Abstract
Objective
To examine a remote method for maintaining long-term contact with Parkinson's disease (PD) patients participating in clinical studies.
Background
Long-term follow-up of PD patients is needed to fill critical information gaps on progression, biomarkers and treatment. Prospective in-person assessment can be costly and may be impossible for some patients. Remote assessment using mail and telephone contact may be a practical follow-up method.
Design/Methods
Patients enrolled in the multi-center LABS-PD in-person follow-up study in 2006 were invited to enroll in FOUND. FOUND is overseen by a single center under a separate, central IRB protocol. FOUND uses mailed questionnaires and telephone interviews to assess PD status. FOUND follow-up continued when LABS-PD in-person visits ended in 2011. Retention and agreement between remote and in-person assessments were determined.
Results
422 /499 (84.5%) of eligible patients volunteered. Ninety-six percent of participants were retained. Of 60 who withdrew consent from LABS-PD, 51 were retained in FOUND. Of 341 active in LABS-PD, 340 were retained in FOUND (99.7%) when in-person visits ceased. Exact agreement between remote and in-person assessments was ≥ 80% for diagnosis, disease features (e.g., dyskinesias) and PD medication. Correlation between expert-rated and self-reported UPDRS and MDS-UPDRS, examined at times separated by several months, was moderate or substantial for most items.
Conclusion
Retention was excellent using remote follow-up of research participants with PD, providing a “safety net” when combined with in-person visits, and is also effective as a stand-alone assessment method, providing a useful alternative when in-person evaluation is not feasible.
Introduction
Prospective follow-up of Parkinson's disease (PD) patients is needed to fill critical information gaps on disease progression, clinical outcomes, biomarkers and treatment effects. Longitudinal population-based studies starting at the onset of PD are limited, and few have multi-year follow-up1-4. Participants in de novo clinical trials may be followed after the interventional phase, taking advantage of the well-characterized baseline information collected during the trial5,6,7. In-person expert assessment is the gold standard. However, long-term in-person follow-up may be limited by financial or logistical considerations and participation may be impossible or undesirable for some patients. Retention becomes more challenging as time passes, and this is particularly true in older populations8. Loss of patients over time can threaten study integrity, since those no longer able to participate are likely different from those continuing participation. This may create bias, and potentially limit the validity of study outcomes.
To address these concerns, we implemented the Follow-up of Persons with Neurologic Diseases (FOUND) remote follow-up protocol using mail and telephone contacts for PD patients enrolled in the LABS-PD study9. FOUND remote follow-up was conducted during the same time period as the in-person LABS-PD study (Phase 1), and continued after the LABS-PD assessments had stopped (Phase 2). Our first goal was to assess feasibility of this follow-up approach in a PD population, and the value in minimizing lost to follow-up and consequent threats to study integrity, such as selection and survivor bias. In Phase 1, we also compared the patient-reported outcomes in FOUND to those obtained at the most proximate in-person visits in order to determine whether valid information on PD status could be collected. In Phase 2, we evaluated whether FOUND participants would continue follow-up after in-person LABS-PD assessments had stopped.
Methods
Patients
Beginning in February 2006, following an interim analysis indicating futility of the interventional agent, participants in the Parkinson Study Group PRECEPT clinical trial10 were invited to participate in a prospective observational study with annual in-person assessments (LABS-PD)9. Fifty-one of 55 LABS-PD sites also offered PRECEPT patients the opportunity to learn about participation in FOUND. Patients could enroll in LABS-PD or FOUND only, or in both.
Study design
The FOUND prospective follow-up study is coordinated through a single site (the Parkinson's Institute, Sunnyvale, CA). Patients are assessed remotely, by mail and telephone.
Informed consent
All participants consented to participate under a centralized protocol approved by the Western Institutional Review Board. PRECEPT participants were provided a brief explanation of the FOUND project by staff at each enrolling site. Patients interested in learning more about FOUND received an informational telephone call. Consent forms were mailed, discussed by telephone, and signed forms were returned by mail.
Assessments
FOUND assessments addressed two goals. The primary objective was to maintain contact with patients. A second goal was to assess PD status. Using standardized self-report questionnaires, information on vital status, current address and telephone number, an alternate informant with contact information, current neurologic diagnosis, PD medication use and side effects were collected (primary information). PD features were assessed using self-reported versions of the Unified Parkinson's Disease Rating Scale (UPDRS) Parts I, II and IV11 and the MDS-UPDRS Parts Ib and II12 (secondary information). Similar information was collected at the annual LABS-PD in-person assessments. Primary information was collected biannually for two years and annually thereafter. Secondary information was collected annually. Patients not responding to mailings were contacted by telephone. Cognitive status was assessed with the modified version of the Telephone Interview for Cognitive Screening (TICS-m)13.
