Abstract
AIM
To investigate the expression of annexin A5 in serum and tumor tissue of patients with colon cancer and to analyze its clinical significance.
METHODS
Ninety-three patients with colon cancer treated at our hospital between February 2013 and March 2016 were included in an observation group, and 40 healthy individuals were included in a control group. Enzyme-linked immunosorbent assay was performed to determine the serum level of annexin A5, while immunohistochemistry was performed to determine the expression of annexin A5 in cancer tissues.
RESULTS
The serum level of annexin A5 was 0.184 ± 0.043 ng/mL in the observation group, which was significantly higher than that in the control group (P < 0.05). Annexin A5 expression was detected in 79.31% of the patients with lymph node metastasis, which was significantly higher than that in patients without lymph node metastasis (P < 0.05). Moreover, annexin A5 expression was detected in 86.96% of the patients with stage III to IV disease, which was significantly higher than that in patients with stage I to II disease (P < 0.05). The serum level of annexin A5 was 0.215 ± 0.044 ng/mL in patients whose tumors were positive for annexin A5 expression, which was significantly higher than that in patients whose tumors were negative for annexin A5 expression (P < 0.05). The serum level of annexin A5 was correlated with annexin A5 expression in colon cancer tissues (r = 0.312, P < 0.05). When a cutoff value of > 0.148 ng/mL for serum level of annexin A5 was used in the diagnosis of colon cancer, the sensitivity was 83.90%, and the specificity was 57.50%.
CONCLUSION
For patients with colon cancer, annexin A5 expression in cancer tissues is related to lymph node metastasis and tumor grade. Serum level of annexin A5 is related to annexin A5 expression in cancer tissues and is of diagnostic relevance.
Keywords: Immunohistochemistry, Annexin A5, Colon cancer, Serum
Core tip: For patients with colon cancer, annexin A5 expression in cancer tissues is related to lymph node metastasis and tumor grade. Serum level of annexin A5 is related to annexin A5 expression in cancer tissues and is of diagnostic relevance.
INTRODUCTION
Colon cancer is a common malignancy of the digestive tract. Studies have shown that the incidence of colon cancer is ≥ 0.005%[1] and that the incidence has continued to trend upwards in recent years because of risk factors such as diet and smoking[2,3] .
Basic cancer research has shown that changes in the levels of certain molecules affect tumor cell proliferation and differentiation, which in turn affect the development and progression of malignant tumors[4]. Annexin A5, first isolated from human placenta, was found to bind to phosphatidylserine in a calcium-dependent manner[5,6]. In the present study, 93 patients with colon cancer who were treated at our hospital between February 2013 and March 2016 were included to investigate the expression of annexin A5 in serum and in cancer tissues, with an aim to investigate its clinical significance.
MATERIALS AND METHODS
General information
Ninety-three patients with colon cancer (observation group) who were treated at our hospital between February 2013 and March 2016 were included in this study. The inclusion criteria were as follows: (1) pathologically confirmed colon cancer; (2) complete clinical and pathological data; and (3) being willing to provide informed consent. The exclusion criterion was incomplete clinical or pathological data. Forty healthy individuals who underwent a routine health checkup at our hospital were included as controls. No significant difference was observed with respect to age or gender between the two groups (Table 1).
Table 1.
General information (mean ± SD)
| Group | n | M/F | Age (yr) |
| Observation group | 93 | 54/39 | 53.29 ± 9.49 |
| Control group | 40 | 27/13 | 52.17 ± 8.14 |
| t/χ2 | 1.046 | 0.65 | |
| P value | > 0.05 | > 0.05 |
Detection of serum level of annexin A5
A fasting venous blood sample was collected from each subject in the morning and centrifuged at 10000 r/min to separate the serum, which was then stored at -20 °C and tested within one week to determine the annexin A5 level. The Roche automated biochemical analyzer E170 module was used for testing, and the assay kit was purchased from Shanghai Taikang Biotechnology Co., Ltd. The assay was performed according to the instructions given in the package insert. Control serum or standard was included with the kit.
