Abstract
Study Objectives:
Sleep variability is a clinically significant variable in understanding and treating insomnia in older adults. The current study examined changes in sleep variability in the course of brief behavioral therapy for insomnia (BBT-I) in older adults who had chronic insomnia. Additionally, the current study examined the mediating mechanisms underlying reductions of sleep variability and the moderating effects of baseline sleep variability on treatment responsiveness.
Methods:
Sixty-two elderly participants were randomly assigned to either BBT-I or self-monitoring and attention control (SMAC). Sleep was assessed by sleep diaries and actigraphy from baseline to posttreatment and at 3-month follow-up. Mixed models were used to examine changes in sleep variability (within-person standard deviations of weekly sleep parameters) and the hypothesized mediation and moderation effects.
Results:
Variabilities in sleep diary-assessed sleep onset latency (SOL) and actigraphy-assessed total sleep time (TST) significantly decreased in BBT-I compared to SMAC (Pseudo R2 = .12, .27; P = .018, .008). These effects were mediated by reductions in bedtime and wake time variability and time in bed. Significant time × group × baseline sleep variability interactions on sleep outcomes indicated that participants who had higher baseline sleep variability were more responsive to BBT-I; their actigraphy-assessed TST, SOL, and sleep efficiency improved to a greater degree (Pseudo R2 = .15 to .66; P < .001 to .044).
Conclusions:
BBT-I is effective in reducing sleep variability in older adults who have chronic insomnia. Increased consistency in bedtime and wake time and decreased time in bed mediate reductions of sleep variability. Baseline sleep variability may serve as a marker of high treatment responsiveness to BBT-I.
Clinical Trial Registration:
ClinicalTrials.gov, Identifier: NCT02967185
Citation:
Chan WS, Williams J, Dautovich ND, McNamara JP, Stripling A, Dzierzewski JM, Berry RB, McCoy KJ, McCrae CS. Night-to-night sleep variability in older adults with chronic insomnia: mediators and moderators in a randomized controlled trial of brief behavioral therapy (BBT-I). J Clin Sleep Med. 2017;13(11):1243–1254.
Keywords: CBT-I, insomnia, mechanisms of change, older adults, sleep variability, treatment efficacy moderator
INTRODUCTION
Chronic insomnia is the most prevalent sleep disorder and affects 15% to 35% of older adults.1–3 Recent research suggests that night-to-night sleep variability is an important clinical characteristic and therapeutic target in treating insomnia in older adults.4,5 Higher objective sleep variability is associated with poorer subjective sleep quality,4,6,7 poorer subjective well-being,7 and greater risks of cardiometabolic diseases5,6 in older adults with chronic insomnia. Although objective sleep is often found to be similar in those with and without insomnia, significantly greater sleep variability was observed in older adults with insomnia than in older adults without insomnia.5,7 Although evidence supports the clinical significance of sleep variability in understanding and treating insomnia, little is known with regard to changes in sleep variability in the treatment of insomnia for older adults, the mechanisms by which changes in sleep variability occur, and the clinical significance of sleep variability in moderating intraindividual changes in sleep outcomes in the course of treatment.
BRIEF SUMMARY
Current Knowledge/Study Rationale: High night-to-night sleep variability is prevalent in older adults who have chronic insomnia, is associated with poorer health outcomes, and may maintain insomnia. It is unclear if and how sleep variability decreases over the course of a brief behavioral therapy for insomnia (BBT-I) and whether pretreatment sleep variability moderates treatment efficacy.
Study Impact: The current findings indicate that BBT-I is efficacious in reducing sleep variability through increasing consistency of bedtime and wake time and reduced time in bed. Baseline sleep variability moderated the efficacy of BBT-I on primary sleep outcomes; individuals who had higher baseline sleep variabilities responded more positively to BBT-I. Sleep variability might be a useful measure for clinicians to identify patients who will or will not benefit from behavioral sleep interventions.
