Abstract
Background
ACC/AHA guidelines advise waiting 5-7 days before operating on P2Y12 inhibitor-treated ACS patients, to allow dissipation of its antiplatelet effects. Platelet transfusion is often used to restore hemostasis during operations but its effectiveness and optimal timing are unclear. We investigated the degree of functional gains obtained from platelet-supplementation after loading and maintenance dual antiplatelet therapy [DAPT]with ticagrelor, and the influence of timing on this strategy.
Methods and Results
Following baseline platelet testing (Multiplate® Analyzer and VerifyNow®), CVD patients (n=20, 56.9±7.9 years, 65% male, 75% diabetic) received DAPT as a single loading-dose [LD: ticagrelor 180mg plus aspirin 325mg] and as daily/maintenance treatment for 5-7 days [MT: ticagrelor 90mg b.i.d. plus aspirin 81mg q.d.]. At 4-, 6-, 24- and 48-hours from (last) dosing, patients’ blood samples were supplemented with concentrated platelets from healthy donors in vitro, raising platelet counts by 0% (un-supplemented ‘control’), 25%, 50% and 75%, and the function retested. Reactivity in supplemented samples was compared with respective 0% sample and with the pre-treatment baseline. Results under LD and MT regimens were nearly identical. Platelet reactivity was higher (p<0.05) in nearly all supplemented samples vs. respective controls. Aggregations with supplementation were 59%-79% of baseline at 24-hours, and equal to baseline at 48-hours.
Conclusions
Platelet reactivity of ticagrelor-treated patients can be restored using concentrated platelets after a loading-dose/maintenance-therapy in a time-dependent manner under in vitro testing. Although statistically significant improvements are evident 6 hours after (last) dosing, up to 24 hours maybe needed for clinically meaningful restoration in platelet function.
Keywords: Antiplatelet therapy, Platelet, Ticagrelor, Transfusion
INTRODUCTION
Dual antiplatelet therapy [DAPT] is the standard of care in the management of acute coronary syndrome [ACS] patients, with aspirin and clopidogrel combination being the current standard of care. Ticagrelor has demonstrated greater benefits than clopidogrel in reducing clinical events in ACS patients.1-3 Although stronger platelet inhibition has clearly proven benefits in preventing ischemic events, it also complicates management of ACS patients by enhancing the risk of bleeding complications and the associated morbidity and mortality.4 Repeat operation to control hemorrhage is almost 6 times more likely if patients receive DAPT prior to coronary artery bypass grafting [CABG] and 20% of such patients require platelet transfusions.5 The ACC/AHA guidelines for ACS patients requiring CABG surgery recommend discontinuation of any P2Y12 receptor inhibitor therapy for at least 5 days before an elective operation, and at least 24 hours before urgent CABG.6 This ‘treatment-devoid’ phase leaves these high-risk patients defenseless against recurrent ischemic events and any means to shorten this vulnerable period would be of clinical value. Platelet transfusions are advised and frequently employed to deal with bleeding complications during CABG surgery in patients on antiplatelet therapy,7, 8 and could potentially be used for the earlier restoration of hemostasis in DAPT treated patients, thereby shortening their waiting time for operation. However, the degree to which the newly infused platelets restore patients’ hemostatic function, and its relationship to the quantity of platelets infused, is unknown. More importantly, the effect of timing of transfusion relative to last drug intake - likely to be an important modulator of any benefits achieved - is also unclear. Timing is a critical variable in urgent scenarios as platelets transfused too soon after antiplatelet dosing may be rendered ineffective by any residual drug in circulation.
We have previously reported that adding treatment-naïve platelets can adequately reverse clopidogrel’s inhibitory effects and restore hemostatic potential,9 and similar results can also be achieved after prasugrel treatment at the appropriate time.10 The possibility of normalizing platelet reactivity after ticagrelor therapy has also been investigated in a few small studies with mixed outcomes, ranging from no recovery at all,11, 12 to some restoration of function.13 Clopidogrel and prasugrel are members of thienopyridine class, binding irreversibly to the P2Y12 receptor and exerting long acting inhibitory effects, whereas ticagrelor is the first member of the cyclo-pentyl-triazolo-pyrimidine class, with reversible binding and short-acting effects, hence the twice-daily administration. Given the limited and conflicting information on the topic, the present study was conducted to investigate the possibility of restoring platelet reactivity of ticagrelor-treated patients, using concentrated platelets from healthy donors, and the effect quantity and timing of infusion has on any functional improvement achieved. The design of the study was based on two clinical scenarios where the need for restoring hemostasis may arise: A) patient having received a loading-dose of DAPT with ticagrelor, and B) patient on maintenance DAPT with ticagrelor. Study aim was to investigate if the platelet function of a patient on DAPT could be restored to pre-treatment levels within 48 hours of dosing, using fresh platelet concentrates.
