food is shared is basically a social-ecological adaptation. For rural farmers in Northeast Thailand, besides glutinous rice, fish from the rice fields and adjacent wetlands have been a staple protein source for generations. Sharing food is a common practice of traditional farming societies [44], as it insures reciprocity. For Isaan people, whose cultural practices generally reflect a history of extracting a living from a particularly harsh and unpredictable environment, food sharing (i.e. mostly fish in this context) is symbolic of sharing their identity [45]. Moreover, people share what they eat and eat what is shared with them. A better understanding is needed of how interventions can reduce risky fish dish consumption without discouraging food-sharing behavior that reinforces social coherence, a well-known positive health factor contributing to resilience and social capital [46,47]. Weighing this against the “physiological risk” of Ov infection (Box 2) could contribute to interventions better aligned with communities’ health development.
Box 2.
Food sharing and infection risk
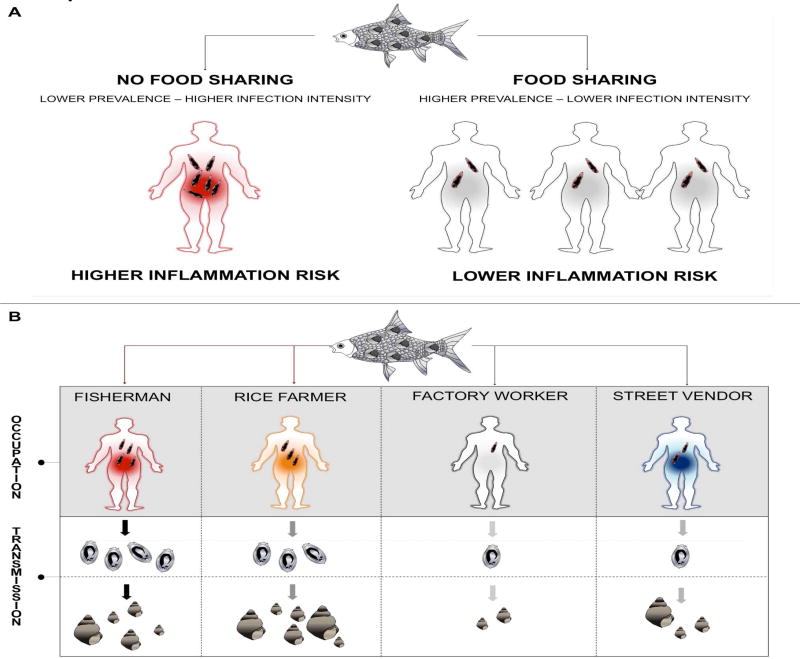
In contrast with micro-parasites and characteristic of helminthes in general, adult liver flukes do not multiply in their human host and are long-lived organisms, with individuals presumably surviving more than ten years in their host [71,72]. Therefore, liver fluke infections tend to be of a persistent nature and the worm burden on an infected individual is directly correlated to the number of metacercariae ingested. However, although it may seem intuitive to relate risk of infection and worm burden to the frequency of raw fish consumption, a certain number of biological and cultural elements contribute to blur this relationship.
First of all, not all cyprinid fish are equally likely to be infected [73]. Additionally, raw fish is consumed in numerous preparations and most often shared among members of the community [40]. It follows that the amount of metacercariae present in the fish preparations will also be shared among members of the community. Consequently, although more individuals are likely to ingest infectious metacercariae and become infected (i.e. higher prevalence), the likelihood for high intensity infection per individuals will be reduced. Considering that high infection intensity is more strongly correlated to clinical disease manifestation [74], food sharing, a deeply embedded cultural behavior and important for community positive health, may also act as an agent of disease risk dilution (Figure IA). Infection risk and transmission may even further be modulated depending on the occupations of the members of the social networks sharing preferentially raw fish dishes and the likelihood for these persons to visit rice fields and adjacent aquatic environments where snails are likely to be found (Figure IB).
Systems thinking, transdisciplinarity and liver fluke sustainable control
Current biomedically-informed liver fluke research and interventions have arguably contributed to significant reduction in infection prevalence and provided powerful tools for diagnostic and treatment purposes in the context of Ov and cholangiocarcinoma (CCA). However, it can also be argued that the clinical benefits associated with infection prevalence reduction may be limited [48] and that current interventions procedures (e.g. mass drug administration, behavioral change campaigns) may also incur hidden health costs when considering the whole social-ecological system, its dynamics, and its emergent properties, including communities’ resilience [6]. These costs may possibly outweigh the direct benefits associated with infection prevalence reduction. Among the most obvious of these potential costs are the disruption of community’s cultural capital possibly associated with top-down interventions targeting behavioral change in relation to food preparation and diet [38]. Systematic deworming may also be related to increase risk of developing metabolic disorders (Box 3), a particularly worrying scenario considering the current public health priorities and concerns towards mitigating the rise of cardiovascular diseases, diabetes and other chronic diseases. Together these and other elements of evidence suggest that disease focused liver fluke interventions, neglecting the integration of broader social, behavioral, environmental and cultural determinants may achieve some successes in reducing Ov prevalence but this may not translate into significantly better health overall and may even conflict with other desirable psychosocial and environmental achievements.