Reduction of sleep variability in the treatment of insomnia has been observed in young and middle-aged adults following cognitive behavioral therapy for insomnia (CBT-I). However, prior studies did not examine whether the reduction of sleep variability was greater in individuals who received CBT-I compared to those who received usual care or sleep education only. Baron et al. examined changes in sleep variability in a randomized controlled trial of a 16-week sleep hygiene plus exercise intervention compared to a sleep hygiene-only control condition in a sample of older adults who had chronic insomnia.4 Although significant reductions in variability in wake after sleep onset (WASO) and sleep efficiency (SE) were found following the sleep hygiene plus exercise intervention, they were not significantly greater than those observed in the sleep hygiene-only control group. It remains unclear whether any treatment of insomnia, especially CBT-I, the recommended treatment of insomnia,8 would be more efficacious than control in reducing sleep variability in older adults who have chronic insomnia.
Behavioral treatment of insomnia has been shown to have comparable effectiveness to CBT-I in older adults.9 In particular, brief behavioral therapy for insomnia (BBT-I), an especially cost-effective intervention that can be easily disseminated in primary care settings, has been shown to be efficacious in reducing sleep onset latency (SOL) and WASO, improving SE and sleep quality, and increasing total sleep time (TST) in older adults who had chronic insomnia.10 However, the impact of BBT-I on sleep variability—an important clinical outcome—has not been examined.
CBT-I is theorized to improve sleep by promoting cognitive and behavioral changes that restore circadian synchrony, balance homeostatic sleep needs, establish positive associations and break negative ones between the sleep setting and sleep, and reduce cognitive and physiological arousal.11 High sleep variability in individuals with insomnia is thought to be the consequence of the interaction between sleep homeostasis and poor stimulus control.12 The greater tendency to engage in “sleep recovery” behavior following poor sleep often leads to elevated sleep-incompatible activities and irregular sleep schedules. Therefore, behavioral techniques such as setting a consistent sleep schedule, reducing time in bed (TIB), and reducing nap duration might be especially relevant in the reduction of sleep variability. The mediating role of these processes in reducing sleep variability has not been previously tested.
In addition to being the therapeutic target in the treatment of insomnia, sleep variability might moderate treatment outcomes. Sanchez-Ortuno and Edinger found that higher baseline sleep variability was associated with worse sleep outcomes following CBT-I,13 but Suh et al. found the opposite.14 These inconsistent results might be due to methodological limitations. Sanchez-Ortuno and Edinger examined the correlations between baseline variability and sleep outcomes at posttreatment. These correlations could not inform how baseline sleep variability affects the change in sleep outcomes. Suh et al. compared the average sleep improvements in patients with chronic insomnia who had high sleep variability and those who had low sleep variability. This analytical approach did not examine intraindividual changes. The use of multilevel modeling is needed to elucidate the effect of baseline sleep variability on the intraindividual changes in sleep outcomes in the course of treatment.
The Current Study
The current study analyzed data from a randomized controlled trial of BBT-I in community-dwelling older adults in whom chronic insomnia was diagnosed. The aims of this study were to examine whether (1) BBT-I was more efficacious in reducing variability in SOL, WASO, TST, and SE than a self-monitoring and attention control (SMAC); (2) changes in the theorized behavioral mediators11—bedtime variability, wake time variability, nap duration, and TIB—mediated changes in sleep variability; and, (3) baseline sleep variability moderated intraindividual changes in sleep outcome (SOL, WASO, TST, and SE). It was hypothesized that (1) there would be significant reductions of sleep variability in BBT-I recipients compared to the SMAC participants; (2) changes in bedtime variability, wake time variability, nap duration, and TIB would partially mediate the reduction of sleep variability; and (3) sleep variability at baseline would moderate intraindividual changes of sleep outcomes.
