Abstract
We studied whether slower community walking speed and whether greater time spent lying down or sleeping were associated with higher mortality in people with lower extremity peripheral artery disease (PAD). Participants with an ankle–brachial index (ABI) < 0.90 were identified from Chicago medical centers. At baseline, participants reported their usual walking speed outside their home and the number of hours they spent lying down or sleeping per day. Cause of death was adjudicated using death certificates and medical record review. Analyses were adjusted for age, sex, race, comorbidities, ABI, and other confounders. Of 1314 PAD participants, 189 (14.4%) died, including 63 cardiovascular disease (CVD) deaths. Mean follow-up was 34.9 months ± 18.1. Relative to average or normal pace (2–3 miles/hour), slower walking speed was associated with greater CVD mortality: no walking at all: hazard ratio (HR) = 4.17, 95% confidence interval (CI) = 1.46–11.89; casual strolling (0–2 miles/hour): HR = 2.24, 95% CI = 1.16–4.32; brisk or striding (>3 miles/hour): HR = 0.55, 95% CI = 0.07–4.30. These associations were not significant after additional adjustment for the six-minute walk. Relative to sleeping or lying down for 8–9 hours, fewer or greater hours sleeping or lying down were associated with higher CVD mortality: 4–7 hours: HR = 2.08, 95% CI = 1.06–4.05; 10–11 hours: HR = 4.07, 95% CI = 1.86–8.89; ⩾12 hours: HR = 3.75, 95% CI = 1.47–9.62. These associations were maintained after adjustment for the six-minute walk. In conclusion, slower walking speed outside the home and less than 8 hours or more than 9 hours lying down per day are potentially modifiable behaviors associated with increased CVD mortality in patients with PAD.
Keywords: cardiovascular mortality, cardiovascular risk factors, intermittent claudication, modifiable behaviors, peripheral artery disease, physical activity, sedentary, six-minute walk test
Introduction
People with lower extremity peripheral artery disease (PAD) have increased rates of all-cause and cardiovascular disease (CVD) mortality compared to people without PAD, even after adjusting for known CVD risk factors.1,2 Statin medications and anti-platelet therapy reduce CVD event rates in patients with PAD.3,4 However, CVD event rates remain high in PAD.5
Supervised treadmill exercise significantly improves walking ability in people with PAD.6,7 However, there is no evidence that supervised exercise reduces mortality rates in PAD.7 Most people with PAD do not have access to or participate in supervised exercise.8 Walking speed during daily life is a measure of less strenuous walking activity, compared to supervised exercise.9 Time spent sleeping or lying down and hours spent sitting per day are behavioral measures of physical inactivity9 that may be targeted with interventions. However, associations of these measures with mortality have not been evaluated in people with PAD. We therefore studied associations of patient-reported walking speed outside the home, the number of hours spent sitting or lying down per day, and the number of hours spent sitting per day with subsequent mortality in patients with PAD. We hypothesized that slower patient-reported walking speed and greater time spent sitting would each be associated with higher all-cause and CVD mortality in people with PAD. Based on prior literature in people without PAD,10,11 we hypothesized that sleeping or lying down for 8–9 hours per day would be associated with lower mortality rates than higher or lower durations of sleeping or lying down.
Methods
Study overview
We combined data from three prospective observational studies of patients with PAD: The Walking and Leg Circulation Study (WALCS) II, WALCS III, and the BRAVO Study.12–14 WALCS II was conducted between 2002 and 2006, WALCS III between 2005 and 2014, and BRAVO between 2009 and 2013. In all three studies, participants were recruited from Chicago-area medical centers and were followed longitudinally. The institutional review boards of Northwestern University and all participating medical centers approved the protocol. All participants gave written informed consent. In the WALCS II and WALCS III cohorts, participants completed baseline testing and returned annually for follow-up visits for up to 4 years. In the BRAVO cohort, participants completed baseline testing and returned every 2 months for up to 3.3 years.
Participant identification
In all three cohorts, PAD participants were identified from among consecutive patients with PAD in Chicago-area vascular surgery and non-invasive vascular laboratories. Participants were also identified from among lists of PAD patients in cardiology, general medicine, endocrinology, and geriatric clinics at Northwestern.
Inclusion criteria
All participants in these analyses had a baseline ankle–brachial index (ABI) < 0.90. Participants with PAD and non-compressible lower extremity vessels and those with prior revascularization with a normal ABI at the time of baseline testing were not included in these analyses.
Exclusion criteria
In all three studies, patients with dementia were excluded because of their inability to answer questions accurately. Nursing home residents were excluded because they had severely impaired functioning at baseline. Non-English-speaking patients were excluded because investigators were not fluent in non-English languages. Patients with recent major surgery were excluded because the surgery may have influenced their walking speed, sitting down time, or lying down time. In the WALCS II and WALCS III cohorts, participants who were wheelchair bound or who had history of leg or foot amputations were excluded because of their severe functional impairment at baseline. In the WALCS III cohort, participants with contraindications to magnetic resonance imaging (MRI) testing were excluded. Participants with critical limb ischemia were not included in any of the cohorts.
Ankle–brachial index measurement
A hand-held Doppler probe (Nicolet Vascular Pocket Dop II; Nicolet Biomedical Inc., Golden, CO, USA) was used to measure systolic pressures in the right and left brachial, dorsalis pedis, and posterior tibial arteries.15 Each pressure was measured twice. For each leg, the ABI was calculated by dividing the mean of the dorsalis pedis and posterior tibial pressures by the mean of the four brachial pressures.16 Average brachial pressures in the arm with the highest pressure were used when one brachial pressure was higher than the opposite brachial pressure in both measurement sets and the two brachial pressures differed by 10 mmHg or more in at least one measurement set. In these cases, subclavian stenosis was possible.17 The leg with the lowest ABI was used in analyses.
