Abstract
The capacity of licensed vaccines to protect the ocular surface against infection is limited. Common ocular pathogens such as herpes simplex virus type 1 (HSV-1) are increasingly recognized as major contributors to visual morbidity worldwide. Humoral immunity is an essential correlate of protection against HSV-1 pathogenesis and ocular pathology, yet the ability of antibody to protect against HSV-1 is deemed limited due to the slow IgG diffusion rate in the healthy cornea. We show that a live-attenuated HSV-1 vaccine elicits humoral immune responses that are unparalleled by a glycoprotein subunit vaccine vis-à-vis antibody persistence and host protection. The live-attenuated vaccine was utilized to assess the impact of immunization route on vaccine efficacy. The hierarchical rankings of primary immunization route with respect to efficacy were: subcutaneous ≥ mucosal > intramuscular. Prime-boost vaccination via sequential subcutaneous and intramuscular administration yielded greater efficacy than any other primary immunization route alone. Moreover, our data also support a role of complement in prophylactic protection as evidenced by intracellular deposition of C3d in the corneal epithelium of vaccinated animals following challenge and delayed viral clearance in C3-deficient mice. We also identify that the neonatal Fc receptor (FcRn) is upregulated in the cornea following infection or injury concomitant with increased antibody perfusion. Lastly, selective siRNA-mediated knockdown of FcRn in the cornea impeded protection against ocular HSV-1 challenge in vaccinated mice. Collectively, these findings establish a novel mechanism of humoral protection in the eye involving FcRn and may facilitate vaccine and therapeutic development for other ocular surface diseases.
INTRODUCTION
The mechanisms by which systemic vaccination strategies induce protective immunity against various mucosal pathogens are incompletely understood. However, the ability of systemic vaccines to protect against mucosal pathogen-associated diseases is contingent on vaccine composition, immunization route, and degree of immunologic or anatomic tissue compartmentalization (1–3). Direct mucosal vaccination as an alternative means to elicit site-specific protection is an area of active research (4). Accordingly, some have suggested that immune responses elicited from systemic vaccination may not faithfully recapitulate protective immune responses generated in a mucosal microenvironment (5). However, in the ocular surface mucosae, robust local immune responses to infection are often detrimental. Even transient inflammatory events in the eye can provoke devastating consequences resulting in permanent vision loss. Therefore, the ocular surface mucosa presents additional complexity to the equation of systemic vaccination.
The cornea is unique among immune privileged tissues due to its direct exposure to the external environment. However, the cornea remains vulnerable to immune-mediated pathological sequelae resulting from injury, toxicity, or infection. These vision-altering sequelae include scarring, neovascularization, and desiccation. Moreover, select pathogens representing essentially every taxonomic classification ranging from bacteria and viruses to yeast, protozoa, and nematodes are associated with infections of the ocular surface mucosae (6). The global burden of infectious external eye disease is formidable in terms of visual morbidity (7). Despite this, no licensed vaccine capable of preventing a single ocular surface infection exists—excluding varicella zoster virus.
Concern remains that boosting immunity by way of vaccination against a common ocular pathogen such as herpes simplex virus type 1 (HSV-1) may exacerbate the severity of ocular pathology (8). While the global incidence of HSV-1 keratitis is estimated at 1.5 million new cases annually, its economic burden in the United States alone is projected to exceed $23 million in treatment-associated costs in 2017 (9, 10).1 Many experimental strategies and approaches have been applied in the preclinical development of prophylactic HSV-1 vaccines with a specific focus on preventing ocular disease (11–17). However, our recent findings comprehensively established that humoral immunity is a strong correlate of protection against HSV-1 pathogenesis and resultant ocular disease in mice using a glycoprotein D (gD-2) subunit and a live-attenuated vaccine designated HSV-1 0ΔNLS (17). This live-attenuated vaccine strain is avirulent and highly immunogenic due to the deletion of the nuclear localization sequence (NLS) in the infected cell protein 0 (ICP0) gene (17, 18). Prophylactic vaccine-induced HSV-specific serum antibody concentration is also recognized as a major correlate of protection against HSV-1-associated disease in humans (19).
Efficient humoral protection against mucosal infections requires a sufficient amount of pathogen-specific antibody at the site of infection to counter replication and dissemination (20). Avascular tissues such as the cornea contain drastically less immunoglobulin during homeostatic conditions compared to other mucosal sites, although an upsurge in the concentration of IgG has been reported in the cornea and tear film during microbial keratitis (20–22). Though humoral immunity is a major correlate of protection against HSV-1 pathogenesis and tissue pathology (17), the mechanism facilitating humoral protection in the cornea remains vague. Here, we explore the dynamics and mechanisms of prophylactic vaccine-mediated humoral protection against HSV-1 in the eye. First, we distinguish that the longevity of humoral protection elicited by a glycoprotein D subunit vaccine is short-lived in mice compared to the recently characterized live-attenuated HSV-1 0ΔNLS vaccine (17, 18). We compare various routes of immunization and identify that the efficacy of the HSV-1 0ΔNLS vaccine is limited following classical intramuscular injection alone. Furthermore, our data support a role of complement fixation in prophylactic protection as evidenced by intracellular deposition of C3d in the corneas of vaccinated animals following ocular HSV-1 challenge. This finding was corroborated by delayed viral clearance in vaccinated C3-deficient animals relative to wild-type upon ocular challenge. We also investigate dynamics of antibody perfusion in the corneas of immunologically naive mice during HSV-1 infection and uncover a novel role of the neonatal Fc receptor (FcRn) in regulating antibody transcytosis and host defense in the corneal epithelium. While FcRn was first characterized for its role in transfer IgG from a mothers milk across the gut lumen in neonates, it exhibits many other important roles involving IgG transport and turnover across the lifespan (23). We anticipate these findings will influence future vaccination strategies targeted against mucosal pathogens at the ocular surface.
MATERIALS AND METHODS
Mice
Male and female outbred CD-1 mice were purchased from Charles River Laboratories (Wilmington, MA). The rationale for utilizing an outbred mouse line for a majority of this vaccine study focuses on the genetic heterozygosity and immunologic diversity of CD-1 mice relative to C57BL/6 (24). Outbred “Swiss” mice such as the CD-1 stock are also more susceptible to HSV-1 neuropathogenesis than C57BL/6 mice (25). However, inbred C57BL/6 wild-type (WT), complement C3 deficient (C3−/−), and Fc gamma receptor 3 deficient (FcγRIII−/−) mice were obtained from the Jackson Laboratory (Bar Harbor, ME) to clarify immunologic mechanisms of antibody-mediated protection. Animals were housed in a specific pathogen-free vivarium at Dean McGee Eye Institute on the University of Oklahoma Health Sciences Center (OUHSC) campus in Oklahoma City. Investigators adhered to procedures approved by the OUHSC Institutional Animal Care and Use Committee (Protocol # 16-087-SSIC-A), and animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Animals were anesthetized for all procedures using an intraperitoneal injection of ketamine (100 mg/kg) and xylazine (6.6 mg/kg). For terminal experiments requiring tissue collection, mice were anesthetized and euthanized by exsanguination via intracardiac perfusion with 10 mL of phosphate buffered saline (PBS).
Vaccines, Immunization Schemes, & Ocular Infection
The HSV-1 0ΔNLS vaccine was used for immunization with each dose consisting of 5×104 plaque forming units (PFU) as described previously (17). Briefly, each dose of the gD-2 subunit vaccine included: 2.5 μg gD-2 protein adjuvanted with 25 μL Imject alum (ThermoFisher Scientific, Waltham, MA) and 10 μg monophosporyl lipid A from Salmonella (Sigma-Alrich, St. Louis, MO) per dose. The gD-2 protein is a truncated form of HSV-2 glycoprotein D and was generated and purified as described previously (26). This formulation has been utilized previously in animal models to mimic the GSK Herpevac vaccine, which was 82% effective against HSV-1-associated genital disease in clinical trials (17, 19, 26). The gD-2 vaccine was utilized as a control in these studies, as the HSV-2 gD glycoprotein immunogen elicits cross-protective neutralizing antibody responses against HSV-1 gD (27). Animals were vaccinated using a two-dose prime-boost regimen in the footpad and flank, respectively, with either the live-attenuated HSV-1 0ΔNLS or gD-2 subunit vaccine as previously described (17). The booster vaccination was given three weeks after the primary. To evaluate the effect of vaccination route on efficacy, animals were vaccinated in the hind footpad alone (subcutaneous), in the hind flank alone (intramuscular), by single intranasal inoculation (mucosal), or with a footpad-flank boost combination regimen as a positive control.
Outbred CD-1 mice were infected by applying 1×103 PFU of HSV-1 McKrae to each cornea following partial epithelial debridement with a 25-gauge needle at the times indicated in each respective figure legend. Inbred C57BL/6 mice were infected with 1×104 PFU of HSV-1 McKrae per eye. Vaccinated animals were challenged seven weeks after the primary vaccination unless indicated otherwise. When necessary, animals were subjected to partial epithelial debridement as a scratch only mock-infection control.
Serological and Virological Assays
Peripheral blood was collected from the facial vein of anesthetized mice at the specified times post-vaccination or challenge and fractionated using Microtainer serum separation tubes (Becton Dickinson, Franklin Lakes, NJ). Serum titers were assessed for virus neutralizing titers in the presence of guinea pig complement (Rockland, Limerick, PA) on confluent Vero cells (American Type Culture Collection, Manassas, VA) as previously described (17). Virus-specific antibody subclass profiling was quantified by ELISA using immobilized HSV-1 virions as a target and alkaline phosphatase-conjugated anti-mouse immunoglobulin subclass-specific detection antibodies (Southern Biotechnology, Birmingham, AL) as described (18). To quantify titers of infectious virus, corneas were swabbed with cotton-tipped applicators to collect free virus shed in the tear film and tissues excised for downstream analysis by standard plaque assay on Vero cells (17). For detection of latent virus, RNA was extracted from cornea-innervating trigeminal ganglia (TG) harvested from mice at day 30 post infection (PI), RNA converted into cDNA, and viral transcript expression evaluated by PCR relative to beta-actin expression and normalized to uninfected control samples as previously described (17). Alternatively, direct quantification of viral genome copy number was performed by PCR on DNA isolated from the TG of challenged mice using a proprietary primer-probe mix according to the manufacturer’s directions (Primerdesign Ltd. Chandler’s Ford, United Kingdom) as described (18).
Spectral Domain Optical Coherence Tomography
Noninvasive in vivo imaging of the anterior segment was performed on anesthetized animals using a Bioptigen spectral domain optical coherence tomography (SD-OCT) system (Leica Microsystems, Triangle Park, NC) to assess corneal structure and inflammation as previously characterized (28, 29).
Immunohistochemistry and Confocal Microscopy
Corneas were cut from enucleated eyes of euthanized animals, fixed in a 4% solution of paraformaldehyde (Sigma-Aldrich) for thirty minutes and washed in 1xPBS containing 1% Triton-X100. Corneas were blocked using anti-CD16/32 Fc block overnight (eBioscience, San Diego, CA), and immunolabeled as described previously to quantify corneal neovascularization (30). Phalloidin (Life Technologies, Carlsbad, CA) and DAPI staining were utilized to identify tissue layers and boundaries. Mast cell granules were stained with FITC-avidin as described (31). Unconjugated goat anti-mouse C3d (catalog no. AF2655; R&D Systems, Minneapolis, MN) and goat anti-mouse FcRn (catalog no. AF6775; R&D Systems) primary antibodies were used to label each respective protein in the in the cornea. Dako polyclonal rabbit anti-HSV-1 antibody was used for viral antigen detection (Agilent Technologies, Santa Clara, CA). Appropriate fluorochrome-conjugated secondary antibodies were utilized for confocal imaging (Jackson Immunoresearch, West Grove, PA). Images were acquired using an Olympus FV1200 confocal microscope in sequential channel scanning mode (Center Valley, PA). Three-dimensional protein fluorescence colocalization analysis was performed using Imaris software (Bitplane USA, Concord, MA).