BOX 3.
Liver fluke infection, health and disease
The evolution of disease tolerance and underlying immuno-modulatory mechanisms by helminthes is widely documented [75–77]. This results in most infections being asymptomatic [78] and lead to the tempering of responses to non-helminth antigens (e.g. allergens), leading to a reduced incidence of allergies and autoimmune diseases in chronically infected individuals [79]. In the case of liver flukes, information is limited regarding the timing and extent of their immunomodulatory capacity in human hosts. However, evidence of immuno-suppression has been provided in animal models where chronic Ov infection was associated with immune evasion [80–82]. The immuno-modulatory capacity of liver flukes and its potential to down regulate overall inflammation is also indirectly supported by results from recent human cohort studies. For instance, chronic O. felineus infection in Siberia has been shown to be associated with lower serum total cholesterol and significant attenuation of atherosclerosis [83,84]. In the case of Ov in Southeast Asia, data suggest increased cholesterol levels and fatty livers in patients following deworming (from 10% to 15% of fatty livers; [85]). Similarly, preliminary observations of nation-wide cardio-vascular diseases (CVD) distribution in Thailand indicate a negative relationship with liver fluke infection, corroborating the observation of cholesterol protection afforded by liver fluke infection as hypercholesterolemia is known to be strongly associated with CVD [83]. Regarding type 2 diabetes, while low levels of cholesterol and fatty livers usually predicts low occurrence of type 2 diabetes [86], nation-wide diabetes distribution mapping in Thailand intriguingly matches liver fluke (and low CVD prevalence) distribution and suggest a positive relationship that warrants further investigation.
These observations have been argued to warrant a refined interpretation of the nature of the interaction between flukes and humans [48]. This may range from parasitic to mutualistic, the latter particularly in endemic areas where people have been persistently exposed to helminthes and other infectious agents. An evolutionary perspective (i.e. dynamic view of host-parasite interactions cf. [87–89]) and a precise understanding of the immune regulatory mechanisms in relation to infection intensity, as well as the influence of certain genetic polymorphisms is key to better identify the thresholds beyond which helminthes infections are prejudicial or beneficial and better assess how the local context modulates these thresholds and how interventions should be adapted.
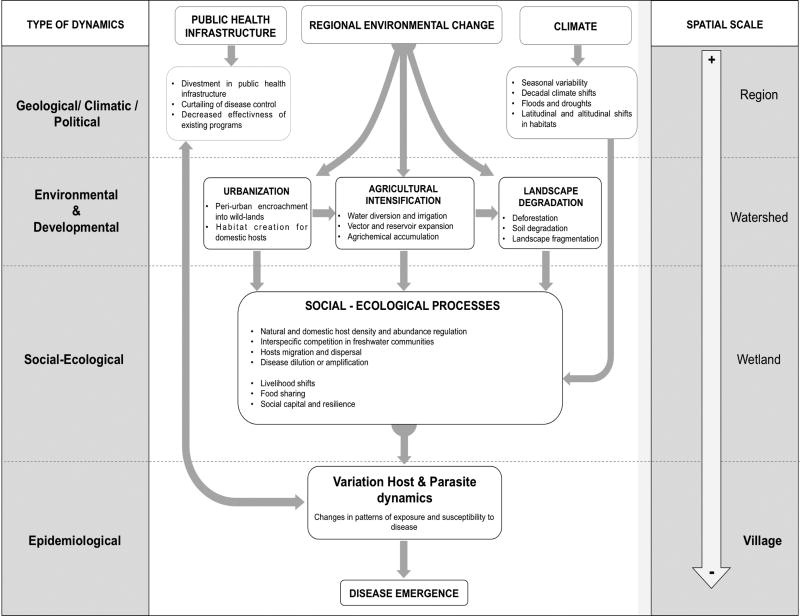
Employing a systems approach with a focus on health (i.e. not disease focused), within which liver fluke disease control can be integrated, is key for sustainable health development. A systems approach and the mapping of health determinants and their scale of influence will help identify and mitigate potentially undesirable consequences associated with un-contextualized disease control initiatives (Fig. 3, key figure).
Figure 3. Blueprint illustrating environmental factors known or suspected to be associated with transmission of Opisthorchis viverrini.