Participant-perceived walking speed outside the home
At baseline, participants were asked to describe their typical walking speed outside their home. Participants were asked: ‘When you walk outside your house, what is your usual walking speed?’.9 Participants selected from one of the following responses: (a) No walking at all; (b) Casual strolling (0–2 miles per hour); (c) average or normal (2–3 miles per hour); (d) brisk or striding (> 3 miles per hour). This measure of community walking speed previously was reported to predict rates of cardiovascular events in community dwelling women9 and decline in calf skeletal muscle density in people with PAD.18
Hours sleeping or lying down and hours spent sitting per day
At baseline, participants were asked: ‘In a typical day, how many hours do you spend sleeping or lying down? Include time spent sleeping, lying down resting, napping and trying to get to sleep’.9 Participants selected from one of the following responses: (a) 4–7 hours; (b) 8–9 hours; (c) 10–11 hours; (d) 12 or more hours.9 This measure of hours spent sleeping or lying down predicted cardiovascular event rates in community dwelling women.9 Participants were also asked: ‘In a typical day, how many hours do you spend sitting? Be sure to include time spent sitting at a desk, riding in a car, eating, and sitting up watching television’.9,19 Participants selected from one of the following responses: (a) Less than 4 hours; (b) 4–7 hours; (c) 8–11 hours; (d) 12 or more hours. This measure of hours spent sitting previously was reported to predict rates of cardiovascular events in community dwelling women9 and rate of decline in walking velocity in people with PAD.18
Six-minute walk
The six-minute walk test was performed at baseline using a standardized and well-validated protocol.20,21 Participants walked up and down a 100-foot hallway for six minutes after instructions to cover as much distance as possible.
Comorbidities
Comorbidities assessed at baseline were diabetes, angina, myocardial infarction, heart failure, cancer, chronic lung disease, and stroke. Disease-specific algorithms that combine data from patient report, medical record review, medications, laboratory values, and a questionnaire completed by the participant’s primary care physician were used to verify and document baseline comorbidities.22
Other measures
Height and weight were measured at baseline. Body mass index (BMI) was calculated as weight (kilograms)/(height (meters))2. Cigarette smoking history was determined with patient report.
Mortality assessment
At baseline, participants provided names of three proxies to assist with ascertaining complete follow-up. Mortality information was obtained from family members, proxies, and primary care physicians. For patients lost to follow-up, we used the Social Security Administration death database to search for deaths. Death certificates were obtained from the State of Illinois or from the patients’ medical records. CVD deaths consisted of deaths due to coronary heart disease, stroke, peripheral vascular disease, and other CVD. The date of death was obtained from the death certificate. Follow-up for participants who were not deceased continued until the date of last contact at a study visit or by telephone.
Statistical analyses
Linear and logistic regressions were used to compare continuous and binary baseline clinical characteristics across categories of participant-reported walking speed outside the home, hours spent lying down or sleeping per day, and hours spent sitting per day, respectively. Pearson correlation coefficients were calculated between the six-minute walk and each patient-reported measure of walking speed, time spent lying down or sleeping, and time spent seated per day.
Proportional hazards analyses were used to compare the all-cause mortality and cardiovascular mortality between the reference group and remaining categories for walking speed outside the home, hours spent lying down or sleeping per day, and hours spent sitting per day, respectively. Analyses were adjusted for age, sex, race, study cohort (WALCS II, WALCS III, or BRAVO), BMI, smoking, and comorbidities, and the ABI. Analyses were repeated with additional adjustment for the baseline six-minute walk. For statistically significant results, separate analyses were performed excluding participants who died during the first 12 months of follow-up, to determine whether the associations were substantively influenced by participants who died soon after the independent measures of interest. Pairwise statistical comparisons were made between a reference category for each independent variable of interest (average or normal walking speed, less than 4 hours sitting, and 8–9 hours lying down or sleeping per day, respectively) with each remaining category of walking speed, hours spent sitting, and hours lying down or sleeping, respectively. The trend test was conducted by coding the independent variable of interest, such as walking speed, as 1, 2, 3, etc. A p-value of < 0.05 was considered statistically significant. The intent of the analyses was to identify overall patterns of associations. Therefore, we did not adjust for multiple comparisons.
Analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA).
Results
A total of 1314 PAD participants (438 from WALCS II, 354 from WALCS III and 522 from BRAVO) met eligibility criteria and were included in analyses (Figure 1). Of the 1314 participants, 189 (14.4%) died during a mean follow-up of 34.9 months ± 18.1. Of the 189 deaths, 63 (33.3%) were CVD deaths, 39 (20.6%) were cancer deaths, 10 (5.3%) were diabetes-related deaths, one (0.62%) was accidental, 29 cause (15.3%) was unknown, and the remainder (n=47, 24.9%) were due to miscellaneous causes.
Figure 1.
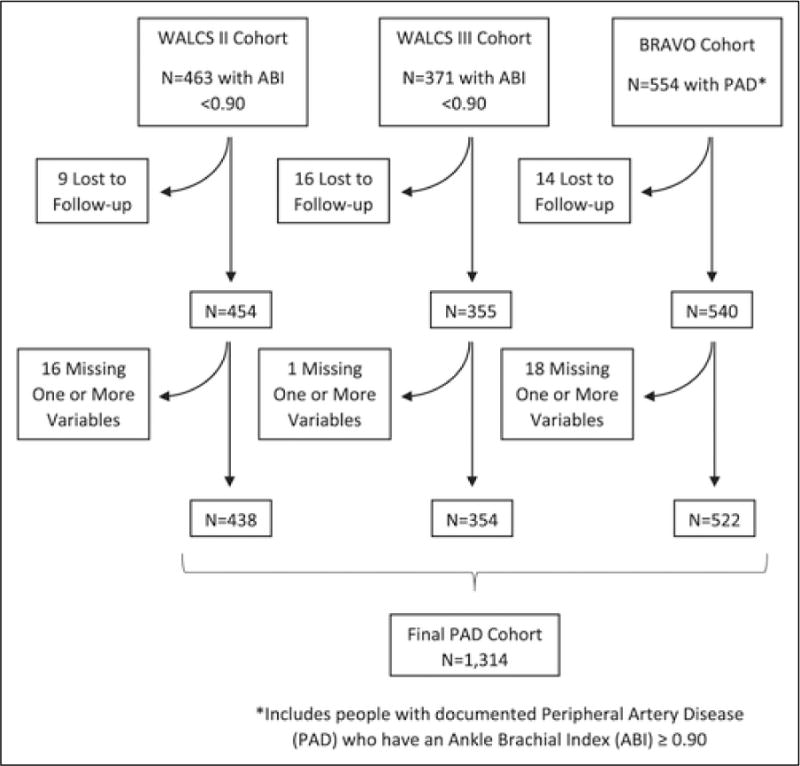
Summary of included participants from three observational cohorts. (WALCS, Walking and Leg Circulation Study.)
