Abstract
Study Objectives:
In heart failure (HF), we observed two patterns of hyperpnea during Cheyne-Stokes respiration with central sleep apnea (CSR-CSA): a positive pattern where end-expiratory lung volume remains at or above functional residual capacity, and a negative pattern where it falls below functional residual capacity. We hypothesized the negative pattern is associated with worse HF.
Methods:
Patients with HF underwent polysomnography. During CSR-CSA, hyperpnea, apnea-hyperpnea cycle, and lung to finger circulation times (LFCT) were measured. Plasma N-terminal prohormone of brain natriuretic peptide (NT-proBNP) concentration and left ventricular ejection fraction (LVEF) were assessed.
Results:
Of 33 patients with CSR-CSA (31 men, mean age 68 years), 9 had a negative hyperpnea pattern. There was no difference in age, body mass index, and apnea-hypopnea index between groups. Patients with a negative pattern had longer hyperpnea time (39.5 ± 6.4 versus 25.8 ± 5.9 seconds, P < .01), longer cycle time (67.8 ± 15.9 versus 51.7 ± 9.9 seconds, P < .01), higher NT-proBNP concentrations (2740 [6769] versus 570 [864] pg/ml, P = .01), and worse New York Heart Association class (P = .02) than those with a positive pattern. LFCT and LVEF did not differ between groups.
Conclusions:
Patients with HF and a negative CSR-CSA pattern have evidence of worse cardiac function than those with a positive pattern. Greater positive expiratory pressure during hyperpnea is likely generated during the negative pattern and might support stroke volume in patients with worse cardiac function.
Commentary:
A commentary on this article appears in this issue on page 1227.
Clinical Trial Registration:
The trial is registered with Current Controlled Trials (www.controlled-trials.com; ISRCTN67500535) and Clinical Trials (www.clinicaltrials.gov; NCT01128816).
Citation:
Perger E, Inami T, Lyons OD, Alshaer H, Smith S, Floras JS, Logan AG, Arzt M, Duran Cantolla J, Delgado D, Fitzpatrick M, Fleetham J, Kasai T, Kimoff RJ, Leung RS, Lorenzi Filho G, Mayer P, Mielniczuk L, Morrison DL, Parati G, Parthasarathy S, Redolfi S, Ryan CM, Series F, Tomlinson GA, Woo A, Bradley TD. Distinct patterns of hyperpnea during Cheyne-Stokes respiration: implication for cardiac function in patients with heart failure. J Clin Sleep Med. 2017;13(11):1235–1241.
Keywords: central sleep apnea, Cheyne-Stokes respiration, heart failure, hyperpnea
INTRODUCTION
Cheyne-Stokes respiration with central sleep apnea (CSRCSA) is a form of periodic breathing commonly observed in patients with heart failure (HF). It is characterized by oscillations of ventilation between central apneas and hyperpneas with a crescendo-decrescendo pattern of hyperpnea. There is controversy on the prognostic significance of CSR-CSA in patients with HF.1 In addition, it remains unclear if CSR-CSA is simply a manifestation of worsening HF, is a cause of HF progression, or is a compensatory mechanism to maintain stroke volume (SV) in severe HF.2 However, it is possible that CSR-CSA may not be entirely homogeneous, but might have different subtypes that might help to explain discrepancies between studies regarding its prognostic significance.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Cheyne-Stokes respiration is common in patients with heart failure, but there are discrepancies between studies regarding its prognostic implications. It is possible that Cheyne-Stokes respiration may not be entirely homogeneous but might have different subtypes.
Study Impact: We report for the first time the observation of two distinct patterns of hyperpnea during Cheyne-Stokes respiration in patients with heart failure, that might be related to underlying cardiac function.
CSR-CSA is attributed to respiratory control instability in which central apneas occur when partial pressure of carbon dioxide (PaCO2) is driven below the apnea threshold by periodic hyperventilation. Several mechanisms can contribute to hyperventilation in HF: stimulation of pulmonary juxtacapillary receptors by pulmonary congestion, increased peripheral and central chemoresponsiveness, and arousals from sleep.3–5 Moreover, the increased lung-to-chemoreceptor circulation delay, typical of HF as a result of decreased cardiac output (CO), may contribute to the ventilatory instability.3–5
The duration of the hyperpnea and apnea-hyperpnea cycle are directly proportional to the lung-to-peripheral chemoreceptor circulation time and inversely proportional to SV and CO.6 Consequently, durations of hyperpnea and apnea-hyper-pnea cycles can be used as indices of SV and CO.
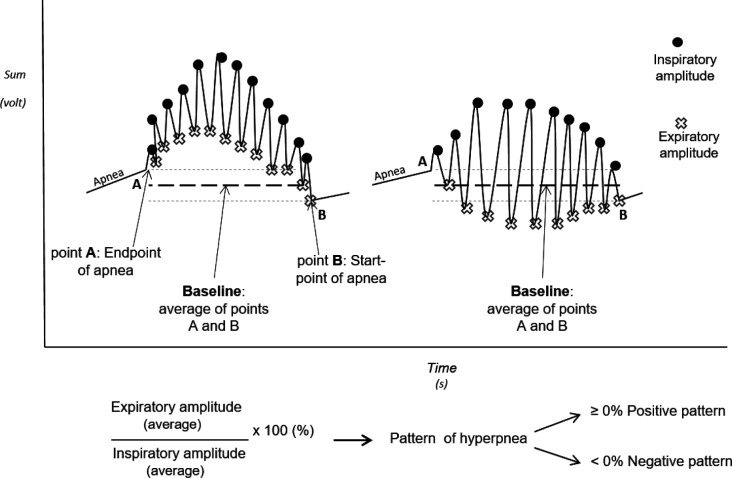
Through careful observation of CSR-CSA in HF patients, we have identified two distinct patterns of hyperpnea: a positive pattern, in which end-expiratory lung volume (EELV) is at or above functional residual capacity (FRC) and a negative pattern in which EELV falls below FRC (Figure 1). The negative pattern implies marked activation of the expiratory muscles to drive EELV below FRC and a greater increase in expiratory intrathoracic pressure (PIT) than during the positive hyperpnea pattern. Such an increase in PIT in patients with HF during the hyperpneic phase of CSR-CSA could be a form of cardiac autoresuscitation similar to the effect of chest compression during cardiopulmonary resuscitation that might augment SV and CO. If that was the case, we would expect that the negative CSR-CSA pattern would be seen in those with worse cardiac function than in those with the positive pattern.