Regional environmental change, which is influenced significantly by population growth, resource consumption, economic development projects, agriculture intensification and waste generation, plays an important role in the emergence of infectious disease in general especially in tropical developing regions. In the case of Opisthorchis viverrini (Ov), although the importance of these broader determinants have been evoked more than a decade ago [2], formal research acknowledging their significance in modulating transmission dynamics and disease manifestation has just begun. Of particular concern in the case of Ov is the unprecedented land use and transformation of resource production (urbanization, agricultural expansion and intensification, and natural habitat alteration) that has intensified during the last decade in the region, and that is increasingly recognized to have produced changes in ecological systems, notably in landscapes and, in turn, their natural communities and ultimately in their parasites, animal host, and human populations. Thus the entire host-parasite dynamics is being modified, including pattern of exposure and susceptibility to disease. Designing research and interventions that integrate these broad scale determinants of transmission and their interactions is key for the proper contextualization of Opisthorchis viverrini control. Overarching systemic factors related to public health infrastructure and climate variability, and their interactions with regional environmental change, also contribute significantly to disease emergence although more diffusely.
For instance, rural livelihoods in northeastern Thailand and Laos, are intimately tied to wetland ecosystems that ultimately determine human health and well-being through a number of characteristic influences, such as: serving as a source of environmental hydration, drinking water and nutrition; being exposed to pollution, toxic agents and infectious pathogens; representing sites of physical hazards, settings for mental health and psycho-social well-being as well as places from which people derive their livelihood and cultural identities [49]. These influences can either enhance or diminish human health depending on the ecological functioning of wetlands and their ability to sustain the relationship between people and their land.
It follows that losses of wetland components and disruptions to wetland ecosystem functions, including cultural functions, inevitably have consequences for human health along any or all of these lines. In this light, problems in which the environment is considered to have been implicated in health outcomes cannot be solved by medical approaches to health alone and a narrow focus on one particular disease. Rather, broader approaches must be utilized, drawing on a wider scientific base, including ecological and social sciences that will help contextualize disease control interventions within a broader health map. Only after key biophysical, psychosocial and environmental (including ecological and evolutionary) components have been taken into account, educated choices and concerted trade-offs agreed upon among stakeholders can be made, allowing for the design of sustainable interventions.
Implementing a systems approach towards integrated liver fluke control (i.e. that accounts for broader sets of determinants and that is more likely to prove sustainable) requires interdisciplinary and often transdisicplinary research, thus an integrative research process, which entails an adaptive conceptual or theoretical framing, including iteratively (repeatedly) reframing of ‘the problem’ throughout the course of research or interventions as new facts and new knowledge arise [50]. A necessary assumption pertaining to the operationalization of transdisciplinary research is epistemological pluralism, allowing the functional assembly of theories, concepts, methods and procedures that originally belonged to clearly defined (and separate) disciplines, towards the creation of integrated meta-languages and reaching consensual decisions. Along the same line of thought and of particular importance towards more sustainable liver fluke interventions, is the integration of beneficiary communities’ health perceptions and development needs within intervention agendas.
A significant step towards this integration was initiated several years ago – the Lawa project [4] – in the form of the implementation of community-based disease prevention and control programme including intentions of creating an understanding of liver fluke infection disease risk with the community. Although significant improvements have been achieved, several barriers and challenges remain including understanding the local culture and broader health and well-being concerns held by communities and ensuring these are aligned with intervention programme approaches and activities. To address these and other issues there is a need to continually review and revise protocols for community engagement at all levels; treatment and education, school science curriculum, etc. drawing on a community participatory approach in which the community, and local knowledge is at least as equally valued to outside experts.
Concluding remarks and future perspectives
On the basis of the above, it is increasingly recognized that social, environmental and cultural considerations are critical elements of sustainable interventions particularly in the case of Ov, as their integration can help contextualize “disease control” (i.e a sub-ordinate system) within a broader health development agenda (i.e. the supra-ordinate system) and consequently design and implement more integrative and sustainable interventions [51] (see also Outstanding Questions). As such, the following elements should be considered carefully and contextualized in any intervention program related to Ov control or other health challenges: 1) protecting and building on local knowledge in the face of outside forces at the national, regional and global levels, 2) balancing top-down and bottom-up approaches to the implementation of intervention programs, 3) reorienting government policies and approaches toward local empowerment adapted to communities’ social, cultural characteristics and needs, including healthy environments and biodiversity. For instance, a promising research avenue would be the investigation of the mechanisms underlying water nutrient enrichment in wetlands in relation to agriculture intensification and how these environmental and ecological imbalances influence intermediate hosts abundance and in turn Ov transmission. Enabling operational dialogue between scientists, policy makers and community stakeholders through the implementation of participatory methodologies should facilitate multiple source of knowledge integration across scales and administrative sectors for liver fluke control that is better aligned with sustainable health development.
Outstanding Questions Box.
How does agricultural intensification and associated nutrient enrichment in wetlands disrupt the ecology freshwater snail and fish communities?
How do environmental and ecological imbalances influence intermediate hosts abundance and influence Ov transmission?
How do current top-down disease control interventions, including campaigns for behavioral change, disrupt rural communities cultural capital and resilience and how do we integrate psychosocial health into intervention design and evaluation?