Pearson correlation coefficients between the six-minute walk and participant-reported walking speed, time spent lying down or sleeping, and time spent sitting per day were 0.470 (p<0.001), –0.142 (p<0.001), and –0.068 (p=0.016), respectively.
Table 1 compares characteristics of survivors and decedents at baseline. Decedents were older, had a lower ABI and had a lower BMI than survivors. Decedents included a smaller proportion of African Americans and had higher prevalences of pulmonary disease, cancer, angina, myocardial infarction, and heart failure compared to survivors.
Table 1.
Comparison of baseline characteristics between participants who died versus survived.
| Overall | Survivors | Deceased | p-valuea | |
|---|---|---|---|---|
| N | 1314 | 1125 | 189 | |
| Age, years | 70.62 (9.95) | 69.83 (9.83) | 75.31 (9.38) | <0.0001 |
| Ankle–brachial index | 0.70 (0.25) | 0.71 (0.25) | 0.65 (0.22) | 0.002 |
| BMI, kg/m2 | 29.01 (5.82) | 29.17 (5.87) | 28.05 (5.39) | 0.015 |
| Male | 61.6% | 61.2% | 63.5% | 0.557 |
| African American | 29.3% | 30.8% | 20.1% | 0.003 |
| Current smoker | 21.8% | 21.9% | 21.2% | 0.829 |
| Diabetes | 39.2% | 38.7% | 42.3% | 0.340 |
| Pulmonary disease | 40.3% | 38.4% | 51.9% | 0.001 |
| Cancer | 17.9% | 17.0% | 23.3% | 0.036 |
| Angina | 26.9% | 25.4% | 35.5% | 0.004 |
| MI | 21.5% | 20.5% | 27.5% | 0.031 |
| CHF | 22.2% | 20.3% | 33.3% | <0.0001 |
| Stroke | 18.9% | 18.2% | 22.8% | 0.141 |
The p-value comparison is between decedents and survivors.
Data shown are mean (standard deviation) unless otherwise specified.
BMI, body mass index; MI, myocardial infarction; CHF, congestive heart failure.
Table 2 shows participant characteristics according to baseline walking speed outside the home, hours spent lying down or sleeping per day, and hours spent sitting per day. Slower self-reported walking speed outside the home was associated with older age, lower ABI values, higher BMI values, a higher prevalence of women and African Americans, and higher prevalences of diabetes, pulmonary disease, angina, myocardial infarction, heart failure, and stroke. Compared to participants who reported sleeping or lying down for 8–9 hours per day, those who reported sleeping or lying down either more or less than 8–9 hours per day were younger and included higher prevalences of current smoking. Duration of sleeping or lying down was also associated with the prevalence of African Americans, males, and stroke. Greater time sitting was associated with younger age, higher BMI and a higher prevalence of pulmonary disease. The prevalences of males and participants with diabetes mellitus also varied significantly across the duration of time sitting (Table 2).
Table 2.
Characteristics of study participants across participant-reported walking speed, sitting/lying down time, and sedentary categories at baseline.
| Group | Walking speed
|
p-trend | |||
|---|---|---|---|---|---|
| No walking at all | Casual strolling (0–2 mph) | Average or normal (2–3 mph) | Brisk or striding (> 3 mph) | ||
| N | 34 | 651 | 523 | 98 | |
| Age, years | 73.91 (10.79) | 71.08 (10.15) | 70.18 (9.56) | 68.10 (9.98) | 0.001 |
| Ankle–brachial index | 0.71 (0.43) | 0.67 (0.21) | 0.73 (0.27) | 0.74 (0.19) | <0.001 |
| BMI, kg/m2 | 29.97 (6.86) | 29.88 (6.34) | 28.17 (5.10) | 27.41 (4.47) | <0.0001 |
| Male | 47.06% | 60.06% | 63.1% | 70.41% | 0.013 |
| African American | 41.2% | 31.0% | 27.7% | 22.5% | 0.020 |
| Current smoker | 17.7% | 23.2% | 20.7% | 20.4% | 0.456 |
| Diabetes | 44.1% | 43.5% | 36.5% | 20.4% | <0.0001 |