Quantification of Tissue Antibody Concentrations
Corneas and TG from healthy, cornea scratch control (24 hours post-injury), and HSV-1 infected (24 and 48 hours post-infection) mice were collected along with ear pinna specimens from healthy or ear-punched mice (24 hours post-injury). Samples were placed in NextAdvance Green bead lysis tubes (Averill Park, NY) containing 150 μL radioimmunoprecipitation assay lysis buffer supplied with protease inhibitor (Santa Cruz Biotechnology, Dallas, TX), homogenized in a NextAdvance Bullet Blender Storm 24 homogenizer for 10 minutes, and subsequently subjected to sonication in a water bath for 10 minutes. Supernatants were clarified by centrifugation at 16,000 g for 10 minutes and used for downstream analysis. Tissue supernatants were surveyed for antibody content using a mouse antibody isotyping multiplex kit from eBioscience on a BioRad Bioplex system (Hercules, CA) according to the manufacturer’s directions. Data are reported as pg immunoglobulin isotype per mg tissue wet weight.
Flow Cytometry
Single cell suspensions were generated from all tissue collected for analysis by flow cytometry. Briefly, lymph nodes were macerated over 40 μm mesh to generate a suspension. Individual corneas were digested in 0.25 Wünsch units of Liberase TL enzyme mix (Roche Diagnostics, Indianapolis, IN) suspended in 500 μL RPMI 1640 media supplemented with 10% heat-inactivated fetal bovine serum, gentamicin, and antibiotic/antimycotic (Invitrogen, Carlsbad, CA) at 37 °C for 2 hours, exposed to mechanical trituration every twenty minutes. Corneal digests were filtered through 40 μm mesh prior to labeling. Peripheral blood was collected from the facial vein and erythrocytes removed through two incubations in hypotonic lysing buffer as described (32). Cell suspensions were blocked with anti-CD16/32 (eBioscience), labeled with target-specific antibodies for twenty-thirty minutes, and washed in 1xPBS containing 1% bovine serum albumin.
Intracellular FcRn labeling was performed with an unlabeled primary antibody (R&D Systems) and fluorochrome-conjugated secondary antibody (Jackson Immunoresearch) using a saponin-based perm/wash buffer (BD Biosciences, San Jose, CA). All samples were analyzed on a Miltenyi Biotec MacsQuant 10 flow cytometer with MacsQuantify software (Bergish Gladbach, Germany). Gating boundaries were established via isotype labeling and/or fluorescence minus one (FMO) controls to ensure antibody specificity and negate spectral overlap, respectively. Biological negative controls were also considered. Gating strategies are shown within this manuscript or are based on those published previously (17, 31, 33).
Western Blot
Supernatants from healthy or HSV-1 infected cornea homogenates were prepared in RIPA buffer as described above. Sample protein concentrations were standardized using a Pierce BCA protein assay kit (ThermoFisher Scientific). Proteins were resolved by electrophoresis on Novex tris-glycine 4–20% gradient polyacrylamide gels (Invitrogen, Carlsbad, CA) and transferred onto nitrocellulose membranes. Membranes were blocked for one hour at room temperature in 5% bovine serum albumin (Sigma-Aldrich) in tris-buffered saline containing 0.2% tween 20 (BSA-TBST). Blots were incubated overnight at 4°C in goat anti-mouse FcRn antibody (catalog no. AF6775; 1:2000 dilution; R&D Systems) or for 90 minutes at room temperature in mouse anti mouse-β actin primary antibody (catalog no. ab6276; 1:10,000 dilution; Abcam, Cambridge, MA). Blots were subsequently incubated with the corresponding HRP-conjugated anti-mouse secondary antibody for FcRn (HRP anti-goat; 1:5000 dilution; R&D systems) or β actin (HRP anti-mouse; 1:4000 dilution; Amersham, GE Healthcare Bio-Sciences, Pittsburgh, PA) for one hour at room temperature and imaged using a Kodak in vivo imaging system F Pro (Rochester, NY) with MI SE version 4.4 software (Carestream Health, Inc., Rochester, NY). Chemiluminescent detection was achieved using SuperSignal extended duration substrate (ThermoFisher Scientific). Band intensity analysis was performed using Image J (National Institutes of Health, Bethesda, MD).
siRNA Transfection
For corneal FcRn knockdown experiments, Ambion Silencer Select siRNA (Invitrogen) was used as previously described with Lipofectamine RNAiMax (Invitrogen) tranfection reagent (33). Briefly, the apical corneal epithelium was partially debrided to facilitate efficient transfection and a drop containing 5 μL lipofectamine, and 3.33 nmol FcRn-specific or nonspecific scramble control siRNAs applied to each cornea in supplement-free Dulbecco’s modified Eagle’s media. siRNA sequences were designed and validated by the manufacturer, although FcRn was targeted with two non-overlapping siRNAs to enhance effectiveness (Table S1). Knockdown efficiency was confirmed by Western blot.
Statistical Analysis
Graphpad Prism 5 software was used for statistical analysis (San Diego, CA). Data reflect mean ± SEM unless indicated otherwise. Statistical tests used for analysis are described in each figure legend. Significance thresholds for comparisons are denoted as follows: * p < 0.05, ** p < 0.01, and *** p < 0.001.
RESULTS
Humoral protection elicited by a gD-2 subunit vaccine is short-lived compared to the live-attenuated HSV-1 0ΔNLS vaccine
Clinical trials for HSV vaccines have favored subunit vaccines over live-attenuated viruses with limited success reported to date (8). Evidence from the Glaxo Smith Kline Herpevac clinical trials using a gD subunit vaccine from HSV-2 demonstrated that gD-2-reactive antibodies exhibit moderate cross-protection against HSV-1 (19, 27). Therefore, we previously utilized a comparable gD-2 subunit vaccine as a measure of comparison for the efficacy of HSV-1 0ΔNLS against ocular challenge in CD-1 mice (17). That study unequivocally demonstrated that HSV-1 0ΔNLS was superior to a gD-2 subunit vaccine in its capacity to elicit high-titer neutralizing antibody responses and prevent ocular pathology, but animals were challenged merely one month after vaccination (17). In order to assess the longevity of prophylactic vaccine-mediated humoral protection comparing HSV-1 0ΔNLS to a gD-2 subunit, CD-1 mice were vaccinated subcutaneously in the footpad, given an intramuscular booster dose in the ipsilateral flank twenty-one days later, and subjected to blood collection at thirty-day intervals over three months for serology (Fig. 1A).
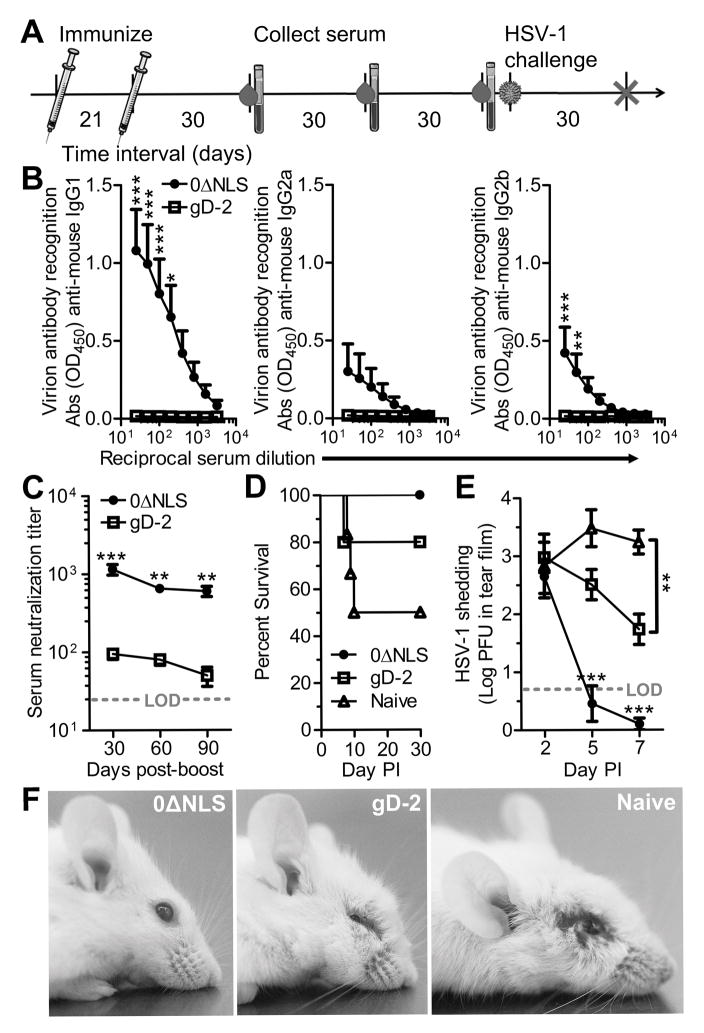
Figure 1. Longevity and efficacy of vaccine-induced humoral immune responses comparing subunit and live-attenuated HSV-1 vaccines.
(A) Experimental timeline detailing vaccination scheme, serial blood draws, and ocular HSV-1 infection. Animals were subcutaneously immunized with HSV-1 0ΔNLS or a gD-2 subunit vaccine followed by an intramuscular boost three weeks later. Blood was collected every thirty days to assess the humoral immune response to HSV-1. Animals were challenged with 1000 PFU HSV-1 McKrae per eye 90 days following the final immunization. (B) ELISA-based characterization of immunoglobulin subtypes binding to immobilized HSV-1 virions including IgG1, IgG2a, and IgG2b in mouse serum collected at day 90 post-boost. Serum samples were diluted on a two-fold dilution series from 1:25 to 1:3200. HSV-specific IgA and IgM were below the limit of detection. (C) HSV-1 neutralization titers recorded from sera taken at days 30, 60, and 90 post-boost. (D) Animal survival following ocular infection with 1000 PFU HSV-1 McKrae. (E) Timecourse of HSV-1 shedding in the tear film of ocularly infected mice, determined by plaque assay. (F) Representative photographs of mice 8 days post-infection (PI). Mice vaccinated with HSV-1 0ΔNLS maintained a healthy appearance, while mice vaccinated with the gD-2 subunit developed periocular edema and head swelling. Naive mice developed viral lesions in the periocular skin in addition to head swelling. Data in panels B-E reflect mean ± SEM for 5–6 mice per group with two independent experiments. Statistical differences were computed by two-way ANOVA with Bonferroni’s multiple comparisons tests; asterisks reflect differences between HSV-1 0ΔNLS and gD-2 vaccine groups unless indicated otherwise.
Mean serum titers of HSV-1 specific antibody were quantified by ELISA on immobilized virions at day 90 post-boost (Fig. 1B). Serum titers of HSV-1 specific IgG1, IgG2a, and IgG2b fell below the limit of detection at day 90 post-boost in gD-2 immunized animals (Fig. 1B). In contrast, HSV-1 specific antibody titers were detected in all mice immunized with HSV-1 0ΔNLS with a predominant IgG1 response (Fig. 1B). Serum titers of HSV-1 specific IgA and IgM were not detected in either group. Virus neutralization titers were also evaluated in sera serially collected at days 30, 60, and 90 post-boost. As previously demonstrated (17), mice immunized with HSV-1 0ΔNLS acquire higher virus neutralizing serum titers than mice immunized with gD-2 by day 30 post-boost (Fig 1C). However, the serum neutralizing capacity of animals immunized with gD-2 was short-lived and declined nearly to the limit of detection (1:25) by day 90 post-boost (Fig. 1C). A modest longitudinal decrease from the peak serum neutralization titer was also observed in HSV-1 0ΔNLS-vaccinated mice, but the titers at days 60 and 90 post-boost remained substantially higher than even the peak titer measured in gD-2-vaccinated animals (Fig. 1C). Collectively, our data show that the live-attenuated HSV-1 0ΔNLS vaccine elicits a sustained high titer neutralizing antibody response unparalleled by a subunit vaccine—as reported for other common vaccines (34).
Vaccinated animals were subsequently subjected to a deferred challenge at day 90 post-boost to investigate whether the discrepancy in antibody titers between mice immunized with HSV-1 0ΔNLS and the gD-2 subunit had an appreciable impact on vaccine efficacy (Fig. 1A). Animals were challenged bilaterally with an inoculum of 1×103 PFU HSV-1 McKrae per eye. Survival proportions for CD-1 mice infected 90 days following the boost vaccination were: 3/6 naive, 4/5 gD-2-vaccinated, and 6/6 HSV-1 0ΔNLS-vaccinated (Fig. 1D). Acute viral shedding in the tear film was equal between all groups at day 2 post infection (PI), but precipitously dropped in animals vaccinated with HSV-1 0ΔNLS by day 5 PI (Fig. 1E). Although gD-2 subunit-vaccinated animals exhibited less viral shedding than naive animals by day 7 PI (Fig. 1E), they were not spared from developing periocular edema or hydrocephalus (Fig. 1F), a pathognomonic sign of viral encephalitis. Likewise, naive animals developed edematous periocular lesions and hydrocephalus by day 8 PI (Fig. 1F).