Analysis
We used descriptive statistics to report enrollment, retention and subject characteristics. We compared characteristics of patients enrolled in FOUND to those in LABS-PD only using χ2 and t-tests. To assess factors associated with retention, odds ratios were calculated using multivariable logistic regression. To assess validity, we compared FOUND self-reported information to in-person LABS-PD expert assessment (defined as the “gold standard”), using exact agreement, Spearman's correlation coefficients and kappa coefficients. For descriptive, diagnostic and UPDRS comparisons, FOUND self- report was compared to the temporally closest LABS-PD in-person assessment. For current medication use and cognition, FOUND assessments occurring within 2 months of a LABS-PD visit were compared. The in-person Montreal Cognitive Assessment (MoCA) was defined as the gold standard screening test for cognitive impairment14,15. In LABS-PD, abnormal values were MoCA < 26 and Mini Mental Status Exam (MMSE) < 2416. For TICS, scores of <31 were abnormal17. Interpretation of kappa values followed Koepsell and Weiss, where κ > 0.80 is almost perfect, κ= 0.61- 0.80 is substantial, k = 0.41-0.60 is moderate, κ = 0.21- 0.40 is fair, κ = .00- 0.20 is slight, and κ <.00 is poor18. Homogeneity of kappa values for like items on the UPDRS and MDS-UPDRS was assessed as described by Donner et al19. All analyses were performed using IBM SPSS Statistics for Windows version 21.0 (IBM Corp., Armonk, New York).
Results
Patients
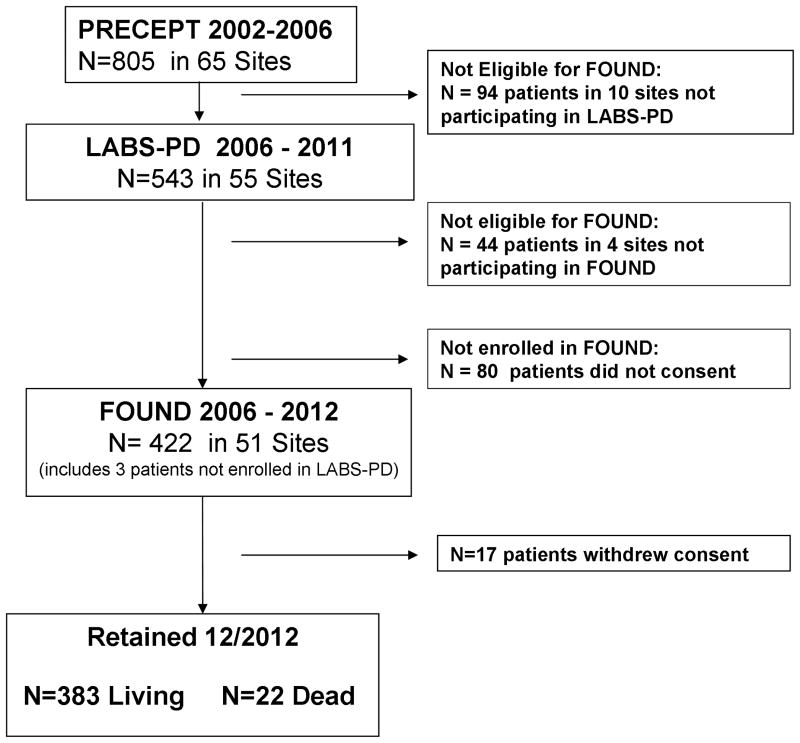
Of 493 patients informed about FOUND, 464 expressed interest and 422 (85%) enrolled (Figure). Patients enrolled were predominantly white (98%) and male (65%), and were similar to LABS-PD (Supplemental Table 1). Most patients reported a diagnosis of PD, but nearly 6% reported other diagnoses (1.9% essential tremor, 0.7% dementia with Lewy bodies, 0.7% multiple system atrophy, 1.4% another movement disorder, 1% no neurologic diagnosis). At FOUND enrollment, 1.2% required a second informant and 11.8% required physical assistance to complete the forms.
Figure. Study flow and Subject Status As of Dec 2012.
Retention
As of December, 2012, mean total follow-up duration since enrollment in the original PRECEPT clinical trial was 9.5 years. Mean duration of follow-up in FOUND was 5.4 years (range 0.5 – 6.3 years). Retention was successful (still enrolled or followed until death) in 96% of FOUND participants. None was lost to follow-up, and only 17 (4%) had withdrawn consent. Patients who withdrew were older, but otherwise similar, to those still participating. Importantly, patients with greater disease severity, longer disease duration, dementia, depression or psychosis were not more likely to withdraw (Supplemental Table 2).