| Pulmonary disease | 47.1% | 46.9% | 32.3% | 36.7% | <0.0001 |
| Cancer | 17.7% | 17.7% | 18.2% | 19.4% | 0.688 |
| Angina | 41.2% | 30.6% | 21.6% | 24.5% | 0.001 |
| Myocardial infarction | 26.5% | 24.1% | 19.3% | 13.3% | 0.004 |
| Heart failure | 38.2% | 27.5% | 16.1% | 12.2% | <0.0001 |
| Stroke | 20.6% | 22.4% | 16.1% | 10.2% | 0.001 |
| Sleeping/lying down hours | |||||
| Group | 4–7 hours | 8–9 hours | 10–11 hours | 12 or more hours | p-value |
| N | 568 | 511 | 145 | 75 | |
| Age, years | 69.65 (9.82) | 71.83 (9.66) | 70.31 (10.14) | 70.48 (11.87) | 0.005 |
| Ankle–brachial index | 0.70 (0.23) | 0.70 (0.26) | 0.70 (0.25) | 0.71 (0.27) | 0.969 |
| BMI, kg/m2 | 29.07 (5.37) | 28.97 (6.16) | 29.17 (6.17) | 28.49 (6.30) | 0.854 |
| Male | 62.3% | 64.4% | 56.6% | 49.3% | 0.043 |
| African American | 35.7% | 22.5% | 20.7% | 38.7% | <0.0001 |
| Current smoker | 23.9% | 17.8% | 20.0% | 29.3% | 0.028 |
| Diabetes | 38.2% | 39.7% | 42.1% | 44.0% | 0.696 |
| Pulmonary disease | 39.6% | 38.2% | 43.5% | 53.3% | 0.073 |
| Cancer | 17.8% | 18.2% | 16.6% | 17.3% | 0.974 |
| Angina | 25.7% | 27.0% | 28.3% | 29.3% | 0.859 |
| MI | 20.4% | 21.5% | 23.5% | 24.0% | 0.804 |
| CHF | 22.7% | 20.7% | 20.7% | 26.7% | 0.629 |
| Stroke | 20.8% | 15.1% | 15.2% | 30.7% | 0.002 |
| Sitting hours | |||||
| Group | Less than 4 hours | 4–7 hours | 8–11 hours | 12 or more hours | p-trend |
| N | 153 | 598 | 380 | 183 | |
| Age, years | 70.46 (9.45) | 71.96 (9.63) | 69.92 (10.02) | 67.78 (10.58) | <0.0001 |
| Ankle–brachial index | 0.69 (0.19) | 0.69 (0.24) | 0.70 (0.27) | 0.72 (0.26) | 0.166 |
| BMI, kg/m2 | 28.11 (5.58) | 28.73 (5.57) | 29.34 (5.94) | 29.98 (6.40) | 0.001 |
| Male | 56.9% | 58.2% | 68.2% | 62.8% | 0.017 |
| African American | 35.9% | 29.9% | 23.9% | 32.8% | 0.217 |
| Current smoker | 26.1% | 18.6% | 23.2% | 25.7% | 0.321 |
| Diabetes | 31.4% | 39.8% | 36.6% | 49.2% | 0.013 |
| Pulmonary disease | 29.4% | 39.8% | 42.6% | 46.5% | 0.002 |
| Cancer | 18.9% | 18.4% | 16.1% | 19.1% | 0.738 |
| Angina | 26.8% | 24.8% | 27.1% | 33.3% | 0.078 |
| MI | 18.3% | 21.2% | 21.6% | 25.1% | 0.159 |
| CHF | 18.3% | 21.7% | 22.9% | 25.1% | 0.134 |
| Stroke | 20.9% | 20.1% | 15.5% | 20.2% | 0.377 |
Data shown are mean (standard deviation) unless otherwise specified.
mph, miles per hour; BMI, body mass index; MI, myocardial infarction; CHF, congestive heart failure.
Adjusting for age, sex, race, BMI, smoking, ABI, and comorbidities, slower outdoor walking speed was associated with higher all-cause mortality (Figure 2A). PAD participants who reported an outdoor walking speed of casual strolling (0–2 miles per hour) had higher all-cause mortality rates than PAD participants who reported an outdoor walking speed of average or normal (2–3 miles per hour) (Figure 2A). There were no other significant pairwise associations between outdoor walking speed and all-cause mortality. Associations in Figure 2A were no longer statistically significant when participants who died during the first 12 months of follow-up were excluded (p-trend = 0.053).
Figure 2.
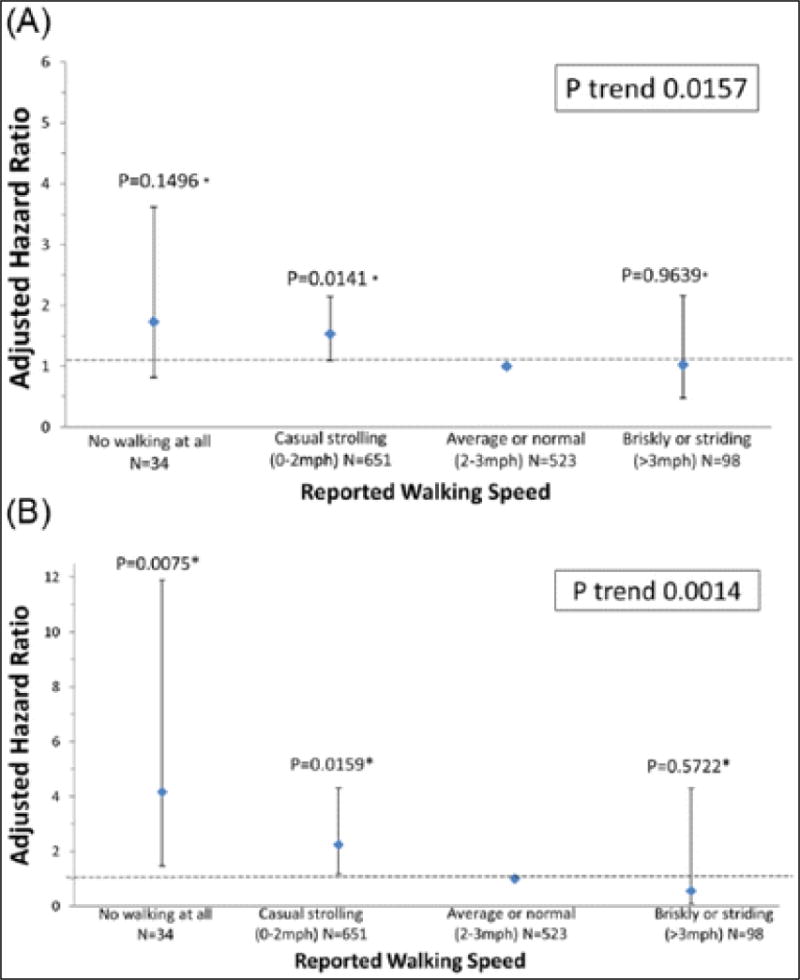
Associations of participant-reported community-based walking speed with (A) all-cause mortality and (B) cardiovascular disease mortality in peripheral artery disease participants. (Models are adjusted for age, sex, race, BMI, smoking status, comorbidities, study cohort, and ankle–brachial index. *Pairwise p-values based on group comparison with referent: average or normal walking speed.)