Figure 1. Different patterns of hyperpnea during Cheyne-Stokes respiration.
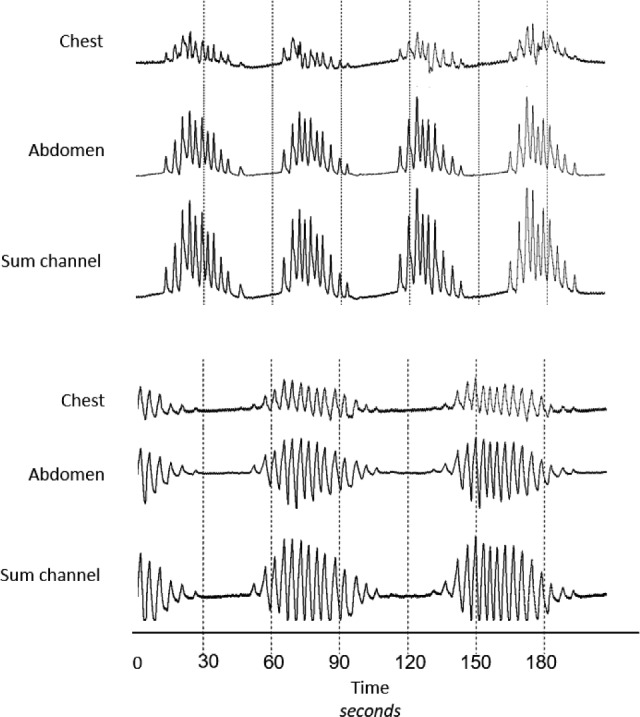
Raw data tracing of chest, abdomen, and sum channels of the respiratory inductance plethysmograph demonstrating two patterns of hyperpnea during Cheyne-Stokes respiration with central sleep apnea. The upper tracings show a positive pattern in which end-expiratory lung volume remains at or above functional residual capacity, and the lower tracings show a negative pattern in which end-expiratory lung volume falls below functional residual capacity.
To test this hypothesis in stable patients with HF and reduced ejection fraction (HFrEF), left ventricular ejection fraction (LVEF), plasma N-terminal prohormone of brain natriuretic peptide (NT-proBNP) concentration, CSR-CSA hyperpnea, cycle and lung to finger circulation times (LFCT), and degree of oxyhemoglobin desaturation following apneas were assessed during overnight polysomnography (PSG). These data were compared between the 2 different patterns of hyperpnea.
METHODS
Subjects
We analyzed the baseline PSG of patients with HFrEF and CSRCSA who were enrolled consecutively at sites meeting our PSG technical requirements in the “Effect of Adaptive Servo Ventilation on Survival and Cardiovascular Hospital Admissions in Patients with Heart Failure and Sleep Apnea” (ADVENT-HF) Trial since the beginning of the trial in January 2010 to May 2016.7 Entry criteria included: (1) men or women 18 years of age of older; (2) American Heart Association stages B, C, and D HF due to ischemic, idiopathic, or hypertensive causes for at least 3 months; (3) reduced LVEF (≤ 45%), as determined by two-dimensional echocardiography; (4) on optimal medical therapy conforming to contemporary national or American Heart Association guidelines, as determined by the patient's cardiolo-gist; (5) presence of CSR-CSA during the baseline sleep study, defined as apnea-hypopnea index (AHI) ≥ 15 with ≥ 50% of events central in nature and a crescendo-decrescendo pattern of hyperpnea. The exclusion criteria were: (1) HF due to primary valvular heart disease, (2) presence of a left ventricular assistive device, (3) transplanted heart or expected to receive a transplanted heart within the next 6 months, (4) pregnancy, (5) current use of adaptive servoventilation, or continuous or bi-level positive airway pressure. The ADVENT-HF trial protocol has received ethics approval from all study sites and all subjects provided written informed consent prior to enrollment.