METHODS
Study design and population
The study was conducted using an open-label, two treatment-regimen design in patients with stable cardiovascular disease [CVD]. Each patient received DAPT as a loading-dose and as maintenance-therapy, and each dosing modality was followed by a protocol for in vitro platelet supplementation and functional assessment performed at 4 pre-specified time-points (Figure 1).
Figure 1. Study design.
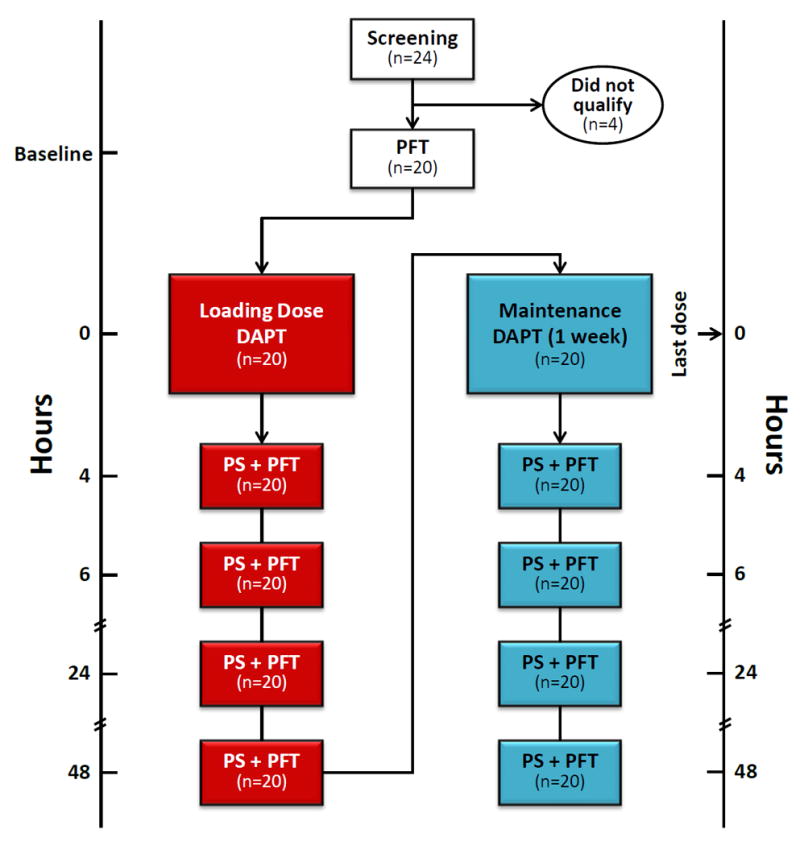
DAPT: Dual Anti-Platelet Therapy, PFT: Platelet function tests (ADPtest in Multiplate® Analyzer and PRUtest in VerifyNow®), PS: Platelet supplementation (addition of concentrated platelets to DAPT-treated patients’ blood samples raising platelet counts by 0% (control), 25%, 50% and 75%).
Study candidates underwent screening that included medical history taking, physical examination, routine blood work, drugs of abuse testing and 12-lead ECG. Patients with unstable or acute CVD, history of stroke or bleeding disorders, clinically significant abnormalities in screening test results, and those on treatments known to affect hemostasis (including anticoagulants, antiplatelets except aspirin, fibrinolytics, NSAIDs) in the month prior to study participation, were excluded. A separate group of healthy volunteers underwent the same screening process and served as donors for freshly prepared concentrated platelets used in supplementation.
On the day of the study, patients reported to the AtheroThrombosis Research Unit [ATRU]where after testing of baseline (pre-treatment) platelet function, they received a Loading Dose [LD] of DAPT (ticagrelor 180 mg plus aspirin 325 mg). At 4, 6, 24 and 48 hours post dosing, patient’s blood samples were collected and supplemented with concentrated platelets to increase the samples’ platelet counts by 0% (control), 25%, 50% and 75%. Platelet reactivity in each sample was tested using two methodologies (Multiplate® Analyzer and VerifyNow® PRU assay). Upon completion of the LD regimen part of the study, patients received DAPT as Maintenance Therapy [MT] (ticagrelor 90 mg b.i.d. plus aspirin 81 mg q.d. × 5-7 days) and the same study protocol was carried out 4, 6, 24 and 48 hours after the last dosing (Figure 1).
The study complied with the Declaration of Helsinki and was approved by the Program for the Protection of Human Subjects (Institutional Review Board) of the Icahn School of Medicine at Mount Sinai. A written informed consent was obtained from each study patient and donor before initiating any study-related procedures.