Animals infected at day 90 post-boost were subsequently assessed for latent viral burden, serology, and tissue pathology at day 30 PI. While gD-2 subunit-vaccinated animals had less latent virus in the trigeminal ganglia (TG) than naive animals, prophylactic vaccination with HSV-1 0ΔNLS diminished the establishment of viral latency in the TG after ocular challenge (Fig. 2A). Serum neutralizing titers for HSV-1 were equivalent among all experimental groups by day 30 PI (Fig. 2B) indicating that the humoral immune response to HSV-1 is not directly governed by the quantity or persistence of viral antigen. Moreover, these levels were similar to serum titers observed in HSV-1 0ΔNLS-vaccinated animals at day 30 post-boost (Fig. 1C).
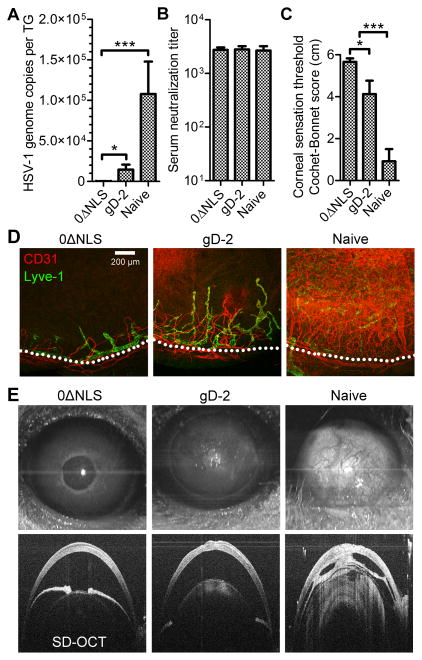
Figure 2. HSV-1 0ΔNLS induces lasting protection that prevents ocular pathology upon challenge.
Mice ocularly challenged with HSV-1 at 90 days post-vaccination as depicted in Fig. 1A were evaluated for latent virus, serostatus, and tissue pathology at day 30 post infection (PI). (A) Quantitative PCR analysis of HSV-1 genome copy number in the trigeminal ganglia (TG). (B) Serum neutralization titers recorded at day 30 PI. (C) Assessment of corneal sensation in vaccinated or naive mice surviving at day 30 PI using standard Cochet-Bonnet esthesiometry scoring. (D) Representative confocal images of corneal neovascularization in vaccinated and naive mice depicting CD31+ blood vessels (red) and Lyve-1+ lymphatic vessels (green) at 10× magnification. Dotted lines represent the anatomic limbal vessels circumscribing the normally avascular cornea; scale bar = 200 μm. (E) Representative anterior projection of SD-OCT images highlighting the cornea at day 30 PI in HSV-1 0ΔNLS and gD-2 immunized or naive mice. Data in panels A-C reflect mean ± SEM for 3–6 mice per group (6–12 corneas or total TG) with two independent experiments. Statistical differences were computed by one-way ANOVA using Student-Newman-Keuls multiple comparisons tests; asterisks reflect differences between the indicated groups. Images in panels D and E are representative of 5–9 corneas per group.
The extent of corneal pathology following ocular challenge was evaluated by assessing corneal sensation, neovascularization, and structural integrity. Corneal sensation loss is a highly sensitive pathological outcome of ocular HSV-1 infection and is useful as a measurement of vaccine efficacy against HSV-associated ocular disease (35, 36). Corneal sensation was preserved in HSV-1 0ΔNLS-vaccinated mice, partially lost in gD-2-immunized mice, and severely diminished in naive mice at day 30 PI (Fig. 2C). Corneal neovascularization is another vaccine-preventable outcome of herpetic keratitis (17). Neovascularization was evaluated by immunolabeling CD31 and Lyve-1 to visualize corneal blood and lymphatic vessels, respectively. The corneas of animals vaccinated with HSV-1 0ΔNLS remained avascular at day 30 PI (Fig. 2D). However, corneal neovascularization was moderate in gD-2-vaccinated animals and severe in naive animals such that the vessels covered the entire cornea (Fig. 2D).
Spectral domain optical coherence tomography (SD-OCT) was utilized to assess the structural integrity of the anterior eye in vivo at day 30 PI. Corneal SD-OCT imaging in mice immunized with HSV-1 0ΔNLS revealed healthy, optically clear corneas with normal light reflexes (Fig. 2E, top left), although a pronounced hyper intensity consistent with leukocyte infiltrate was observed in the central corneas (Fig. 2E, bottom left). In contrast, corneas of mice immunized with gD-2 exhibited moderate corneal opacity (Fig. 2E, top center) consistent with alterations in the corneal structure (Fig. 2E, bottom center). Cellular infiltrate was also observed in the lens of gD-2 immunized mice (Fig. 2E, bottom center). Moreover, post-infectious mydriasis (dilated pupil) was observed in half of the eyes of gD-2 immunized mice (Fig. 2E, center). Stark corneal opacity (Fig. 2E, top right) and lens infiltrate (Fig. 2E, bottom right) were also detected in the eyes of naive animals. In contrast to the other groups, adhesions formed between the iris and cornea and/or iris and lens (designated clinically as anterior and posterior synechiae, respectively) of naive animals (Fig. 2E, bottom right). Both post-infectious mydriasis and development of iris adhesions are consistent with the development of anterior uveitis during acute HSV-1 infection (37). Taken together, the data confirm our previous finding that the HSV-1 0ΔNLS vaccine is superior to a gD-2 subunit in terms of enabling acute viral clearance, limiting latent infection, and preventing ocular pathology (17). However, data here utilizing a delayed challenge model show that the humoral immune response elicited by HSV-1 0ΔNLS sustains its protective effect over time, while humoral immunity elicited by gD-2 subunit vaccination wanes quickly.
Routes of immunization influence IgG subclass profiles and vaccine efficacy
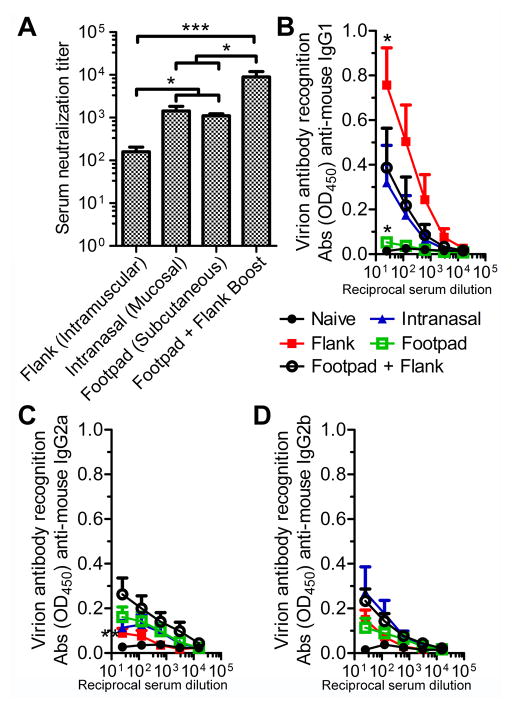
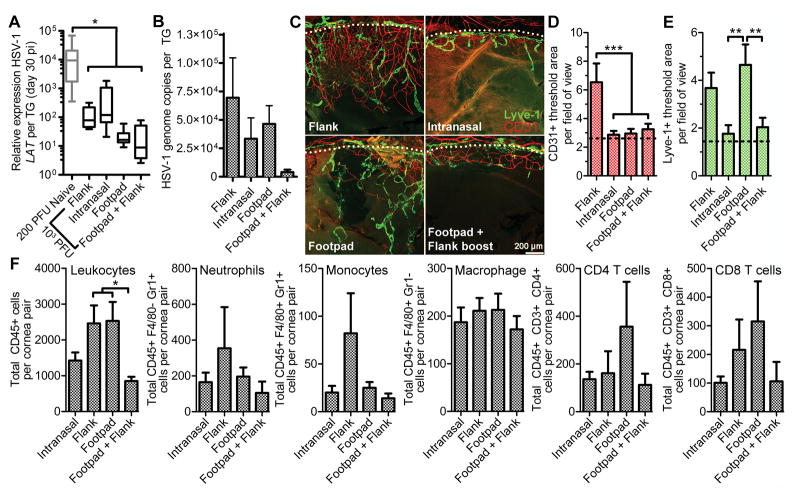
Routes of immunization are known to distinctively influence vaccine responsiveness despite the routine clinical use of intramuscular injection. Mice were immunized with HSV-1 0ΔNLS in various sites to assess how unique tissue microenvironments impact immunogenicity and efficacy. Intramuscular, mucosal, and subcutaneous routes of immunization were modeled by administering equivalent titers of HSV-1 0ΔNLS in the hind-leg flank, intranasally, or in the footpad of CD-1 mice, respectively. Post-vaccination serum neutralizing antibody titers were lowest in the flank only immunization group, similar between intranasal and footpad vaccination groups, but no single vaccination site generated as strong of a neutralizing antibody response as was observed using a dual prime-boost immunization strategy in the footpad and flank (Fig. 3A). Flank-only vaccination elicited the highest titers of HSV-specific IgG1 (Fig. 3B) despite the reduced virus neutralization capacity in this group (Fig. 3A). The HSV-specific IgG1 response was negligible following footpad-only vaccination (Fig. 3B). All routes of vaccination elicited IgG2a (Fig. 3C) and IgG2b (Fig. 3D) responses, but titers were not statistically different between groups following primary vaccination alone.
Figure 3. Immunization route impacts the humoral response to HSV-1 0ΔNLS.
Mice were vaccinated with 5×104 PFU HSV-1 0ΔNLS in the flank, footpad, or intranasally using a primary immunization only. Mice receiving a combined footpad vaccination followed by a flank-boost vaccination three weeks later were utilized as a control. Serum was collected from animals seven weeks after the primary immunization to assess virus neutralization titers (A) or IgG isotype recognition of HSV-1 by ELISA (B, C, D). All data reflect mean ± SEM with 3–4 independent experiments; n = 11 mice per group in panel A; n= 7–8 mice per vaccinated group and n = 4 naive mice per group in panels B–D. Differences were calculated using a nonparametric Kruskal-Wallis test with Dunn’s multiple comparisons tests for panel A and by two-way ANOVA with Bonferroni’s multiple comparisons tests for panels B–D. Asterisks reflect differences from the mean value of the footpad + flank boost group unless indicated otherwise.
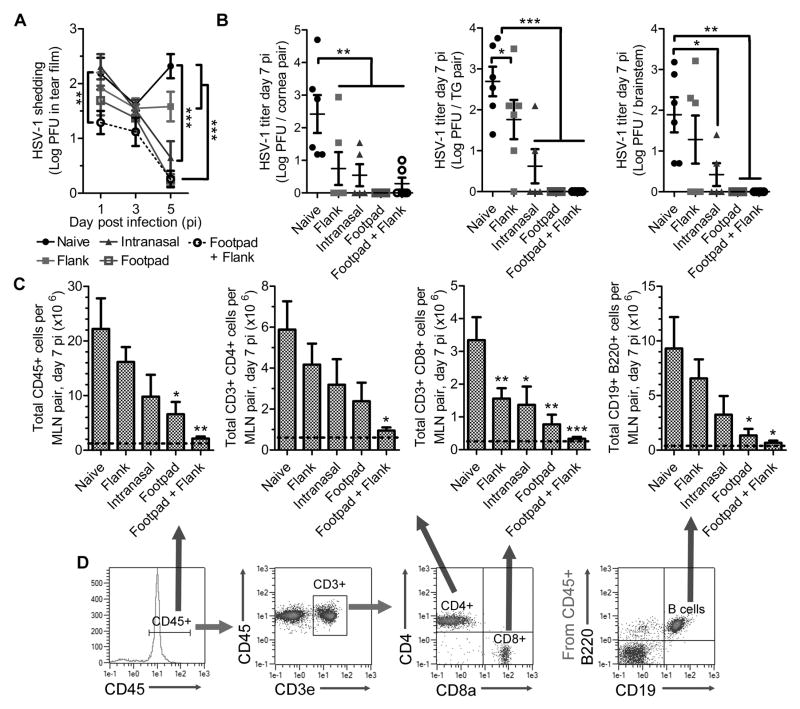
The route of immunization had a clear impact on the efficacy of protection following HSV-1 challenge. It was suspected that intranasal vaccination could have an advantage for ocular protection relative to the other sites by establishing a population of tissue-resident memory CD8+ T cells within the cornea-innervating trigeminal ganglia (TG). However, no increase in the number of CD69+ CD103+ CD8+ T cells was observed in TG from intranasally immunized mice at 30 days post-vaccination relative to healthy non-vaccinated controls (data not shown). Viral shedding in the ocular tear film was sustained for a longer period of time in mice immunized in the flank only (intramuscular) relative to other vaccination sites (Fig. 4A). However, virus was largely cleared from the corneas of all vaccinated mice by day 7 PI (Fig. 4B). A higher tendency in viral trafficking to the TG and brainstem was observed in mice immunized in the flank or intranasally relative to the other sites in which neuro-dissemination was absent (Fig. 4B). Trends in delayed viral clearance in the cornea or nervous system (Fig. 4B) correlated with amplified adaptive immune responses in the cornea-draining mandibular lymph nodes (MLN) at day 7 PI (Fig. 4C, D). Therefore, the lack of lymphocyte expansion in the MLN upon ocular challenge is a useful prognostic correlate of vaccine efficacy in terms of acute viral clearance, as reported previously (17).