Polysomnography and Clinical Data
Overnight PSG was performed in all patients using standard techniques and scoring criteria for sleep stages.8,9 Thoracoabdominal motion was measured by respiratory inductance plethysmography (Inductotrace RIP; Ambulatory Monitoring, White Plains, New York, United States), the electronic sum of which was taken as an index of tidal volume (VT).10 Apneas and hypopneas were defined as a reduction in airflow from intra-nasal pressure of at least 90%, or between 50% and 90%, respectively, for at least 10 seconds. If there was evidence that the nasal pressure signal was artifactual (eg, if the signal was attenuated due to mouth breathing), then the sum of thoracoabdominal motion was used instead to detect apneas and hypopneas. Apneas were classified as obstructive if thoracoabdominal motion was present, and central if it was absent.8,11 Hypopneas were classified as obstructive if thoracoabdominal motion was out of phase or if airflow limitation was observed on the nasal pressure signal, whereas central hypopneas were those in which thoracoabdominal motion was in phase, and there was no evidence of airflow limitation on the nasal pressure signal. Mixed apneas were those that began as central for a minimum of 10 seconds and ended as obstructive, with a minimum of three obstructive efforts. The frequency of apneas and hypopneas per hour of sleep (AHI) was calculated. Arousals were defined according to the American Academy of Sleep Medicine criteria9 and the arousal index was calculated as the total number of arousals per hour of sleep. Arterial oxyhemoglobin saturation (SaO2) was measured continuously with a finger oximeter and the minimum SaO2 after an apnea (SaO2 nadir) was determined. The degree of oxygen desaturation was calculated as the difference between the highest SaO2 before the onset of events and the SaO2 nadir (ΔSaO2). Respiratory rate during hyperpnea was also calculated. To avoid the potential confounding influence of sleep stage on respiratory pattern, analysis of CSR-CSA was confined to stage N2 sleep. For consistency of the respiratory signal, we included only the sites participating in the ADVENT-HF trial that used the Inductotrace RIP, which allows an accurate analysis of the thoracoabdominal sum channel, because it is not influenced by nonmodifiable low/high frequency filters. Among the sites included in this study, the frequency changes in RIP were converted to a direct current signal that was directly analyzed for the determination of the hyperpnea pattern. Indeed, the signal we analyzed was free from inherent or applied high or low frequency filters.
We analyzed only episodes of CSR-CSA that occurred in the supine position to control for potential influence of body position on the respiratory pattern. LFCT was used as an index of circulation time and was measured from the end of the apnea to the subsequent nadir of SaO2 from the finger oximeter. CSR-CSA cycle time (CT) was measured as the time from the first breath following a central apnea to the first breath of the following apnea on the same CSR-CSA cycle used to determine LFCT. Hyperpnea time (HT) was calculated as the time between the beginning of inspiration during the ventilatory phase and the onset of the subsequent apnea. Apnea time (AT) was calculated as the difference between CT and HT.6 Mean CT, HT, AT, LFCT, ΔSaO2, and SaO2 nadir were calculated by averaging data from at least five consecutive apnea-hyperpnea cycles. NT-proBNP plasma concentration was assessed.
CSR-CSA Hyperpnea Pattern Analysis
The differences in the hyperpnea phase were evaluated comparing relative changes in EELV, as the magnitude of EELV changes was not quantifiable in absolute terms. To be sure that the signals were comparable, each trace was evaluated in the same conditions: in the absence of applied low/high filters and only through the use of Inductotrace RIP that provides a signal without any intrinsic filter. To take into account potential changes in EELV from one apnea to the next, so that baseline EELV could be determined, a computer algorithm was developed. First, manual scoring of PSG data was used to identify the end and start points of apneas from the sum channel of the RIP (Figure 2). The average of the end and start points was taken as the baseline (zero) for each CSR-CSA cycle. The amplitudes of all end-inspiratory and end-expiratory lung volumes during hyperpnea were measured in volts relative to baseline (Figure 2). End-expiratory amplitudes below the baseline were assigned negative values. For a given hyperpnea, the integral of all end-expiratory amplitudes (Aexp) was calculated as:
where ∑ denotes summation, Exp_ampl(n) is the relative amplitude of the nth breath and n is the number of breaths in the hyperpnea. Similarly, the integral of end-inspiratory amplitudes (Ains) was calculated as:
Finally, for each hyperpnea, the ratio of:
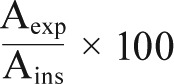 |
represents the percentage of average fluctuation in expira-tory amplitudes relative to the average changes in inspiratory amplitudes of each hyperpnea. This index can be compared among different CSR-CSA cycles during the night and among different patients.
Figure 2. Analysis of the positive and negative patterns of Cheyne-Stokes respiration.
The end of an apnea was taken as the first reference (point A) and the start of the following apnea as the second reference (point B). The baseline endexpiratory lung volume was calculated as the average between point A and point B. For every hyperpnea all end-inspiratory amplitudes (circles) were averaged and all end-expiratory amplitudes (crosses) were averaged referenced to baseline. The ratio between the averaged end-expiratory amplitudes and the averaged end-inspiratory amplitudes determined the pattern of each apnea-hyperpnea cycle: a ratio ≥ 0% was defined as positive pattern, a ratio < 0% was defined as a negative pattern.
Each hyperpnea was qualitatively classified as being positive if this ratio was ≥ 0% and negative if it was < 0%. A patient was defined as having a predominantly negative pattern if > 50% of the hyperpneas analyzed were negative and positive if ≥ 50% of the cycles were positive.
Statistical Analysis
Data were summarized with numbers and percentages, mean ± standard deviation, or median [interquartile range], as appropriate. Continuous variables were compared between positive and negative groups using a two-tailed unpaired t test or, for asymmetrically distributed data, the Mann-Whitney U test. Fisher exact test was used to compare categorical variables. Relationships among variables were assessed by logistic regression. A value of P < .05 was considered statistically significant. Statistical analyses were performed using SPSS 19.00 (IBM, Armonk, New York, United States) and GraphPad Prism 6.0 (McKiev Software, Boston, Massachusetts, United States).