Blood Sampling
Blood samples were drawn without stasis by clean venipuncture from an antecubital vein using a 21-gauge butterfly cannula system (Vacutainer®; Becton Dickinson, Franklin Lakes, NJ). Samples from patients were collected in siliconized vacutainer tubes containing 3.2% sodium citrate at baseline before the administration of ticagrelor, and at 4, 6, 24 and 48 hours after the loading dose /last maintenance dose.
Blood samples for isolating concentrated platelets for supplementation were drawn from non-treated, healthy donors (3 per subject) into acid citrate dextrose [ACD] and 3.2% sodium citrate tubes. Platelets were isolated from the ACD samples using a two-step centrifugation protocol and resuspended in citrated platelet-poor plasma as previously reported.10
Platelet supplementation
Cell counts in the concentrated platelets used for supplementation averaged between 2200 - 2500 × 103/μl at the four assessment time-points. At each post-dose time-point, the concentrates were added to treated patients’ blood in vitro targeting increases in the samples platelet counts of 25%, 50% and 75%. For example, targeted counts for a blood sample with 200 × 103/μl platelets would be 250 (↑25%), 300 (↑50%) and 350 (↑75%) × 103/μl, respectively. In clinical use, transfusion of one platelets apheresis unit is expected to increase platelet count by as much as 50 × 103/μl in an average adult,14 or approximately by 25% in an adult patient with a platelet count of 200 × 103/μl.
Volume of added concentrate was always kept at <10% of the sample-volume to which it was being added, thereby preventing undue sample dilution. After gentle mixing by inversion, samples were allowed to stand at room temperature for 15 minutes before testing and all experiments were completed within 2 hours of the collection of blood.
Assessment of Platelet reactivity
Platelet function was assessed using two separate techniques: Impedance aggregometry and light transmission aggregometry.
Multiplate® Analyzer (DiaPharma - West Chester, OH) measures platelet function by impedance aggregometry and is one of the most widely used methodologies for platelet functional assessment in research. In addition to testing platelet reactivity in response to a wide variety of agonists, this methodology can also be used in the diagnosis of certain platelet disorders. Testing for study purposes was carried out using the ADPtest assay, which stimulates platelet activation by adenosine diphosphate [ADP] at a final concentration of 6.5 μM, according to manufacturer’s instructions. The most important ADP receptor (P2Y12) is blocked by clopidogrel, ticagrelor and prasugrel. Tests runs were for 6 minutes and the parameter of area under the aggregation curve was recorded as Units (U).
VerifyNow® system (Accriva Diagnostics - San Diego, CA) is an FDA-approved, point-of-care device used in many catheterization laboratories. It is designed specifically for the testing of platelet inhibition by aspirin, P2Y12-receptor inhibitors and GP-IIb/IIIa inhibitors, and is not suitable for diagnosing platelet disorders. VerifyNow®, like the Multiplate®, measures platelet aggregation in whole blood, but utilizes light transmission instead of impedance aggregometry for its assessment. The PRUtest kit, specific for assessing P2Y12 receptor inhibition, was used for this study as per manufacturer’s instructions and the output of P2Y12 Reaction Units (PRU) was recorded.
Statistical analysis
The primary parameter was the platelet aggregation result ‘area under the curve (U)’generated by the Multiplate® Analyzer. The pharmacodynamic parameters were listed and summarized using the standard statistics: mean ± standard deviation separately by each time-point and the quantity of concentrated platelets added, unless specified otherwise.
Comparisons of platelet function across time-points were performed using longitudinal mixed-effect model. Comparisons between supplementation levels within time-points were tested using one-way ANOVA. After testing for normality of distribution, pairwise comparisons were made using paired t-test or Wilcoxon signed-rank test as appropriate, with Bonferroni correction. Baseline results were compared with each of the post-treatment time-point means (in supplemented and non-supplemented samples)using paired t-test or Wilcoxon signed-rank test as appropriate. The last set of analyses was not corrected for multiple comparisons as the aim was to identify the earliest time-point when a lack of statistically significance was observed.
The differences between the supplemented samples at each time point were visualized versus 100%, 90%, 80%, 70% and 60% of baseline, using histograms. The threshold for statistical significance was set at the nominal p=0.05 level. All analyses were performed using STATA 14.2 software (StataCorp, College Station, TX).
RESULTS
Twenty four patients underwent screening out of which 4 were excluded for not meeting the inclusion/exclusion criteria. A total of 20 patients with cardiovascular disease (mean age of 56.9 years, 65% males and 75% diabetics) were enrolled in and completed both LD and MT parts of the study. Their demographic information is summarized in Table 1. No adverse events were reported by any of the study participants in the study.
Table 1.