The transition from traditional food and eating practices to more westernized food consumption patterns, inadvertently encouraged by current Ov interventions, leads to a de facto reduction of Ov infection risk but also an increased risk for metabolic disorders as a “richer” diet is replacing a more balanced one. How do we address this situation?
Does Ov infection provide protection against allergies and cardiovascular disease? Then, is deworming associated with metabolic disorder risk?
If infection intensity is more strongly correlated to clinical disease manifestation than infection prevalence, would it be more cost-effective and epidemiologically relevant to refine intervention risk group and subsequent drug administration based on infection intensity?
How do we integrate liver fluke control within a broader sustainable health development agenda?
How do we achieve the functional assembly of theories, concepts, methods and procedures, the health perceptions of beneficiary communities and development needs, towards the creation of integrated, context-sensitive disease control?
Figure I.
* Food sharing and infection. A) All else being equal, sharing raw fish dishes among community members may contribute to increase the number of individuals infected (prevalence) but also dilute disease risk associated with inflammation as infection intensity per capita will be lower on average. B) Depending on social network group member occupations, likelihood for consumption of raw fish dishes varies. For example, rice farmers and fishermen are more likely to consume raw fish dishes and defecate in or near wetlands and rice fields due to their daily activities. Accordingly, the quantity of Ov eggs to be released in environments where Bithynia snails are found (wetlands, rice fields), thus transmission risk, will also vary among individuals having different occupations.
Trends.
The distinct socio-economic, cultural and environmental history of northeastern Thailand can provide insights on the intransigence of Ov infection prevalence and disease incidence in the region
Agriculture intensification is associated with significant socio-economic and ecological changes affecting intermediate hosts patterns of abundance and diversity in turn influencing transmission to humans
Recent modeling studies suggest that Ov transmission is seasonal and that control should be practiced seasonally rather than year-round in order to maximize resource utility
A better understanding is needed of how interventions can reduce raw-fish consumption without discouraging food-sharing behavior that reinforces social coherence, a factor well-known to positively influence human health
Acknowledgments
The authors wish to thank Bruce A. Wilcox and John F. Smith for numerous discussions that helped better understand the complexities underlying Ov transmission and related health challenges. This work was supported by Khon Kaen University; the Thailand Research Fund (TRF) under the TRF Senior Scholar (RTA 5680006); the National Research Council of Thailand; the Ecohealth Emerging Infectious Diseases Initiative (EcoEID) [funded by Canada’s International Development Research Centre; Foreign Affairs, Trade and Development Canada (through the Global Health Research Initiative); and the Australian Agency for International Development]; the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS); the Grand Challenges Canada (grant no. 0221-01); the National Institute of Allergy and Infectious Diseases (NIAID), award number P50AI098639; and the National Health Security Office, Thailand. BS is a TRF Senior Research Scholar. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIAID or the NIH or other funders.
Glossary
- Adaptive management
a structured, iterative process of robust decision making in the face of uncertainty, with an aim to reducing uncertainty over time via system monitoring
- Agricultural intensification
The process of utilizing an existing resource to a greater extent, by increasing the efficiency of resource exploitation (e.g. utilizing fossil fuels to produce plant fertilizers).
- Biodiversity
The variety of life forms, the ecological roles they play, and the genetic diversity they contain
- Biomedical model
A medical framework that considers illness to be caused by identifiable agents. The model's focus on the physical processes, such as the pathology, the biochemistry and the physiology of a disease, does not take into account the role of social or ecological factors.
- Deagrarianization
a term to describe the process of moving the societal structure away from an agrarian mode toward something else
- Disease prevention
specific, population-based and individual-based interventions for primary and secondary (early detection) prevention, aiming to minimize the burden of diseases and associated risk factors.
- Epistemological pluralism
a term used in philosophy, economics, and virtually any field of study to refer to different ways of knowing things, including conceptual frameworks and methodologies
- Eutrophication
excessive richness of nutrients in a lake or other body of water, frequently due to runoff from the land, which causes a dense growth of plant life and death of animal life from lack of oxygen.
- Exposure assessment
Measuring or estimating how often humans are exposed to an agent, for how long, and the intensity of exposure. This can involve methods such as asking subjects about their lifestyles and habits; taking environmental samples; and screening subjects' physical samples to measure concentrations of the agents in their bodies.
- Health promotion
the process of empowering people to increase control over their health and its determinants through health literacy efforts and multisectoral action to increase healthy behaviors.
- Incidence
A measure of the probability of occurrence of a given medical condition in a population within a specified period of time. Incidence conveys information about the risk of contracting the disease.
- Interdisciplinary research
a form of integrative research that involves academic disciplines whose different or even contrasting methods, models, and research paradigms (e.g., natural versus social sciences) are combined to create new knowledge and theory and address a common research goal
- Participatory research
research that involves academic researchers and non-academic participants working together to solve a problem
- Prevalence
Prevalence is a measurement of all individuals affected by the disease at a particular time. Prevalence indicates how widespread the disease is.