RESULTS
Characteristics of the Subjects
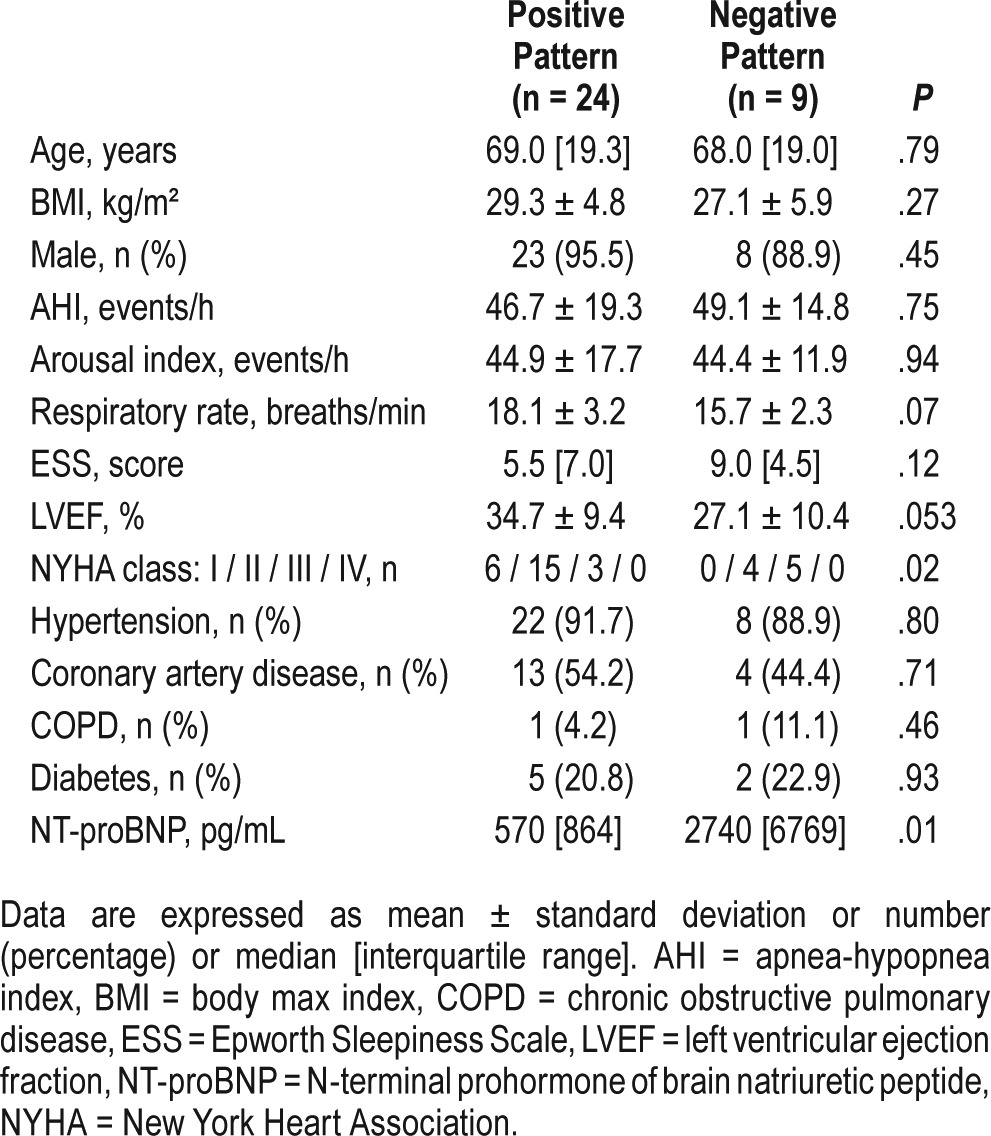
Table 1 shows the characteristics of the 33 patients meeting entry criteria, 24 of whom had a positive pattern and 9 of whom had a negative pattern. Most of the subjects in both groups were male and were comparable for age, body mass index, AHI, Epworth Sleepiness Scale, and the prevalences of comorbidi-ties. There was a nonsignificant trend for a lower LVEF in the negative than in the positive pattern group (P = .053). Among the negative pattern group, the New York Heart Association functional class was worse (P = .02), and NT-proBNP concentration was higher than in the positive pattern group (P = .01).
Table 1.
Baseline characteristics of the subjects.
Polysomnographic data
Patients in the negative pattern group had significantly longer HT and CT than those in the positive pattern group (P < .01 for both), but AT did not differ (Figure 3). Although the LFCT tended to be greater in the negative compared to the positive pattern group, the difference was not statistically significant (P = .12, Figure 3). Arousal index and respiratory rate did not differ between the 2 groups. ΔSaO2 and SaO2 nadir did not differ significantly between the negative and positive pattern groups (6.6 [6.1] versus 7.5 [4.4]%, P = .6 and 91.6 [6.0] versus 90.2 [4.2]%, P = .27, respectively).
Figure 3. Comparison of hyperpnea time, apnea time, cycle time, and lung to finger circulation time between the groups.