Adjusting for age, sex, race, BMI, smoking, ABI, and comorbidities, slower outdoor walking speed was associated with higher CVD mortality (Figure 2B). PAD participants who reported no outdoor walking and those who reported an outdoor walking speed of casual strolling had higher CVD mortality rates compared to PAD participants who reported an outdoor walking speed of average or normal. Associations in Figure 2B remained statistically significant when participants who died during the first 12 months of follow-up were excluded (p-trend = 0.009).
Adjusting for age, sex, race, BMI, smoking, ABI, and comorbidities, time spent sleeping or lying down was associated significantly with all-cause mortality and CVD mortality (Figure 3). Compared to PAD participants who reported lying down or sleeping 8–9 hours per day, PAD participants who reported lying down or sleeping 10–11 hours per day and those who reported lying down or sleeping 12 or more hours per day had higher all-cause mortality (Figure 3A). Results in Figure 3A remained statistically significant when participants who died during the first 12 months of follow-up were excluded (p-value = 0.003).
Figure 3.
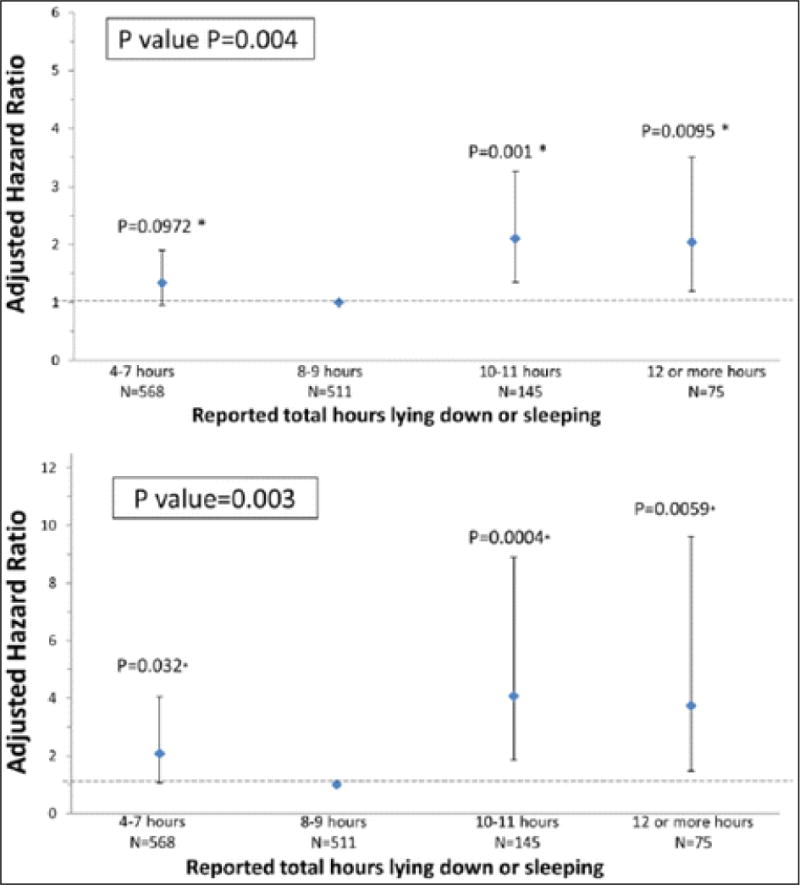
Associations of total lying down or sleeping time with (A) all-cause mortality and (B) cardiovascular disease mortality in peripheral artery disease. (Models are adjusted for age, sex, race, BMI, smoking status, comorbidities, study cohort, and ankle–brachial index. *Pairwise p-values based on group comparison with referent: 8–9 hours.)
Compared to PAD participants who reported sleeping 8–9 hours per day, those who reported lying down or sleeping 10–11 hours per day, 12 or more hours per day, and 4–7 hours per day had higher CVD mortality (Figure 3B). Results in Figure 3B remained statistically significant when participants who died during the first 12 months of follow-up were excluded (p-value = 0.006).
After adjusting for age, sex, race, BMI, ABI, smoking, and comorbidities, there were no associations of time sitting per day with all-cause or CVD mortality (Table 3).
Table 3.
Associations of participant-reported time spent sitting with all-cause and cardiovascular disease mortality in peripheral artery disease participants (N=1314).