- Resilience
capacity of individuals, families, organizations and whole communities to respond effectively to significant adversity and risk.
- Salutogenesis
describes an approach focusing on factors that support human health and well-being, rather than on factors that cause disease (pathogenesis).
- Social capital
the networks of relationships among people who live and work in a particular society, enabling that society to function effectively.
- Social-ecological system
A socio-ecological system consists of 'a bio-geo-physical' unit and its associated social actors and institutions. Socio-ecological systems are complex and adaptive and delimited by spatial or functional boundaries surrounding particular ecosystems and their problem context
- Systems thinking: (or holism)
a scientific approach that recognizes that complex systems possess emergent properties (more than the simple sum of multiple parts)
- Transdisciplinary research
a form of integrative research employing a systems and participatory approach, combines knowledge from outside (such as from communities) as well as with academia to address a ‘real world’ problem with a common goal, creating new knowledge and theory.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sithithaworn P, et al. The current status of opisthorchiasis and clonorchiasis in the Mekong Basin. Parasitol. Int. 2012;61:10–16. doi: 10.1016/j.parint.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Petney TN. Environmental, cultural and social changes and their influence on parasite infections. Int. J. Parasitol. 2001;31:919–932. doi: 10.1016/s0020-7519(01)00196-5. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox BA, Colwell RR. Emerging and Reemerging Infectious Diseases: Biocomplexity as an Interdisciplinary Paradigm. EcoHealth. 2005;2:244–257. [Google Scholar]
- 4.Sripa B, et al. Toward integrated opisthorchiasis control in northeast Thailand: The Lawa project. Acta Trop. 2015;141:361–367. doi: 10.1016/j.actatropica.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang Y-C, et al. Social Capital and Health-Protective Behavior Intentions in an Influenza Pandemic. PLOS ONE. 2015;10:e0122970. doi: 10.1371/journal.pone.0122970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilcox BA, Echaubard P. Balancing Biomedical and Ecological Perspectives in Research Framing of Liver Fluke and Cholangiocarcinoma in NE Thailand. Parasitol. Int. doi: 10.1016/j.parint.2016.10.002. (In press) [DOI] [PubMed] [Google Scholar]
- 7.Rigg J. Land, farming, livelihoods, and poverty: Rethinking the links in the Rural South. World Dev. 2006;34:180–202. [Google Scholar]
- 8.Rigg J, Nattapoolwat S. Embracing the global in Thailand: activism and pragmatism in an era of deagrarianization. World Dev. 2001;29:945–960. [Google Scholar]
- 9.Bryceson DF, Jamal V. Farewell to Farms: De-agrarianisation and Employment in Africa. Ashgate; 1997. [Google Scholar]
- 10.Yaro JA. Is deagrarianisation real? A study of livelihood activities in rural northern Ghana. J. Mod. Afr. Stud. 2006;44:125. [Google Scholar]
- 11.Bradley DJ. An Exploration of Chronotones: A Concept for Understanding the Health Processes of Changing Ecosystems. EcoHealth. 2004;1:165–171. [Google Scholar]
- 12.Sithithaworn P, et al. Changes to the life cycle of liver flukes: dams, roads, and ponds. Lancet Infect. Dis. 2012;12:588. doi: 10.1016/S1473-3099(12)70174-3. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler AD, et al. Dams and Disease Triggers on the Lower Mekong River. PLoS Negl Trop Dis. 2013;7:e2166. doi: 10.1371/journal.pntd.0002166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sri-Aroon P, et al. Freshwater mollusks of medical importance in Kalasin Province, northeast Thailand. Southeast Asian J. Trop. Med. Public Health. 2005;36:653–657. [PubMed] [Google Scholar]
- 15.Pavlovsky EN, Levine ND. Natural nidality of transmissible diseases: with special reference to the landscape epidemiology of zooanthroponoses. University of Illinois Press; 1966. [Google Scholar]
- 16.Lambin EF, et al. Pathogenic landscapes: Interactions between land, people, disease vectors, and their animal hosts. Int. J. Health Geogr. 2010;9:54. doi: 10.1186/1476-072X-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CS, et al. Seasonal and Spatial Environmental Influence on Opisthorchis viverrini Intermediate Hosts, Abundance, and Distribution: Insights on Transmission Dynamics and Sustainable Control. PLoS Negl. Trop. Dis. 2016;10:e0005121. doi: 10.1371/journal.pntd.0005121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y-C, et al. An ecological study of Bithynia snails, the first intermediate host of Opisthorchis viverrini in northeast Thailand. Acta Trop. 2015;141:244–252. doi: 10.1016/j.actatropica.