Baseline demographic characteristics of the study population
| Baseline characteristics (n=20)
| |
|---|---|
| Age (years) | 56.9 ± 7.9 |
| Male | 65 % |
| Body Mass Index (BMI - kg/m2) | 30.7 ± 4.5 |
| Type-II diabetes mellitus | 75 % |
| Hypertension | 80 % |
| Systolic blood pressure (mmHG) | 127.2 ± 17.5 |
| Diastolic blood pressure (mmHG) | 70.6 ± 9.0 |
| Hypercholesterolemia | 95 % |
| Cholesterol (mg/dL) | 158.7 ± 62.0 |
| Triglycerides (mg/dL) | 165.4 ± 91.4 |
| LDL (mg/dL) | 85.6 ± 51.7 |
| HDL (mg/dL) | 42.5 ± 12.6 |
| Smoking | |
| Current | 10 % |
| Past | 40 % |
| Never | 50 % |
| Alcohol use | |
| Current | 45 % |
| Past | 15 % |
| Never | 40 % |
Platelet effects of ticagrelor loading versus maintenance-dosing
Platelet reactivity was significantly reduced after both LD and MT regimens and showed a nearly identical inhibitory pattern over the 48 hour testing period. The inhibitions were significant versus baseline at all four time-points, with both testing methodologies (Figure 2). Peak inhibition was seen at 6 hours in both LD and MT dosing regimens with 82% and 80% inhibitions in Multiplate® results and 91% and 92% inhibitions in PRU, respectively. Thereafter, a natural, time-dependent recovery in function was observed.
Figure 2. Time-dependent Recovery of Platelet Function without Supplementation after Ticagrelor Loading and Maintenance Therapy.

Platelet reactivity (mean ± SEM) is shown before (Baseline) and after ticagrelor administration as a single loading dose and after maintenance therapy for one week. A natural recovery in platelet reactivity was observed from 6 hours onwards, in both Multiplate® and VerifyNow® testing. *p<0.05 vs. Baseline.
Platelet function normalization after ticagrelor loading dose
Increases in platelet counts in the patients’ samples following supplementation were consistent and close to specified targets of 25%, 50% and 75% at each time-point (see supplemental Table).
The addition of concentrated platelets produced a step-wise increase in platelet reactivity at each time-point in Multiplate® testing (Figure 3). These gains were statistically significant versus respective control (0%) samples even at the lowest supplementation level. Despite the statistically significant increases, platelet reactivity at 4 and 6 hours remained substantially lower than pre-treatment value, peaking at 35% of the baseline aggregation. From 24 hours onwards, improvements in function from the addition of fresh platelets were more sizeable; with aggregations in 25%, 50% and 75% supplemented samples reaching 59%, 74% and 79% of baseline (Figure 3). By 48 hours, addition of fresh platelets even at the lowest tested level restored aggregation to where it was 85% of baseline (Table 2).
Figure 3. Restoring Platelet Function after Ticagrelor Loading Dose.

Platelet aggregation (mean ± SEM) before (Baseline) and after ticagrelor loading dose, with (25%, 50% and 75%) and without (0%) platelet supplementation is shown. Reactivity was measured using Multiplate® Analyzer ADPtest (U) and VerifyNow® (PRU). Aggregation was significantly higher in all supplemented samples vs. corresponding 0% sample except at 48 hours in VerifyNow® testing. At 48 hours, Multiplate® testing showed aggregation in all supplemented samples to be statistically no different from Baseline. *p<0.05 vs. corresponding 0% sample, NS: p>0.05 vs. Baseline.
Table 2.
Platelet normalization assessed using Multiplate® Analyzer (U)
| LOADING DOSE
|
MAINTENANCE THERAPY
|
|
|---|---|---|
| Aggregation (U) (mean ± SD) | Aggregation (U) (mean ± SD) | |
| Baseline | 67.0 ± 14.3 | 67.0 ± 14.3 |
| Post-dose | ||
| 4 hours 0% | 13.6 ± 4.6 | 15.7 ± 6.7 |
| 25% | 18.7 ± 5.2 * | 20.9 ± 6.1 * |
| 50% | 21.4 ± 7.7 * | 22.1 ± 5.9 * |
| 75% | 23.6 ± 8.4 * | 24.2 ± 6.3 * |
| 6 hours 0% | 12.3 ± 5.9 | 13.4 ± 6.0 |
| 25% | 17.3 ± 6.1 * | 16.5 ± 5.5 * |
| 50% | 20.7 ± 6.5 * | 18.9 ± 6.3 * |
| 75% | 22.3 ± 7.5 * | 20.4 ± 6.2 * |
| 24 hours 0% | 24.9 ± 14.0 | 25.6 ± 15.6 |
| 25% | 39.7 ± 18.1 * | 35.5 ± 15.1 * |
| 50% | 49.3 ± 17.9 * | 43.3 ± 16.7 * |
| 75% | 52.8 ± 22.7 * | 47.5 ± 18.2 * |
| 48 hours 0% | 41.3 ± 17.7 | 39.1 ± 21.3 |
| 25% | 57.7 ± 17.6 *† | 56.8 ± 19.5 *† |
| 50% | 68.6 ± 20.4 *† | 63.8 ± 19.1 *† |
| 75% | 72.8 ± 18.4 *† | 70.3 ± 23.0 *† |
p<0.05 vs. corresponding 0%,
p is not significant vs. Baseline
Results obtained with VerifyNow® testing were less consistent than those with Multiplate®. Platelet function after supplementation increased significantly versus respective control at 4, 6 and 24 hours, but not at 48 hours, and did not exhibit the consistent step-wise pattern observed with Multiplate® testing (Figure 3). At 48 hours, PRU reached 90% of baseline in the control (0%) sample and the incremental addition of concentrated platelets produced an inverse effect on platelet reactivity (mean PRU of 251, 250, 226 and 204 with 0%, 25%, 50% and 75% supplementations, respectively). Platelet function tested with VerifyNow® could not be restored to baseline levels at any of the tested time-points (Table 3).