In Phase 1, 60 of 419 cases enrolled in both FOUND and LABS-PD had withdrawn from LABS-PD. Of these, 51 (85%) remained active in FOUND. In Phase 2, 340 of 341 patients active in LABS-PD remained active in FOUND through December, 2012.
Validity analysis
Diagnosis and anti-parkinsonian treatment at baseline was compared to LABS-PD baseline for all patients in both studies. The FOUND baseline self-report version of the UPDRS was compared to the LABS-PD baseline UPDRS in 387 patients. The self-report portion of the MDS-UPDRS collected in 308 patients at FOUND year one was compared to LABS-PD year one data. The mean interval between the LABS-PD and FOUND baseline and year one assessments was 2.7 months (range: 0 to 65 months) and 2.5 months (range: 0 to 53 months), respectively. All FOUND assessments followed the LABS-PD baseline. Agreement on primary information at FOUND enrollment was higher than 90% (Table 1). Agreement on individual disease features at enrollment or year 1 follow-up, as measured by the UPDRS or MDS-UPDRS, was fair to substantial (Table 2). Comparing like measures on the UPDRS and MDS-UPDRS, agreement appeared to be better for most MDS-UPDRS measures, but these differences were not statistically significant.
Table 1. Remotely Collected Self-Reported Information Compared to Investigator-Reported Information.
| N | Agreement | kappa | |
|---|---|---|---|
| Diagnosis: PD/nonPD1 | 418 | 95.0% | 0.562 |
| Medication: On PD medication or not2 |
273 | 96.0% | 0.77 |
| Medication: Type of PD medication | 244 | 91.9% | 0.893 |
| Ldopa preparations | 864 | 97.6% | |
| DA agonists | 654 | 90.5% | |
| Ldopa preparations plus DA agonists | 744 | 91.0% | |
| Other PD medications3 | 194 | 86.5% | |
| Dyskinesias: Present/absent | 87.5% | 0.502 |
Mean interval between reports is 6.55 months
Interval between reports is ≤ 2 months
Other PD medications include monoamine oxidase B inhibitors, catechol-o-methyl transferase inhibitors, amantadine, anticholinergics.
Investigator's report of medication at baseline is gold standard.
Table 2. UPDRS and MDS UPDRS: Comparison of Expert In-Person and Patient-Reported Remote Assessments.
| UPDRS2 (n=387) | MDS UPDRS3 (n=308) | |||||
|---|---|---|---|---|---|---|
| LABS-PD Investigator |
FOUND Patient Report |
LABS-PD Investigator |
FOUND Patient Report |
|||
|
| ||||||
| Questions assessed in both UPDRS and MDS UPDRS1 | Mean Score | Mean score | Kappa4 | Mean score | Mean score | Kappa4 |
| 2.1/2.1 Speech | 0.73 | 0.80 | 0.496 | 0.88 | 0.80 | 0.484 |
| 2.2/2.2 Salivation | 0.66 | 0.68 | 0.595 | 1.06 | 1.10 | 0.639 |
| 2.3/2.3 Swallowing | 0.20 | 0.42 | 0.430 | 0.26 | 0.27 | 0.439 |
| 2.4/2.7 Handwriting | 1.35 | 1.47 | 0.699 | 1.14 | 1.13 | 0.579 |
| 2.5/2.4 Cutting food | 0.63 | 0.68 | 0.584 | 0.56 | 0.60 | 0.597 |
| 2.6/2.5 Dressing | 0.80 | 0.82 | 0.468 | 0.75 | 0.75 | 0.533 |
| 2.7/2.6 Hygiene | 0.44 | 0.54 | 0.455 | 0.39 | 0.45 | 0.478 |
| 2.8/2.9 Turning in bed | 0.52 | 0.59 | 0.592 | 0.60 | 0.57 | 0.609 |
| 2.10/2.13 Freezing | 0.21 | 0.23 | 0.573 | 0.20 | 0.20 | 0.644 |
| 2.11/2.12 Walking | 0.78 | 0.84 | 0.364 | 0.71 | 0.74 | 0.471 |
| 2.12/2.10 Tremor | 1.26 | 1.19 | 0.504 | 1.24 | 1.11 | 0.559 |
| 2.13/1.9 Painful sensation | 0.46 | 0.66 | 0.364 | 0.95 | 0.81 | 0.402 |
|
| ||||||
| Questions assessed in only one scale | Mean Score | Mean score | kappa | Mean Score | Mean score | kappa |
|
| ||||||
| 1.1 Intellectual impairment | 0.43 | 0.40 | 0.400 | |||
| 1.2 Thought disorder | 0.36 | 0.35 | 0.389 | |||
| 1.3 Depression | 0.32 | 0.46 | 0.393 | |||
| 1.4 Motivation | 0.44 | 0.46 | 0.306 | |||
| 2.9 Falling | 0.07 | 0.29 | 0.238 | |||
| 4a1 Dyskinesia | 0.15 | 0.18 | 0.534 | |||
| 4c2 Trouble falling asleep or sleeping too much | 0.41 | 0.44 | 0.412 | |||
| 1.7 Trouble falling asleep | 1.27 | 1.30 | 0.488 | |||
| 1.8 Daytime sleepiness | 1.13 | 1.22 | 0.433 | |||
| 1.10 Urinary problems | 0.71 | 0.80 | 0.589 | |||
| 1.11 Constipation | 0.62 | 0.72 | 0.606 | |||
| 1.12 Faint | 0.46 | 0.43 | 0.390 | |||
| 1.13 Fatigue | 0.88 | 1.02 | 0.425 | |||
| 2.8 Hobby | 0.81 | 0.90 | 0.365 | |||