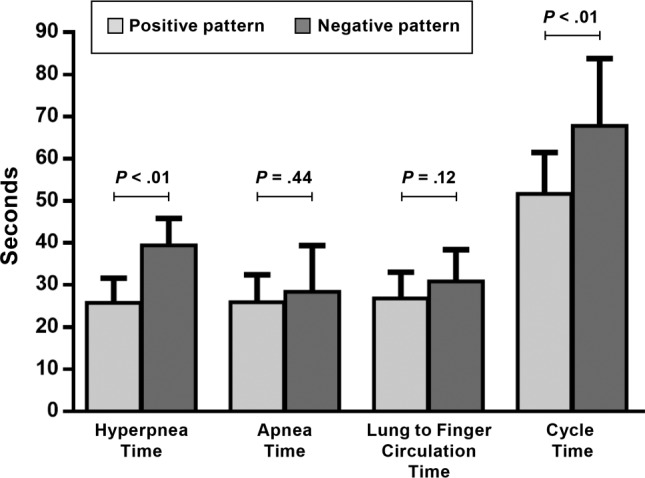
Hyperpnea and cycle times were significantly longer (39.5 ± 6.5 versus 25.8 ± 5.9 seconds and 67.8 ± 15.9 versus 51.7 ± 9.9 seconds, respectively), whereas apnea time (negative pattern 28.4 ± 11.0 versus 25.9 ± 6.6 seconds) and lung to finger circulation time (negative pattern 30.9 ± 7.5 versus 26.8 ± 6.2) did not differ during the negative compared to the positive pattern of hyperpnea.
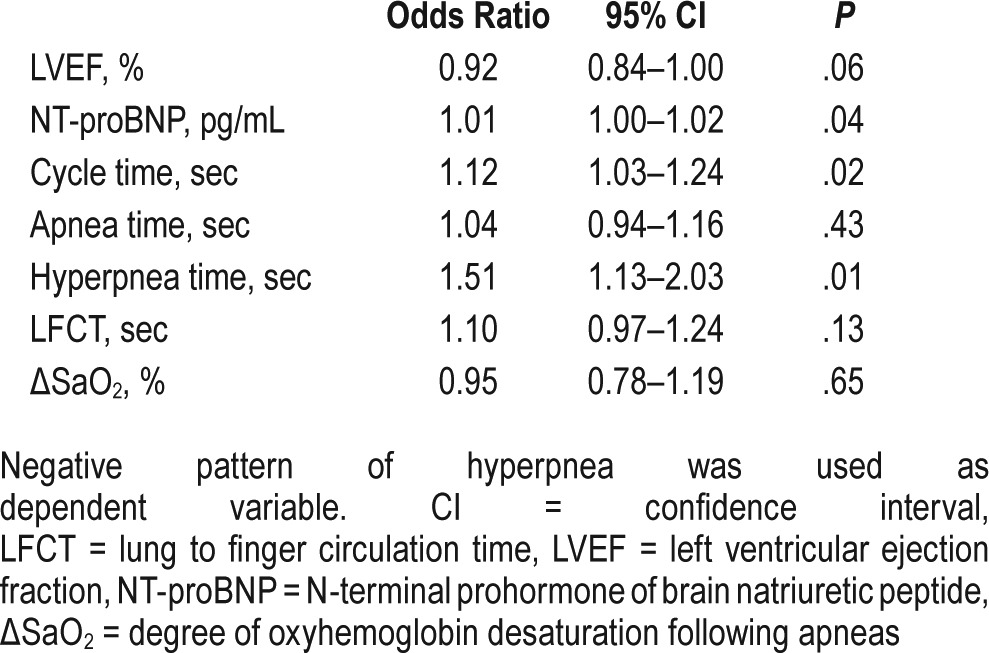
On univariate analysis, the variables associated significantly with the presence of a negative pattern were NT-proBNP concentration, CT, and HT (Table 2). LVEF had a nonsignificant tendency to be associated with a negative pattern (P = .06).
Table 2.
Univariate analyses of factors associated with the negative pattern.
DISCUSSION
The current study has given rise to 2 novel observations regarding CSR-CSA in patients with HF. First, we describe 2 different patterns of hyperpnea: a positive pattern during which EELV remains at or above FRC, and a negative pattern during which EELV falls below FRC. Second, we demonstrated that patients with a negative pattern of hyperpnea have longer hyperpnea and CSR-CSA cycle durations, indicating lower cardiac output,6 and higher NT-proBNP concentration, implying greater left ventricular wall tension12 than those with the positive pattern. These observations suggest that patients with CSR-CSA and a negative hyperpnea pattern have worse cardiac function than those with CSR-CSA and a positive hyper-pnea pattern.
Adoption of the negative pattern of hyperpnea would produce a greater degree of positive PIT during expiration than the positive pattern through force generated by the expiratory muscles. Such positive PIT could support cardiac function during expiration through two distinct mechanisms. First, by impeding venous return and reducing right ventricular preload, there may be a shift of the interventricular septum to the right, allowing more complete filling of the left ventricle through attenuation of adverse ventricular interactions.12–15 Second, positive PIT will reduce left ventricular afterload by reducing the difference between intracardiac left ventricular and extracardiac pressure (ie, PIT).16 Because the failing heart is very sensitive to changes in afterload, such an effect could also boost SV and CO by recruiting the respiratory system to assist left ventricular ejection in the face of deteriorating cardiac function.17,18 This mechanism might be comparable to that from chest compressions during cardio-pulmonary resuscitation for cardiac arrest,19,20 and to voluntary coughing during conscious cardiac arrest in the cardiac catheterization laboratory where generation of positive PIT augments SV and CO.21 By this means, in patients with HFrEF and very compromised cardiac function, the respiratory system may be recruited to assist cardiac pumping activity via active expiration and generation of positive PIT during the hyperpnea phase of CSR-CSA.