Table 3.
Platelet normalization assessed using VerifyNow® (P2Y12 Reaction Units - PRU)
| LOADING DOSE
|
MAINTENANCE THERAPY
|
|
|---|---|---|
| Aggregation (PRU) (mean ± SD) | Aggregation (PRU) (mean ± SD) | |
| Baseline | 284.1 ± 42.2 | 284.1 ± 42.2 |
| Post-dose | ||
| 4 hours 0% | 39.1 ± 25.9 | 37.5 ± 32.4 |
| 25% | 69.6 ± 35.6 * | 72.2 ± 38.5 * |
| 50% | 100.3 ± 49.6 * | 88.4 ± 42.9 * |
| 75% | 98.6 ± 54.4 * | 85.9 ± 49.4 * |
| 6 hours 0% | 25.9 ± 28.9 | 24.0 ± 30.3 |
| 25% | 55.3 ± 50.2 * | 46.0 ± 37.3 * |
| 50% | 67.8 ± 53.8 * | 55.4 ± 41.8 * |
| 75% | 71.2 ± 42.8 * | 67.7 ± 45.3 * |
| 24 hours 0% | 127.6 ± 58.8 | 119.0 ± 78.0 |
| 25% | 157.3 ± 52.5 * | 147.6 ± 65.2 * |
| 50% | 165.9 ± 40.5 * | 158.0 ± 43.5 * |
| 75% | 162.5 ± 29.4 * | 154.0 ± 51.0 |
| 48 hours 0% | 250.6 ± 45.8 | 214.8 ± 74.0 |
| 25% | 249.5 ± 35.1 | 219.7 ± 52.2 |
| 50% | 226.2 ± 51.6 | 221.2 ± 44.2 |
| 75% | 204.2 ± 45.9 | 203.9 ± 41.5 |
p<0.05 vs. corresponding 0%,
p is not significant vs. Baseline
Platelet function normalization after ticagrelor maintenance therapy
Increases in cell counts after the addition of concentrated platelets were consistently close to targets (supplemental Table). Functional improvements obtained with supplementation were step-wise and statistically significant versus the respective control at each time-point in Multiplate® testing (Figure 4). Supplementation did not improve function beyond 36% of baseline in the initial 6 hours. At 24 hours, addition of 25%, 50% and 75% platelets produced results that reached 53%, 65% and 71% of baseline (Table 2). By 48 hours, all three supplementation levels were able to restore function to baseline levels (mean aggregations of 56.8, 63.8 and 70.3 vs. 67.0; p = 0.051, 0.503 and 0.723, respectively; Figure 4, Table 2).
Figure 4. Restoring Platelet Function after Ticagrelor Maintenance Therapy.

Platelet aggregation (mean ± SEM) before (Baseline) and after one week of ticagrelor maintenance therapy, with (25%, 50% and 75%) and without (0%) platelet supplementation is shown. Reactivity was measured using Multiplate® Analyzer ADPtest (U) and VerifyNow® (PRU). Aggregation was significantly higher in all supplemented samples vs. corresponding 0% sample except at 48 hours in VerifyNow® testing. At 48 hours Multiplate® testing showed aggregation in all supplemented samples to be statistically no different from Baseline. * p<0.05 vs. corresponding 0% sample, NS: p>0.05 vs. Baseline.
In VerifyNow® testing, platelet function at 48hours returned to 76% of baseline without the addition of any concentrated platelets. No amount of platelet supplementation could restore PRU to baseline levels at any of the tested time-points (Figure 4, Table 3).