Immunohistochemistry
Paraffin sections were deparaffinized, rehydrated, and cut into 3 mm sections. The sections were incubated in 3% H2O2 at room temperature for 5 min, rinsed with deionized water (3 min × 3 times), blocked with 10% milk protein (1 g protein in 100 mL of purified water), and incubated at room temperature for 5 min. Next, the sections were incubated with a mouse anti-annexin A5 antibody (Nanjing Biyuntian Biotechnology Co., Ltd.) for 2 h at 37 °C, followed by a PBS wash (5 min × 3 times). Then, the slides were incubated with a horseradish peroxidase-labeled rabbit secondary antibody (Roche) for 30 min at 37 °C, followed by a PBS wash (5 min × 3 times). After that, the slides were incubated with NBT/BCIP reagent, which was used to develop the reaction, for 5 min. Finally, the sections were counterstained, dehydrated, cleared, mounted, and observed under an OLIPICS microscope (Shanghai Precision Instrument Co., Ltd). All the required reagents were purchased from Nanjing Taikang Biotechnology Co., Ltd.
Evaluation criteria for immunohistochemical staining
Immunohistochemical staining was considered positive if yellow granules were present in the cytoplasm of tumor cells or stromal cells. The staining intensity was graded as follows: 0, no staining; 1, light yellow; 2, yellow; and 3, brown. The percentage of positive cells was scored as follows: 0, < 5%; 1, 5% to 24%; 2, 25% to 50%; 3, 51% to 74%; and 4, and ≥ 75%. The product of the staining intensity and the percentage of positive cells was either < 2 (negative) or ≥ 2 (positive).
Statistical analysis
SPSS v19.0 was used for statistical analyses. Measurement data are expressed as mean ± SD and were analyzed by the t-test. Count data were analyzed by the χ2 test. Spearman rank correlation analysis was performed to analyze potential correlations between variables. A receiver operating characteristic curve was used to analyze the diagnostic value of serum annexin A5 level. P < 0.05 was considered statistically significant.
RESULTS
Annexin A5 expression in cancer tissue
No significant difference was observed in the positive expression rates of annexin A5 among patients of different ages or genders, or those with different tumor diameters. Moreover, 79.31% of the patients with lymph node metastasis expressed annexin A5, which was significantly higher than the percentage of patients without lymph node metastasis (P < 0.05); 86.96% of the patients with stage III to IV disease expressed annexin A5, which was significantly higher than the percentage of patients with stage I to II disease (P < 0.05) (Table 2).
Table 2.
Relationship between Annexin A5 and clinicopathological features of patients with colon cancer n (%)
| Clinicopathological feature | n | Positive | χ2 | P value |
| Gender | ||||
| M | 54 | 31 (57.41) | 0.023 | > 0.05 |
| F | 39 | 23 (58.97) | ||
| Age, yr | ||||
| ≥ 55 | 47 | 29 (61.70) | 0.516 | > 0.05 |
| < 50 | 46 | 25 (54.35) | ||
| Lymph node metastasis | ||||
| Yes | 29 | 23 (79.31) | 7.812 | < 0.05 |
| No | 64 | 31 (48.44) | ||
| Tumor diameter (cm) | ||||
| ≥ 5 | 51 | 29 (56.86) | 0.067 | > 0.05 |
| < 5 | 42 | 25 (59.52) | ||
| Tumor stage | ||||
| I to II | 47 | 14 (29.79) | 31.204 | < 0.05 |
| III to IV | 46 | 40 (86.96) |
Serum levels of annexin A5 in the two groups
The serum level of annexin A5 was significantly higher in the observation group than in the control group (P < 0.05) (Table 3).
Table 3.
Serum levels of Annexin A5 in the two groups (mean ± SD, ng/mL)
| Group | n | Annexin A5 | t | P value |
| Observation group | 93 | 0.184 ± 0.043 | 2.904 | < 0.05 |
| Control group | 40 | 0.159 ± 0.051 |
Correlation between serum level of annexin A5 and expression of annexin A5 in tumor tissue
The serum level of annexin A5 was 0.215 ± 0.044 ng/mL in patients whose colon tumors were positive for annexin A5 expression, which was significantly higher than the corresponding value in patients whose colon tumors were negative for annexin A5 (0.180 ± 0.021 ng/mL) (t = 4.599, P < 0.05). A Spearman rank correlation analysis showed that the serum level of annexin A5 was related to the expression of annexin A5 in tumor tissues (r = 0.312, P < 0.05).
Diagnostic value of serum level of annexin A5
The ROC curve for the serum level of annexin A5 in the diagnosis of colon cancer showed an area under the curve of 0.732 (P < 0.05). At a cutoff value of 0.148 ng/mL, the sensitivity was 83.90%, and the specificity was 57.50% (Figure 1).