| 2.11 Getting out of bed | 0.82 | 0.72 | 0.476 | |||
|
| ||||||
| Subscale Scores | Mean Score | Mean score | Spearman R | Mean Score | Mean score | Spearman R |
|
| ||||||
| sum of part I | 1.55 | 1.67 | 0.478 | 6.01 | 6.24 | 0.662 |
| sum of part II | 8.11 | 9.32 | 0.681 | 9.43 | 9.30 | 0.724 |
| sum of partial IV | 0.96 | 2.54 | 0.542 | |||
| Total score | 15.44 | 15.54 | 0.719 | |||
Question number in UPDRS/question number in MDS-UPDRS
Compariing baseline for LABS-PD to baseline for FOUND, mean interval between reports: 2.7 months
Comparing one year follow-up for LABS-PD to 1 year follow-up in FOUND, mean interval between reports: 2.5 month.
Interpretation of kappa (Koepsell & Weiss 2003): kappa > 0.60: Substantial to almost perfect agreement; kappa .41-.60 moderate agreement; kappa 0.40 – 0.2: fair agreement 4, p = 0.002
At year one, 217 patients had TICS assessments within 2 months of LABS-PD visits, 47 of whom were abnormal on the MoCA and 3 on the MMSE. The TICS identified all patients abnormal using the MMSE and 24 abnormal using the MoCA. Using the in person MoCA as the gold standard, TICS sensitivity was 51%, and specificity 83%, compared to 6% sensitivity and 100% specificity for the MMSE. An additional 29 screened abnormal on the TICS but were normal on the MoCA (17% false positives). For identical questions on the TICS and MoCA, exact agreement ranged from 73% for serial subtractions to 99% for day of the week.
Discussion
We report one of the largest populations of PD patients with systematic follow-up starting before the onset of disability requiring dopaminergic therapy. Parallel follow-up of more than 400 patients using in-person (LABS-PD) and remotely obtained patient-reported (FOUND) assessments allowed us to take advantage of the rich information collected during a clinical trial of early, untreated PD, and to continue systematic assessments over an average of nearly 10 years. Remote follow-up of this large, geographically-dispersed group of PD patients was successful even after in-person visits had ended, with no patients lost to follow-up.
The ideal study of disease progression includes regular systematic, prospective, expert assessments of community-based PD populations, starting with disease onset and ending with post-mortem assessment. In contrast, most prospective studies of PD studied prevalent cases, and may have under-represented certain patients, for example, those with shorter survival, limited access to care or unwillingness to participate20,21. Investigations of incident cases in comprehensive health care systems minimize bias, but typically lack repeated systematic assessments23. In the few prospective studies of incident PD conducting multiple in-person assessments, patient numbers have been small and long-term retention limited1,4,22.
Clinical trials populations can provide large numbers of systematically-assessed patients. The Sydney Multicenter Study conducted systematic in-person follow-up of 136 PD patients who were initially enrolled in a clinical trial22. After 10 years, only 59% continued in-person assessments. The DATATOP cohort provided systematic follow-up of early, untreated PD patients, with more than half retained after 8 years5. These and other studies have made key contributions to our understanding of PD, yet are limited by low retention at later stages of illness. In FOUND, retention of more than 400 former clinical trials participants was excellent after nearly 10 years, with none lost to follow-up and 4% who withdrew consent from the remote clinical assessment. Retention success has been attributed to frequent contact with participants and use of incentives23-26. In FOUND, despite relatively infrequent contacts and no financial incentives, participants' interest remained high. Anecdotally, FOUND participants appreciated the opportunity to contribute to science, an altruistic motivation observed in other trials27. Increasing motor disability, depression, psychosis or cognitive decline can limit study participation26,28, but in FOUND only advanced age was associated with non-retention.
