Abstract
ATP-dependent chromatin remodeling enzymes play essential biological roles by mobilizing nucleosomal DNA. Yet how DNA is mobilized despite the steric constraints placed by the histone octamer remains unknown. Here, using methyl transverse-relaxation-optimized NMR spectroscopy on a 450 kDa complex, we show that the chromatin remodeler, SNF2h, distorts the histone octamer. Binding of SNF2h in an activated ATP state changes dynamics of buried histone residues. Preventing octamer distortion by site-specific disulfide linkages inhibits nucleosome sliding by SNF2h, while promoting octamer eviction by the SWI/SNF complex, RSC. Our findings indicate that the histone core of a nucleosome is more plastic than previously imagined and that octamer deformation plays different roles based on the type of chromatin remodeler. Octamer plasticity may contribute to chromatin regulation beyond ATP-dependent remodeling.
Introduction
The establishment of specific gene expression states during the course of development as well as their maintenance through the disruptive events of transcription, DNA replication and DNA repair requires rapid rearrangements of chromatin structure (1, 2). ATP-dependent chromatin remodeling motors are the workhorses that enable dynamic changes in chromatin structure. Different families of chromatin remodeling motors have diverse biological roles ranging from gene activation to DNA repair (2, 3). Yet compared to other essential motors such as myosins and helicases, the biochemical mechanisms of chromatin remodeling motors are poorly understood, limiting an understanding of how their functions are regulated.
Chromatin remodeling motors have the formidable task of mobilizing DNA in the context of a nucleosome, which contains ~150 bp of DNA tightly wrapped around an octamer of histone proteins(4). Two classes of chromatin remodeling motors, the ISWI class and the SWI/SNF class have proved to be powerful model systems for asking mechanistic questions. Previous work has shown that motors from both these classes can translocate on naked DNA (5–8). However it is not known how such translocation occurs in the context of the structural constraints placed by the histone octamer. Remarkably, both the ISWI and SWI/SNF family motors can move DNA without dis-assembling the histone octamer (2, 3). It has been suggested that these motors can feed in DNA and propagate a DNA loop across the histone octamer (2). However, recent studies indicate that ISWI family motors translocate DNA out of the nucleosome before feeding DNA into the nucleosome, a result that is difficult to reconcile with the loop propagation model (9). It has also been proposed that chromatin remodelers may be able to drive a conformational change in the octamer itself (10–16). If such distortions can be demonstrated, this would provide a new class of models to explain remodeling mechanisms. Yet it has been difficult to investigate the existence of conformational changes within the histone core largely due to the limitations of available methods. For example, to date there are no high-resolution structures of a chromatin remodeling motor bound to a nucleosomal substrate.
Recent advances in high-resolution 1H-13C Transverse Relaxation Optimized NMR Spectroscopy (methyl-TROSY NMR) provide an opportunity to directly investigate conformational changes within the histone octamer. Methyl-TROSY NMR is a powerful method that has been used to investigate dynamics at residue level resolution in complexes as large as the ~670-KDa Proteosome and the ~200 KDa nucleosome (17–20). Here we report the results from applying this method to the ~450 KDa complex of a nucleosome with the major ISWI family remodeling motor from humans, SNF2h, bound to an activated state of ATP. We also report the results of biochemical experiments derived from the NMR data to test the functional roles of octamer distortion.
SNF2h alters environment of buried histone residues in an activated ATP state
Our previous work has indicated that SNF2h functions most optimally as dimer with one protomer binding on either side of a nucleosome (21). Additionally in the presence of the ATP analog, ADP-BeFx, SNF2h engages the histone H4 tail, an allosteric activator of ISWI enzymes (22–25). This analog also promotes a restricted conformation of the ATPase active site (26). We therefore reasoned that NMR studies carried out in the presence of ADP-BeFx would mimic either an activated ground state or a transition state. We queried histone H4 in a nucleosome by methyl-TROSY NMR spectroscopy in the presence of ADP-BeFx. This method employs selective 13C methyl group labeling of Ile, Leu of Val (ILV) residues in an otherwise deuterated protein environment in order to exploit the line-narrowing afforded by cross-correlated relaxation of CH dipolar couplings in an HMQC experiment (17, 27). The assembled nucleosomes contain ILV labeled H4 and fully deuterated H3, H2A and H2B. The methyl-TROSY spectrum of ILV labeled H4 is well resolved, and a high degree of overlap was observed between the published spectra of Drosophila nucleosomes and our spectrum as observed by the small Δδ (ppm) values (Fig. S2). It was therefore possible to assign all the cross-peaks based on the previously published spectra of Drosophila nucleosomes (20) (Fig. 1A, Fig. S1, S2).
Figure 1. Methyl-TROSY spectra of nucleosome bound to SNF2h.
(A) 2D methyl-TROSY spectra of ILV-methyl labeled H4 in a nucleosome in the presence of ADP-BeFx (blue cross-peaks, left panel), and SNF2h + ADP-BeFx (orange cross-peaks, right panel). High conservation of amino acid sequence between Drosophila and Xenopus H4 allowed the assignments to be transferred from the published assignments of Drosophila nucleosome (20) (Fig S1). Top part of the spectrum shows Ile δ1-methyl groups, whereas the bottom half shows Leu and Val methyl cross-peaks. Stereospecific assignments for Leu δ1/δ2 have been shown where available. The cross-peaks for which stereospecific assignment was not made have been arbitrarily assigned as “a” or “b”. Cross-peaks that show small changes (e.g. L10, V21, V60), cross-peaks that show decreased intensity due to resonance broadening (e.g. I50, L58, V57) can be seen in the spectrum of the complex. Nucleosome structure in the right panel (64) shows the location of the Cα of all the ILV residues in H4 (light blue); dark blue spheres: residues that do not change much upon binding of SNF2h; red spheres: residues that get broadened upon SNF2h binding (based on 1B). A surface representation of nucleosome structure shows how most of the ILVs (red) in H4 (blue) are buried. The location of the superhelix location 2 (SHL2) is shown in green on the DNA.