HF patients with CSR-CSA intermittently hyperventilate to the extent that PaCO2 falls cyclically below the apnea threshold giving rise to central apneas.22,23 Hyperventilation in patients with HF and CSR-CSA occurs in response to stimulation of pulmonary juxtacapillary receptors by pulmonary venous congestion24,25 and by increased central and peripheral chemo-responsiveness.26 In any case, hyperventilation indicates an increased load on and energy expenditure by the inspiratory muscles. However, the inspiratory muscles of patients with HF are weaker than normal, and thus are chronically operating at a greater proportion of their maximum output than normal and might be susceptible to fatigue.27 Indeed, it has been shown that resting weakened inspiratory muscles by continuous positive airway pressure in patients with HF and CSR-CSA increases their maximum pressure- generating capacity, providing evidence for a state of chronic inspiratory muscle fatigue.28
One way to unload inspiratory muscles under such conditions would be to recruit expiratory muscles to maintain ventilation. Reduction in EELV below FRC during the negative hyperpnea pattern means that the initial phase of inspiration is passive due to the outward elastic recoil of the chest wall. In addition, for a given inspiratory volume, a lower proportion will be above FRC so that peak pressure generation by the inspiratory muscles should also decline in the negative compared to the positive hyperpnea. Both these factors should unload the already weakened diaphragm and other inspiratory muscles. Thus, unloading of inspiratory muscles may be another factor responsible for the genesis of a negative pattern of hyperpnea during CSR-CSA. Although it is possible that recruiting expiratory muscles during CSR-CSA will increase the overall work of breathing, it is equally possible that by “spreading the work around” between inspiratory muscles and expiratory muscles that the potential to fatigue the inspiratory muscles might be ameliorated. The persistent activity of the inspiratory muscles throughout expiration can contribute to an increase in EELV. Due to the absence in our study of electromyography evaluations, it is not possible to analyze differences in postinspiratory activity of the inspiratory muscles between the positive and negative pattern. However, because EELV fell below FRC during the negative pattern it is very likely that there was less postinspiratory activation of the inspiratory muscles during the negative than the positive pattern.
To our knowledge, this is the first time that different patterns of hyperpnea during CSR-CSA have been described. Previous studies analyzing periodic breathing have shown figures with positive,29–31 negative,32,33 or both patterns of hyperpnea34 but there was no mention of these different patterns or their potential physiological significance. Moreover, there was no description of the precise means for monitoring breathing in these studies. As mentioned previously, it is essential to use the Inductotrace RIP for this purpose because it is not influenced by nonmodifiable low-/high-frequency filters that will mask the presence of the two distinct patterns. The bio-calibrated Inductotrace RIP was also appropriate because our analysis was confined to relative changes in lung volume from FRC without the measurements of absolute magnitudes of the volumes.
Brack et al. previously demonstrated a periodic increase in EELV during hyperpnea in 12 patients with HF and periodic breathing.35 However, in that study periodic breathing was examined during wakefulness both while breathing spontaneously and during hypoxic gas inhalation. For this reason, those results may not be comparable to ours. Nevertheless, most of our patients demonstrated a positive hyperpnea pattern that is not inconsistent with the findings of Brack et al.
There are some limitations of our study. The reason why expiratory muscles are activated sufficiently to reduce EELV in the negative hyperpnea group cannot be determined from our data. Because we did not measure absolute lung volume we cannot determine the absolute differences in inspiratory and expiratory lung volumes between the 2 groups. Moreover, the absence of an electromyographic measurement of the expiratory muscles did not allow us to quantify their activity. Similarly, because we did not measure PIT with an esophageal balloon, we were not able to determine the magnitude of the positive PIT generated at EELV or the difference in this variable between the 2 groups. The need to evaluate changes in EELV restricted the analysis to the few ADVENT-HF trial centers that used Inductotrace RIP and this explains the limited number of subjects reported. Finally, because we had no direct measure of SV or CO, especially during the hyperpnea phase, we could not directly assess potential differences in these variables between the 2 groups. Nevertheless, the longer cycle and hyperpnea times in the negative group are consistent with lower SV and CO than in the positive group.6
In conclusion, we have made the novel observation of two distinct patterns of hyperpnea during Cheyne-Stokes respiration: one in which EELV remains at or above FRC, and one in which it falls below FRC. When EELV falls below FRC, this pattern is associated with longer CSR-CSA cycle and hyper-pnea times, higher NT-proBNP, and worse New York Heart Association functional class, and therefore, probably lower SV and CO, and higher left ventricular wall tension than when EELV remains at or above FRC. Accordingly, there appears to be some heterogeneity of CSR-CSA whose manifestation may be at least partly related to underlying cardiac function in two respects. First, the negative pattern probably generates greater positive expiratory pressure that might cause an autoresuscitative effect to maintain SV and CO in those with more severely impaired cardiac function. Second, the negative pattern may unload weak inspiratory muscles, at least during the initial part of inspiration. These observations provide some support for a potential compensatory role of CSR-CSA, at least in relation to the negative pattern, in HFrEF.2 Further studies in which lung volumes, PIT, SV, and CO are assessed will be required to determine more precisely the respiratory and hemodynamic manifestations of these two variants of CSR-CSA.
DISCLOSURE STATEMENT
This was a substudy of the ADVENT-HF trial that was jointly funded by the Canadian Institutes of Health Research (CIHR) and an unrestricted grant from Philips Respironics according to the regulations of the CIHR's University Industry Program. M.A. was supported by a grant from ResMed. T.I. was supported by unrestricted research fellowships from Nippon Medical School, Tokyo, Japan, Phillips Respironics, Japan and Fukuda Foundation for Medical Technology. S.P was supported by research grants from NIH/NHLBI, Patient Centered Outcomes Research Institute, US Department of Defense and NIH (National Cancer Institute) NCI. J.S.F. was supported by a Canada Research Chair in Integrative Cardiovascular Biology. T.D.B. was supported by the Clifford Nordal Chair in Sleep Apnea and Rehabilitation Research and the Godfrey S. Pettit Chair in Respiratory Medicine. This research was performed at the University Health Network Toronto General Hospital, Toronto, Canada. All the authors have seen and approved the manuscript.