A secondary goal of this study was to determine the quality of remotely-collected patient-reported outcomes about PD status. Because this was not the primary goal, FOUND participants completed the assessments at home as long as 6 months before or after the clinic visit; thus, comparisons must be judged cautiously. “Correct” responses reflecting actual changes in PD status may appear to be incorrect. Agreement for characteristics less prone to short-term change, such as diagnosis and medication use, exceeded 95%. Agreement for disease status measured by the UPDRS or the MDS-UPDRS was moderate to substantial, despite an average of nearly 3 months between in-person and remote assessments. Importantly, agreement was good for many individual symptoms, that may trigger an adjustment in treatment, or might serve as outcomes in clinical trials, including swallowing, freezing of gait, falling, and dyskinesia. Louis29 reported agreement between same day, in-person self- and interviewer-administered UPDRS subscales. Cubo and colleagues compared office-based to web-based UPDRS assessments and found good agreement of the two methods30. Goetz and colleagues previously demonstrated internal validity and consistency of the MDS-UPDRS by interviewers12, and sensitivity to longitudinal change was observed in the LABS-PD in-person assessments31. In the current study, FOUND assessments occurred after the in-person LABS-PD assessment, and the expert in-person ratings may conceivably have influenced the patients' self-report. However, assessments were separated, on average, by 3 months, and it is equally likely that any bias introduced by the patient's knowledge of the expert rating would have waned. Agreement was substantial for the MDS-UPDRS Parts I and IIb, and the UPDRS Part II, and moderate for the UPDRS Part I, measuring cognitive and psychiatric symptoms, suggesting that remotely assessed self-reported measures of PD symptoms are valid.
Screening for cognitive impairment was determined by telephone interview using the TICS. Using the in person MoCA as a gold standard, the TICS missed more than half of those cognitively impaired, but still out-performed the in person MMSE, which missed 94% of those classified as cognitively impaired at the same in person visit. The TICS also falsely identified 17% as having cognitive impairment, while the MMSE did not. These differences may reflect the different domains tested by two instruments, as well as difficulties inherent in telephone testing, such as inability to compensate for subjects' hearing difficulties or distracting environments, or may represent true differences in cognitive status as the TICS was obtained at a second time point. Agreement for identical questions on the TICS and both the MMSE and the MoCA was good (73% to 99%), indicating that telephone interview can supplement in person cognitive assessment in some domains. Our results suggest that telephone screening for cognitive impairment can be useful in identifying some cognitive changes in PD. Future work to develop a remote assessment instrument incorporating domains more sensitive to cognitive changes in PD would be useful.
Previous evaluations of multicenter trials have demonstrated enormous cost and inconsistent protocol reviews as major limitations to use of on-site IRBs32-34. Our experience adds uninterrupted data collection and excellent subject retention to the time and cost savings associated with the use of a central IRB. Use of a central IRB is not without limitations. Institutional review boards at one U.S. site and 3 Canadian sites did not allow participation under a central IRB and those subjects were lost to remote follow-up. Close work with regulatory bodies to allow efficient methods such as a central IRB while respecting local regulatory requirements will be an important task for the future.
There are limitations to this study. The population was originally recruited for a clinical trial, and cannot be considered representative of a community-based population of PD patients. The usefulness of remote assessment in community settings remains to be assessed. However, the FOUND population is demographically similar to other participants in PD clinical trials35-38, suggesting this remote method of self-reported assessment may provide a useful alternative in clinical trials, reducing subject burden and costs of in-person assessments.
Retention of study participants is key to the scientific integrity of prospective studies. FOUND provided a “safety net” for retention, even after the in-person assessments stopped. We also demonstrated good agreement between patient-reported outcomes and expert clinical assessments. Remote assessment methods may provide an alternative to in-person prospective follow-up, although the type of assessments possible will be limited. Future applications include active monitoring of patients through use of frequent, automated telephone surveillance, such as for falls39. Recent studies have demonstrated the utility of telemedicine in remote assessment of PD patients receiving clinical care40. Incorporating telemedicine into this remote assessment protocol would be expected to result in even greater validity of clinical assessments.
Supplementary Material
Acknowledgments
Support: Parkinson's Disease Foundation, NIH, Cephalon, Inc., H. Lundbeck A/S, James & Sharron Clark
Footnotes
Disclosure: Mr. Elliott is an employee at the Parkinson's Disease Foundation.
Full financial disclosure: Dr. Tanner has served as an advisor for Abbvie and Adamas Pharmaceuticals. She has received research support from National Institutes of Health (NINDS, NIEHS), Michael J Fox Foundation, the Parkinson's Disease Foundation, DOD, Brin foundation and James and Sharron Clark.
Dr. Ravina is an Employee of Biogen Idec.