(B) Quantification of cross-peak broadening for H4 residues where cross-peaks in the bound state have not disappeared. Left panel: distribution of ILV residues showing peak broadening depicted on nucleosome structure. The intensity of red color represents extent of broadening. Residues shown as deep red spheres disappear completely, and hence, are broadened to a greater extent. Right panel: red bars (left y-axis) represent relative peak volumes of residues in the nucleosome-SNF2h complex (Ibound) compared to in the nucleosome alone (Ifree) for residues that give observable signals in both spectra. The two sets of spectra were normalized using the volumes of their respective L10δ2 peak, which does not show broadening upon SNF2h binding. The blue dashed line (right y-axis) shows solvent accessible surface area (SASA) of the corresponding ILV residues. SASA was calculated from the pdb structure file 1kx5 using the program POPS (65). The SASA values are for the entire residue and represent fraction of exposed surface area. The location of different ILV residues in H4 can be read from the cartoon below showing the H4 polypeptide chain with its structural elements (loops - L1-L5, helices – αN-α3) (64).
(C) 2D Methyl-TROSY spectra of Ile-methyl labeled H2A within a nucleosome showing the Ile region of the spectrum. The spectrum of the SNF2h bound form of the nucleosome (brown) with ADP-BeFx shows peak broadening as well as the appearance of a new peak when overlaid on the spectrum of nucleosome alone with ADP-BeFx (green). The two versions of nucleosome structures on right show H2A in orange, and the Ile residues in H2A as green spheres.
Numerous cross-peaks were perturbed upon addition of saturating concentrations of SNF2h to the nucleosome. These perturbations included reductions in resonance intensities and/or changes in chemical shift as well as the appearance of new cross-peaks. The new cross-peaks can in principle arise from the natural abundance of 13C in the unlabeled SNF2h. An NMR spectrum of unlabeled SNF2h bound to unlabeled nucleosomes did not show any cross-peaks that overlap with these new cross-peaks (Fig. S3). This result is consistent with the new cross-peaks in Fig. 1 arising from the ILV labeled H4 instead of SNF2h. Yet, to be conservative we only investigate the origins for the reduction in resonance intensities of the ILV cross-peaks in H4. In this context we first tested if the observed perturbations arise from disassembly of nucleosomes by comparing the NMR spectra of free histone ILV-labelled H4 with that of the SNF2h-nucleosome complex (Fig. S4). We found no significant overlap in cross-peaks indicating that the observed NMR changes in the SNF2h-nucleosome complex spectrum are not due to dissociation of histone H4. Dynamic Light Scattering experiments carried out under NMR conditions also confirmed a largely homogenous SNF2h-nucleosome complex (Fig. S5).
Given the above controls, we interpret the reductions in resonance intensities as reflecting a change in magnetic environment of the H4 residues within an intact nucleosome. Such resonance broadening results from an increase in dephasing of transverse magnetization. This could be a manifestation of (i) an increase in molecular weight, (ii) exchange between bound and unbound states of the nucleosome, (iii) proximity of histone residues to non-deuterated SNF2h, or (iv) induction of protein dynamics on a ms-μs timescale in the vicinity of the labeled residues (28, 29). Below we investigate these possibilities:
An increase in molecular mass is expected to show largely uniform broadening of all the H4 residues. However, a quantification of the relative resonance broadening associated with the H4 residues showed that the residues that were broadened were not uniformly distributed (Fig. 1B). Some residues buried in the core of the nucleosome are only marginally affected by addition of SNF2h (e.g. Ile 26, Val 60 and Leu 90), whereas other residues are significantly affected (e.g. Ile 29, Ile 50, and V65). These results suggest that an increase in mass is not the sole source of resonance broadening.
The Kd of SNF2h for binding nucleosomes under these conditions is ~150 nM (Fig. S6). Given a range of diffusion limited binding rates (104–106 M−1sec−1) (30) for protein-protein interactions the dissociation rate constants are slow (<0.15 sec−1) relative to the NMR chemical shift timescale, suggesting that the broadening does not arise from exchange between the bound and unbound states of the nucleosome.
Substantial previous work has suggested that ISWI enzymes bind near the superhelical location 2 (SHL2) on the nucleosome (Fig. 1A)(31–33). It is therefore possible that H4 ILV residues proximal to SHL2 are broadened by direct contacts with protonated SNF2h. Consistent with this possibility, I29, which is closest to SHL2 (Fig. S7; ~ 10.6 Å from phosphate group of dT17 in SHL2), is significantly broadened. However, residues L49, I50 and V57, which are much further away (>20 Å from phosphate group of dT17 in SHL2) and substantially buried, also show comparable extent of broadening (Fig. 1B). Direct contact between SNF2h and these residues would not be possible in a canonical nucleosome structure. We therefore interpret the data to suggest that either (i) binding of SNF2h on the exterior allosterically induces protein dynamics on a ms-μs timescale in buried regions of the octamer or (ii) SNF2h opens up the octamer to directly contact residues that are normally buried. In either interpretation, the octamer would have to undergo a structural change that results in a change in the environment of buried histone residues.
To test if the SNF2h-induced changes are confined to a defined interface between histones H4 and H3 or affect a broader region of nucleosome, we looked at changes in H2A residues by NMR.
Unlike H4 it was not possible to transfer the assignments for H2A ILV cross-peaks from previously published spectra of Drosophila nucleosomes to Xenopus nucleosomes because of low sequence homology between Xenopus and Drosophila H2A (Fig. S1). Isoleucine residue cross-peaks show up in a spectral region that is distinct from Leucine and Valine (67). Accordingly we focused on the Ile region of the Xenopus H2A spectrum, which has well resolved cross-peaks (Fig. 1C, left panel). Like H4, broadening and chemical shift changes are observed for resonances of H2A in the isoleucine region of the spectrum upon binding of SNF2h. While we have not assigned the H2A cross-peaks, the Ile residues in H2A are mostly buried suggesting that the effect of SNF2h on the histone core is not confined to H4 residues (Fig. 1C, right panel). H2A makes minimal contacts with the H3-H4 tetramer in the nucleosome structure (4). Therefore, the effect of SNF2h is either relayed to it via H2B or, possibly, SNF2h also makes direct contacts with H2A.
We next tested the functional consequences of altering histone regions that show perturbed resonances within the solvent inaccessible octamer core as well as near the histone-DNA interface.