The platelet inhibitory effects of ticagrelor and the functional restoration achieved with supplementation after MT dosing regimen were nearly identical to those seen after LD regimen.
DISCUSSION
Patients on DAPT with ticagrelor requiring surgical intervention are advised to stop treatment and wait 5 days for its antiplatelet effects to dissipate.6 The ability of platelet concentrate supplementation to improve hemostasis in patients treated with clopidogrel and prasugrel has been previously reported.9, 10 In the present exploratory study, we found that concentrated platelets from healthy donors have the ability to restore platelet reactivity of ticagrelor-treated patients to pre-treatment levels within 48 hours of loading-dose administration or daily-therapy cessation.
The pharmacodynamic profile of ticagrelor after a loading versus maintenance dosing regimen appears almost identical in our study. Peak inhibitory effect was observed 6 hours after the dose administration in LD regimen and 6 hours after the last dose in MT regimen. In both regimens, an unaided physiological recovery in platelet function was evident beyond the 6 hour time-point, resulting from a combination of gradual drug clearance from the body and the release of new platelets into circulation from the bone marrow.The pattern of natural recovery in platelet function observed in our study fits well with the guideline’s recommendation regarding urgent CABG in P2Y12-inhibitor treated patients.
The addition of increasing levels of concentrated platelets from un-treated, healthy donors caused a stepwise increase in platelet reactivity of ticagrelor-treated patients’ blood. Although these gains were statistically significant at all tested time-point, the clinical benefits of using platelet concentrates within the initial 6 hours of ticagrelor administration appear doubtful, given the diminutive absolute recovery attained this close to dosing. The lowest supplementation level (25%) used in this study corresponds to the increase in platelet count expected from transfusion of one platelets apheresis unit in an adult patient with a platelet count of 200 × 103/μl.14 Even at 75% - a level impractical for most clinical scenarios -platelet supplementation fails to improve function beyond 36% of pre-treatment baseline when administered within 6 hours of dosing. In other words, platelet inhibition at 6 hours even after supplementation is greater than what is typically observed after a 600 mg loading dose of clopidogrel.15 This was equally true after both loading and maintenance dosing regimens. The muted hemostatic recovery within 4-6 hours of ticagrelor administration fits well with the half-life of ticagrelor at around 6.5 hours and its active metabolite AR-C124910XX at approximately 9 hours.16 It indicates inhibition of the newly added platelets by residual drug metabolite and is in concordance with the findings of earlier studies that had different patient populations and testing methodologies, but assessment times identical or very close to the 4 and 6 hour time-points of our study.11, 13 By 24 hours, the improvements attained from adding fresh platelets appear much more substantial. The residual drug metabolite concentration around this time is too low to significantly affect new platelets16 and as a result, gains attained at 24 hours shift platelet function safely away from the cutoff associated with increased bleeding risk as per the Working Group on On-Treatment Platelet Reactivity.17 By 48 hours, the substantial natural recovery in patients own platelet function combined with miniscule levels of drug metabolites in plasma, allow platelet supplementation to restore reactivity back to pre-treatment levels.
The overall results of the two testing methodologies employed in our study - Multiplate® and VerifyNow® -were essentially analogous, but displayed some differential characteristics that confirm previous findings.18 VerifyNow®,designed primarily as a point-of-care device for assessing the inhibitory effects of antiplatelet agents, may not be ideally suited for measuring restoration of function using platelet concentrates, as it employs light transmittance for its testing and may be susceptible to interpreting sample dilutions as functional changes. This assumption is supported by our data from the 48-hour time-point, when the patients’ PRU has recovered on its own to a level where platelet supplementation contributes no additional functionality in VerifyNow® testing. With no opposing functional changes to mask it, the effects of dilution thus become apparent and the addition of increasing levels (volumes) of platelet concentrates produces corresponding decreases in PRU. Impedance aggregometry in the Multiplate® analyzer seem impervious to the effects of dilution and in fact makes it part of its standard testing protocol.
Based on the results of our study it is apparent there is a short refractory period after ticagrelor treatment during which the ability of platelet transfusion to restore functionality is substantially lower. The availability of an antidote for ticagrelor would be desirable for the restoration of hemostasis in emergency situations.19
CONCLUSIONS
Platelet reactivity of patients treated with ticagrelor can be restored using concentrated platelets from healthy donors in a dose-dependent manner in in vitro testing. This is true whether the drug is administered as a loading dose or maintenance therapy. Restoration of function is strongly dependent upon the time elapsed since (last) dosing. Although statistically significant improvements in platelet function can be attained as early as 6 hours from treatment, these gains appear unlikely to be of substantial clinical value; therefore a ticagrelor antidote could prove valuable for restoring platelet function in this period. The benefit quotient of platelet transfusion is likely to be significantly higher if administered 24 hours after the last drug intake, be it loading or maintenance therapy.