Dr. Lang has served as an advisor for Abbott, Abbvie, Allon Therapeutics, Avanir Pharmaceuticals, Biogen Idec, Boerhinger-Ingelheim, Ceregene, Medtronic, Merck, Novartis, NeuroPhage Pharmaceuticals, Teva and UCB. He received grants from Brain Canada, Canadian Institutes of Health Research, Edmond J Safra Philanthropic Foundation, Michael J. Fox Foundation, National Parkinson Foundation, Parkinson Society Canada, Tourette Syndrome Association, W. Garfield Weston Foundation; received publishing royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press; and has served as an expert witness in cases related to the welding industry.
Dr. Kurlan has received research support from Otsuka, Astra Zeneca and Phytopharm and speakers bureau for Teva.
Dr. Marek has ownership in Molecular NeuroImaging, LLC. He has served as a consultant for Pfizer, GE Healthcare, Merck, Lilly, BMS, Piramal, Prothena, Neurophage, nLife, and Roche. He received research support from DOD, Michael J. Fox Foundation, and the Parkinson's Disease Foundation.
Dr. Oakes has received research support from NIH, DOD, Michael J Fox Foundation, Auspex, Prana and Biogen. He has received consultancy fees from Sanofi Pharmaceuticals.
Dr. Seibyl has received research support from Molecular Neuroimaging, LLC, Michael J Fox Foundation, and DOD/USMRMC.
Dr. Goetz has received honoraria from AOP Orphan, Addex Pharma, Advanced Studies of Medicine, Boston Scientific, CHDI, Health Advances, ICON Clinical Research, Ingenix (i3 Research), National Institutes of Health, Neurocrine, Oxford Biomedica, Synthonics, Movement Disorder Society, American Academy of Neurology, Movement Disorder Society, University of Pennsylvania, University of Chicago, University of Luxembourg. He has received grants/research support from NIH, Michael J. Fox Foundation, the Parkinson's Disease Foundation, and MDS.
Dr. Kieburtz has served as a consultant for NIH, NINDS, FDA, VA, Abbott, Acorda, Aptiv, AstraZeneca, Auspex, Biogen Idee, Biotie, Biovail, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Ceregene, CHDI, Civitas, Clintrex, Cynapsus, Endo, Impax, Intec, Ipsen, Isis, Knopp, Lilly, Lundbeck, LZ Therapeutics, Medivation, Merck, Merz, Neotope/Elan Pharmaceutical, Novartis, Orion, Otsuka ,Pharma2B, Phytopharm, Roche, Siena Biotech, Sofinnova, Synagile, Synosia, Teva, UCB Pharma, Upsher-Smith, US WorldMeds, Vaccinex, Vectura, and Xenoport. He has received research support from National Institutes of Health (NEI, NINDS, NIA, NICHD), Michael J Fox Foundation, Neurosearch, and Medivation. He also serves as legal consultant for Thompson Hine.
Dr. Fahn has received honoraria from Merz Pharma, Genervon Biotechnology, American Academy of Neurology, Columbia University, Sun Pharmaceuticals India, Springer Publishers for serving as co-editor of Current Neurology and Neurosurgery Report (annual); Elsevier Publishers for co-authorship of book Principles and Practice of Movement Disorders. He has received research support from the Smart Family Foundation and Genervon Biotechnology.
Dr Shoulson has received Consulting and Advisory Board Membership with honoraria from Auspex Pharmaceuticals, AZTherapies, Biogen Idec, Caravel Group, Curry Rockefeller Group, LLC, Edison Pharmaceuticals, Facilitate Limited on behalf of Lundbeck (SAB), Innovation Consulting Group, LLC, JAMA Neurology, Knopp Biosciences LLC, Medtronic, Inc., Neuroglobe Ltd., Omeros Corporation, Partners Health Care, Pick Research Solutions, Prana Biotechnology, Salamandra LLC, Seneb Biosciences, Inc., Sofinnova Venture Partners, Third Rock Ventures, and Velocity Pharmaceutical Development. He received honoraria from University of California, Irvine, University of Rochester, and Grants/Research support from Food and Drug Administration (FDA), Johns Hopkins University, National Institutes of Health (NHGRI, NINDS), and Parkinson Disease Foundation.
Ms Meng, Flagg, Drs DiEuliis, Gauger, and Guest have nothing to disclose.