Destabilizing DNA-histone contacts modestly enhances remodeling by SNF2h
The cross peak for the H4-I46 terminal methyl group is broadened in the SNF2h-nucleosome complex. This residue is proximal to H4-R45, which makes contacts with nucleosomal DNA and is a SIN mutant location(4, 34). These mutations were found in budding yeast as suppressors of defects in the SWI/SNF family of motors (34). The side chain of R45 inserts into the DNA minor groove and is thought to participate in the stability of histone-DNA interactions (4). Indeed mutating R45 has been shown to increase thermally driven nucleosome mobilization (35, 36). This raised the possibility that SNF2h mobilizes nucleosomes in part by destabilizing the interaction between H4-R45 and nucleosomal DNA, thereby resulting in altered dynamics of H4-I46. To test this possibility we measured the effect of an H4-R45A mutation on nucleosome remodeling by SNF2h. SNF2h moves nucleosomes positioned on one end of a short piece of DNA towards the center. We used a native gel based assay that allows detection of such movement of nucleosomes. The rate constant for centering the mutant nucleosome was ~2-fold faster than for the WT nucleosome (Fig. S7B). The small magnitude of the effect with SNF2h is similar to previous observations with the SWI/SNF family of chromatin remodeling enzymes. The 2-fold faster rate constant could reflect 2-fold faster remodeling by SNF2h or faster non-enzymatic re-equilibration of the centered R45A nucleosomes back to the end-position. Both possibilities imply that the interaction between R45 and DNA represents a relatively modest barrier for remodeling (36, 37).
Restricting internal histone movements inhibits nucleosome sliding by SNF2h
We next investigated the functional significance of the resonance perturbations observed in the octamer core. We reasoned that if we could restrict the flexibility of a large region of the buried H3-H4 interface, we could test if and how such restriction affects nucleosome sliding. Accordingly, using the crystal structure as a guide (PDB code: 1kx5), we introduced cysteine mutations that would lock the H3-H4 interface at two different locations by means of disulfide bridges (Fig. 2A), H3F104C-H4V43C and H3L82C-H4V81C. The mutation V43C in H4 is proximal to residues I46 and L37, for whom the cross-peaks disappeared in presence of SNF2h. Likewise, the cross peak for V81C in H4 also disappeared in the presence of SNF2h. We chose the cysteine pairs such that the two side chains in H3 and H4 were oriented towards each other away from solvent, and were at most 5 Å apart in the structure. Due to this design, the disulfide bonds formed efficiently within a pre-assembled histone octamer in the presence of ambient oxygen upon removal of reducing agent (Fig. S8A). Our design allowed formation of a largely uniform population of nucleosomes with dual disulfide linked H3-H4 (referred to as dCX, Fig. 2B).
Figure 2. Functional impact of restricting H3-H4 interface on SNF2h.
(A) Location of residues mutated to Cysteines. Residues L82 and F104 in H3 and, V43 and V81 in H4 were mutated to Cys. (B) Comparison of SNF2h ATPase rates in the presence of WT (purple), dCX (green), and no nucleosomes (red). Error bars represent standard deviation (s.d.) from three independent experiments (n = 3). (C) Comparison of remodeling of WT and dCX nucleosomes by SNF2h. Left panels: representative gel for remodeling kinetics for WT (top panel) and dCX (bottom panel) respectively. Right panels: Quantification of fraction centered nucleosomes (top panel) and fraction end positioned nucleosomes (bottom panel) as a function of time. WT data are in green and dCX data are in red. Time points (in minutes): 0, 1, 3, 5, 10, 20, and 60. Error bars are standard deviation (s.d.) from three independent experiments (n = 3). In the top right panel, the observed rate constant, kobs for WT is 0.18 ± 0.03 min−1. The rate constant for the minor fast phase of dCX (k1fixed) was fixed to be the same as the kobs for WT. The rate constant for the major slow phase (k2obs) with the constraint of k1fixed = 0.18 min−1, was obtained to be 0.003 ± 0.001 min−1. Fitting the entire time-course for dCX to a single exponential gives a value of kobs= 0.01 ± 0.001 min−1. For all experiments with Cysteine cross-linked nucleosomes, Cy3-labeled 601+60 DNA was externally added to WT nucleosome samples in order to make the reaction conditions comparable between WT and cross-linked nucleosomes as the cross-linked nucleosomes had with some free DNA. The free DNA inhibits rates of remodeling in a dose-dependent manner (see Methods for details).
We first tested the ability of dCX nucleosomes to stimulate ATP hydrolysis by SNF2h because nucleosome stimulated ATP hydrolysis is a defining feature of ISWI enzymes (38–41). We found that dCX nucleosomes also stimulated ATP hydrolysis although slightly less (<2-fold) than wild-type nucleosomes (Fig. 2B). This result suggested that SNF2h can bind and recognize dCX nucleosomes.
Next, we investigated the effect of restricting octamer flexibility on nucleosome sliding using the native gel based assay. We used single-turnover conditions with excess and saturating concentrations of SNF2h over nucleosomes to measure maximal rate constants. We found that in contrast to the small effects seen on ATP hydrolysis, sliding of the vast majority of dCX nucleosomes (>90%) by SNF2h was severely inhibited (Fig. 2C, ~60–fold inhibition). A small population (<10%) of the dCX nucleosomes appeared to be centered with comparable rates as wild-type nucleosomes. This population is consistent with the small population of uncross-linked histones (Fig. S8A). To control for the effects of introducing the cysteine mutations, we measured the ability of SNF2h to remodel nucleosomes containing the cysteine mutants assembled under reducing conditions (dCH nucleosomes). SNF2h remodeled these nucleosomes with efficiency similar to that of WT nucleosomes (Fig. S8B). Together, these results suggest that the observed inhibition of nucleosome sliding is due to the disulfide cross-links.
Of the two cross-links, H3L82C-H4V81C, which we term sCX2, is in the vicinity of SHL2, the DNA region where the ATPase domain of SNF2h is proposed to bind, while the other cross-link, H3F104C-H4V43C, which we term sCX1, is closer to the vicinity of the dyad. We wondered if restricting the nucleosome with each of these cross-links separately would have similar or different effects than the doubly cross-linked nucleosome, dCX. We therefore compared the effects of each cross-link individually. The sCX2 cross-link, dramatically inhibits nucleosome sliding, similar to effects observed with the dCX nucleosomes (Fig. 3A). On first glance the sCX1 cross-link also appears to inhibit nucleosome sliding as seen by the reduction in the amount of centered nucleosomes. However closer inspection of the data reveal some interesting differences. In contrast to sCX2, the sCX1 cross-link does not significantly inhibit nucleosome sliding away from the end position [Compare WT vs. sCX2 in Fig. 3A, right-most panel to WT vs. sCX1 (~4-fold inhibition) in Fig. 3B, right-most panel]. Instead, sCX1 reduces the fraction of centered nucleosomes because its remodeling leads to a greater proportion of alternative products (Fig. 3B, products denoted by “*”) that migrate faster than the end positioned starting material on a native gel.