Figure 4. Immunization route impacts the protective efficacy of HSV-1 0ΔNLS.
Animals vaccinated in various tissues as described in Fig. 3 were challenged with 1000 PFU HSV-1 McKrae per eye to evaluate viral shedding in the ocular tear film (A) during the first 5 days PI and subsequently euthanized at day 7 PI to measure HSV-1 titers in the cornea, TG, and brainstem (B). Additionally, the cornea-draining mandibular lymph nodes (MLN) were collected and processed by flow cytometry to assess lymphocyte proliferation (C) with representative plots shown in (D). Dotted lines reflect the mean value observed in MLN from uninfected mice. Data reflect mean ± SEM and statistical differences were determined by two-way ANOVA with Bonferroni’s multiple comparisons tests in (A) where n = 6–16/group, 4 independent experiments. One-way ANOVA with Student-Newman-Keuls multiple comparisons tests were utilized in (B) and (C), where n = 5–6 mice per group, 3 independent experiments. Asterisks reflect differences from the mean value of the naive control group unless indicated otherwise.
Efficient viral clearance is a goal of prophylactic HSV-1 vaccination, but the ultimate measure of vaccine efficacy is its ability to reduce the amount of latent virus in the TG and prevent ocular disease. All routes of immunization reduced the total amount of latent HSV-1 in the TG as assessed by latency associated transcript (LAT) RNA expression levels (Fig. 5A) relative to levels previously reported in naive control CD-1 mice infected with a 200 PFU challenge inoculum (17). However, animals that received the two-dose prime-boost vaccination had the lowest levels of latent HSV-1 in the TG after a high-titer ocular 1×103 PFU challenge in terms of both LAT expression and viral genome copy number (Fig. 5A,B).
Figure 5. Routes of immunization govern the degree of protection against viral latency and tissue pathology upon ocular challenge.
Animals were vaccinated in various tissues as described in Fig. 3 and challenged with 1000 PFU HSV-1 McKrae per eye to evaluate impact of immunization route on viral latency by LAT expression (A) or viral genome copy number (B) in the TG at day 30 PI. Corneas were imaged to evaluate neovascularization (C) and quantify areas of hemangiogenesis (D) and lympangiogenesis (E). Leukocyte populations in the cornea were also assessed by flow cytometry at day 30 PI (F). Data reflect mean ± SEM except for the standard quartile-based box plot depicted in (A) where n = 7–8 TG per group with two independent experiments. Note that the values reflected for naive animals in (A) reflect mice infected with only 200 PFU per eye. Statistical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests; for panel (A), n = 8 TG per group with 2–3 independent experiments; for panel (B), n = 8–15 TG per group with 2–3 independent experiments, panels (C), (D), and (E) reflect 24–32 images from quadrants of 6–8 flat-mounted corneas per group spanning 2–3 independent experiments. For panel (F), n = 7–10 mice per group with 3 independent experiments.
Corneas of immunized and challenged mice were evaluated for corneal neovascularization at day 30 PI. Corneas from mice vaccinated intranasally or by prime-boost footpad-flank vaccination maintained a healthy avascular appearance (Fig. 5C, D, E). Although mice vaccinated in the footpad alone were spared from corneal hemangiogenesis (Fig. 5D), corneal lymphangiogenesis was observed (Fig. 5E). Mice vaccinated in the flank only developed moderate corneal neovascularization involving both lymphangiogenesis and hemangiogenesis (Fig. 5C, D, E). Lastly, the number of leukocytes in the corneas of each group was assessed at day 30 PI (Fig. 5F). No differences in cell numbers were observed that correlated with the tissue neovascularization status aside from a tendency for elevated numbers of CD45+ leukocytes within vascularized corneas (Fig. 5F). Based on acute viral clearance, viral latency, and ocular pathology data, it is apparent that intramuscular vaccination alone is not suitable for eliciting the full protective efficacy of the HSV-1 0ΔNLS vaccine.
Antibody biodistribution and effector function in herpetic keratitis
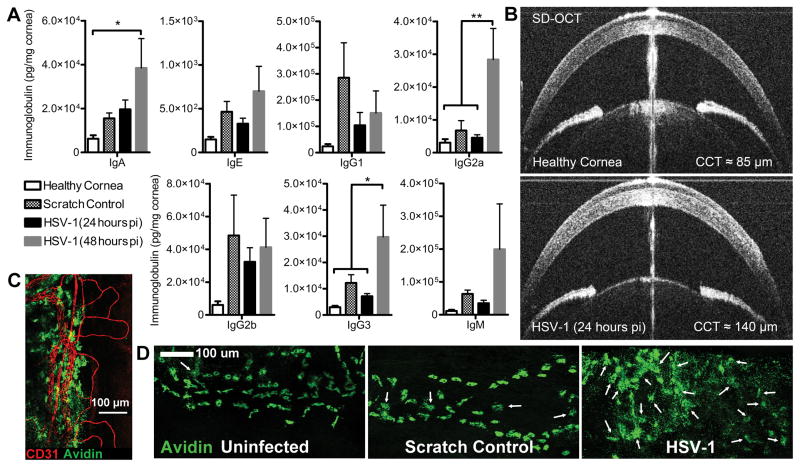
Paracellular diffusion of large macromolecules including immunoglobulins and many therapeutic drugs is restricted in the cornea under homeostatic conditions due to the densely organized tissue architecture and physiological mechanisms that maintain transparency (38–40). However, the dynamics of humoral immunity in the cornea during inflammation or infection are not well characterized. In order to address antibody biodistribution in the cornea during inflammation, we measured immunoglobulin concentrations following infection or mock infection using a bead-based multiplex array on tissue homogenates derived from immunologically naive CD-1 mice. Concentrations of virtually all immunoglobulin sub-classes broadly increased two-fold or greater in corneal buttons within 24–48 hours after scratching the corneal epithelium (mock infection) or HSV-1 infection (Fig. 6A). Increases were statistically significant in HSV-1 infected corneas at 48 hours PI for IgA, IgG2a, and IgG3 (Fig. 6A). Notably, immunoglobulin concentrations in the avascular cornea prior to trauma or infection were an order of magnitude lower than levels observed in healthy vascularized tissues including the TG and ear pinna after exsanguination (Sup. Fig. 1A, B). Moreover, elevations in total antibody concentration in the TG after infection (Sup. Fig. 1A) or the ear following a hole-punch injury (Sup. Fig. 1B) were subdued relative to the fluctuations observed in the cornea.
Figure 6. Antibody diffusion dynamics in the cornea following injury or infection.
(A) Concentration of antibody by isotype in corneas from immunologically naive CD-1 mice reflecting healthy, scratch control (24 hours), and HSV-1 infected corneas (n = 6–7 cornea pairs/group; 2–3 independent experiments). Statistical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests. (B) Representative photos from SD-OCT imaging of the anterior segment of healthy and HSV-1-infected eyes showing corneal edema and prominent hyperintensities (leukocytic infiltrates) at 24 hours PI. The central vertical line artifact is a Purkinje-reflection indicative of alignment with the corneal apex, which was used to control for consistency between eyes during image acquisition. (C) Avidin labeled mast cells (green) reside along the cornea-sclera junction in close association with the CD31+ limbal blood vessels (red), image was captured at 20× magnification, scale bar = 100 μm. (D) Confocal images of peri-corneal mast cells at 20× magnification in healthy, scratch control, and HSV-1 infected corneas at 48 hours PI. White arrows point to degranulated mast cells to highlight the widespread degranulation observed following HSV-1 infection; scale bar = 100 μm.
Sharp increases in total immunoglobulin within the cornea are consistent with our previous observations of transient edema following ocular HSV-1 infection by ultrasound pachymetry (17, 31). Here, we show that edema is prevalent within the corneal stroma at 24 hours PI using in vivo SD-OCT imaging (Fig. 6B). Mast cells are associated with the peri-corneal vasculature (Fig. 6C) and contribute to activation of the vascular endothelium through degranulation of potent vasoactive mediators such as histamine and TNFα—resulting in increased antibody perfusion (31, 41). Therefore, we evaluated the peri-corneal mast cell activation status as evidenced by degranulation in naive CD-1 mice via confocal microscopy. Few degranulated mast cells were observed in healthy corneas and intermittent degranulation was noted in mock-infected corneas; however, mast cell degranulation was widespread in HSV-1 infected corneas by 48 hours PI (Fig 6D) as previously reported in C57BL/6 mice (31). We interpret these observations to suggest that the increased perfusion of antibody in the cornea following infection is an active physiological response to ocular surface injury or infection.
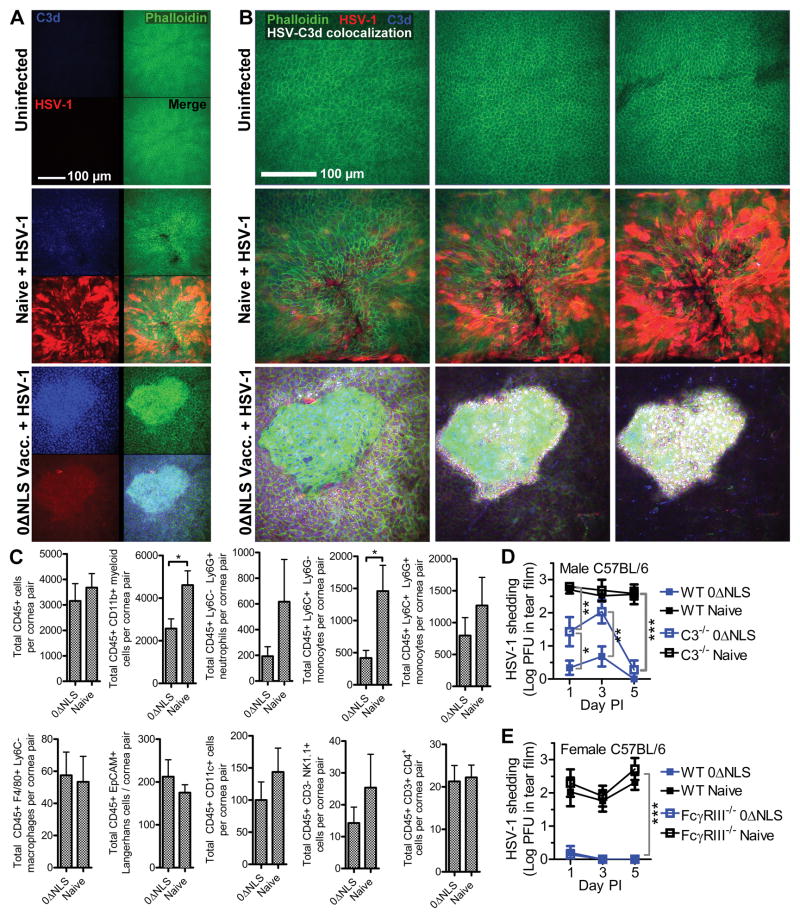
Although antibody has been identified as a correlate of protection against HSV-1, the mechanism of the antibody effector function contributing to virus neutralization and clearance is not established for ocular infection. To further dissect this mechanism, we evaluated the relative contributions of complement fixation and antibody-dependent cell cytotoxicity (ADCC) with respect to viral clearance in vaccinated animals. Confocal microscopy was utilized to visualize complement activation in the corneas of CD-1 mice by colocalization of viral antigen and C3d—the final cleavage product of the complement cascade. C3d deposition is an established indicator of complement activation associated with antibody-mediated pathogen neutralization or opsonization (42, 43). Fewer viral lesions were observed in corneas from vaccinated animals compared to naive at 48 hours PI (data not shown). Moreover, viral lesions were smaller in the corneas of HSV-1 0ΔNLS-vaccinated mice compared to immunologically naive mice following ocular HSV-1 challenge (Fig. 7A, B). While the immunofluorescence signal of viral antigen was also diminished in vaccinated animals, this likely reflects epitope masking by endogenous HSV-specific antibodies (Fig. 7A). Although strong C3d labeling was evident in corneas from vaccinated mice, labeling was absent in healthy corneas and sparse in corneas from naive mice at 48 hours PI (Fig. 7A, B). Extensive colocalization between viral antigen and C3d was observed exclusively in corneas from animals vaccinated with HSV-1 0ΔNLS (Fig. 7B). Contrary to our expectations of finding typical surface-bound deposits of C3d, the C3d signal clustered within intracellular foci of corneal epithelial cells (Fig. 7A, B; Supplementary Movie 1).