STUDY LIMITATIONS
The study employed ex vivo platelet supplementation as a proxy to gauge the functional gains obtained with in vivo platelet transfusions. Our overall study design is as close as one can get to the clinical scenario of a patient population receiving platelet transfusion, but it cannot truly reflect the actual clinical setting. This limitation however, is more a part of the clinical setting than the study itself. A DAPT-treated surgical patient receiving platelet transfusion represents a serious situation, where clinical indications and ethical considerations hamper the standardization of critical study variables of timing and quantity of platelet infusion. The heterogeneity of variables associated with such a setup substantially raise the likelihood of inconclusive findings, leaving our chosen study design as the most viable option to investigate the specific aims of this study.
Despite the use of a surrogate measure, the findings of this study are significant for clinical practice. A previous study with in vivo platelet transfusion reported results consistent with this study, but due to the limitations inherent to a setup with in vivo transfusion, included no assessment of the effect of timing or quantity of transfusion.13 Taken together, it is safe to assume that the overall findings of this study would be applicable in a clinical setting.
Supplementary Material
CLINICAL PERSPECTIVE SUMMARY.
What is Known
The strategy of infusing platelets to reduce bleeding risk is frequently employed during surgery with patients on antiplatelet therapy.
Previous reports exploring the usefulness of platelet transfusion in countering the antiplatelet effects of ticagrelor show mixed results.
What the Study Adds
Use of homologous platelets to counter the antiplatelet effects of ticagrelor therapy in cardiovascular patients is a viable strategy.
Time elapsed since ticagrelor dose administration/treatment cessation plays a critical role in determining the degree of functional gains obtained with platelet supplementations.
Acknowledgments
The authors thank Jose C. Rodriguez, MD for his critical role in conducting this study. We also thank the dedicated nurses and staff of the Mount Sinai Clinical Research Center.
SOURCES OF FUNDING
This study was funded in part by a research grant from AstraZeneca. The Clinical Research Center of Mount Sinai is supported by a grant (#UL1TR000067) from the National Center for Advancing Translational Sciences [NCATS], a component of the NIH.
Footnotes
Clinical Trial Registration Information: Unique Identifier: NCT02201394
URL: https://clinicaltrials.gov/ct2/show/NCT02201394?term=NCT02201394&rank=1
DISCLOSURES
Juan Badimon - Research Grant (significant) AstraZeneca Pharmaceuticals LP.
Valentin Fuster - Consultant (modest) AstraZeneca Pharmaceuticals LP.
Remaining authors have nothing to disclose.
References
- 1.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–57. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 2.Steg PG, James S, Harrington RA, Ardissino D, Becker RC, Cannon CP, Emanuelsson H, Finkelstein A, Husted S, Katus H, Kilhamn J, Olofsson S, Storey RF, Weaver WD, Wallentin L Group PS. Ticagrelor versus clopidogrel in patients with ST-elevation acute coronary syndromes intended for reperfusion with primary percutaneous coronary intervention: A Platelet Inhibition and Patient Outcomes (PLATO) trial subgroup analysis. Circulation. 2010;122:2131–41. doi: 10.1161/CIRCULATIONAHA.109.927582. [DOI] [PubMed] [Google Scholar]
- 3.Steg PG, Harrington RA, Emanuelsson M, Katus HA, Mahaffey KW, Meier B, Storey RF, Wojdyla DM, Lewis BS, Maurer G, Wallentin L, James SK for the PSG. Stent Thrombosis With Ticagrelor Versus Clopidogrel in Patients With Acute Coronary Syndromes: An Analysis From the Prospective, Randomized PLATO Trial. Circulation. 2013;128:1055–1065. doi: 10.1161/CIRCULATIONAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 4.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 5.Yende S, Wunderink RG. Effect of clopidogrel on bleeding after coronary artery bypass surgery. Critical care medicine. 2001;29:2271–5. doi: 10.1097/00003246-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Hillis LD, Smith PK, Anderson JL, Bittl JA, Bridges CR, Byrne JG, Cigarroa JE, Disesa VJ, Hiratzka LF, Hutter AM, Jr, Jessen ME, Keeley EC, Lahey SJ, Lange RA, London MJ, Mack MJ, Patel MR, Puskas JD, Sabik JF, Selnes O, Shahian DM, Trost JC, Winniford MD American College of Cardiology F, American Heart Association Task Force on Practice G, American Association for Thoracic S, Society of Cardiovascular A and Society of Thoracic S. 