Figure 3. Investigating the extent of H3-H4 flexibility required for remodeling by SNF2h and ACF.
(A) Comparison of remodeling of WT and sCX2 nucleosomes by SNF2h and quantification of fraction centered nucleosomes (middle panel) and fraction end positioned nucleosomes (right panel) as a function of time. For the middle panel, WT: kobs = 0.9 ± 0.1 min−1 and, sCX2: because of the absence of clearly detectable product a straight line has been drawn through the data points obtained from quantifying the signal at the locations where the centered nucleosomes migrate on the gel. Error bars are average deviation from three independent repetitions of the experiment (n = 3) (B) Comparison of remodeling of WT and sCX1 nucleosomes by SNF2h and quantification of fraction centered nucleosomes (middle panel) and fraction end positioned nucleosomes (right panel) as a function of time. For the middle panel (fraction centered), Wt: kobs = 1.5 ± 0.02±min−1; sCx1: kobs = 0.2 ± 0.05 min−1. For the right panel (fraction end-positioned), Wt: kobs = 3 ± 0.6 min−1; sCx1: kobs = 0.7 ± 0.3 min−1. Error bars are average deviation from two independent repetitions of the experiment (n = 2). (C) Comparison of remodeling of WT and sCX1 nucleosomes by ACF and quantification of fraction centered nucleosomes (middle panel) and fraction end positioned nucleosomes (right panel) as a function of time. For the middle panel (fraction centered), Wt: kobs = 0.64 ± 0.11 min−1; sCx1: kobs = 3.6 ± 1 min−1. For the right panel (fraction end-positioned), Wt: kobs = 1.5 ± 0.3 min−1; sCx1: kobs = 2.2 ± 0.1 min−1. Error bars represent average deviation from two independent experiments (n = 2).
In vivo, SNF2h is found in larger complexes. A key SNF2h containing complex is ACF, which consists of the subunit Acf1 in addition to SNF2h. The Acf1 subunit has been shown to extend the ability of SNF2h to sense flanking DNA length and increase its ability to space nucleosomes. It was therefore informative to test if the nucleosome centering ability of ACF was also affected by the SCX1 cross-link. ACF generated a slightly higher proportion of centered sCX1 nucleosomes compared to SNF2h. However compared to its action on WT nucleosomes, ACF generated a much smaller fraction of centered sCX1 nucleosomes and a much larger fraction of alternative products (Fig. 3C, products denoted by “*”). These results suggest that octamer distortion proximal to the H3F104 and H4V43 residues is required for both SNF2h and ACF to effectively center nucleosomes.
To test if constraining octamer conformation inhibits nucleosome sliding by another class of remodeling enzymes, we investigated how the yeast INO80 complex acts on the doubly cross-linked dCX nucleosome. The INO80 remodeling complex is known to play important roles in double strand break repair (42,43), and is thought to act by mechanism that is distinct from the ISWI class of remodeling enzymes (44–46). Interestingly, INO80 activity was not inhibited on the dCX nucleosomes but instead showed a small (2-fold) rate enhancement compared to WT nucleosomes (Fig. 4A). That the dCX nucleosomes are remodeled comparable to WT by INO80 also indicates that the cross-links do not introduce major defects in nucleosome structure.
Figure 4. Effects of constraining H3-H4 interface on additional remodeling complexes.
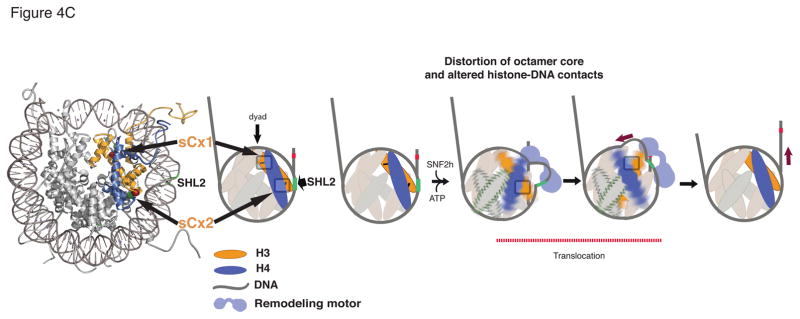
(A) Comparison of remodeling rates of INO80 for WT (right panel: green squares, kobs= 0.08 ± 0.006 min−1) and dCX (right panel: red squares, kobs= 0.22 ± 0.04 min−1) nucleosomes. Left panels: representative gels for remodeling kinetics. Time points (in minutes): 0, 2, 5, 10, 20, and 40. Error bars represent standard deviation from three independent experiments (n = 3). (B) Comparison of RSC remodeling rates and outcomes for WT (green symbols) and dCX (red symbols) nucleosomes. For disappearance of centered nucleosomes, kWT=1.28 ± 0.02 min−1, kdCX= 1.8 ± 0.07 min−1; For DNA eviction from dCX kEvic = 1.4 ± 0.2 min−1; For appearance of end-positioned nucleosomes in WT, kEndpos = 1.18 ± 0.18 min−1. Error bars represent average deviation from two independent experiments (n = 2). The nucleosomal band that migrates faster than the end–positioned nucleosome is consistent with a product in which the octamer has moved beyond the DNA end as seen previously (66). (C) Model for role of octamer distortion in DNA mobilization by SNF2h. Histone octamer deformation proximal to SHL2 is coupled to translocation from SHL2 while deformation proximal to the dyad enables movement may allow the nucleosome to accommodate less DNA thereby preventing the accumulation of any strain in the structure. These coupled conformational changes lead to a net translation of DNA from the exit site before any DNA is drawn in from the entry site. The location of the sCx1 and sCx2 cross-links is highlighted by the grey rectangles.