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Wu J-Y, et al. Three Gorges Dam: Impact of Water Level Changes on the Density of Schistosome-Transmitting Snail Oncomelania hupensis in Dongting Lake Area, China. PLoS Negl. Trop. Dis. 2015;9:e0003882. doi: 10.1371/journal.pntd.0003882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ong X, et al. Uncovering the Pathogenic Landscape of Helminth (Opisthorchis viverrini) Infections: A Cross-Sectional Study on Contributions of Physical and Social Environment and Healthcare Interventions. PLoS Negl. Trop. Dis. 2016;10:e0005175. doi: 10.1371/journal.pntd.0005175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y-C, et al. Assessing the role of landscape connectivity on Opisthorchis viverrini transmission dynamics. Parasitol. Int. doi: 10.1016/j.parint.2016.06.002. (In press) [DOI] [PubMed] [Google Scholar]
- 22.Panuwet P, et al. Agricultural pesticide management in Thailand: status and population health risk. Environ. Sci. Policy. 2012;17:72–81. doi: 10.1016/j.envsci.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prasopdee S, et al. Temperature dependence of Opisthorchis viverrini infection in first intermediate host snail, Bithynia siamensis goniomphalos. Acta Trop. 2015;141:112–117. doi: 10.1016/j.actatropica.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Echaubard P, et al. Experimental and modeling investigations of Opisthorchis Viverrini miracidium transmission over time and across temperatures: implications for control. Int. J. Parasitol. 2017;47:257–270. doi: 10.1016/j.ijpara.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiatsopit N, et al. Seasonal cercarial emergence patterns of Opisthorchis viverrini infecting Bithynia siamensis goniomphalos from Vientiane Province, Lao PDR. Parasit. Vectors. 2014;7:551. doi: 10.1186/s13071-014-0551-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Namsanor J, et al. Seasonal Transmission of Opisthorchis viverrini sensu lato and a Lecithodendriid Trematode Species in Bithynia siamensis goniomphalos Snails in Northeast Thailand. Am. J. Trop. Med. Hyg. 2015;93:87–93. doi: 10.4269/ajtmh.14-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaewkes W, et al. Fecal bacterial contamination in natural water reservoirs as an indicator of seasonal infection by Opisthorchis viverrini in snail intermediate hosts. Parasitol. Int. 2012;61:49–51. doi: 10.1016/j.parint.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Petney T, et al. The ecology of the Bithynia first intermediate hosts of Opisthorchis viverrini. Parasitol. Int. 2012;61:38–45. doi: 10.1016/j.parint.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Suwannatrai A, et al. Modeling impacts of climate change on the potential distribution of the carcinogenic liver fluke, Opisthorchis viverrini in Thailand. Parasitol. Res. 2017;116:243–250. doi: 10.1007/s00436-016-5285-x. [DOI] [PubMed] [Google Scholar]
- 30.Hinz E, et al. Opisthorchiasis control in northeast Thailand: proposal for a new approach. Appl. Parasitol. 1994;35:118–124. [PubMed] [Google Scholar]
- 31.Babel M, et al. Evaluation of climate change impacts and adaptation measures for rice cultivation in Northeast Thailand. Clim. Res. 2011;46:137–146. [Google Scholar]
- 32.Mas-Coma S, et al. Effects of climate change on animal and zoonotic helminthiases. Rev Sci Tech Int Epiz. 2008;27:443–57. [PubMed] [Google Scholar]
- 33.Lafferty KD. The ecology of climate change and infectious diseases. Ecology. 2009;90:888–900. doi: 10.1890/08-0079.1. [DOI] [PubMed] [Google Scholar]
- 34.Lafferty KD, Mordecai EA. The rise and fall of infectious disease in a warmer world. F1000Research. 2016;5:2040. doi: 10.12688/f1000research.8766.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phongluxa K, et al. Helminth infection in southern Laos: high prevalence and low awareness. Parasit. Vectors. 2013;6:328. doi: 10.1186/1756-3305-6-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229–232. doi: 10.1016/j.actatropica.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Grundy-Warr C, et al. Raw attitudes, wetland cultures, life-cycles: Socio-cultural dynamics relating to Opisthorchis viverrini in the Mekong Basin. Parasitol. Int. 2012;61:65–70. doi: 10.1016/j.parint.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 38.Kim CS, et al. Role of socio-cultural and economic factors in cyprinid fish distribution networks and consumption in Lawa Lake region, Northeast Thailand: Novel perspectives on Opisthorchis viverrini transmission dynamics. Acta Trop. 2017;170:85–94. doi: 10.1016/j.actatropica.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MRC Fisheries Unit. Mekong Fish Catch and Culture, The Family Cyprinidae. MRC Fisheries Unit; 1998. [Google Scholar]