Figure 7. Complement mediates humoral protection against ocular HSV-1 infection.
(A) Representative confocal images centered above apparent viral lesions at 40× magnification showing C3d (blue) and HSV-1 antigen (red) in healthy or HSV-1 infected corneas at 48 hours PI with phalloidin (green) staining to delineate cell boundaries. “Naive” reflects HSV-infected immunologically naive mice. “0ΔNLS Vacc.” reflects mice that were immunized in the footpad, boosted in the flank with HSV-1 0ΔNLS, and infected 30 days later with 1000 PFU HSV-1 McKrae in the eye. (B) Individual z-slices of representative composite image shown in (A) with colocalization of HSV-1 and C3d calculated using Imaris displayed as white overlay. Images are representative of 4–6 corneas per group. (C) Flow cytometry was utilized to characterize various leukocyte populations in the corneas of immunologically naive and HSV-1 0ΔNLS-vaccinated mice immunized at 48 hours post infection. Populations represented include total CD45+ leukocytes, CD11b+ myeloid cells, Ly6G+ neutrophils, Ly6C+ monocytes, F4/80+ macrophages, CD45+ EpCAM+ Langerhans cells, CD11c+ dendritic cells, CD3− NK1.1+ NK cells, and CD4+ T cells. Data reflect mean ± SEM for 7–8 mice per group; 3 independent experiments. Differences were determined by Student’s T test. Panels (A), (B), and (C) reflect CD-1 mice. Given the evidence of complement activation and presence of ADCC effector cells in the corneas of vaccinated CD-1 mice, C57BL/6 mice were subsequently utilized to assess viral clearance/shedding and the effector mechanism of humoral protection in the tear film of naive and HSV-1 0ΔNLS-vaccinated WT and C3−/− mice (D) or WT and FcγRIII−/− mice (E); data reflect 5 mice per group; 2 independent experiments. Differences were determined by two-way ANOVA with Bonferroni’s multiple comparisons tests.
In addition to complement fixation analysis, flow cytometry was used to assess cornea-infiltrating and tissue-resident leukocyte populations due to their potential to mediate antibody-dependent cellular toxicity (ADCC). Limited differences were observed in leukocyte populations in the corneas of naive and immunized mice at 48 hours PI (Fig. 7C). Fewer CD11b+ myeloid cells and specifically inflammatory monocytes were detected in corneas from HSV-1 0ΔNLS-immunized mice, although no differences were observed in the total number of macrophages, Langerhans cells, dendritic cells, or NK cells (Fig. 7C).
Given the apparent complement activation and presence of ADCC effector cells in the corneas of CD-1 mice vaccinated with HSV-1 0ΔNLS, we utilized C57BL/6 mice to further dissect the mechanism of protection. For these studies, wild-type (WT), C3−/−, and FcγRIII−/− mice were vaccinated with HSV-1 0ΔNLS using a prime-boost immunization scheme in the footpad and flank, respectively, and subsequently challenged with 1×104 PFU HSV-1 strain McKrae per eye at day 30 post-boost. Serum neutralization titers were equivalent between sex-matched WT and C3−/− or FcγRIII−/− mice prior to ocular challenge (data not shown). Viral shedding in the tear film was assessed following HSV-1 challenge. A substantial delay in viral clearance was observed in vaccinated C3−/− mice relative to WT (Fig. 7D). Viral clearance was equivalent between vaccinated WT and FcγRIII−/− mice (Fig. 7E), thus downplaying the importance of classical FcγRIII (CD16)-dependent ADCC effector cells in prophylactic protection against HSV-1 in the cornea. Likewise, shedding was equivalent between immunologically naive WT and knockout mice—albeit at higher titers than observed in their respective vaccinated counterparts (Fig 7D, E). While the contributions of vaccine-induced humoral immunity in host defense against HSV-1 in the ocular mucosae are undoubtedly multifactorial, our data support a prominent role of the complement pathway in efficient prophylactic protection against HSV-1.
FcRn augments vaccine efficacy through IgG transport in the corneal epithelium
The barrier integrity of the cornea is maintained, in part, through tight junctional complexes between epithelial cells (39, 44). While the intrinsic barrier property of the corneal epithelium protects the eye from the external environment, it concomitantly impedes macromolecule diffusion. Thus, the ability of antibody to passively diffuse into the corneal epithelium is restricted. Given the importance of maintaining the barrier integrity of the ocular surface, we explored the hypothesis that the neonatal Fc receptor (FcRn) mediates antibody transcytosis within the corneal epithelium to facilitate humoral protection against HSV-1. While FcRn is reportedly expressed at low levels in the cornea, its function remains enigmatic (45, 46).
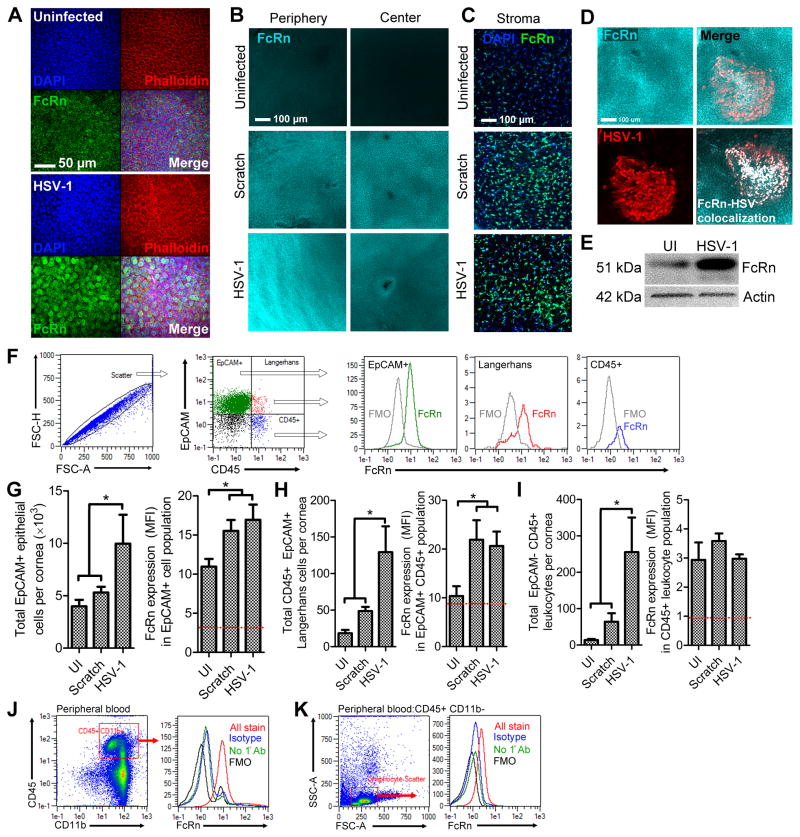
The expression profile of FcRn was first evaluated by confocal microscopy in cornea whole mounts from naive CD-1 mice before and after infection. FcRn expression was detected in the epithelium of healthy corneas, although expression was more conspicuous following HSV-1 infection (Fig. 8A). Intracellular FcRn expression was confirmed based on localization relative to actin filaments and nuclei in the corneal epithelium (Fig. 8A). Low magnification imaging revealed that FcRn expression in the epithelium of healthy corneas decreased in a centripetal fashion from the periphery towards the center (Fig. 8B, top panel). In contrast, robust FcRn immunoreactivity was noted across the entire corneal epithelium in scratch control and HSV-1 infected corneas at 24 hours PI (Fig. 8B). Similar patterns of FcRn expression were observed in the corneal stroma (Fig. 8C). Strong co-localization of FcRn and HSV-1 antigen was observed within viral lesions on corneas from naive animals (Fig. 8D), suggesting that HSV-1 does not inhibit murine FcRn expression within infected cells. The specificity of the FcRn antibody for the p51 subunit was confirmed by Western blot (Fig. 8E) on lysates from uninfected and HSV-1 infected corneas (47).
Figure 8. FcRn is upregulated in the cornea following injury or infection.
(A) Representative confocal images of FcRn (green) labeling in the epithelium of healthy uninfected and HSV-1 infected corneas at 48 hours PI. Images were captured at 40× magnification, scale bar = 50 μm. DAPI (blue) and phalloidin (red) staining were utilized to identify tissue boundaries and borders. (B) Representative low magnification confocal images showing the radial patterning of FcRn expression (cyan) in the healthy corneal epithelium (peripheral expression > central) and its widespread elevated expression in mock infected (scratch control) or HSV-1 infected corneas. (C) FcRn expression (cyan) in the deeper stromal keratocyte layer is also upregulated following corneal injury or infection. (D) Colocalization of FcRn (cyan) and HSV-1 antigen (red) was observed within the corneal epithelium of naive CD-1 mice and digitally confirmed using Imaris software to verify pixel overlap (white overlay, bottom right). Images shown in panels (B), (C), and (D) are representative of 4–6 labeled corneas per group and were captured at 20× magnification, scale bars = 100 μm. (E) Western blot confirmation of FcRn antibody specificity showing the p51 subunit of FcRn in digests from healthy and HSV-1 infected corneal digests (48 hours PI). Actin labeling was used as a loading control with 20 μg of total protein input per sample. (F) Gating strategy for FcRn analysis by flow cytometry including scatter profile for doublet discrimination, selection of cell subsets (EpCAM+ CD45− epithelial cells, EpCAM+ CD45+ Langerhans cells, or EpCAM− CD45+ leukocytes), and downstream quantification of FcRn MFI relative to fluorescence minus one (FMO) background controls. Summary of flow cytometry data quantifying the total number of cells and median fluorescence intensity (MFI) of FcRn expression for epithelial cells (G), Langerhans cells (H), and other leukocytes (I) in corneal digests; (n = 5–6 corneas per group, 3 independent experiments). Data in panels G, H, and I reflect mean ± SEM. Statistical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests. Flow cytometry was used to assess FcRn expression in peripheral blood CD45+ CD11b+myeloid cells (J) and CD45+ lymphocytes based on scatter profile (K); data are representative of 3 independent experiments.