ACKNOWLEDGMENTS
See Appendix 1 and Appendix 2 in the supplemental material for trial personnel and complete list of author affiliations, trial sites, and investigators. All the investigators of the ADVENT-HF trial are listed in Appendix 2.
ABBREVIATIONS
- Aexp
end-expiratory amplitude
- Ains
end-inspiratory amplitude
- ADVENT-HF
Effect of Adaptive Servo Ventilation on Survival and Cardiovascular Hospital Admissions in Patients with Heart Failure and Sleep Apnea
- AHI
apnea-hypopnea index
- AT
apnea time
- BMI
body mass index
- CO
cardiac output
- COPD
chronic obstructive pulmonary disease
- CSR-CSA
Cheyne-Stokes respiration with central sleep apnea
- CT
cycle time
- EELV
end-expiratory lung volume
- ESS
Epworth Sleepiness Scale
- FRC
functional residual capacity
- HFrEF
heart failure with reduced ejection fraction
- HF
heart failure
- HT
hyperpnea time
- LFCT
lung-to-finger circulation time
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
- NT-proBNP
N-terminal prohormone of brain natriuretic peptide
- PaCO2
partial pressure of carbon dioxide
- PIT
intrathoracic pressure
- PSG
polysomnography
- RIP
respiratory inductance plethysmography
- SaO2
arterial oxyhemoglobin saturation
- SaO2 nadir
minimum arterial oxyhemoglobin saturation after an apnea
- SV
stroke volume
- VT
tidal volume
- ΔSaO2
difference between the highest arterial oxyhemoglobin saturation before the onset of apnea and the minimum arterial oxyhemoglobin saturation after an apnea
- ∑
summation
REFERENCES
- 1.Lanfranchi P, Braghiroli A, Bosimini E, et al. Prognostic value of nocturnal Cheyne-Stokes respiration in chronic heart failure. Circulation. 1999;99(11):1435–1440. doi: 10.1161/01.cir.99.11.1435. [DOI] [PubMed] [Google Scholar]
- 2.Naughton MT. Cheyne-Stokes respiration: friend or foe? Thorax. 2012;67(4):357–360. doi: 10.1136/thoraxjnl-2011-200927. [DOI] [PubMed] [Google Scholar]
- 3.Yumino D, Bradley TD. Central sleep apnea and Cheyne-Stokes respiration. Proc Am Thorac Soc. 2008;5(2):226–236. doi: 10.1513/pats.200708-129MG. [DOI] [PubMed] [Google Scholar]
- 4.Sands SA, Owens RL. Congestive heart failure and central sleep apnea. Crit Care Clin. 2015;31(3):473–495. doi: 10.1016/j.ccc.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyons OD, Bradley TD. Heart failure and sleep apnea. Can J Cardiol. 2015;31(7):898–908. doi: 10.1016/j.cjca.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 6.Hall MJ, Xie A, Rutherford R, Ando S, Floras JS, Bradley TD. Cycle length of periodic breathing in patients with and without heart failure. Am J Respir Crit Care Med. 1996;154(2 Pt 1):376–381. doi: 10.1164/ajrccm.154.2.8756809. [DOI] [PubMed] [Google Scholar]
- 7.Lyons OD, Floras JS, Logan AG, et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. Eur J Heart Fail. 2017;19(4):579–587. doi: 10.1002/ejhf.790. [DOI] [PubMed] [Google Scholar]
- 8.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 9.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 10.Chadha TS, Watson H, Birch S, et al. Validation of respiratory inductive plethysmography using different calibration procedures. Am Rev Respir Dis. 1982;125(6):644–649. doi: 10.1164/arrd.1982.125.6.644. [DOI] [PubMed] [Google Scholar]
- 11.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine task force. Sleep. 1999;22(5):667–689. [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Circulation. 2013;128(16):e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 13.Miro AM, Pinsky MR. Heart-lung Interactions. In: Tobin MJ, editor. Principles and Practice of Mechanical Ventilation. New York, NY: McGraw-Hill; 1994. [Google Scholar]
- 14.Weber KT, Janicki JS, Hunter WC, Shroff S, Pearlman ES, Fishman AP. The contractile behavior of the heart and its functional coupling to the circulation. Prog Cardiovasc Dis. 1982;24(5):375–400. doi: 10.1016/0033-0620(82)90020-2. [DOI] [PubMed] [Google Scholar]
- 15.Cassidy SS, Mitchell JH. Effects of positive pressure breathing on right and left ventricular preload and afterload. Fed Proc. 1981;40(8):2178–2181. [PubMed] [Google Scholar]
- 16.Naughton MT, Rahman MA, Hara K, Floras JS, Bradley TD. Effect of continuous positive airway pressure on intrathoracic and left ventricular transmural pressures in patients with congestive heart failure. Circulation. 1995;91(6):1725–1731. doi: 10.1161/01.cir.91.6.1725. [DOI] [PubMed] [Google Scholar]
- 17.Bradley TD, Holloway RM, McLaughlin PR, Ross BL, Walters J, Liu PP. Cardiac output response to continuous positive airway pressure in congestive heart failure. Am Rev Respir Dis. 1992;145(2 Pt 1):377–382. doi: 10.1164/ajrccm/145.2_Pt_1.377. [DOI] [PubMed] [Google Scholar]