- 40.Saenna P, et al. Fish sharing as a risk factor for Opisthorchis viverrini infection: evidence from two villages in north-eastern Thailand. Infect. Dis. Poverty. 2017;6:66. doi: 10.1186/s40249-017-0281-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macpherson CNL. Human behaviour and the epidemiology of parasitic zoonoses. Int. J. Parasitol. 2005;35:1319–1331. doi: 10.1016/j.ijpara.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 42.Parnwell MJ. The power to change: Rebuilding sustainable livelihoods in North-East Thailand. J. Transdiscipl. Environ. Stud. 2005;4:1–21. [Google Scholar]
- 43.Gadgil M, Guha R. Ecological Conflicts and the Environmental Movement in India. Dev. Change. 1994;25:101–136. [Google Scholar]
- 44.Gurven M. To give and to give not: the behavioral ecology of human food transfers. Behav. Brain Sci. 2004;27:543–559. [Google Scholar]
- 45.Klausner WJ. Reflections on Thai Culture: Collected Writings. The Siam Society; 1993. [Google Scholar]
- 46.Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196:129–136. doi: 10.1126/science.847460. [DOI] [PubMed] [Google Scholar]
- 47.Antonovsky A. Unraveling the mystery of health: how people manage stress and stay well. Jossey-Bass; 1987. [Google Scholar]
- 48.Echaubard P, et al. The role of evolutionary biology in research and control of liver flukes in Southeast Asia. Infect. Genet. Evol. 2016;43:381–397. doi: 10.1016/j.meegid.2016.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horwitz P, et al. Healthy wetlands, healthy people: a review of wetlands and human health interactions. Secretariat of the Ramsar Convention on Wetlands, Gland, Switzerland, & The World Health Organization; 2012. [Google Scholar]
- 50.Richter CH, et al. Toward Operational Criteria for Ecosystem Approaches to Health. EcoHealth. 2015 doi: 10.1007/s10393-015-1028-1. [DOI] [PubMed] [Google Scholar]
- 51.Bardosh K. Global aspirations, local realities: the role of social science research in controlling neglected tropical diseases. Infect. Dis. Poverty. 2014;3:35. doi: 10.1186/2049-9957-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molle F, et al., editors. Contested waterscapes in the Mekong Region: hydropower, livelihoods and governance. Earthscan; 2009. [Google Scholar]
- 53.Grumbine RE, Xu J. Mekong Hydropower Development. Science. 2011;332:178–179. doi: 10.1126/science.1200990. [DOI] [PubMed] [Google Scholar]
- 54.Arikan OA, et al. Occurrence of antibiotics and hormones in a major agricultural watershed. Desalination. 2008;226:121–133. [Google Scholar]
- 55.Zhang X, et al. Prevalence of Veterinary Antibiotics and Antibiotic-Resistant Escherichia coli in the Surface Water of a Livestock Production Region in Northern China. PLoS ONE. 2014;9:e111026. doi: 10.1371/journal.pone.0111026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y-X, et al. The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ. Monit. Assess. 2013;185:2211–2220. doi: 10.1007/s10661-012-2702-1. [DOI] [PubMed] [Google Scholar]
- 57.Zhao L, et al. Residues of veterinary antibiotics in manures from feedlot livestock in eight provinces of China. Sci. Total Environ. 2010;408:1069–1075. doi: 10.1016/j.scitotenv.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Matson PA. Agricultural Intensification and Ecosystem Properties. Science. 1997;277:504–509. doi: 10.1126/science.277.5325.504. [DOI] [PubMed] [Google Scholar]
- 59.Pimm SL, Raven P. Extinction by numbers. Nature. 2000;403:843–845. doi: 10.1038/35002708. [DOI] [PubMed] [Google Scholar]
- 60.Gomiero T. Soil Degradation, Land Scarcity and Food Security: Reviewing a Complex Challenge. Sustainability. 2016;8:281. [Google Scholar]
- 61.Bennett EM, et al. Human Impact on Erodable Phosphorus and Eutrophication: A Global Perspective Increasing accumulation of phosphorus in soil threatens rivers, lakes, and coastal oceans with eutrophication. BioScience. 2001;51:227–234. [Google Scholar]
- 62.Alauddin M, Quiggin J. Agricultural intensification, irrigation and the environment in South Asia: Issues and policy options. Ecol. Econ. 2008;65:111–124. [Google Scholar]
- 63.Vitousek PM, et al. Human Domination of Earth’s Ecosystems. Science. 1997;277:494–499. [Google Scholar]
- 64.Johnson PT, et al. Linking environmental nutrient enrichment and disease emergence in humans and wildlife. Ecol. Appl. 2010;20:16–29. doi: 10.1890/08-0633.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones BA, et al. Zoonosis emergence linked to agricultural intensification and environmental change. Proc. Natl. Acad. Sci. 2013;110:8399–8404. doi: 10.1073/pnas.1208059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morand S, et al. Infectious Diseases and Their Outbreaks in Asia-Pacific: Biodiversity and Its Regulation Loss Matter. PLoS ONE. 2014;9:e90032. doi: 10.1371/journal.pone.0090032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richter CH, et al. Intensified food production and correlated risks to human health in the Greater Mekong Subregion: a systematic review. Environ. Health. 2015;14:43. doi: 10.1186/s12940-015-0033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Croft AC, et al. Update on the antibacterial resistance crisis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2007;13:RA103–118. [PubMed] [Google Scholar]