FcRn expression profiles were subsequently evaluated quantitatively by flow cytometry in epithelial cells, Langerhans cells, and other leukocytes in cornea digests at 48 hours PI (Fig 8F). Basal FcRn expression was detected in CD45− EpCAM+ (epithelial cell adhesion molecule) corneal epithelial cells in uninfected mice based on median fluorescence intensity (MFI) relative to fluorescence minus one (FMO)-background control levels (Fig 8F, G). Consistent with the confocal data, FcRn expression levels increased by 30 percent in epithelial cells following ocular surface injury (mock infection scratch control) or HSV-1 infection (Fig. 8G). In addition, the total number of epithelial cells expressing FcRn increased nearly two-fold following HSV-1 infection (Fig. 8G). Increases in basal FcRn expression were also noted in the CD45+ EpCAM+ Langerhans cell population following corneal surface injury or infection (Fig. 8H). The observed FcRn MFI was comparatively negligible in the CD45+ EpCAM− population composed of resident and infiltrating leukocytes (Fig. 8I). This finding contrasts with the observation that myeloid lineage leukocytes in circulation express FcRn (48). To address these divergent findings, we evaluated the FcRn expression with the same instrument settings in peripheral blood (Fig. 8J,K). Indeed, CD11b+ myeloid cells in circulation expressed much higher levels of FcRn (Fig. 8J) than was observed in the corneal CD45+ EpCAM− leukocyte population (Fig. 8I). Peripheral blood lymphocytes were evaluated in parallel and found to express insignificant levels of FcRn (Fig. 8K). Peripheral blood CD11b+ myeloid cells were further profiled using Ly6C and Ly6G antigens to specifically confirm FcRn expression in circulating monocytes and neutrophils (Sup. Fig. 2) —the major population of cornea infiltrating cells during acute HSV-1 infection (49). Collectively, the confirmed up-regulation of FcRn in the corneal epithelium provides a plausible mechanism to explain the protective capacity of antibody against HSV-1 in the cornea.
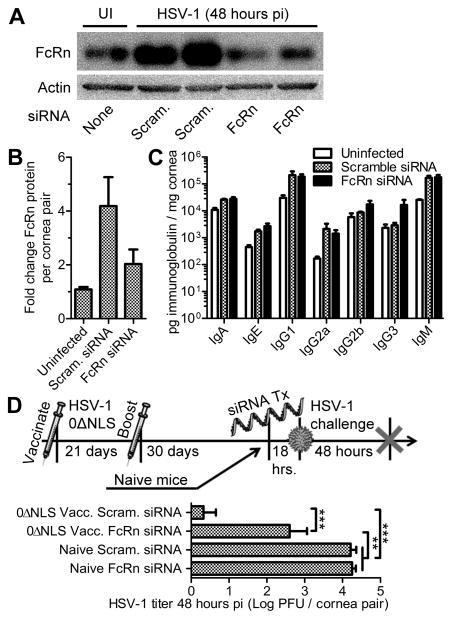
The protective contributions of FcRn were investigated by employing siRNA to specifically target FcRn in the cornea. First, the knockdown efficiency of the topical FcRn siRNA treatment was confirmed in the corneas of naive CD-1 mice at 48 hours PI relative to scramble control-treated animals by Western blot (Fig. 9A). Densitometry analysis indicated that FcRn protein levels were reduced in corneas treated with siRNA targeting FcRn compared to scramble control siRNA treated mice (Fig. 9B). Local siRNA-mediated FcRn knockdown did not have an appreciable impact on total immunoglobulin concentrations within the corneas of naive mice following HSV-1 infection (Fig. 9C). However, FcRn knockdown in the corneas of mice vaccinated with HSV-1 0ΔNLS severely compromised the protective efficacy of the vaccine compared to the scramble control group (Fig. 9D). Explicitly, virus was detected in corneal homogenates by plaque assay in 100% of the FcRn siRNA-treated mice and only 25% of the scramble siRNA-treated mice (Fig. 9D). Nevertheless, the level of protection afforded to mice vaccinated with HSV-1 0ΔNLS after FcRn knockdown remained comparatively better than naive mice receiving either siRNA treatment in terms of viral titer in the cornea at 48 hours PI (Fig. 9D). Taken together, our data reveal a novel mechanism of vaccine-mediated protection against viral infection at the ocular surface involving FcRn. Although FcRn does not appear to be directly responsible for the observed influx of IgG into the cornea as a whole, its established role in antibody transcytosis is now appreciated to be a major factor in humoral immunity against infection within the mucosal epithelium of the ocular surface.
Figure 9. FcRn is essential for humoral protection against HSV-1 in the cornea.
(A) Corneas of naive CD-1 mice were topically treated with non-specific scramble control siRNA (scram.) or FcRn-specific siRNA, infected with HSV-1, and tissue assessed at 48 hours PI for FcRn protein content by Western blot to confirm knockdown efficienty. (B) Quantitative summary of FcRn knockdown efficiency using densitometric analysis of FcRn blots relative to actin loading controls and normalized to healthy uninfected corneas (UI); n = 4 naive, 7 scram. siRNA treated, and 8 FcRn siRNA treated mice per group (3 independent experiments). (C) Tissue lysates from panel A were also evaluated for total immunoglobulin content as described in Fig. 6 to determine the contributions of FcRn on antibody perfusion into the cornea. (D) Timeline depicts siRNA treatment in naive and vaccinated (HSV-1 0ΔNLS footpad-flank prime-boost regimen) CD-1 mice, ocular HSV-1 challenge (1000 PFU HSV-1 McKrae), and tissue collection at 48 hours PI. Corneas were assessed for viral burden by plaque assay; n = 4–5 mice per group (2 independent experiments). Statistical differences were determined by one-way ANOVA with Student-Newman-Keuls multiple comparisons tests.
DISCUSSION
Vaccine composition strongly influences the longevity of protection elicited by immunization, and this is likely dependent upon how a given vaccine “imprints” the immune system during the initial encounter to support plasma cell generation or memory B cell development (50). Epidemiological studies evaluating the kinetics of humoral responses to common vaccines in humans indicate that live-attenuated vaccines often offer life-long humoral protection against infection while humoral responses elicited by subunit vaccines generally decay over time (34). Our recent work highlights that HSV-1 0ΔNLS was much more efficacious than a gD-2 subunit vaccine similar to that used in multiple clinical trials in limiting viral pathogenesis and preventing HSV-1 associated ocular disease in vaccinated mice challenged with HSV-1 (17). However, the kinetics of the humoral immune response was not evaluated in our previous study. Here, we show that antibody titers elicited by a gD-2 subunit vaccine decay substantially within three months in mice, but HSV-1 0ΔNLS elicits sustained high titers of HSV-specific neutralizing antibody. Interestingly, data from multiple clinical trials with gD-2 subunit vaccines show that the cumulative mean HSV-specific and neutralizing antibody concentrations decay by approximately 85% and 77%, respectively, from peak titers within one year of the last dose (51). Furthermore, even with a live-attenuated virus, the site of vaccination has an important effect on the induction of humoral immunity and efficacy (Figs. 3–5). Despite intramuscular injection being the most common route of vaccination clinically, HSV-1 0ΔNLS was least effective following intramuscular vaccination alone. On the other hand, near complete protection against acute disease and viral latency was achieved with HSV-1 0ΔNLS following primary subcutaneous primary immunization with an intramuscular boost. However, vaccine efficacy and safety must be weighed in light of the status of the recipient’s immune system. Subunit vaccines may be preferred over attenuated vaccines for immunocompromised patients due to safety concerns. Additonally, immunoscenescence is documented to limit the efficacy of Zostavax, a therapeutic live-attenuated shingles vaccine, relative to a novel subunit vaccine in older adults (52).
In addition to their limited antigenic breadth, the ephemeral nature of humoral responses elicited by past HSV subunit vaccines is a likely reason for the widespread failure of such vaccines in multiple clinical trials (8, 53). Subunit vaccines such as the alum-adjuvanted hepatitis B surface antigen vaccine can be highly effective against disease despite waning or undetectable antibody titers through anamnestic responses. A meta-analysis of subjects vaccinated with hepatitis B surface antigen clearly links anamnestic responses to protection against disease in patients who seroconvert for other hepatitis B antigens (54). The partial yet incomplete protective effect elicited by a gD-2 subunit vaccine presumably functions through an anamnestic response. However, anamnestic responses are likely too little too late in the case of HSV-1 infection, as the virus can quickly transfer from mucosal epithelial cells to nerve ganglia where the virus persists indefinitely. Moreover, anamnestic responses against the gD-2 subunit reflect approximately 1% of the HSV-1 proteome, whereas the antigenic coverage of the hepatitis B surface antigen is 12% (53). In contrast, the HSV-1 0ΔNLS vaccine encodes nearly 99% of the native viral proteome and elicits humoral immune responses similar to that observed during natural infection (17, 18). Results herein from a delayed challenge model mirror previous data showing that the HSV-1 0ΔNLS vaccine can prevent both corneal sensation loss and neovascularization; however, data here show that the immune response elicited by HSV-1 0ΔNLS sustains its protective effect over time (Fig. 1C).
While IgG is found throughout the cornea, the distribution of IgM and IgA are restricted to the corneal periphery and apical surface epithelium, respectively (21, 55). Concentrations of IgG in the central region of healthy corneas are approximately two-thirds of that found in the periphery, but the basal diffusion rate of IgG in the cornea is low (40, 56, 57). This is evidenced by the limited clinical success of IgG-based therapeutics in the cornea. While the general mechanisms of ocular surface protection have been reviewed elsewhere (58–60), secretory IgA is commonly associated with humoral protection against microbial infection in the tear film and in other mucosal sites (61, 62). Clinical and basic research data confirm that serum titers of HSV-specific IgA do not correlate with either susceptibility to primary HSV infection after vaccination or viral reactivation in latently infected hosts (63–66).
Protein diffusion in the cornea has largely been calculated experimentally using IgG and albumin (56, 57, 67, 68); however, FcRn is responsible for the bidirectional transport of both proteins (69, 70). Coupling the results from the present study identifying FcRn expression changes during viral infection or surface injury, we postulate that FcRn expression levels largely regulate IgG (and albumin) diffusion in the corneal epithelium. This insight into the kinetics of IgG perfusion in the cornea may enhance the utility of antibody-based therapies that normally have low corneal penetration and efficacy if combined with neoadjuvant therapy to modulate FcRn expression in the surface epithelium barrier. Furthermore, this approach could enhance the “depot effect” of IgG-based therapeutics in the cornea described by Osusky et al (57) to increase drug half-life and local efficacy.
The peri-corneal limbal vasculature is well documented as the source of antibody within the cornea given the comparatively low concentrations of IgG in the adjacent tear film and aqueous humor (40, 71). It is estimated that it takes 50–70 days for serum IgG to establish a concentration equilibrium with IgG in the cornea under normal homeostatic conditions (40, 57). Given this low basal rate of diffusion, it is ostensibly clear that a prophylactic HSV-1 vaccine must elicit and sustain high serum concentrations of protective IgG to provide a tenable level of protection in the cornea. However, this limitation may be partially overcome by physiological mechanisms that enhance IgG diffusion in the cornea during infection—particularly up-regulation of FcRn in the epithelium. Here, we demonstrate that the antibody concentration in the cornea rises sharply following corneal infection or injury, and that this phenomenon is concomitant with FcRn up-regulation in various cornea-resident cell populations (Fig. 6, Fig. 8). Indeed, local FcRn knockdown compromises the efficacy of humoral protection elicited by HSV-1 0ΔNLS (Fig. 9). Previous studies show that FcRn facilitates intracellular neutralization of viruses within mucosal epithelial cells in passively immunized animals (72). FcRn expression has also been correlated with passive humoral protection against HSV-2 infection in the vaginal mucosae (73). There are caveats, however, to using global FcRn deficient mice for passive immunization studies, as antibody half-life is severely impacted (23). Accordingly, our studies utilized localized FcRn knockdown to determine the effect of FcRn on viral clearance (Fig. 9). It remains unclear why cornea-infiltrating leukocytes express much lower levels of FcRn than is observed in circulating leukocytes (Fig. 8I, J); it tempting to speculate that leukocytes store antibody in circulation via FcRn-mediated retention for discharge upon activation and extravasation into inflamed tissues as they are proposed to do with soluble complement components (74).
Consistent with the role of FcRn in intracellular trafficking of IgG, we also observed intracellular deposition of C3d in the corneal epithelium of HSV-1 0ΔNLS-vaccinated mice following ocular challenge (Fig. 7A, B; Supplemental Movie 1). While complement deposition classically occurs on the cell surface, evidence is mounting that intracellular complement signaling (dubbed the ‘complosome’) serves multiple functions (75–78). Our data also show that prophylactic protection against HSV-1 is compromised in the absence of a functional complement pathway in the cornea (Fig. 7D). While the complement pathway reportedly enhances humoral immunity during primary HSV-1 infection, the role of complement in prophylactic protection against HSV-1 infection in the eye has not been previously reported (79–82). The ability of complement to enhance neutralization of HSV-1 virions is thought to be limited due to the viral glycoproteins that mimic the IgG Fc receptor and interact with C3 to modulate complement activation (82, 83). Despite these viral immune evasion mechanisms, antibody retains its capacity to neutralize HSV-1 in patients vaccinated with a gD-2 subunit vaccine (27). However, the role of complement may be dependent upon the site of virus entry, as others have demonstrated that ADCC is central to protection against HSV-1 using a skin infection model (84).