Figure 1.
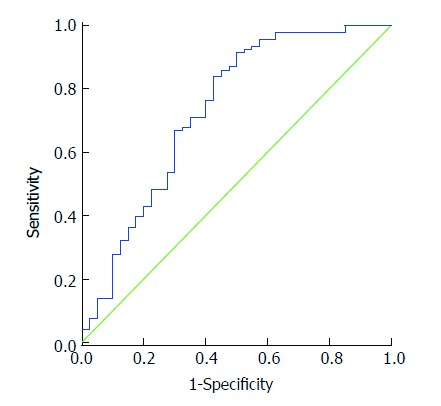
Receiver operating characteristic curve for the value of serum level of annexin A5 in the diagnosis of colon cancer.
DISCUSSION
Changes in diet, excessive alcohol consumption, and genetic susceptibility factors promote the development and progression of colon cancer. In particular, among elderly male smokers aged 45 or older, the incidence of colon cancer is 0.005% or higher and has continued to trend upwards in recent years[7,8]. For colon cancer, the incidence of early metastasis is high, which results in poor patient outcomes: the five years survival rate is < 35%, and the median survival time is < 32 mo[9-11]. Studies on the genetic and biological mechanisms of the development and progression of colon cancer may provide new targets for immune therapy or strategies of comprehensive biological therapy for colon cancer[12,13].
Molecular changes play an important regulatory role in the development of malignant tumors. Cell surface Connexins or membrane proteins can induce the transcription initiation activity of downstream oncogenes, which promotes aberrant activation of the cell cycle in colonic epithelial cells and leads to excessive proliferation of cancer cells[14]. Accumulating experimental data indicate that phosphatidylserine exposition is associated with apoptosis and other cell death programs[15-17], which renders it an attractive target in imaging overall cell death. Annexin A5 is identified in blood vessel as a blood anticoagulation factor and it builds voltage-dependent calcium channel in phosphatidylserine bilayers[18,19]. Corsten et al[20] showed that through binding with strong affinity to phosphatidylserine, annexin A5 offers an interesting opportunity for visualization of aggregate cell death[21,22], thus providing a fit benchmark for in vivo monitoring of anticancer treatment[23-26]. Recently, annexin A5 has been reported as a new mediator of cisplatin-induced apoptosis by inducing voltage-dependent anion channel oligomerization in human kidney epithelial cells[27,28]. Annexin A5 forms N6-acetyllysine at specific positions of the amino-terminal region of the membrane protein, and as a result, it affects the formation of a transcriptional co-inhibitory complex and participates in transcriptional repression and silencing of tumor suppressor genes via H1 phosphorylation[29,30]. Previous studies have investigated the relationship between annexin A5 and liver cancer or esophageal cancer and showed that uH2B-related monotone generalization increased the risk of malignant digestive tumors and promoted clinical progression[31-34]. This study explored not only the expression of uH2B in colon cancer tissue but also the diagnostic value of its serum level in the diagnosis of colon cancer.
In this study, immunohistochemical staining showed significantly high expression of annexin A5 in colon cancer tissue and demonstrated that the positive expression rate of annexin A5 was significantly higher in patients with lymph node metastasis than in those without. This suggests that annexin A5 may play a role in the promotion of the invasion of lymph nodes by colon cancer cells. Furthermore, approximately 80% of the patients with late-stage (III and IV) colon cancer expressed annexin A5, which was significantly higher than the percentage of patients with stage I or II disease, which suggests that annexin A5 significantly promotes the clinical progression and worsening of colon cancer. Annexin A5 induces the activation of second messengers in cancer cells, which promotes the production of cancer cell differentiation antigens, the proliferation and differentiation of colon cancer cells, and clinical progression.
In conclusion, annexin A5 is highly expressed in serum and tumor tissues of patients with colon cancer, and its expression is closely related to the clinical stage and presence of lymph node metastasis in patients with colon cancer. Nevertheless, this study has certain limitations. For instance, we did not investigate the relationship between the expression of annexin A5 and the long-term survival of patients with colon cancer.
COMMENTS
Background
Colon cancer is a common malignancy of the digestive tract. Studies have shown that the incidence of colon cancer is ≥ 0.005% and that the incidence has continued to trend upwards in recent years because of risk factors such as diet and smoking.