METHODS
Participants and Procedures
This study investigates changes in sleep variability in older adults with insomnia over the course of a 4-week BBT-I. BBT-I included 4 main components: sleep hygiene, stimulus control, sleep restriction, and relaxation. The University of Florida's Institutional Review Board (UF IRB-02) approved the protocol, and all participants signed informed consent forms prior to participating. Inclusion criteria were: (1) individual reports insomnia (sleep onset or awake time during night > 30 minutes) for more than 6 months15; (2) sleep diary confirms insomnia (sleep onset or awake time during night > 30 minutes) at least 6 nights during 2-week baseline period; (3) daytime dysfunction due to insomnia (mood, cognitive, social, or occupational impairment); and (4) no prescribed or over-the-counter sleep medication for at least 1 month, or stabilized on medication for at least 6 months. Exclusion criteria were: (1) significant medical or neurological disorder; (2) major psychopathology; (3) other sleep disorders (eg, sleep apnea, periodic limb movements) assessed through single-night ambulatory monitoring and structured interview; (4) severe depressive symptomatology based on a score of 24 or higher on the Beck Depression Inventory-II16 or a score of 13 or higher on the Geriatric Depression Scale17; (5) cognitive impairment based on Mini-Mental State Examination (MMSE) score lower than 23 (ninth-grade education or higher) or lower than 18 (less than ninth-grade education); and (6) suspected sleep-disordered breathing based on a single-night ambulatory monitoring (Compass F10; Embla, Broomfield, Colorado, United States) of blood oxygen saturation and respiration indicating an apnea-hypopnea index greater than 15.1 and minimum oxygen desaturation less than 93%. A board-certified sleep medicine physician (RBB) reviewed all ambulatory monitoring records.
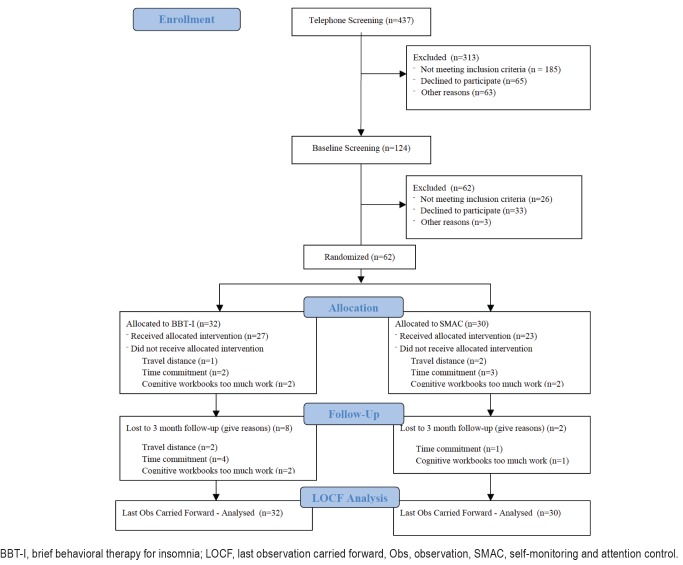
A total of 62 older adults with insomnia (age: mean = 69.45, standard deviation = 7.71; sex: 68% female; ethnicity: 82.26% non-Hispanic White, 6.45% Hispanic White, 3.23% Black, 3.23% Asian, and 4.84% multiracial) were recruited from Gainesville and surrounding areas through newspaper and other community advertisements for treatment of late-life insomnia. Participants were randomly assigned to either the BBTI or SMAC group. Participants in the SMAC group completed the same set of assessments as BBT-I participants, including completing daily diaries and wearing an actigraph throughout the study. Hence, any effects of self-monitoring of sleep were held equal in both groups. To control for the weekly attention received from the therapist, SMAC participants also met with the therapist once a week for an hour. During that hour, SMAC participants and the therapist engaged in social conversations unrelated to sleep or any aspect of BBT-I. The CONSORT diagram is presented in Figure 1. Details of screening and randomization procedures and procedures taken to ensure treatment fidelity are included in the supplemental material. This clinical trial is registered on ClinicalTrials.gov (NCT02967185). Table 1 presents the demographic and clinical characteristics of the entire sample and those of the BBT-I and SMAC groups. The BBT-I and SMAC groups did not differ significantly on any of the demographic and clinical characteristics except the duration of insomnia. The SMAC group had significantly longer duration of insomnia. The primary outcomes of this trial (the mean levels of sleep diary-assessed SOL, WASO, and SE) significantly improved in BBT-I compared to SMAC.18 In the BBT-I group, SOL improved from 51 minutes to 29 minutes at 3-month follow-up; WASO from 67 minutes to 42 minutes; and SE from 70% to 81%. TST improved marginally from 350 minutes to 372 minutes (P = .08). The current study examined improvements in sleep variability across treatment, controlling for the improvements in the primary mean-level outcomes.