- 69.Rossolini GM, et al. Update on the antibiotic resistance crisis. Curr. Opin. Pharmacol. 2014;18:56–60. doi: 10.1016/j.coph.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 70.Gilchrist MJ, et al. The Potential Role of Concentrated Animal Feeding Operations in Infectious Disease Epidemics and Antibiotic Resistance. Environ. Health Perspect. 2007;115:313–316. doi: 10.1289/ehp.8837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harinasuta C, Harinasuta T. Opisthorchis viverrini: life cycle, intermediate hosts, transmission to man and geographical distribution in Thailand. Arzneimittelforschung. 1984;34:1164–1167. [PubMed] [Google Scholar]
- 72.Kaewpitoon N, et al. Opisthorchis viverrini: The carcinogenic human liver fluke. World J. Gastroenterol. WJG. 2008;14:666–674. doi: 10.3748/wjg.14.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Petney TN, et al. The zoonotic, fish-borne liver flukes Clonorchis sinensis, Opisthorchis felineus and Opisthorchis viverrini. Int. J. Parasitol. 2013;43:1031–1046. doi: 10.1016/j.ijpara.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Upatham ES, et al. Relationship between prevalence and intensity of Opisthorchis viverrini infection, and clinical symptoms and signs in a rural community in north-east Thailand. Bull. World Health Organ. 1984;62:451–461. [PMC free article] [PubMed] [Google Scholar]
- 75.Allen JE, Wynn TA. Evolution of Th2 Immunity: A Rapid Repair Response to Tissue Destructive Pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Little TJ, et al. The Coevolution of Virulence: Tolerance in Perspective. PLoS Pathog. 2010;6:e1001006. doi: 10.1371/journal.ppat.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Medzhitov R, et al. Disease Tolerance as a Defense Strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McSorley HJ, Maizels RM. Helminth Infections and Host Immune Regulation. Clin. Microbiol. Rev. 2012;25:585–608. doi: 10.1128/CMR.05040-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yazdanbakhsh M, et al. Th2 responses without atopy: immunoregulation in chronic helminth infections and reduced allergic disease. Trends Immunol. 2001;22:372–377. doi: 10.1016/s1471-4906(01)01958-5. [DOI] [PubMed] [Google Scholar]
- 80.Sripa B, Kaewkes S. Relationship between parasite-specific antibody responses and intensity of Opisthorchis viverrini infection in hamsters. Parasite Immunol. 2000;22:139–145. doi: 10.1046/j.1365-3024.2000.00286.x. [DOI] [PubMed] [Google Scholar]
- 81.Jittimanee J, et al. Protective immunization of hamsters against Opisthorchis viverrini infection is associated with the reduction of TGF-β expression. Acta Trop. 2012;122:189–195. doi: 10.1016/j.actatropica.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 82.Wongratanacheewin S, et al. Immunodepression in hamsters experimentally infected with Opisthorchis viverrini. J. Helminthol. 1987;61:151–156. doi: 10.1017/s0022149x00009913. [DOI] [PubMed] [Google Scholar]
- 83.Magen E, et al. Chronic Opisthorchis felineus infection attenuates atherosclerosis – An autopsy study. Int. J. Parasitol. 2013;43:819–824. doi: 10.1016/j.ijpara.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 84.Magen E, et al. Can worms defend our hearts? Chronic helminthic infections may attenuate the development of cardiovascular diseases. Med. Hypotheses. 2005;64:904–909. doi: 10.1016/j.mehy.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 85.Mairiang E, et al. Ultrasonography assessment of hepatobiliary abnormalities in 3,359 subjects with Opisthorchis viverrini infection in endemic areas of Thailand. Parasitol. Int. 2012;61:208–211. doi: 10.1016/j.parint.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sung K-C, Kim SH. Interrelationship between fatty liver and insulin resistance in the development of type 2 diabetes. J. Clin. Endocrinol. Metab. 2011;96:1093–1097. doi: 10.1210/jc.2010-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Renaud F, de Meeüs T. A simple model of host-parasite evolutionary relationships. Parasitism: Compromise or conflict? J. Theor. Biol. 1991;152:319–327. doi: 10.1016/s0022-5193(05)80197-3. [DOI] [PubMed] [Google Scholar]
- 88.Fellous S, Salvaudon L. How can your parasites become your allies? Trends Parasitol. 2009;25:62–66. doi: 10.1016/j.pt.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 89.Horwitz P, Wilcox BA. Parasites, ecosystems and sustainability: an ecological and complex systems perspective. Int. J. Parasitol. 2005;35:725–732. doi: 10.1016/j.ijpara.2005.03.002. [DOI] [PubMed] [Google Scholar]