Restricting internal histone movements increases octamer eviction by yeast RSC complex
Previous work has shown that the SWI/SNF family of complexes and the ISWI family complexes differ in the types of remodeled products generated (11). For example, unlike most ISWI complexes, which move nucleosomes away from DNA ends, SWI/SNF complexes move nucleosomes towards DNA ends and sometimes beyond the DNA end (66). In addition, a small fraction of SWI/SNF products arise from eviction of the histone octamer, whereas ISWI complexes do not evict the histone octamer (11). The two types of complexes also differ in their sensitivity to DNA super-coiling and in the amounts of DNA translocated within a remodeling cycle: (11, 15, 32, 47, 48). These and other results have suggested that SWI/SNF and ISWI complexes use distinct mechanisms to mobilize nucleosomes. We therefore wondered if the SWI/SNF family of complexes would respond differently than the ISWI complex to constraining the H3-H4 interface. To test this possibility we used RSC, the major SWI/SNF complex from budding yeast. We found that RSC slides both WT and dCX nucleosomes towards the DNA ends with similar rates (Fig. 4B). However, a larger fraction of the centered dCX nucleosomes had their octamers evicted (Fig. 4B, lowermost right panel; fraction nucleosomes evicted at 2 min = ~0.4 vs 0.1 for dCX vs. WT nucleosomes respectively). One implication of these results is that inhibiting octamer distortion biases the RSC products towards octamer eviction. It is also possible that these cross-links contribute to increased octamer eviction through reducing the stability of histone-DNA interactions though such increased eviction is not observed with SNF2h and INO80.
Conclusions and Implications
Our results suggest that the octamer core of a nucleosome is structurally plastic and that this plasticity can be exploited by chromatin remodeling motors to mobilize DNA within chromatin. Below we discuss the basis for this conclusion and the biological implications.
Here we have leveraged the power of Methyl-TROSY NMR to ask how a chromatin remodeling motor alters histone dynamics within the nucleosome at residue-level resolution. With this technique we observed that binding of an activated form of SNF2h induced broadening of several cross-peaks corresponding to buried residues, consistent with a conformational change in the nucleosome core. Constraining the movement of buried histone residues in the vicinity of the DNA binding site of SNF2h strongly inhibited nucleosome sliding, but had modest effects on ATP hydrolysis. Together, these observations suggest that octamer deformation plays a major role in allowing SNF2h to use its intrinsic nucleosome-stimulated ATP hydrolysis activity to drive nucleosome sliding.
In the simplest model we can imagine, nucleosome stimulated ATP hydrolysis generates an intermediate in which DNA deformation is thermodynamically coupled with octamer deformation. In such a model, deformation of DNA by SNF2h would stabilize deformation of the octamer and correspondingly deformation of the octamer would promote deformation of DNA by SNF2h. The molecular basis for octamer deformation could then have two mutually compatible possibilities: (i) octamer deformation could arise indirectly due to changes propagated from remodeler-DNA interactions or; (ii) the octamer could be directly deformed by SNF2h. Indeed previous cross-linking, foot printing and low-resolution EM studies suggest that ISWI enzymes make several contacts with the DNA and surface exposed histone residues (21, 31, 50, 51). It is also possible that SNF2h stabilizes a deformed octamer by making direct contacts with histone residues that are buried in the crystal structure.
The ATPase domain of ISWI enzymes has been mapped to bind near SHL2 (Fig. 4C) and this requires that ISWI enzymes like SNF2h translocate on DNA from a location where the DNA is tightly bound by histones (31). We find that constraining octamer flexibility in the vicinity of SHL2 via the H3L82C-H4V81C cross-link almost completely blocks nucleosome sliding by SNF2h. The simplest interpretation of this result suggests that initiation of DNA translocation from SHL2 relies on deforming the octamer proximal to this location. It however remains formally possible that local alterations in nucleosome structure caused by the H3L82C-H4V81C cross-link inhibit some other aspect of SNF2h function prior to the actual sliding step, such as a conformational change in SNF2h that is required for translocation from SHL2. Our studies also help rationalize more recent observations with ISWI enzymes that are difficult to explain based on the canonical nucleosome structure. These studies, which were carried out with the yeast ISW2 complex, implied that ~7 bp of DNA is translocated from SHL2 towards the dyad and eventually out from the exit side before any DNA is translocated inwards from the entry site (9) (Fig. 4C). Models using the canonical nucleosome structure require substantial stretching of the nucleosomal DNA, which is thought to impose a large strain on the nucleosome structure (9). We find that constraining octamer flexibility in the vicinity of the dyad via the H3F104C-H4V43C cross-link allows nucleosome sliding but substantially reduces the proportion of centered nucleosomes, generating instead a larger proportion of products that migrate faster on a native gel. This result suggests that the strain generated by translocation of DNA from SHL2 towards the dyad is alleviated by octamer deformation proximal to the dyad. In such a model preventing octamer deformation near the dyad would lead to collapse of the partially remodeled nucleosomes to alternative positions on the DNA.
Our observations that the RSC and INO80 complexes respond differently than SNF2h to constraining octamer flexibility suggest that different remodeler families use the plasticity of the histone octamer is distinct ways. For INO80, it is not known where the ATPase subunit of the INO80 complex binds on a nucleosome (46). Therefore it is possible that INO80 binds at a location where it is easier to break histone-DNA contacts, making its reaction less reliant on octamer deformation. Interestingly, the ATPase subunit of RSC, Sth1 has been suggested to bind near SHL2 analogous to the ISWI ATPase. However, unlike with SNF2h, the dCX double cross-links do not inhibit nucleosome sliding by RSC but instead greatly increase octamer eviction. A recent RSC study has suggested that faster DNA translocation correlates with higher levels of octamer eviction, while slower translocation favors nucleosome sliding (52). Our results suggest that partitioning between sliding and eviction is further regulated by the distortability of the histone octamer. A distortable histone octamer may allow the DNA translocation activity of RSC to be accommodated in a manner that avoids octamer eviction. Overall, consistent with the previously observed mechanistic differences between SWI/SNF and ISWI (reviewed in reference 15), our results suggest different roles for octamer distortion in the SWI/SNF and ISWI reactions.