| Time spent sitting (self-reported) | N | Number of deaths | Hazard ratio (95% confidence interval) | Pairwise p-value |
p-trend |
|---|---|---|---|---|---|
| All-cause mortality | |||||
| < 4 hours | 153 | 22 | 1.00 (reference) | N/A | 0.157 |
| 4–7 hours | 598 | 80 | 0.94 (0.58–1.54) | 0.811 | |
| 8–11 hours | 380 | 58 | 1.15 (0.69–1.91) | 0.589 | |
| ⩾12 hours | 183 | 29 | 1.30 (0.73–2.30) | 0.371 | |
| Cardiovascular mortality | |||||
| < 4 hours | 153 | 11 | 1.00 (reference) | N/A | 0.824 |
| 4–7 hours | 598 | 24 | 0.65 (0.31–1.36) | 0.256 | |
| 8–11 hours | 380 | 20 | 0.83 (0.39–1.79) | 0.644 | |
| ⩾12 hours | 183 | 8 | 0.73 (0.28–1.87) | 0.506 |
Table 4 shows associations of outdoor walking speed and hours lying down or sleeping with all-cause and CVD mortality before and after adjusting for six-minute walk performance. Of the 1314 participants, 1234 (93.9%) had data for both the six-minute walk and outdoor walking speed at baseline, and 1227 (93.4%) had data for both the six-minute walk and hours lying down or sleeping. Associations of outdoor walking speed with all-cause and CVD mortality were no longer statistically significant after adjusting for the six-minute walk in addition to age, sex, race, BMI, ABI, smoking, and comorbidities (Table 4). The association of 10–11 hours lying down or sleeping per day with higher all-cause mortality remained statistically significant compared to sleeping or lying down 8–9 hours/day, even after adjusting for the six-minute walk (hazard ratio (HR) = 1.99, 95% confidence interval (CI) = 1.26–3.15, p=0.003) (Table 4). Associations of hours lying down or sleeping with cardiovascular mortality were attenuated but remained statistically significant even after adjusting for the six-minute walk in addition to age, sex, race, BMI, ABI, smoking, and comorbidities (Table 4). In this fully adjusted model, the six-minute walk was significantly associated with both all-cause mortality and CVD mortality (HR = 0.898, 95% CI = 0.859–0.939/100 feet, p-value <0.0001 and HR = 0.890, 95% CI = 0.822–0.965/100 feet, p-value = 0.004, respectively).
Table 4.
Associations of outdoor walking speed and time spent lying down or sleeping with mortality, with and without the adjustment for baseline six-minute walk performance in peripheral artery disease participants.
| Walking speed (self-reported) | N | No. of deaths | Hazard ratio (95% CI) |
Pairwise p-value |
p-trend | Hazard ratio (95% CI) |
Pairwise p-value |
p-trend |
|---|---|---|---|---|---|---|---|---|
| Not adjusted for 6-minute walk | Includes adjustment for 6-minute walk | |||||||
| All-cause mortality | ||||||||
| No walking at all | 21 | 5 | 1.20 (0.46–3.14) | 0.705 | 0.021 | 0.58 (0.21–1.61) | 0.297 | 0.814 |
| Casual strolling (0–2 mph) | 615 | 112 | 1.51 (1.07–2.13) | 0.019 | 1.12 (0.78–1.63) | 0.538 | ||
| Average or normal (2–3 mph) | 505 | 51 | 1.00 (reference) | Reference | 1.00 (reference) | Reference | ||
| Brisk or striding (>3 mph) | 93 | 6 | 0.80 (0.34–1.87) | 0.599 | 0.87 (0.37–2.04) | 0.744 | ||
| Cardiovascular mortality | ||||||||
| No walking at all | 21 | 4 | 3.89 (1.16–13.09) | 0.028 | 0.005 | 2.46 (0.65–9.28) | 0.184 | 0.064 |
| Casual strolling (0–2 mph) | 615 | 40 | 2.13 (1.10–4.14) | 0.025 | 1.76 (0.87–3.54) | 0.115 | ||
| Average or normal (2–3 mph) | 505 | 12 | 1.00 (reference) | Reference | 1.00 (reference) | |||
| Brisk or striding (>3 mph) | 93 | 1 | 0.57 (0.07–4.48) | 0.597 | 0.62 (0.08–4.86) | 0.652 | ||
|
| ||||||||
| Hours lying down or sleeping (self-reported) | N | No. of deaths | Hazard ratio (95% CI) |
Pairwise p-value |
p-value | Hazard ratio (95% CI) |
Pairwise p-value |
p-value |
| Not adjusted for 6-minute walk | Includes adjustment for 6-minute walk | |||||||
|
| ||||||||
| All-cause mortality | ||||||||
| 4–7 hours | 536 | 69 | 1.33 (0.93–1.90) | 0.124 | 0.004 | 1.39 (0.97–2.00) | 0.071 | 0.021 |
| 8–9 hours | 486 | 57 | 1.00 (reference) | Reference | 1.0 (reference) | Reference | ||
| 10–11 hours | 138 | 29 | 2.15 (1.36–3.40) | 0.001 | 1.99 (1.26–3.15) | 0.003 | ||
| 12 or more hours | 67 | 18 | 2.07 (1.16–3.68) | 0.013 | 1.73 (0.97–3.09) | 0.066 | ||
| Cardiovascular mortality | ||||||||
| 4–7 hours | 536 | 22 | 1.94 (0.96–3.93) | 0.064 | 0.002 | 2.05 (1.01–4.16) | 0.047 | 0.004 |
| 8–9 hours | 486 | 13 | 1.00 (reference) | Reference | 1.00 (reference) | Reference | ||
| 10–11 hours | 138 | 13 | 4.44 (2.00–9.88) | <0.001 | 4.17 (1.88–9.26) | <0.001 | ||
| 12 or more hours | 67 | 7 | 3.83 (1.39–10.57) | 0.010 | 3.43 (1.24–9.47) | 0.017 | ||
CI, confidence interval; mph, miles per hour.
Discussion
Among 1314 men and women with PAD followed for nearly 3 years, slower patient-reported outdoor community walking speed was associated with higher all-cause and CVD mortality, even after adjusting for comorbidities, age, and other confounders. These results were independent of PAD severity, measured by the ABI. However, these associations were no longer statistically significant after additional adjustment for the six-minute walk. PAD participants who reported sleeping or lying down either more or less than 8–9 hours per day had higher all-cause and CVD mortality compared to people who reported sleeping or lying down 8–9 hours per day. These associations of lying down or sleeping time per day with all-cause and CVD mortality were independent of PAD severity and were not significantly attenuated after adjustment for six-minute walk performance.