Figure 1. CONSORT flow diagram.
BBT-I, brief behavioral therapy for insomnia; LOCF, last observation carried forward, Obs, observation, SMAC, self-monitoring and attention control.
Table 1.
Demographics and clinical characteristics of participants.
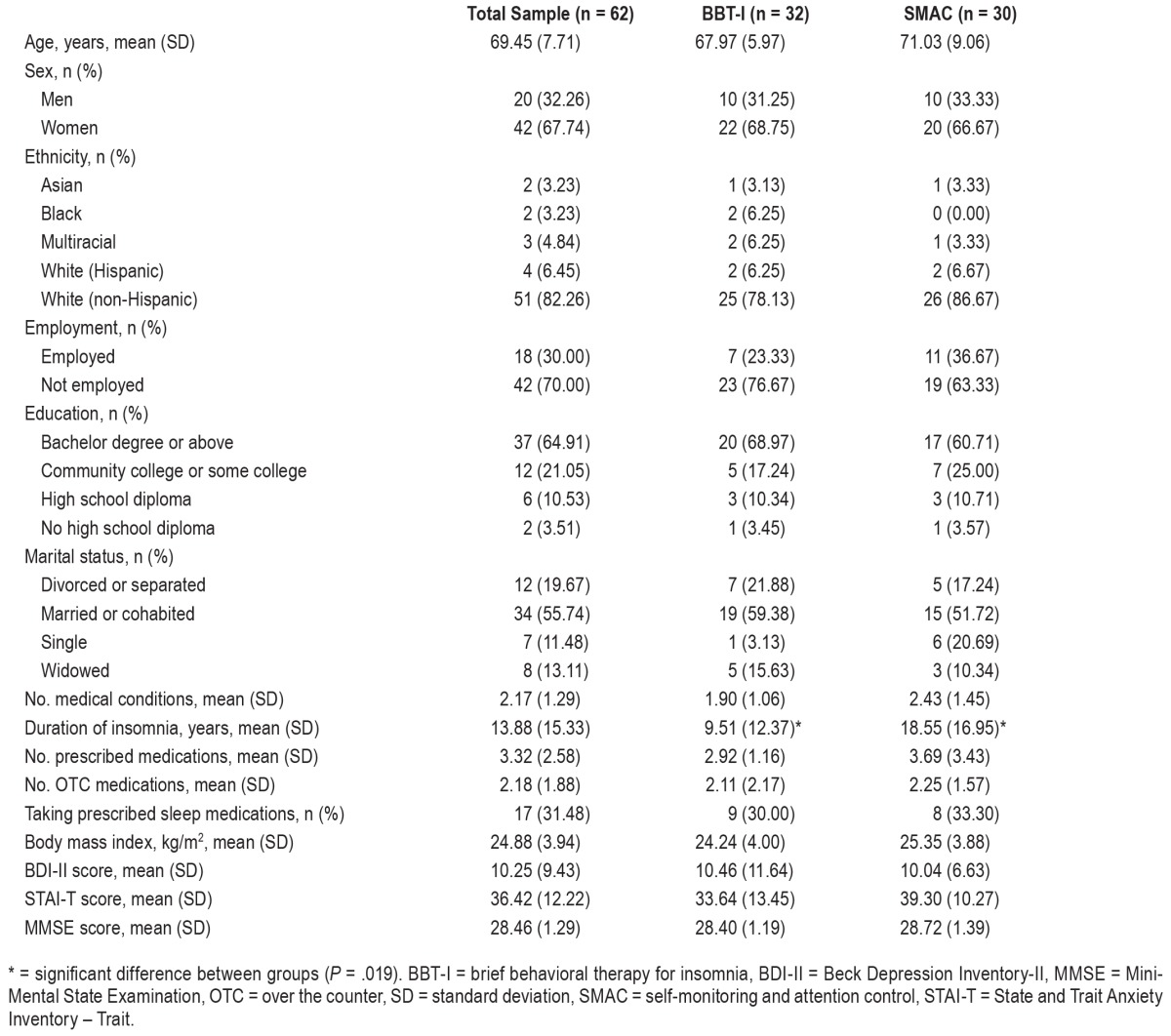
Most of the sample and clinical characteristics were not significantly different between the groups with higher baseline sleep variability and the groups with lower baseline sleep variability, except that the groups with higher baseline variability in diary-assessed SOL, TST, or SE were more likely taking medications for sleep than the groups with lower baseline variability (48% to 53% versus 21% to 23%, P = .02 to .03). Additionally, the group with higher baseline diary-assessed SOL variability were less likely to have received a bachelor's or graduate degree (43% versus 68%, P = .03). Controlling for the duration of insomnia, education, and taking sleep medications in the subsequent analyses did not alter the patterns or significance of results.
Measures
Sleep Diaries
Participants were instructed to complete a sleep diary upon arising each morning throughout the study (10 weeks total = 2 weeks at baseline, 4 weeks of treatment, 2 weeks at posttreatment, and 2 weeks at 3-month follow up). The sleep diaries provided the following variables: (1) SOL—time from initial lights-out until sleep onset, (2) WASO—time spent awake after initial sleep onset until last awakening; and, (3) TST—time between sleep onset and the last awakening, and, (4) SE— computed by dividing TST by total TIB and multiplying it by 100. The sleep outcome variables were the weekly averages of SOL, WASO, TST, and SE throughout the 10-week assessment period. The sleep variability variables were the within-person standard deviations of 1-week measures of SOL, WASO, TST, and SE (varSOL, varWASO, varTST, and varSE). The sleep variables derived from sleep diaries are denoted by an “s” at the end of each variable (ie, SOLs, WASOs, TSTs, and SEs). The sleep variability variables were varSOLs, varWASOs, var-TSTs, and varSEs.