Early work indicated that human SWI/SNF causes long lived but reversible changes in the topology of nucleosomal arrays assembled on closed circular DNA templates (53, 54). SWI/SNF action also generates mono and di-nucleosomes with altered nuclease accessibility that cannot be explained simply by nucleosome sliding or eviction (10, 12, 13). Based on the results here we propose that some of the stably altered nucleosome conformations implied by previous studies may involve an altered octamer conformation. Previous work has also shown that a disulfide cross-link between two H3 molecules at residue 110 does not inhibit remodeling by SWI/SNF nor does general cross-linking of the histone octamer by dimethyl suberimidate (55, 56). While these previous studies did not directly investigate effects on octamer eviction the published results are qualitatively consistent with our results suggesting that nucleosome sliding by RSC is not inhibited by constraining octamer flexibility.
Most models for altering nucleosome structure are based on Lego block like conceptions, where the individual histones can be removed or exchanged, but the octamer conformation is largely rigid. Indeed in the absence of other protein factors, the canonical nucleosome conformation observed in crystal structures appears to be the most highly populated conformation in solution (57–59). At the same time, the possibility of alternative nucleosome conformations during nucleosome assembly and transcription has also been raised previously (60, 61). Our results provide additional evidence that the octamer adopts more than one conformation and further demonstrate that octamer distortability is functionally relevant. Similar to the effects of SNF2h in the presence of ADP-BeFx, other stably bound chromatin regulators, specific histone modifications, and histone variants may also stabilize alternative octamer conformations. Indeed several histone modifications are being discovered on core histone residues raising the possibility that some of these alter the equilibrium between different octamer conformations within a nucleosome (62). A distortable histone octamer may provide molecular explanations for how pioneer factors like FoxA and hormone receptors preferentially recognize DNA sequences buried within nucleosomes (63). In general given the primary role of nucleosomes in regulating DNA accessibility, it is tempting to speculate that there are many nucleosome plasticity based mechanisms exploited by the cell, which can be uncovered by future mechanistic studies.
Supplementary Material
Acknowledgments
We thank Julia Tretyakova for her tireless effort in preparing histones, Coral Zhou for generously providing yeast INO80 complex, Nathan Gamarra for generously providing ACF and Jeffrey Pelton and the QB3 NMR Facility at University of California Berkeley, for help with collecting NMR data. We thank Dr. Tim Richmond for the plasmid containing repeats of the 601+20 sequence. We thank Coral Zhou and John Leonard for helpful comments on the manuscript and members of the Narlikar laboratory for stimulating discussions. This work was supported by a grant from the Program for Breakthrough Biomedical Research at UCSF to G.J.N and J.D.G, a grant from the NIH to G.J.N (R01GM073767) and a Postdoctoral Fellowship from the Human Frontiers Science Program (HFSP) to K.K.S.
Footnotes
Methods, Supplementary Figures and Supplementary Figure Legends are described in the Supplementary Information.
Author Contributions
K.K.S. and G.J.N identified, developed the core mechanistic questions and J.D.G identified NMR as a powerful approach for addressing this question. K.K.S. performed all the experiments, J.D.G guided the NMR experiments and helped K.K.S. and G.J.N interpret the NMR data. K.K.S., J.D.G and G.J.N wrote the manuscript. G.J.N oversaw the project.
References
- 1.Ho L, Crabtree GR. Chromatin remodelling during development. Nature. 2010;463:474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clapier CR, Cairns BR. The Biology of Chromatin Remodelling Complexes. Annu Rev Biochem. 2009;78:273–304. doi: 10.1146/annurev.biochem.77.062706.153223. [DOI] [PubMed] [Google Scholar]
- 3.Narlikar GJ, Sundaramoorthy R, Owen-Hughes T. Mechanisms and functions of ATP-dependent chromatin-remodeling enzymes. Cell. 2013;154:490–503. doi: 10.1016/j.cell.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 5.Saha A, Wittmeyer J, Cairns BR. Chromatin remodeling by RSC involves ATP-dependent DNA translocation. Genes Dev. 2002;16:2120–2134. doi: 10.1101/gad.995002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lia G, et al. Direct observation of DNA distortion by the RSC complex. Mol Cell. 2006;21:417–425. doi: 10.1016/j.molcel.2005.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whitehouse I, Stockdale C, Flaus A, Szczelkun MD, Owen-Hughes T. Evidence for DNA translocation by the ISWI chromatin-remodeling enzyme. Mol Cell Biol. 2003;23:1935–1945. doi: 10.1128/MCB.23.6.1935-1945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang Y, et al. DNA translocation and loop formation mechanism of chromatin remodeling by SWI/SNF and RSC. Mol Cell. 2006;24:559–568. doi: 10.1016/j.molcel.2006.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deindl S, et al. ISWI remodelers slide nucleosomes with coordinated multi-base-pair entry steps and single-base-pair exit steps. Cell. 2013;152:442–452. doi: 10.1016/j.cell.2012.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fan HY, He X, Kingston RE, Narlikar GJ. Distinct strategies to make nucleosomal DNA accessible. Mol Cell. 2003;11:1311–1322. doi: 10.1016/s1097-2765(03)00192-8. [DOI] [PubMed] [Google Scholar]
- 11.Narlikar GJ, Fan HY, Kingston RE. Cooperation between complexes that regulate chromatin structure and transcription. Cell. 2002;108:475–487. doi: 10.1016/s0092-8674(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 12.Schnitzler G, Sif S, Kingston RE. Human SWI/SNF interconverts a nucleosome between its base state and a stable remodeled state. Cell. 1998;94:17–27. doi: 10.1016/s0092-8674(00)81217-9. [DOI] [PubMed] [Google Scholar]