Previous work demonstrated that poorer six-minute walk performance, slower four-meter walking velocity, poorer treadmill-measured exercise capacity, poorer patient-reported stair climbing ability measured with the Walking Impairment Questionnaire (WIQ), and greater declines in patient-reported walking ability measured by the WIQ were each associated with higher all-cause and CVD mortality in people with PAD.20,23–26 However, objective measures of walking are distinct from participants’ perception of their outdoor walking speed and hours spent lying down or sleeping. The WIQ assesses participants’ perception of the ease with which they walk increasingly greater distances, faster speeds, or up flights of stairs. In contrast, we used a single question to assess PAD participants’ perception of their outdoor walking speed.9,18 We also used a single question to assess the number of hours spent lying down or sleeping.9,18 Based on our results, further study is warranted to determine whether interventions to increase walking speed outside the home may reduce mortality in people with PAD. Identifying biologic pathways associated with mortality in PAD is important in order to establish potential therapeutic targets to reduce mortality in people with PAD.
The lack of association between time spent sitting and mortality is unexpected. Prior study showed that greater quantities of accelerometer-measured physical activity were associated with lower all-cause and CVD mortality among people with PAD.27 There are at least three potential explanations for the lack of association between hours spent sitting and mortality. First, it may be true that only vigorous activity (that is not measured by time spent seated) is associated with reduced mortality. Second, the scale used to measure time sitting may not be ideally suited for PAD participants. PAD participants are known to be more inactive than non-PAD participants.28 Our question about time spent sitting categorized all participants with time spent sitting of > 12 hours into one group.9,18 The single question did not allow us to distinguish between people sitting for 13 versus 15 hours per day, for example. Third, PAD participants may not accurately report the time they spend seated.
Our results suggest that the optimal combined duration of sleeping or lying down at night is 8–9 hours per day for people with PAD. Both shorter and longer time periods of sleeping or lying down per day were associated with higher rates of CVD mortality. These findings are consistent with some studies of sleep duration in community dwelling men and women without PAD.9–11,29,30 However, a recent systematic review of people without PAD reported that a ‘U’ shape association of sleep with all-cause mortality has not been consistently demonstrated.31 Potential mechanisms by which a short sleep duration may be associated with increased mortality include higher levels of cortisol or increased inflammation in people with shorter sleep duration.31 Potential mechanisms by which prolonged sleep duration may be associated with increased mortality include altered immune function, depression, or underlying disease processes in people with longer sleep duration.
The six-minute walk is a well-validated measure of walking endurance in patients with PAD.20,21,32 Previous studies showed that poorer performance on the six-minute walk test was associated with higher rates of mobility loss and mortality in patients with PAD.20,32 Our results showed that the association of walking speed in the community with all-cause and CVD mortality was attenuated and no longer statistically significant after additional adjustment for the six-minute walk test. This may be related to the moderately strong correlation between the six-minute walk and walking speed in the community. However, this finding may also be related to the fact that the six-minute walk measure is an objective assessment, whereas patient-reported walking speed outside the home is a subjective assessment. Subjective assessments are more susceptible to reporting bias and are based on a participant’s perception rather than a direct objective measurement. The six-minute walk test requires the presence of a 100-foot hallway, a stopwatch, and a coordinator to conduct the test. Six-minute walk testing may not be feasible in all clinical settings. When the six-minute walk test, a treadmill test, or a multi-component questionnaire is not feasible in clinical practice, obtaining information from patients with a single question about their walking speed in the community and/or about the number of hours they spend lying down or sleeping may be useful alternates. In contrast to patient-reported walking speed, the associations of patient-reported time spent lying down or sleeping with all-cause and CVD mortality were not attenuated, even after adjustment for the six-minute walk.
Self-reported outdoor walking speed and time spent lying down or sleeping are likely to be strongly associated with overall health, and thereby be proxies for mortality risk. PAD patients with more severe or greater numbers of comorbidities may sleep more hours per day and also have higher mortality. The association of outdoor walking speed with all-cause mortality was no longer statistically significant, but associations of time spent lying down or sleeping remained statistically significant, even after participants who died during the first 12 months of follow-up were excluded from analyses. These results suggest that our results may not be overly influenced by the greater burden of comorbidities that increase near-term mortality among PAD participants who slept more.
Limitations
Our study has limitations. First, our study was observational and findings should not be misconstrued as causal. Second, because of the observational study design, we cannot rule out the possibility of residual confounding. Third, our study design does not allow us to discern the biological explanation for the significant associations identified. For example, further study is needed to determine whether very long or very short durations of sleeping alter levels of inflammation, immune function, or cortisol levels, thereby increasing mortality. Fourth, our findings may not be generalizable to PAD patients who did not meet study inclusion criteria. Fifth, the mean follow-up for our study was 34 months. Our findings may not be generalizable to shorter or longer term follow-up. Sixth, we did not adjust for multiple comparisons, and therefore some of the associations may have been significant due to chance. Seventh, our analyses did not include accelerometer-measured physical activity, a more precise measure of vigorous activity.