Actigraphy-Assessed Sleep
Participants wore an Actiwatch-L (ACTL) (Mini Mitter Co., Inc., Bend, Oregon, United States) on their nondominant wrists continuously throughout the study (10 weeks); the device contains an omnidirectional piezoelectric accelerometer with sensitivity of 0.01 λg force or greater. The sensor of the ACTL is sampled 32 times per second and records peak values for each second. These peak values are then summed into 30-second “activity” counts. These activity counts are downloaded to a personal computer and analyzed using Actiware-Sleep v. 3.3 (Philips Respironics, Murrysville, Pennsylvania), which uses a validated algorithm to identify periods of sleep or wake. Actiware-Sleep determined sleep start automatically by searching for the first 10 minutes during which no more than one epoch was scored as wake. Likewise, sleep end was the last 10 minutes during which no more than one epoch was scored as wake. Bedtime and time out of bed in the morning were based on sleep diary entries as recommended in the software manual. They were used to compute the total TIB for subsequent calculations of SOL, WASO, TST, and SE. If there was a discrepancy greater than 30 minutes between diary and actigraphy values of bedtime and time out of bed, a 3-step procedure was used to determine the bedtime and time out of bed. (1) If diary times fall within 15 minutes of a sharp decrease/increase of activity (50% change of activity), the diary times were used. (2) If diary times did not fall within 15 minutes of a sharp change of activity, but actigraphy times did, the actigraphy times were used. (3) If both diary and actigraphy times did not fall within 15 minutes of a sharp change of activity, the times at which there was a sharp change of activity closest to the diary times were used. The same set of variables provided by the sleep dairies were provided by the actigraphy. The sleep variables that were derived from actigraphy were denoted by an “a” at the end of each variable (ie, SOLa, WASOa, TSTa, and SEa). The sleep variability variables were varSOLa, varWASOa, varTSTa, and varSEa.