- 13.Lorch Y, Cairns BR, Zhang M, Kornberg RD. Activated RSC-nucleosome complex and persistently altered form of the nucleosome. Cell. 1998;94:29–34. doi: 10.1016/s0092-8674(00)81218-0. [DOI] [PubMed] [Google Scholar]
- 14.Côté J, Peterson CL, Workman JL. Perturbation of nucleosome core structure by the SWI/SNF complex persists after its detachment, enhancing subsequent transcription factor binding. Proc Natl Acad Sci U S A. 1998;95:4947–4952. doi: 10.1073/pnas.95.9.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou CY, Johnson SL, Gamarra NI, Narlikar GJ. Mechanisms of ATP-Dependent Chromatin Remodeling Motors. Annu Rev Biophys. 2016;45:153–181. doi: 10.1146/annurev-biophys-051013-022819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alilat M, Sivolob A, Révet B, Prunell A. Nucleosome dynamics. Protein and DNA contributions in the chiral transition of the tetrasome, the histone (H3-H4)2 tetramer-DNA particle. J Mol Biol. 1999;291:815–41. doi: 10.1006/jmbi.1999.2988. [DOI] [PubMed] [Google Scholar]
- 17.Rosenzweig R, Kay LE. Bringing Dynamic Molecular Machines into Focus by Methyl-TROSY NMR. Annu Rev Biochem. 2014;83:291–315. doi: 10.1146/annurev-biochem-060713-035829. [DOI] [PubMed] [Google Scholar]
- 18.Sprangers R, Gribun A, Hwang PM, Houry WA, Kay LE. Quantitative NMR spectroscopy of supramolecular complexes: dynamic side pores in ClpP are important for product release. Proc Natl Acad Sci U S A. 2005;102:16678–16683. doi: 10.1073/pnas.0507370102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprangers R, Kay LE. Quantitative dynamics and binding studies of the 20S proteasome by NMR. Nature. 2007;445:618–622. doi: 10.1038/nature05512. [DOI] [PubMed] [Google Scholar]
- 20.Kato H, et al. Architecture of the high mobility group nucleosomal protein 2-nucleosome complex as revealed by methyl-based NMR. Proc Natl Acad Sci U S A. 2011;108:12283–8. doi: 10.1073/pnas.1105848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Racki LR, et al. The chromatin remodeller ACF acts as a dimeric motor to space nucleosomes. Nature. 2009;462:1016–1021. doi: 10.1038/nature08621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clapier CR, Längst G, Corona DF, Becker PB, Nightingale KP. Critical role for the histone H4 N terminus in nucleosome remodeling by ISWI. Mol Cell Biol. 2001;21:875–883. doi: 10.1128/MCB.21.3.875-883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamiche A, Kang JG, Dennis C, Xiao H, Wu C. Histone tails modulate nucleosome mobility and regulate ATP-dependent nucleosome sliding by NURF. Proc Natl Acad Sci U S A. 2001;98:14316–14321. doi: 10.1073/pnas.251421398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clapier CR, Nightingale KP, Becker PB. A critical epitope for substrate recognition by the nucleosome remodeling ATPase ISWI. Nucleic Acids Res. 2002;30:649–655. doi: 10.1093/nar/30.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dang W, Kagalwala MN, Bartholomew B. Regulation of ISW2 by concerted action of histone H4 tail and extranucleosomal DNA. Mol Cell Biol. 2006;26:7388–7396. doi: 10.1128/MCB.01159-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Racki LR, et al. The Histone H4 Tail Regulates the Conformation of the ATP-Binding Pocket in the SNF2h Chromatin Remodeling Enzyme. J Mol Biol. 2014 doi: 10.1016/j.jmb.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tugarinov V, Hwang PM, Ollerenshaw JE, Kay LE. Cross-correlated relaxation enhanced 1H[bond]13C NMR spectroscopy of methyl groups in very high molecular weight proteins and protein complexes. J Am Chem Soc. 2003;125:10420–10428. doi: 10.1021/ja030153x. [DOI] [PubMed] [Google Scholar]
- 28.Mittermaier A, Kay LE. New tools provide new insights in NMR studies of protein dynamics. Science. 2006;312:224–228. doi: 10.1126/science.1124964. [DOI] [PubMed] [Google Scholar]
- 29.Hamel DJ, Dahlquist FW. The contact interface of a 120 kD CheA-CheW complex by methyl TROSY interaction spectroscopy. J Am Chem Soc. 2005;127:9676–9677. doi: 10.1021/ja052517m. [DOI] [PubMed] [Google Scholar]
- 30.Schlosshauer M, Baker D. Realistic protein-protein association rates from a simple diffusional model neglecting long-range interactions, free energy barriers, and landscape ruggedness. Protein Sci. 2004;13:1660–9. doi: 10.1110/ps.03517304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang W, Bartholomew B. Domain architecture of the catalytic subunit in the ISW2-nucleosome complex. Mol Cell Biol. 2007;27:8306–8317. doi: 10.1128/MCB.01351-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zofall M, Persinger J, Kassabov SR, Bartholomew B. Chromatin remodeling by ISW2 and SWI/SNF requires DNA translocation inside the nucleosome. Nat Struct Mol Biol. 2006;13:339–346. doi: 10.1038/nsmb1071. [DOI] [PubMed] [Google Scholar]
- 33.Kagalwala MN, Glaus BJ, Dang W, Zofall M, Bartholomew B. Topography of the ISW2-nucleosome complex: insights into nucleosome spacing and chromatin remodeling. EMBO J. 2004;23:2092–2104. doi: 10.1038/sj.emboj.7600220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kruger W, et al. Amino acid substitutions in the structured domains of histones H3 and H4 partially relieve the requirement of the yeast SWI/SNF complex for transcription. Genes Dev. 1995;9:2770–9. doi: 10.1101/gad.9.22.2770. [DOI] [PubMed] [Google Scholar]
- 35.Muthurajan UM, et al. Crystal structures of histone Sin mutant nucleosomes reveal altered protein-DNA interactions. EMBO J. 2004;23:260–71. doi: 10.1038/sj.emboj.7600046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flaus A, Rencurel C, Ferreira H, Wiechens N, Owen-Hughes T. Sin mutations alter inherent nucleosome mobility. EMBO J. 2004;23:343–53. doi: 10.1038/sj.emboj.7600047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horn PJ, Crowley Ka, Carruthers LM, Hansen JC, Peterson CL. The SIN domain of the histone octamer is essential for intramolecular folding of nucleosomal arrays. Nat Struct Biol. 2002;9:167–71. doi: 10.1038/nsb762. [DOI] [PubMed] [Google Scholar]
- 38.Tsukiyama T, Wu C. Purification and properties of an ATP-dependent nucleosome remodeling factor. Cell. 1995;83:1011–1020. doi: 10.1016/0092-8674(95)90216-3. [DOI] [PubMed] [Google Scholar]