Research frontiers
Basic cancer research has shown that changes in the levels of certain molecules affect tumor cell proliferation and differentiation, which in turn affect the development and progression of malignant tumors. Annexin A5 is a glycoprotein that contains a multiplex carboxyl terminus binding domain, which influences the differentiation of surface antigens on cancer cells and promotes tumor proliferation and invasion.
Innovations and breakthroughs
The objective was to investigate the clinical significance of annexin A5 expression in colon cancer.
Applications
For patients with colon cancer, annexin A5 expression in cancer tissues is related to lymph node metastasis and tumor grade. Serum level of annexin A5 is related to annexin A5 expression in cancer tissues and is of diagnostic relevance.
Peer-review
In this study, the authors investigated the expression of annexin A5 in serum and tumor tissues of patients with colon cancer and its clinical significance.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: This study is approved by the Local Hospital Review Board.
Informed consent statement: All cases enrolled have signed the consent statement.
Conflict-of-interest statement: No conflict of interest.
Data sharing statement: No additional data are available.
Peer-review started: February 27, 2017
First decision: April 10, 2017
Article in press: August 8, 2017
P- Reviewer: Faerch K, GordonLG S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Huang Y
Contributor Information
Chong-Bing Sun, Department of General Surgery, Weifang People’s Hospital, Weifang 261000, Shandong Province, China.
Ai-Yan Zhao, Department of General Surgery, Weifang People’s Hospital, Weifang 261000, Shandong Province, China.
Shuai Ji, Department of Anorectal Surgery, Linqu People’s Hospital, Weifang 261000, Shandong Province, China.
Xiao-Qing Han, Department of General Surgery, Weifang People’s Hospital, Weifang 261000, Shandong Province, China.
Zuo-Cheng Sun, Department of General Surgery, Weifang People’s Hospital, Weifang 261000, Shandong Province, China.
Meng-Chun Wang, Department of General Surgery, Weifang People’s Hospital, Weifang 261000, Shandong Province, China.
Fu-Chang Zheng, Department of General Surgery, Weifang People’s Hospital, Weifang 261000, Shandong Province, China. zhengfc@yeah.net.
References
- 1.Chan M, Hugh-Yeun K, Gresham G, Speers CH, Kennecke HF, Cheung WY. Population-Based Patterns and Factors Associated With Underuse of Palliative Systemic Therapy in Elderly Patients With Metastatic Colon Cancer. Clin Colorectal Cancer. 2017;16:147–153. doi: 10.1016/j.clcc.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Kornmann M, Formentini A, Ette C, Henne-Bruns D, Kron M, Sander S, Baumann W, Kreuser ED, Staib L, Link KH. Prognostic factors influencing the survival of patients with colon cancer receiving adjuvant 5-FU treatment. Eur J Surg Oncol. 2008;34:1316–1321. doi: 10.1016/j.ejso.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Ueno K, Hazama S, Mitomori S, Nishioka M, Suehiro Y, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br J Cancer. 2009;101:1374–1381. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ashktorab H, Shakoori A, Zarnogi S, Sun X, Varma S, Lee E, Shokrani B, Laiyemo AO, Washington K, Brim H. Reduced Representation Bisulfite Sequencing Determination of Distinctive DNA Hypermethylated Genes in the Progression to Colon Cancer in African Americans. Gastroenterol Res Pract. 2016;2016:2102674. doi: 10.1155/2016/2102674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohn H, Kraus W. [Isolation and characterization of a new placenta specific protein (PP10) (author’s transl)] Arch Gynecol. 1979;227:125–134. doi: 10.1007/BF02103286. [DOI] [PubMed] [Google Scholar]
- 6.Boersma HH, Kietselaer BL, Stolk LM, Bennaghmouch A, Hofstra L, Narula J, Heidendal GA, Reutelingsperger CP. Past, present, and future of annexin A5: from protein discovery to clinical applications. J Nucl Med. 2005;46:2035–2050. [PubMed] [Google Scholar]