Recent findings demonstrate that intracellular antibody-bound viral components are shuttled to the proteasome by the intracellular effector TRIM21 (tripartite motif-containing 21) for non-cytolytic viral clearance (85). FcRn recycling may enable sufficient internalization of antibody within mucosal epithelial cells to facilitate targeting and degradation of diverse viral proteins. This concept challenges the dogma that antibody mediates protective effects exclusively in the extracellular space. Additionally, the concept of antibody actively targeting viral proteins intracellularly also begs the question of whether intracellular epitopes should be targeted in vaccine development against other mucosal pathogens to enhance humoral protection. These questions will need to be addressed in future studies.
Although antibody mediates prophylactic protection against primary ocular HSV-1 infection, it remains unknown why antibody responses fail to protect patients who experience frequent HSV-1 reactivation in the eye. Uncovering the central role of FcRn in antibody-mediated protection against HSV-1 may provide insight into this conundrum. Ocular immunopathology associated with recurrent corneal HSV-1 reactivation in humans is driven primarily by TLR signaling and CD4 T cells (9). In vitro studies have demonstrated that FcRn expression is upregulated by TNFα and repressed by IFNγ in polarized epithelial cells (86, 87). Therefore, the establishment of tissue resident CD4 T cells in the corneas of patients with frequent HSV-1 reactivation may in fact limit the expression of FcRn via IFNγ expression and consequently usurp the local protective functions of antibody. While HSV-1 0ΔNLS has strong translational potential, diverse vaccine strategies will undoubtedly emerge and continue to augment HSV-1 vaccine development. It is now evident that a successful prophylactic HSV-1 vaccine must be capable of eliciting a long-lived, high-titer humoral immune response to effectively combat ocular disease and limit neuroinvasion. Irrespective of whether the antibody-mediated effector function protects via complement, ADCC, intracellular targeting, or a combination thereof, antibody persistence following vaccination is a critical limiting factor of prophylactic vaccine efficacy against HSV-1 infection (88).
Supplementary Material
Acknowledgments
The authors express gratitude to the Dean McGee Eye Institute vivarium staff for maintaining our animal colonies, Edward Gershburg for supplying the recombinant gD-2 protein, and the late Brian Gebhardt for contributing the original stock of HSV-1 McKrae.
D.J.R. designed and conducted experiments, analyzed data, and prepared the manuscript; H.R.G. acquired confocal images in Fig. 5C; M.M.C. assisted with vaccinating animals, tissue processing, and image quantification; W.P.H. designed the HSV-1 0ΔNLS virus and critiqued the manuscript; D.J.J.C. conducted experiments and supervised all work. The content of the manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Eye Institute.
D.J.R., H.R.G., and M.M.C. have no conflicts of interest to report. W.P.H. is a coauthor on U.S. patent 8802109, which describes the uses of herpes simplex virus mutant ICP0 in the design of a live attenuated HSV-2 vaccine strain. In addition, W.P.H. is a cofounder of Rational Vaccines, Inc., which has licensed U.S. patents 77856605 and 8802109. D.J.J.C is a member of the advisory board of Rational Vaccines, Inc.
Grant support: This work was supported by National Institutes of Health (NIH) Grants R01 EY021238, P30 EY021725, and T32 EY023202. Additional support was provided by an unrestricted grant from Research to Prevent Blindness. Funding for the IMARIS software was provided by a grant from the Presbyterian Health Foundation.
Footnotes
Treatment cost projection is based upon consumer price index inflation calculations reported by the United States Bureau of Labor Statistics (available online at https://www.bls.gov/data/inflation_calculator.htm) relative to the original cost estimate reported in 2003 by Lairson et. al. (10).
References
- 1.Iwasaki A. Exploiting Mucosal Immunity for Antiviral Vaccines. Annu Rev Immunol. 2016;34:575–608. doi: 10.1146/annurev-immunol-032414-112315. [DOI] [PubMed] [Google Scholar]
- 2.Su F, Patel GB, Hu S, Chen W. Induction of mucosal immunity through systemic immunization: Phantom or reality? Hum Vaccines Immunother. 2016;12:1070–1079. doi: 10.1080/21645515.2015.1114195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czerkinsky C, Holmgren J. Topical immunization strategies. Mucosal Immunol. 2010;3:545–555. doi: 10.1038/mi.2010.55. [DOI] [PubMed] [Google Scholar]
- 4.Neutra MR, Kozlowski PA. Mucosal vaccines: the promise and the challenge. Nat Rev Immunol. 2006;6:148–158. doi: 10.1038/nri1777. [DOI] [PubMed] [Google Scholar]
- 5.Belyakov IM, Ahlers JD. What Role Does the Route of Immunization Play in the Generation of Protective Immunity against Mucosal Pathogens. J Immunol. 2009;183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 6.Forrester JV, Dick AD, McMenamin PG, Roberts F, Pearlman E. The eye basic sciences in practice 2016 [Google Scholar]
- 7.Whitcher JP, Srinivasan M, Upadhyay MP. Corneal blindness: a global perspective. Bull World Health Organ. 2001;79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 8.Royer DJ, Cohen AW, Carr DJ. The current state of vaccine development for ocular HSV-1 infection. Expert Rev Ophthalmol. 2015;10:113–126. doi: 10.1586/17469899.2015.1004315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lairson DR, Begley CE, Reynolds TF, Wilhelmus KR. Prevention of herpes simplex virus eye disease: a cost-effectiveness analysis. Arch Ophthalmol. 2003;121:108–112. doi: 10.1001/archopht.121.1.108. [DOI] [PubMed] [Google Scholar]
- 11.Caselli E, Balboni PG, Incorvaia C, Argnani R, Parmeggiani F, Cassai E, Manservigi R. Local and systemic inoculation of DNA or protein gB1s-based vaccines induce a protective immunity against rabbit ocular HSV-1 infection. Vaccine. 2000;19:1225–1231. doi: 10.1016/s0264-410x(00)00242-5. [DOI] [PubMed] [Google Scholar]
- 12.Keadle TL, Laycock KA, Miller JK, Hook KK, Fenoglio ED, Francotte M, Slaoui M, Stuart PM, Pepose JS. Efficacy of a recombinant glycoprotein D subunit vaccine on the development of primary and recurrent ocular infection with herpes simplex virus type 1 in mice. J Infect Dis. 1997;176:331–338. doi: 10.1086/514049. [DOI] [PubMed] [Google Scholar]
- 13.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol Baltim Md 1950. 2010;184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walker J, Leib DA. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine. 1998;16:1–5. doi: 10.1016/s0264-410x(97)00164-3. [DOI] [PubMed] [Google Scholar]
- 15.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine–phosphate–guanine adjuvant. Vaccine. 2005;23:873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Bettahi I, Nesburn AB, Yoon S, Zhang X, Mohebbi A, Sue V, Vanderberg A, Wechsler SL, BenMohamed L. Protective Immunity against Ocular Herpes Infection and Disease Induced by Highly Immunogenic Self-Adjuvanting Glycoprotein D Lipopeptide Vaccines. Investig Opthalmology Vis Sci. 2007;48:4643. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 17.Royer DJ, Gurung HR, Jinkins JK, Geltz JJ, Wu JL, Halford WP, Carr DJJ. A Highly Efficacious Herpes Simplex Virus 1 Vaccine Blocks Viral Pathogenesis and Prevents Corneal Immunopathology via Humoral Immunity. J Virol. 2016;90:5514–5529. doi: 10.1128/JVI.00517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Royer DJ, Carr MM, Chucair-Elliott AJ, Halford WP, Carr DJJ. Impact of type 1 interferon on the safety and immunogenicity of an experimental live-attenuated herpes simplex virus type 1 vaccine in mice. J Virol. 2017;91:e02342–16. doi: 10.1128/JVI.02342-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD. Correlate of Immune Protection Against HSV-1 Genital Disease in Vaccinated Women. J Infect Dis. 2014;209:828–836. doi: 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6. Elsevier Saunders; Philadelphia, Pa: 2013. [Google Scholar]
- 21.Allansmith MR, McClellan BH. Immunoglobulins in the Human Cornea. Am J Ophthalmol. 1975;80:123–132. doi: 10.1016/0002-9394(75)90882-x. [DOI] [PubMed] [Google Scholar]
- 22.Preston MJ, Kernacki KA, Berk JM, Hazlett LD, Berk RS. Kinetics of serum, tear, and corneal antibody responses in resistant and susceptible mice intracorneally infected with Pseudomonas aeruginosa. Infect Immun. 1992;60:885–891. doi: 10.1128/iai.60.3.885-891.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 24.Aldinger KA, Sokoloff G, Rosenberg DM, Palmer AA, Millen KJ. Genetic variation and population substructure in outbred CD-1 mice: implications for genome-wide association studies. PloS One. 2009;4:e4729. doi: 10.1371/journal.pone.0004729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yao HW, Ling P, Chen SH, Tung YY, Chen SH. Factors affecting herpes simplex virus reactivation from the explanted mouse brain. Virology. 2012;433:116–123. doi: 10.1016/j.virol.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 26.Halford WP, Püschel R, Gershburg E, Wilber A, Gershburg S, Rakowski B. A Live-Attenuated HSV-2 ICP0ΔVirus Elicits 10 to 100 Times Greater Protection against Genital Herpes than a Glycoprotein D Subunit Vaccine. PLoS ONE. 2011;6:e17748. doi: 10.1371/journal.pone.0017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Awasthi S, Belshe RB, Friedman HM. Better neutralization of herpes simplex virus type 1 (HSV-1) than HSV-2 by antibody from recipients of GlaxoSmithKline HSV-2 glycoprotein D2 subunit vaccine. J Infect Dis. 2014;210:571–575. doi: 10.1093/infdis/jiu177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryant-Hudson KM, Carr DJJ. CXCL1-Deficient Mice Are Highly Sensitive to Pseudomonas aeruginosa but Not Herpes Simplex Virus Type 1 Corneal Infection. Investig Opthalmology Vis Sci. 2012;53:6785. doi: 10.1167/iovs.12-10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downie LE, Stainer MJ, Chinnery HR. Monitoring of Strain-Dependent Responsiveness to TLR Activation in the Mouse Anterior Segment Using SD-OCT. Invest Ophthalmol Vis Sci. 2014;55:8189–8199. doi: 10.1167/iovs.14-15595. [DOI] [PubMed] [Google Scholar]
- 30.Wuest TR, Carr DJJ. VEGF-A expression by HSV-1-infected cells drives corneal lymphangiogenesis. J Exp Med. 2010;207:101–115. doi: 10.1084/jem.20091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royer DJ, Zheng M, Conrady CD, Carr DJJ. Granulocytes in Ocular HSV-1 Infection: Opposing Roles of Mast Cells and Neutrophils. Invest Ophthalmol Vis Sci. 2015;56:3763–3775. doi: 10.1167/iovs.15-16900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royer DJ, Conrady CD, Carr DJJ. Herpesvirus-Associated Lymphadenitis Distorts Fibroblastic Reticular Cell Microarchitecture and Attenuates CD8 T Cell Responses to Neurotropic Infection in Mice Lacking the STING-IFNα/β Defense Pathways. J Immunol Baltim Md 1950. 2016;197:2338–2352. doi: 10.4049/jimmunol.1600574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Royer DJ, Carr DJJ. A STING-dependent innate-sensing pathway mediates resistance to corneal HSV-1 infection via upregulation of the antiviral effector tetherin. Mucosal Immunol. 2016;9:1065–1075. doi: 10.1038/mi.2015.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amanna IJ, Carlson NE, Slifka MK. Duration of Humoral Immunity to Common Viral and Vaccine Antigens. N Engl J Med. 2007;357:1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- 35.Hamrah P, Cruzat A, Dastjerdi MH, Zheng L, Shahatit BM, Bayhan HA, Dana R, Pavan-Langston D. Corneal sensation and subbasal nerve alterations in patients with herpes simplex keratitis: an in vivo confocal microscopy study. Ophthalmology. 2010;117:1930–1936. doi: 10.1016/j.ophtha.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chucair-Elliott AJ, Zheng M, Carr DJJ. Degeneration and regeneration of corneal nerves in response to HSV-1 infection. Invest Ophthalmol Vis Sci. 2015;56:1097–1107. doi: 10.1167/iovs.14-15596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldstein DA, Mis AA, Oh FS, Deschenes JG. Persistent pupillary dilation in herpes simplex uveitis. Can J Ophthalmol J Can Ophtalmol. 2009;44:314–316. doi: 10.3129/i09-018. [DOI] [PubMed] [Google Scholar]