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, Society of Cardiovascular Anesthesiologists, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e123–210. doi: 10.1016/j.jacc.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 7.British Committee for Standards in H. Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–41. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 8.Sousa-Uva M, Storey R, Huber K, Falk V, Leite-Moreira AF, Amour J, Al-Attar N, Ascione R, Taggart D, Collet JP Surgery ESCWGoC and Thrombosis ESCWGo. Expert position paper on the management of antiplatelet therapy in patients undergoing coronary artery bypass graft surgery. Eur Heart J. 2014;35:1510–4. doi: 10.1093/eurheartj/ehu158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vilahur G, Choi BG, Zafar MU, Viles-Gonzalez JF, Vorchheimer DA, Fuster V, Badimon JJ. Normalization of platelet reactivity in clopidogrel-treated subjects. J Thromb Haemost. 2007;5:82–90. doi: 10.1111/j.1538-7836.2006.02245.x. [DOI] [PubMed] [Google Scholar]
- 10.Zafar MU, Santos-Gallego C, Vorchheimer DA, Viles-Gonzalez JF, Elmariah S, Giannarelli C, Sartori S, Small DS, Jakubowski JA, Fuster V, Badimon JJ. Platelet function normalization after a prasugrel loading-dose: time-dependent effect of platelet supplementation. J Thromb Haemost. 2013;11:100–6. doi: 10.1111/jth.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonhomme F, Bonvini R, Reny JL, Poncet A, Fontana P. Impact of non-inhibited platelet supplementation on platelet reactivity in patients treated with prasugrel or ticagrelor for an acute coronary syndrome: An ex vivo study. Platelets. 2015;26:324–30. doi: 10.3109/09537104.2015.1035247. [DOI] [PubMed] [Google Scholar]
- 12.Martin AC, Berndt C, Calmette L, Philip I, Decouture B, Gaussem P, Gouin-Thibault I, Samama CM, Bachelot-Loza C, Godier A. The effectiveness of platelet supplementation for the reversal of ticagrelor-induced inhibition of platelet aggregation: An in-vitro study. Eur J Anaesthesiol. 2016;33:361–7. doi: 10.1097/EJA.0000000000000348. [DOI] [PubMed] [Google Scholar]
- 13.O’Connor SA, Amour J, Mercadier A, Martin R, Kerneis M, Abtan J, Brugier D, Silvain J, Barthelemy O, Leprince P, Montalescot G, Collet JP, Group AS. Efficacy of ex vivo autologous and in vivo platelet transfusion in the reversal of P2Y12 inhibition by clopidogrel, prasugrel, and ticagrelor: the APTITUDE study. Circulation Cardiovascular interventions. 2015;8:e002786. doi: 10.1161/CIRCINTERVENTIONS.115.002786. [DOI] [PubMed] [Google Scholar]
- 14.Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. Fresh-Frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. JAMA. 1994;271:777–81. [PubMed] [Google Scholar]
- 15.L’Allier PL, Ducrocq G, Pranno N, Noble S, Ibrahim R, Gregoire JC, Azzari F, Nozza A, Berry C, Doucet S, Labarthe B, Theroux P, Tardif JC Investigators PS. Clopidogrel 600-mg double loading dose achieves stronger platelet inhibition than conventional regimens: results from the PREPAIR randomized study. J Am Coll Cardiol. 2008;51:1066–72. doi: 10.1016/j.jacc.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Butler K, Teng R. Pharmacokinetics, pharmacodynamics, safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol. 2010;70:65–77. doi: 10.1111/j.1365-2125.2010.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Ten Berg J, Kirtane A, Siller-Matula J, Mahla E, Becker RC, Bhatt DL, Waksman R, Rao SV, Alexopoulos D, Marcucci R, Reny JL, Trenk D, Sibbing D, Gurbel PA and Working Group on On-Treatment Platelet R. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62:2261–73. doi: 10.1016/j.jacc.2013.07.101. [DOI] [PubMed] [Google Scholar]
- 18.Vries MJ, Bouman HJ, Olie RH, Veenstra LF, Zwaveling S, Verhezen PW, Ten Cate-Hoek AJ, Ten Cate H, Henskens YM, van der Meijden PE. Determinants of agreement between proposed therapeutic windows of platelet function tests in vulnerable patients. Eur Heart J Cardiovasc Pharmacother. 2017;3:11–17. doi: 10.1093/ehjcvp/pvw026. [DOI] [PubMed] [Google Scholar]
- 19.Buchanan A, Newton P, Pehrsson S, Inghardt T, Antonsson T, Svensson P, Sjogren T, Oster L, Janefeldt A, Sandinge AS, Keyes F, Austin M, Spooner J, Gennemark P, Penney M, Howells G, Vaughan T, Nylander S. Structural and functional characterization of a specific antidote for ticagrelor. Blood. 2015;125:3484–90. doi: 10.1182/blood-2015-01-622928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