- 7.Weixler B, Warschkow R, Güller U, Zettl A, von Holzen U, Schmied BM, Zuber M. Isolated tumor cells in stage I & II colon cancer patients are associated with significantly worse disease-free and overall survival. BMC Cancer. 2016;16:106. doi: 10.1186/s12885-016-2130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rivera CA, Ahlberg NC, Taglia L, Kumar M, Blunier A, Benya RV. Expression of GRP and its receptor is associated with improved survival in patients with colon cancer. Clin Exp Metastasis. 2009;26:663–671. doi: 10.1007/s10585-009-9265-8. [DOI] [PubMed] [Google Scholar]
- 9.McArdle CS, McMillan DC, Hole DJ. The impact of blood loss, obstruction and perforation on survival in patients undergoing curative resection for colon cancer. Br J Surg. 2006;93:483–488. doi: 10.1002/bjs.5269. [DOI] [PubMed] [Google Scholar]
- 10.Mesker WE, Liefers GJ, Junggeburt JM, van Pelt GW, Alberici P, Kuppen PJ, Miranda NF, van Leeuwen KA, Morreau H, Szuhai K, et al. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cell Oncol. 2009;31:169–178. doi: 10.3233/CLO-2009-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grande R, Corsi D, Mancini R, Gemma D, Ciancola F, Sperduti I, Rossi L, Fabbri A, Diodoro MG, Ruggeri E, et al. Evaluation of relapse-free survival in T3N0 colon cancer: the role of chemotherapy, a multicentric retrospective analysis. PLoS One. 2013;8:e80188. doi: 10.1371/journal.pone.0080188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang DQ, Wang K, Yan DW, Liu J, Wang B, Li MX, Wang XW, Liu J, Peng ZH, Li GX, et al. Ciz1 is a novel predictor of survival in human colon cancer. Exp Biol Med (Maywood) 2014;239:862–870. doi: 10.1177/1535370213520113. [DOI] [PubMed] [Google Scholar]
- 13.Lederer A, Herrmann P, Seehofer D, Dietel M, Pratschke J, Schlag P, Stein U. Metastasis-associated in colon cancer 1 is an independent prognostic biomarker for survival in Klatskin tumor patients. Hepatology. 2015;62:841–850. doi: 10.1002/hep.27885. [DOI] [PubMed] [Google Scholar]
- 14.Kazmierczak PM, Burian E, Eschbach R, Hirner-Eppeneder H, Moser M, Havla L, Eisenblätter M, Reiser MF, Nikolaou K, Cyran CC. Monitoring Cell Death in Regorafenib-Treated Experimental Colon Carcinomas Using Annexin-Based Optical Fluorescence Imaging Validated by Perfusion MRI. PLoS One. 2015;10:e0138452. doi: 10.1371/journal.pone.0138452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung S, Gumienny TL, Hengartner MO, Driscoll M. A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat Cell Biol. 2000;2:931–937. doi: 10.1038/35046585. [DOI] [PubMed] [Google Scholar]
- 16.Krysko O, De Ridder L, Cornelissen M. Phosphatidylserine exposure during early primary necrosis (oncosis) in JB6 cells as evidenced by immunogold labeling technique. Apoptosis. 2004;9:495–500. doi: 10.1023/B:APPT.0000031452.75162.75. [DOI] [PubMed] [Google Scholar]
- 17.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, Bodmer JL, Schneider P, Seed B, Tschopp J. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 18.Reutelingsperger CP, Hornstra G, Hemker HC. Isolation and partial purification of a novel anticoagulant from arteries of human umbilical cord. Eur J Biochem. 1985;151:625–629. doi: 10.1111/j.1432-1033.1985.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 19.Demange P, Voges D, Benz J, Liemann S, Göttig P, Berendes R, Burger A, Huber R. Annexin V: the key to understanding ion selectivity and voltage regulation? Trends Biochem Sci. 1994;19:272–276. doi: 10.1016/0968-0004(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 20.Corsten MF, Hofstra L, Narula J, Reutelingsperger CP. Counting heads in the war against cancer: defining the role of annexin A5 imaging in cancer treatment and surveillance. Cancer Res. 2006;66:1255–1260. doi: 10.1158/0008-5472.CAN-05-3000. [DOI] [PubMed] [Google Scholar]
- 21.Park N, Chun YJ. Auranofin promotes mitochondrial apoptosis by inducing annexin A5 expression and translocation in human prostate cancer cells. J Toxicol Environ Health A. 2014;77:1467–1476. doi: 10.1080/15287394.2014.955834. [DOI] [PubMed] [Google Scholar]