- 18.De Hoyos A, Liu PP, Benard DC, Bradley TD. Haemodynamic effects of continuous positive airway pressure in humans with normal and impaired left ventricular function. Clin Sci (Lond) 1995;88(2):173–178. doi: 10.1042/cs0880173. [DOI] [PubMed] [Google Scholar]
- 19.Weisfeldt ML, Chandra N. Physiology of cardiopulmonary resuscitation. Annu Rev Med. 1981;32:435–442. doi: 10.1146/annurev.me.32.020181.002251. [DOI] [PubMed] [Google Scholar]
- 20.Werner JA, Greene HL, Janko CL, Cobb LA. Visualization of cardiac valve motion in man during external chest compression using two-dimensional echocardiography. Implications regarding the mechanism of blood flow. Circulation. 1981;63(6):1417–1421. doi: 10.1161/01.cir.63.6.1417. [DOI] [PubMed] [Google Scholar]
- 21.Criley JM, Blaufuss AH, Kissel GL. Cough-induced cardiac compression. Self-administered from of cardiopulmonary resuscitation. JAMA. 1976;236(11):1246–1250. [PubMed] [Google Scholar]
- 22.Naughton M, Benard D, Tam A, Rutherford R, Bradley TD. Role of hyperventilation in the pathogenesis of central sleep apneas in patients with congestive heart failure. Am Rev Respir Dis. 1993;148(2):330–338. doi: 10.1164/ajrccm/148.2.330. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzi-Filho G, Rankin F, Bies I, Bradley TD. Effects of inhaled carbon dioxide and oxygen on Cheyne-Stokes respiration in patients with heart failure. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1490–1498. doi: 10.1164/ajrccm.159.5.9810040. [DOI] [PubMed] [Google Scholar]
- 24.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99(12):1574–1579. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 25.Yu J, Zhang JF, Fletcher EC. Stimulation of breathing by activation of pulmonary peripheral afferents in rabbits. J Appl Physiol. 1998;85(4):1485–1492. doi: 10.1152/jappl.1998.85.4.1485. [DOI] [PubMed] [Google Scholar]
- 26.Solin P, Roebuck T, Johns DP, Walters EH, Naughton MT. Peripheral and central ventilatory responses in central sleep apnea with and without congestive heart failure. Am J Respir Crit Care Med. 2000;162(6):2194–2200. doi: 10.1164/ajrccm.162.6.2002024. [DOI] [PubMed] [Google Scholar]
- 27.Mancini DM, Henson D, LaManca J, Levine S. Respiratory muscle function and dyspnea in patients with chronic congestive heart failure. Circulation. 1992;86(3):909–918. doi: 10.1161/01.cir.86.3.909. [DOI] [PubMed] [Google Scholar]
- 28.Granton JT, Naughton MT, Benard DC, Liu PP, Goldstein RS, Bradley TD. CPAP improves inspiratory muscle strength in patients with heart failure and central sleep apnea. Am J Respir Crit Care Med. 1996;153(1):277–282. doi: 10.1164/ajrccm.153.1.8542129. [DOI] [PubMed] [Google Scholar]
- 29.La Rovere MT, Pinna GD, Maestri R, et al. Clinical relevance of short-term day-time breathing disorders in chronic heart failure patients. Eur J Heart Fail. 2007;9(9):949–954. doi: 10.1016/j.ejheart.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Leung RST, Bowman ME, Diep T, Lorenzi-Filho G, Floras JS, Bradley TD. Influence of Cheyne-Stokes respiration on ventricular response to atrial fibrillation in heart failure. J Appl Physiol. 2005;99(5):1689–1696. doi: 10.1152/japplphysiol.00027.2005. [DOI] [PubMed] [Google Scholar]
- 31.Tkacova R, Niroumand M, Lorenzi-Filho G, Bradley TD. Overnight shift from obstructive to central apneas in patients with heart failure: role of PCO2 and circulatory delay. Circulation. 2001;103(2):238–243. doi: 10.1161/01.cir.103.2.238. [DOI] [PubMed] [Google Scholar]
- 32.Jobin V, Rigau J, Beauregard J, et al. Evaluation of upper airway patency during Cheyne-Stokes breathing in heart failure patients. Eur Respir J. 2012;40(6):1523–1530. doi: 10.1183/09031936.00060311. [DOI] [PubMed] [Google Scholar]
- 33.Oldenburg O. Cheyne-stokes respiration in chronic heart failure. Treatment with adaptive servoventilation therapy. Circ J. 2012;76(10):2305–2317. doi: 10.1253/circj.cj-12-0689. [DOI] [PubMed] [Google Scholar]
- 34.Leung RS, Floras JS, Lorenzi-Filho G, Rankin F, Picton P, Bradley TD. Influence of Cheyne-Stokes respiration on cardiovascular oscillations in heart failure. Am J Respir Crit Care Med. 2003;167(11):1534–1539. doi: 10.1164/rccm.200208-793OC. [DOI] [PubMed] [Google Scholar]
- 35.Brack T, Jubran A, Laghi F, Tobin MJ. Fluctuations in end-expiratory lung volume during Cheyne-Stokes respiration. Am J Respir Crit Care Med. 2005;171(12):1408–1413. doi: 10.1164/rccm.200503-409OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.