References
- 1.Evans JR, Mason SL, Williams-Gray CH, et al. The natural history of treated Parkinson's disease in an incident, community based cohort. Journal of neurology, neurosurgery, and psychiatry. 2011 Oct;82(10):1112–1118. doi: 10.1136/jnnp.2011.240366. [DOI] [PubMed] [Google Scholar]
- 2.Lo RY, Tanner CM, Albers KB, et al. Clinical features in early Parkinson disease and survival. Arch Neurol. 2009 Nov;66(11):1353–1358. doi: 10.1001/archneurol.2009.221. [DOI] [PubMed] [Google Scholar]
- 3.Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA. Incidence and Pathology of Synucleinopathies and Tauopathies Related to Parkinsonism. JAMA neurology. 2013 May 20;:1–7. doi: 10.1001/jamaneurol.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Post B, Muslimovic D, van Geloven N, Speelman JD, Schmand B, de Haan RJ. Progression and prognostic factors of motor impairment, disability and quality of life in newly diagnosed Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2011 Feb 15;26(3):449–456. doi: 10.1002/mds.23467. [DOI] [PubMed] [Google Scholar]
- 5.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Predictors of deterioration in health-related quality of life in Parkinson's disease: results from the DATATOP trial. Movement disorders : official journal of the Movement Disorder Society. 2008 Apr 15;23(5):653–659. doi: 10.1002/mds.21853. quiz 776. [DOI] [PubMed] [Google Scholar]
- 6.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Movement disorders : official journal of the Movement Disorder Society. 2008 Apr 30;23(6):837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 7.Biglan KM, Holloway RG, Jr, McDermott MP, Richard IH. Risk factors for somnolence, edema, and hallucinations in early Parkinson disease. Neurology. 2007 Jul 10;69(2):187–195. doi: 10.1212/01.wnl.0000265593.34438.00. [DOI] [PubMed] [Google Scholar]
- 8.Strotmeyer ES, Arnold AM, Boudreau RM, et al. Long-term retention of older adults in the Cardiovascular Health Study: implications for studies of the oldest old. J Am Geriatr Soc. 2010 Apr;58(4):696–701. doi: 10.1111/j.1532-5415.2010.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ravina B, Tanner C, Dieuliis D, et al. A longitudinal program for biomarker development in Parkinson's disease: a feasibility study. Movement disorders : official journal of the Movement Disorder Society. 2009 Oct 30;24(14):2081–2090. doi: 10.1002/mds.22690. [DOI] [PubMed] [Google Scholar]
- 10.Parkinson Study Group PI. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007 Oct 9;69(15):1480–1490. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 11.Fahn S, Elton RL. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease, Volume II. Vol. 1987. Florham Park, New Jersey: Macmillan Healthcare Information; pp. 153–163. [Google Scholar]
- 12.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement disorders : official journal of the Movement Disorder Society. 2008 Nov 15;23(15):2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 13.Gallo JJ, Breitner JC. Alzheimer's disease in the NAS-NRC Registry of aging twin veterans, IV. Performance characteristics of a two-stage telephone screening procedure for Alzheimer's dementia. Psychol Med. 1995 Nov;25(6):1211–1219. doi: 10.1017/s0033291700033183. [DOI] [PubMed] [Google Scholar]
- 14.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010 Nov 9;75(19):1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- 15.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2008 Jan 30;23(2):297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 16.Ravina B, Marek K, Eberly S, et al. Dopamine transporter imaging is associated with long-term outcomes in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2012 Sep 15;27(11):1392–1397. doi: 10.1002/mds.25157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh KA, Breitner JC, Magruder-Habib KM. Detection of Dementia in the Elderly Usng Telephone Screening of Cognitive Status. Neuropsychiatry, Neuropsychology and Behavioral neurology. 1993;6(2):103–110. [Google Scholar]
- 18.Koepsell TD, Weiss NS. Epidemiologic Methods: Studying the Occurrence of Illness. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 19.Donner A, Eliasziw M, Klar N. Testing the homogeneity of kappa statistics. Biometrics. 1996 Mar;52(1):176–183. [PubMed] [Google Scholar]
- 20.Schrag A, Dodel R, Spottke A, Bornschein B, Siebert U, Quinn NP. Rate of clinical progression in Parkinson's disease. A prospective study. Movement disorders : official journal of the Movement Disorder Society. 2007 May 15;22(7):938–945. doi: 10.1002/mds.21429. [DOI] [PubMed] [Google Scholar]
- 21.Alves G, Wentzel-Larsen T, Aarsland D, Larsen JP. Progression of motor impairment and disability in Parkinson disease: a population-based study. Neurology. 2005 Nov 8;65(9):1436–1441. doi: 10.1212/01.wnl.0000183359.50822.f2. [DOI] [PubMed] [Google Scholar]