Conclusion
In conclusion, self-reported outdoor walking speed in the community and the number of hours spent lying down or sleeping were associated with rates of all-cause and CVD mortality in patients with PAD. However, the association of outdoor walking speed with mortality was no longer significant after adjusting for the six-minute walk. Further study is needed to determine whether interventions that reverse or improve these potentially modifiable risk factors lower rates of mortality in patients with PAD.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: grants # R01-HL071223, R01-HL076298, R01-HL109244, R01-HL083064, R01-HL64739 and R01-HL089619 from the National Heart, Lung, and Blood Institute. Supported in part by the Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ankle Brachial Index Collaboration. Fowkes FG, Murray GD, Butcher I. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: A meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy TP, Reynolds MR, Cohen D. Correlation of patient-reported symptom outcomes and treadmill test outcomes after treatment for aortoiliac claudication. J Vasc Interv Radiol. 2013;24:1427–1435. doi: 10.1016/j.jvir.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 4.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerjee S, Vinas A, Mohammad A. Significance of an abnormal ankle-brachial index in patients with established coronary artery disease with and without associated diabetes mellitus. Am J Cardiol. 2014;113:1280–1284. doi: 10.1016/j.amjcard.2014.01.403. [DOI] [PubMed] [Google Scholar]
- 6.McDermott MM, Ades P, Guralnik JM. Treadmill training and resistance exercise in patients with peripheral arterial disease with and without intermittent claudication: A randomized trial. JAMA. 2009;301:165–174. doi: 10.1001/jama.2008.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lane R, Ellis B, Watson L. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2014;7:CD000990. doi: 10.1002/14651858.CD000990.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Regensteiner JG. Exercise rehabilitation for the patient with intermittent claudication: A highly effective yet underutilized treatment. Curr Drug Targets Cardiovasc Haematol Disord. 2004;4:233–239. doi: 10.2174/1568006043336195. [DOI] [PubMed] [Google Scholar]
- 9.Manson JE, Greenland P, LaCroix AZ. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. N Engl J Med. 2002;347:716–725. doi: 10.1056/NEJMoa021067. [DOI] [PubMed] [Google Scholar]
- 10.Cappuccio FP, D’Elia L, Strazzullo P. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallichino L, Kalesan B. Sleep duration and mortality: A systematic review and meta-analysis. J Sleep Res. 2009;18:148–158. doi: 10.1111/j.1365-2869.2008.00732.x. [DOI] [PubMed] [Google Scholar]
- 12.McDermott MM, Ferrucci L, Guralnik J. Pathophysiological changes in calf muscle predict mobility loss at 2-year follow-up in men and women with peripheral arterial disease. Circulation. 2009;120:1048–1055. doi: 10.1161/CIRCULATIONAHA.108.842328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDermott MM, Carroll TJ, Kibbe M. Proximal superficial femoral artery occlusion, collateral vessels, and walking performance in peripheral artery disease. JACC Cardiovasc Imaging. 2013;6:687–694. doi: 10.1016/j.jcmg.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott MM, Greenland P, Liu K. Vulnerable blood in high risk vascular patients: Study design and methods. Contemp Clin Trials. 2014;38:121–129. doi: 10.1016/j.cct.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aboyans V, Criqui MH, Abraham P. Measurement and interpretation of the ankle-brachial index: A scientific statement from the American Heart Association. Circulation. 2012;126:2890–2909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 16.McDermott MM, Criqui MH, Liu K. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. J Vasc Surg. 2000;32:1164–1171. doi: 10.1067/mva.2000.108640. [DOI] [PubMed] [Google Scholar]
- 17.Shadman R, Criqui MH, Bundens WP. Subclavian artery stenosis: Prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–623. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 18.McDermott MM, Liu K, Ferrucci L. Greater sedentary hours and slower walking speed outside the home predict faster declines in functioning and adverse calf muscle changes in peripheral arterial disease. J Am Coll Cardiol. 2011;57:2356–2364. doi: 10.1016/j.jacc.2010.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu FB, Li TY, Colditz GA. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 20.McDermott MM, Tian L, Liu K. Prognostic value of functional performance for mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2008;51:1482–1489. doi: 10.1016/j.jacc.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott MM, Guralnik JM, Criqui MH. Six-minute walk is a better outcome measure than treadmill walking tests in therapeutic trials of patients with peripheral artery disease. Circulation. 2014;130:61–68. doi: 10.1161/CIRCULATIONAHA.114.007002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried LP, Kasper JD, Williamson JD. Disease ascertainment algorithms. In: Guralnik JM, Fried LP, Simonsick EM, editors. The Women’s Health and Aging Study: Health and Social Characteristics of Older Women with Disability. Bethesda, MD: National Institute on Aging; 1995. Appendix E, NIH Pub. No. 95-4009. [Google Scholar]
- 23.Leeper N, Myers J, Zhou M. Exercise capacity is the strongest predictor of mortality in patients with peripheral arterial disease. J Vasc Surg. 2013;57:728–733. doi: 10.1016/j.jvs.2012.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jain A, Liu K, Ferrucci L. The walking impairment questionnaire stair-climbing score predicts mortality in men and women with peripheral arterial disease. J Vasc Surg. 2012;55:1662–1673. doi: 10.1016/j.jvs.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jain A, Liu K, Ferrucci L. Declining walking impairment questionnaire scores are associated with subsequent increased mortality in peripheral artery disease. J Am Coll Cardiol. 2013;30:1820–1829. doi: 10.1016/j.jacc.2013.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris DR, Rodriguiez AJ, Moxon JV. Association of lower extremity performance with cardiovascular and all-cause mortality in patients with peripheral artery disease: A systematic review and meta-analysis. J Am Heart Assoc. 2014;3:e001105. doi: 10.1161/JAHA.114.001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garg PK, Tian L, Criqui MH. Physical activity during daily life and mortality in patients with peripheral arterial disease. Circulation. 2006;114:242–248. doi: 10.1161/CIRCULATIONAHA.105.605246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDermott MM, Greenland P, Liu K. The ankle brachial index is associated with leg function and physical activity: The Walking and Leg Circulation Study. Ann Intern Med. 2002;136:873–883. doi: 10.7326/0003-4819-136-12-200206180-00008. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Q, Keadle SK, Hollenbeck AR. Sleep duration and total and cause-specific mortality in a large US cohort: Interrelationships with physical activity, sedentary behavior, and body mass index. Am J Epidemiol. 2014;180:997–1006. doi: 10.1093/aje/kwu222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holtermann A, Mork PJ, Nilsen TI. Hours lying down per day and mortality from all-causes and cardiovascular disease: The HUNT Study, Norway. Eur J Epidemiol. 2014;29:559–565. doi: 10.1007/s10654-014-9939-7. [DOI] [PubMed] [Google Scholar]
- 31.Kurina LM, McClintock MK, Chen JH. Sleep duration and all-cause mortality: A critical review of measurement and associations. Ann Epidemiol. 2013;23:361–370. doi: 10.1016/j.annepidem.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDermott MM, Guralnik JM, Tian L. Baseline functional performance predicts the rate of mobility loss in persons with peripheral arterial disease. J Am Coll Cardiol. 2007;50:974–982. doi: 10.1016/j.jacc.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