- 39.Ito T, Bulger M, Pazin MJ, Kobayashi R, Kadonaga JT. ACF, an ISWI-containing and ATP-utilizing chromatin assembly and remodeling factor. Cell. 1997;90:145–155. doi: 10.1016/s0092-8674(00)80321-9. [DOI] [PubMed] [Google Scholar]
- 40.Aalfs JD, Narlikar GJ, Kingston RE. Functional Differences between the Human ATP-dependent Nucleosome Remodeling Proteins BRG1 and SNF2H. J Biol Chem. 2001;276:34270–34278. doi: 10.1074/jbc.M104163200. [DOI] [PubMed] [Google Scholar]
- 41.Corona DFV, et al. ISWI is an ATP-dependent nucleosome remodeling factor. Mol Cell. 1999;3:239–245. doi: 10.1016/s1097-2765(00)80314-7. [DOI] [PubMed] [Google Scholar]
- 42.Morrison AJ, Shen X. Chromatin remodelling beyond transcription: the INO80 and SWR1 complexes. Nat Rev Mol Cell Biol. 2009;10:373–384. doi: 10.1038/nrm2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horigome C, et al. SWR1 and INO80 Chromatin Remodelers Contribute to DNA Double-Strand Break Perinuclear Anchorage Site Choice. Mol Cell. 2014 doi: 10.1016/j.molcel.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 44.Shen X, Mizuguchi G, Hamiche A, Wu C. A chromatin remodelling complex involved in transcription and DNA processing. Nature. 2000;406:541–544. doi: 10.1038/35020123. [DOI] [PubMed] [Google Scholar]
- 45.Udugama M, Sabri A, Bartholomew B. The INO80 ATP-dependent chromatin remodeling complex is a nucleosome spacing factor. Mol Cell Biol. 2011;31:662–673. doi: 10.1128/MCB.01035-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tosi A, et al. Structure and subunit topology of the INO80 chromatin remodeler and its nucleosome complex. Cell. 2013;154 doi: 10.1016/j.cell.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 47.Blosser TR, Yang JG, Stone MD, Narlikar GJ, Zhuang X. Dynamics of nucleosome remodelling by individual ACF complexes. Nature. 2009;462:1022–7. doi: 10.1038/nature08627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harada BT, et al. Stepwise nucleosome translocation by RSC remodeling complexes. Elife. 2016;5 doi: 10.7554/eLife.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hota SK, et al. Nucleosome mobilization by ISW2 requires the concerted action of the ATPase and SLIDE domains. Nat Struct Mol Biol. 2013;20:222–9. doi: 10.1038/nsmb.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gangaraju VK, Prasad P, Srour A, Kagalwala MN, Bartholomew B. Conformational Changes Associated with Template Commitment in ATP-Dependent Chromatin Remodeling by ISW2. Mol Cell. 2009;35:58–69. doi: 10.1016/j.molcel.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leonard JD, Narlikar GJ. A Nucleotide-Driven Switch Regulates Flanking DNA Length Sensing by a Dimeric Chromatin Remodeler. Mol Cell. 2015;57:850–859. doi: 10.1016/j.molcel.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Clapier CR, et al. Regulation of DNA Translocation Efficiency within the Chromatin Remodeler RSC/Sth1 Potentiates Nucleosome Sliding and Ejection. Mol Cell. 2016;62:453–461. doi: 10.1016/j.molcel.2016.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guyon JR, Narlikar GJ, Sullivan EK, Kingston RE. Stability of a human SWI-SNF remodeled nucleosomal array. Mol Cell Biol. 2001;21:1132–1144. doi: 10.1128/MCB.21.4.1132-1144.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Imbalzano AN, Schnitzler GR, Kingston RE. Nucleosome disruption by human SWI/SNF is maintained in the absence of continued ATP hydrolysis. J Biol Chem. 1996;271:20726–20733. doi: 10.1074/jbc.271.34.20726. [DOI] [PubMed] [Google Scholar]
- 55.Boyer LA, Shao X, Ebright RH, Peterson CL. Roles of the histone H2A-H2B dimers and the (H3-H4)2 tetramer in nucleosome remodeling by the SWI-SNF complex. J Biol Chem. 2000;275:11545–11552. doi: 10.1074/jbc.275.16.11545. [DOI] [PubMed] [Google Scholar]
- 56.Bazett-Jones DP, Côté J, Landel CC, Peterson CL, Workman JL. The SWI/SNF complex creates loop domains in DNA and polynucleosome arrays and can disrupt DNA-histone contacts within these domains. Mol Cell Biol. 1999;19:1470–1478. doi: 10.1128/mcb.19.2.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brehove M, et al. Histone core phosphorylation regulates DNA accessibility. J Biol Chem. 2015;290:22612–22621. doi: 10.1074/jbc.M115.661363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falk SJ, et al. Chromosomes. CENP-C reshapes and stabilizes CENP-A nucleosomes at the centromere. Science. 2015;348:699–703. doi: 10.1126/science.1259308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrews AJ, Luger K. Nucleosome Structure (s) and Stability: Variations on a Theme. Biochemistry. 2011:99–117. doi: 10.1146/annurev-biophys-042910-155329. [DOI] [PubMed] [Google Scholar]
- 60.Prior CP, Cantor CR, Johnson EM, Littau VC, Allfrey VG. Reversible changes in nucleosome structure and histone H3 accessibility in transcriptionally active and inactive states of rDNA chromatin. Cell. 1983;34:1033–1042. doi: 10.1016/0092-8674(83)90561-5. [DOI] [PubMed] [Google Scholar]
- 61.Fei J, et al. The prenucleosome, a stable conformational isomer of the nucleosome. Genes Dev. 2015;29:2563–2575. doi: 10.1101/gad.272633.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cosgrove MS, Boeke JD, Wolberger C. Regulated nucleosome mobility and the histone code. Nat Struct Mol Biol. 2004;11:1037–1043. doi: 10.1038/nsmb851. [DOI] [PubMed] [Google Scholar]
- 63.Zaret KS, Carroll JS. Pioneer transcription factors: Establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 Å resolution. J Mol Biol. 2002;319:1097–1113. doi: 10.1016/S0022-2836(02)00386-8. [DOI] [PubMed] [Google Scholar]
- 65.Cavallo L, Kleinjung J, Fraternali F. POPS: A fast algorithm for solvent accessible surface areas at atomic and residue level. Nucleic Acids Res. 2003;31:3364–3366. doi: 10.1093/nar/gkg601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bruno M, et al. Histone H2A/H2B dimer exchange by ATP-dependent chromatin remodeling activities. Mol Cell. 2003;12:1599–606. doi: 10.1016/s1097-2765(03)00499-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Biological Magnetic Resonance Data Bank. http://www.bmrb.wisc.edu/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.