- 22.Hely MA, Morris JG, Traficante R, Reid WG, O'Sullivan DJ, Williamson PM. The sydney multicentre study of Parkinson's disease: progression and mortality at 10 years. Journal of neurology, neurosurgery, and psychiatry. 1999 Sep;67(3):300–307. doi: 10.1136/jnnp.67.3.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilliss CL, Lee KA, Gutierrez Y, et al. Recruitment and retention of healthy minority women into community-based longitudinal research. Journal of women's health & gender-based medicine. 2001 Jan-Feb;10(1):77–85. doi: 10.1089/152460901750067142. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson LM, Schwirian PM, Klein EG, et al. Recruitment and retention strategies in longitudinal clinical studies with low-income populations. Contemp Clin Trials. 2011 May;32(3):353–362. doi: 10.1016/j.cct.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parra-Medina D, D'Antonio A, Smith SM, Levin S, Kirkner G, Mayer-Davis E. Successful recruitment and retention strategies for a randomized weight management trial for people with diabetes living in rural, medically underserved counties of South Carolina: the POWER study. J Am Diet Assoc. 2004 Jan;104(1):70–75. doi: 10.1016/j.jada.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Steinhauser KE, Clipp EC, Hays JC, et al. Identifying, recruiting, and retaining seriously-ill patients and their caregivers in longitudinal research. Palliative medicine. 2006 Dec;20(8):745–754. doi: 10.1177/0269216306073112. [DOI] [PubMed] [Google Scholar]
- 27.Fearn P, Avenell A, McCann S, Milne AC, Maclennan G. Factors influencing the participation of older people in clinical trials - data analysis from the MAVIS trial. The journal of nutrition, health & aging. 2010 Jan;14(1):51–56. doi: 10.1007/s12603-010-0009-x. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE, Katzen HL, Klein B, Llabre ML. Cognitive decline affects subject attrition in longitudinal research. Journal of clinical and experimental neuropsychology. 2000 Oct;22(5):580–586. doi: 10.1076/1380-3395(200010)22:5;1-9;FT580. [DOI] [PubMed] [Google Scholar]
- 29.Louis ED, Lynch T, Marder K, Fahn S. Reliability of patient completion of the historical section of the Unified Parkinson's Disease Rating Scale. Movement disorders : official journal of the Movement Disorder Society. 1996 Mar;11(2):185–192. doi: 10.1002/mds.870110212. [DOI] [PubMed] [Google Scholar]
- 30.Cubo E, Gabriel-Galan JM, Martinez JS, et al. Comparison of office-based versus home Web-based clinical assessments for Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2012 Feb;27(2):308–311. doi: 10.1002/mds.24028. [DOI] [PubMed] [Google Scholar]
- 31.Lang AE, Eberly S, Goetz CG, et al. Movement Disorder Society Unified Parkinson Disease Rating Scale experiences in daily living: Longitudinal changes and correlation with other assessments. Movement disorders : official journal of the Movement Disorder Society. 2013 Oct 9; doi: 10.1002/mds.25671. [DOI] [PubMed] [Google Scholar]
- 32.McWilliams R, Hoover-Fong J, Hamosh A, Beck S, Beaty T, Cutting G. Problematic variation in local institutional review of a multicenter genetic epidemiology study. JAMA : the journal of the American Medical Association. 2003 Jul 16;290(3):360–366. doi: 10.1001/jama.290.3.360. [DOI] [PubMed] [Google Scholar]
- 33.Ravina B, Deuel L, Siderowf A, Dorsey ER. Local institutional review board (IRB) review of a multicenter trial: local costs without local context. Annals of neurology. 2010 Feb;67(2):258–260. doi: 10.1002/ana.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christian MC, Goldberg JL, Killen J, et al. A central institutional review board for multi-institutional trials. The New England journal of medicine. 2002 May 2;346(18):1405–1408. doi: 10.1056/NEJM200205023461814. [DOI] [PubMed] [Google Scholar]
- 35.Holloway RG, Shoulson I, Fahn S, et al. Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol. 2004 Jul;61(7):1044–1053. doi: 10.1001/archneur.61.7.1044. [DOI] [PubMed] [Google Scholar]
- 36.Shoulson I. Deprenyl and tocopherol antioxidative therapy of parkinsonism (DATATOP). Parkinson Study Group. Acta neurologica Scandinavica. Supplementum. 1989;126:171–175. doi: 10.1111/j.1600-0404.1989.tb01798.x. [DOI] [PubMed] [Google Scholar]
- 37.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson's disease. The New England journal of medicine. 2004 Dec 9;351(24):2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 38.The NINDS NET-PD Investigators. A Randomized, Double-blind, Futility Clinical Trial of Creatine and Minocycline in Early Parkinson Disease. Neurology. 2006;66:664–671. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 39.Bloem BR, Grimbergen YA, Cramer M, Willemsen M, Zwinderman AH. Prospective assessment of falls in Parkinson's disease. J Neurol. 2001 Nov;248(11):950–958. doi: 10.1007/s004150170047. [DOI] [PubMed] [Google Scholar]
- 40.Dorsey ER, Venkataraman V, Grana MJ, et al. Randomized controlled clinical trial of “virtual house calls” for Parkinson disease. JAMA neurology. 2013 May;70(5):565–570. doi: 10.1001/jamaneurol.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.