- 38.Thiel MA, Coster DJ, Standfield SD, Brereton HM, Mavrangelos C, Zola H, Taylor S, Yusim A, Williams KA. Penetration of engineered antibody fragments into the eye. Clin Exp Immunol. 2002;128:67–74. doi: 10.1046/j.1365-2249.2002.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaudana R, Ananthula HK, Parenky A, Mitra AK. Ocular Drug Delivery. AAPS J. 2010;12:348–360. doi: 10.1208/s12248-010-9183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhagen C, Breeboart AC, Kijlstra A. Diffusion of immunoglobulin G from the vascular compartment into the normal rabbit cornea. Invest Ophthalmol Vis Sci. 1990;31:1519–1525. [PubMed] [Google Scholar]
- 41.Frossi B, Mion F, Tripodo C, Colombo MP, Pucillo CE. Rheostatic Functions of Mast Cells in the Control of Innate and Adaptive Immune Responses. Trends Immunol. 2017 doi: 10.1016/j.it.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Toapanta FR, Ross TM. Complement-Mediated Activation of the Adaptive Immune Responses: Role of C3d in Linking the Innate and Adaptive Immunity. Immunol Res. 2006;36:197–210. doi: 10.1385/IR:36:1:197. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R, Zheng Z, Lin J, Qu L, Zheng F. The continual presence of C3d but not IgG glomerular capillary deposition in stage I idiopathic membranous nephropathy in patients receiving corticosteroid treatment. Diagn Pathol. 2012;7:109. doi: 10.1186/1746-1596-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zihni C, Mills C, Matter K, Balda MS. Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol. 2016;17:564–580. doi: 10.1038/nrm.2016.80. [DOI] [PubMed] [Google Scholar]
- 45.Kim H, Fariss RN, Zhang C, Robinson SB, Thill M, Csaky KG. Mapping of the Neonatal Fc Receptor in the Rodent Eye. Investig Opthalmology Vis Sci. 2008;49:2025. doi: 10.1167/iovs.07-0871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Powner MB, McKenzie JAG, Christianson GJ, Roopenian DC, Fruttiger M. Expression of Neonatal Fc Receptor in the Eye. Investig Opthalmology Vis Sci. 2014;55:1607. doi: 10.1167/iovs.13-12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- 48.Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, Wang Y, Robert C, Wu B, Smith PD, Lencer WI, Blumberg RS. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol Baltim Md 1950. 2001;166:3266–3276. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Conrady CD, Zheng M, Mandal NA, van Rooijen N, Carr DJJ. IFN-α-driven CCL2 production recruits inflammatory monocytes to infection site in mice. Mucosal Immunol. 2013;6:45–55. doi: 10.1038/mi.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belshe RB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang S-J, Levin JM, McElhaney JE, Poder A, Puig-Barberà J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N Engl J Med. 2015;372:2087–2096. doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 53.Halford WP. Antigenic breadth: a missing ingredient in HSV-2 subunit vaccines? Expert Rev Vaccines. 2014;13:691–710. doi: 10.1586/14760584.2014.910121. [DOI] [PubMed] [Google Scholar]
- 54.Banatvala JE, Van Damme P. Hepatitis B vaccine -- do we need boosters? J Viral Hepat. 2003;10:1–6. doi: 10.1046/j.1365-2893.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- 55.Hazlett LD, Wells P, Berk RS. Immunocytochemical localization of IgA in the mouse cornea. Exp Eye Res. 1981;32:97–104. doi: 10.1016/s0014-4835(81)80043-7. [DOI] [PubMed] [Google Scholar]
- 56.Allansmith M, de Ramus A, Maurice D. The dynamics of IgG in the cornea. Invest Ophthalmol Vis Sci. 1979;18:947–955. [PubMed] [Google Scholar]
- 57.Osusky R, Morell A, Imbach P, Lerch PG. Diffusion of immunoglobulins into rabbit cornea after subconjunctival injection: Experimental demonstration and mathematical model. Graefes Arch Clin Exp Ophthalmol. 1993;231:122–128. doi: 10.1007/BF00920226. [DOI] [PubMed] [Google Scholar]
- 58.Gipson IK. The Ocular Surface: The Challenge to Enable and Protect Vision: The Friedenwald Lecture. Investig Opthalmology Vis Sci. 2007;48:4391. doi: 10.1167/iovs.07-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Akpek EK, Gottsch JD. Immune defense at the ocular surface. Eye. 2003;17:949–956. doi: 10.1038/sj.eye.6700617. [DOI] [PubMed] [Google Scholar]
- 60.Knop E, Knop N. The role of eye-associated lymphoid tissue in corneal immune protection. J Anat. 2005;206:271–285. doi: 10.1111/j.1469-7580.2005.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hazlett LD, Berk RS. Kinetics of immunoglobulin appearance at the ocular surface. Reg Immunol. 1989;2:294–299. [PubMed] [Google Scholar]
- 62.Masinick SA, Montgomery CP, Montgomery PC, Hazlett LD. Secretory IgA inhibits Pseudomonas aeruginosa binding to cornea and protects against keratitis. Invest Ophthalmol Vis Sci. 1997;38:910–918. [PubMed] [Google Scholar]
- 63.Friedman MG, Kimmel N. Herpes simplex virus-specific serum immunoglobulin a: detection in patients with primary or recurrent herpes infections and in healthy adults. Infect Immun. 1982;37:374–377. doi: 10.1128/iai.37.1.374-377.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ashley RL, Crisostomo FM, Doss M, Sekulovich RE, Burke RL, Shaughnessy M, Corey L, Polissar NL, Langenberg AG. Cervical antibody responses to a herpes simplex virus type 2 glycoprotein subunit vaccine. J Infect Dis. 1998;178:1–7. doi: 10.1086/515611. [DOI] [PubMed] [Google Scholar]
- 65.Belshe RB, Heineman TC, Bernstein DI, Bellamy AR, Ewell M, van der Most R, Deal CD. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J Infect Dis. 2014;209:828–836. doi: 10.1093/infdis/jit651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petro C, González PA, Cheshenko N, Jandl T, Khajoueinejad N, Bénard A, Sengupta M, Herold BC, Jacobs WR. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife. 2015:4. doi: 10.7554/eLife.06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Karring H, I, Thøgersen B, Klintworth GK, Møller-Pedersen T, Enghild JJ. The human cornea proteome: bioinformatic analyses indicate import of plasma proteins into the cornea. Mol Vis. 2006;12:451–460. [PubMed] [Google Scholar]
- 68.Maurice DM, Watson PG. The distribution and movement of serum albumin in the cornea. Exp Eye Res. 1965;4:355–363. doi: 10.1016/s0014-4835(65)80052-5. [DOI] [PubMed] [Google Scholar]
- 69.Chaudhury C, Brooks CL, Carter DC, Robinson JM, Anderson CL. Albumin binding to FcRn: distinct from the FcRn-IgG interaction. Biochemistry (Mosc) 2006;45:4983–4990. doi: 10.1021/bi052628y. [DOI] [PubMed] [Google Scholar]
- 70.Dickinson BL, Badizadegan K, Wu Z, Ahouse JC, Zhu X, Simister NE, Blumberg RS, Lencer WI. Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line. J Clin Invest. 1999;104:903–911. doi: 10.1172/JCI6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Waldrep JC, Noe RL, Stulting RD. Analysis of human corneal IgG by isoelectric focusing. Invest Ophthalmol Vis Sci. 1988;29:1538–1543. [PubMed] [Google Scholar]
- 72.Bai Y, Ye L, Tesar DB, Song H, Zhao D, Bjorkman PJ, Roopenian DC, Zhu X. Intracellular neutralization of viral infection in polarized epithelial cells by neonatal Fc receptor (FcRn)-mediated IgG transport. Proc Natl Acad Sci. 2011;108:18406–18411. doi: 10.1073/pnas.1115348108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li Z, Palaniyandi S, Zeng R, Tuo W, Roopenian DC, Zhu X. Transfer of IgG in the female genital tract by MHC class I-related neonatal Fc receptor (FcRn) confers protective immunity to vaginal infection. Proc Natl Acad Sci. 2011;108:4388–4393. doi: 10.1073/pnas.1012861108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liszewski MK, Elvington M, Kulkarni HS, Atkinson JP. Complement’s hidden arsenal: New insights and novel functions inside the cell. Mol Immunol. 2017;84:2–9. doi: 10.1016/j.molimm.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kolev M, Friec GL, Kemper C. Complement - tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 76.Tam JCH, Bidgood SR, McEwan WA, James LC. Intracellular sensing of complement C3 activates cell autonomous immunity. Science. 2014;345:1256070–1256070. doi: 10.1126/science.1256070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Elvington M, Liszewski MK, Bertram P, Kulkarni HS, Atkinson JP. A C3(H20) recycling pathway is a component of the intracellular complement system. J Clin Invest. 2017;127:970–981. doi: 10.1172/JCI89412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kolev M, Kemper C. Keeping It All Going?Complement Meets Metabolism. Front Immunol. 2017:8. doi: 10.3389/fimmu.2017.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Da Costa XJ, Brockman MA, Alicot E, Ma M, Fischer MB, Zhou X, Knipe DM, Carroll MC. Humoral response to herpes simplex virus is complement-dependent. Proc Natl Acad Sci U S A. 1999;96:12708–12712. doi: 10.1073/pnas.96.22.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Verschoor A, Brockman MA, Gadjeva M, Knipe DM, Carroll MC. Myeloid C3 determines induction of humoral responses to peripheral herpes simplex virus infection. J Immunol Baltim Md 1950. 2003;171:5363–5371. doi: 10.4049/jimmunol.171.10.5363. [DOI] [PubMed] [Google Scholar]
- 81.Awasthi S, Lubinski JM, Friedman HM. Immunization with HSV-1 glycoprotein C prevents immune evasion from complement and enhances the efficacy of an HSV-1 glycoprotein D subunit vaccine. Vaccine. 2009;27:6845–6853. doi: 10.1016/j.vaccine.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lubinski JM, Lazear HM, Awasthi S, Wang F, Friedman HM. The herpes simplex virus 1 IgG fc receptor blocks antibody-mediated complement activation and antibody-dependent cellular cytotoxicity in vivo. J Virol. 2011;85:3239–3249. doi: 10.1128/JVI.02509-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lubinski J, Wang L, Mastellos D, Sahu A, Lambris JD, Friedman HM. In vivo role of complement-interacting domains of herpes simplex virus type 1 glycoprotein gC. J Exp Med. 1999;190:1637–1646. doi: 10.1084/jem.190.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Petro CD, Weinrick B, Khajoueinejad N, Burn C, Sellers R, Jacobs WR, Herold BC. HSV-2 ΔgD elicits FcγR-effector antibodies that protect against clinical isolates. JCI Insight. 2016:1. doi: 10.1172/jci.insight.88529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21) Proc Natl Acad Sci. 2010;107:19985–19990. doi: 10.1073/pnas.1014074107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu X, Ye L, Christianson GJ, Yang JQ, Roopenian DC, Zhu X. NF-kappaB signaling regulates functional expression of the MHC class I-related neonatal Fc receptor for IgG via intronic binding sequences. J Immunol Baltim Md 1950. 2007;179:2999–3011. doi: 10.4049/jimmunol.179.5.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu X, Ye L, Bai Y, Mojidi H, Simister NE, Zhu X. Activation of the JAK/STAT-1 signaling pathway by IFN-gamma can down-regulate functional expression of the MHC class I-related neonatal Fc receptor for IgG. J Immunol Baltim Md 1950. 2008;181:449–463. doi: 10.4049/jimmunol.181.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lewis GK, DeVico AL, Gallo RC. Antibody persistence and T-cell balance: two key factors confronting HIV vaccine development. Proc Natl Acad Sci U S A. 2014;111:15614–15621. doi: 10.1073/pnas.1413550111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.