- 22.Hong M, Park N, Chun YJ. Role of annexin a5 on mitochondria-dependent apoptosis induced by tetramethoxystilbene in human breast cancer cells. Biomol Ther (Seoul) 2014;22:519–524. doi: 10.4062/biomolther.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vangestel C, Van de Wiele C, Mees G, Mertens K, Staelens S, Reutelingsperger C, Pauwels P, Van Damme N, Peeters M. Single-photon emission computed tomographic imaging of the early time course of therapy-induced cell death using technetium 99m tricarbonyl His-annexin A5 in a colorectal cancer xenograft model. Mol Imaging. 2012;11:135–147. [PubMed] [Google Scholar]
- 24.Shin DW, Kwon YJ, Ye DJ, Baek HS, Lee JE, Chun YJ. Auranofin Suppresses Plasminogen Activator Inhibitor-2 Expression through Annexin A5 Induction in Human Prostate Cancer Cells. Biomol Ther (Seoul) 2017;25:177–185. doi: 10.4062/biomolther.2016.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng S, Wang J, Hou L, Li J, Chen G, Jing B, Zhang X, Yang Z. Annexin A1, A2, A4 and A5 play important roles in breast cancer, pancreatic cancer and laryngeal carcinoma, alone and/or synergistically. Oncol Lett. 2013;5:107–112. doi: 10.3892/ol.2012.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schaper FL, Reutelingsperger CP. 99mTc-HYNIC-Annexin A5 in Oncology: Evaluating Efficacy of Anti-Cancer Therapies. Cancers (Basel) 2013;5:550–568. doi: 10.3390/cancers5020550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwon YJ, Jung JJ, Park NH, Ye DJ, Kim D, Moon A, Chun YJ. Annexin a5 as a new potential biomarker for Cisplatin-induced toxicity in human kidney epithelial cells. Biomol Ther (Seoul) 2013;21:190–195. doi: 10.4062/biomolther.2013.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong JJ, Park N, Kwon YJ, Ye DJ, Moon A, Chun YJ. Role of annexin A5 in cisplatin-induced toxicity in renal cells: molecular mechanism of apoptosis. J Biol Chem. 2014;289:2469–2481. doi: 10.1074/jbc.M113.450163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsukamoto H, Tanida S, Ozeki K, Ebi M, Mizoshita T, Shimura T, Mori Y, Kataoka H, Kamiya T, Fukuda S, et al. Annexin A2 regulates a disintegrin and metalloproteinase 17-mediated ectodomain shedding of pro-tumor necrosis factor-α in monocytes and colon epithelial cells. Inflamm Bowel Dis. 2013;19:1365–1373. doi: 10.1097/MIB.0b013e318281f43a. [DOI] [PubMed] [Google Scholar]
- 30.Tristante E, Martínez CM, Jiménez S, Mora L, Carballo F, Martínez-Lacaci I, de Torre-Minguela C. Association of a characteristic membrane pattern of annexin A2 with high invasiveness and nodal status in colon adenocarcinoma. Transl Res. 2015;166:196–206. doi: 10.1016/j.trsl.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 31.Schurgers LJ, Burgmaier M, Ueland T, Schutters K, Aakhus S, Hofstra L, Gullestad L, Aukrust P, Hellmich M, Narula J, et al. Circulating annexin A5 predicts mortality in patients with heart failure. J Intern Med. 2016;279:89–97. doi: 10.1111/joim.12396. [DOI] [PubMed] [Google Scholar]
- 32.Ogawa K, Ohtsuki K, Shibata T, Aoki M, Nakayama M, Kitamura Y, Ono M, Ueda M, Doue T, Onoguchi M, et al. Development and evaluation of a novel (99m)tc-labeled annexin A5 for early detection of response to chemotherapy. PLoS One. 2013;8:e81191. doi: 10.1371/journal.pone.0081191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paweletz CP, Ornstein DK, Roth MJ, Bichsel VE, Gillespie JW, Calvert VS, Vocke CD, Hewitt SM, Duray PH, Herring J, et al. Loss of annexin 1 correlates with early onset of tumorigenesis in esophageal and prostate carcinoma. Cancer Res. 2000;60:6293–6297. [PubMed] [Google Scholar]
- 34.Zaidi AH, Gopalakrishnan V, Kasi PM, Zeng X, Malhotra U, Balasubramanian J, Visweswaran S, Sun M, Flint MS, Davison JM, et al. Evaluation of a 4-protein serum biomarker panel-biglycan, annexin-A6, myeloperoxidase, and protein S100-A9 (B-AMP)-for the detection of esophageal adenocarcinoma. Cancer. 2014;120:3902–3913. doi: 10.1002/cncr.28963. [DOI] [PMC free article] [PubMed] [Google Scholar]