Behavioral Mediators
The behavioral mediator variables included bedtime variability (varBT) and wake time variability (varWT)—the standard deviations of the weekly measures of bedtime and wake time based on sleep diaries, the weekly averages of nap duration, and the 2-week average of TIB.
Data Analysis
The intent-to-treat approach was used in all analyses. Weekly averages of sleep outcome variables were computed when there were 4 days or more data for that week. Weekly sleep variability variables were computed only when there were 7 days of data for that week, given that the estimate of variability (within-person standard deviation) would be strongly affected by number of days included in the computation. The last observation carried forward technique was used for the missing weekly variables due to dropout. Missing data in assessments not due to dropout were handled using mixed modeling—all available data were analyzed.19
Multilevel modeling was used to model intraindividual changes in sleep variability measured by sleep diaries (var-SOLs, varWASOs, varTSTs, and varSEs) and actigraphy (var-SOLa, varWASOa, varTSTa, and varSEa) from baseline to posttreatment and follow-up using the lmer package20 in RStudio version 0.99.486 (RStudio, Boston, Massachusetts). As recommended by Enders and Tofighi,21 time-varying variables (sleep variability variables, sleep outcome variables, behavioral mediators) were centered by their group means to capture within-individual variability. Between-individual variables (baseline sleep variability variables) were centered by their grand means to capture between-individual variability.
Hypothesis 1: Sleep Variability Would Decrease Across Time in BBT-I Versus SMAC
An initial model with a random intercept, a random linear slope of change, and the fixed effects of both linear and quadratic slopes to model changes across time was examined for each sleep variability variable. The quadratic slope remained in the following interaction models only if it was significant. To examine whether there were significantly greater changes in sleep variability for BBT-I than SMAC, each sleep variability variable was regressed on the fixed effects of the intercept, time (linear slope of change), group (BBT-I versus SMAC), and the time × group interaction. Pseudo R2 was computed to indicate the proportion of random effect of the linear slope of change (ie, between-individual variability in the intra-individual change of the outcome) explained by the time × group interaction.
Hypothesis 2: Changes in the Behavioral Mediators Would Mediate the Reduction of Sleep Variability in BBT-I Versus SMAC
For variables with significant reductions in variability, mediation analyses were conducted to test whether those reductions were mediated by varBT, varWT, TIB, and nap duration. In other words, the indirect effect of BBT-I on sleep variability through each behavioral mediator was estimated. The indirect effect was the product of path a (the effect of BBT-I on the mediator) and path b (the effect of the mediator on the sleep variability variable). The distribution-of-the-product method implemented in the RMediation package was used to estimate the 95% confidence interval of the indirect effect, so as to evaluate the significance of the indirect effect.22 This method has been shown to be less biased than the traditional Sobel significance test, even in small samples with a sample size of 50.22,23
In order to evaluate the effect of BBT-I on the intra-individual changes of sleep variability, path a was estimated by regressing the intraindividual change of the mediator on BBTI and path b was estimated by regressing the intraindividual change of the sleep variability variable on the intraindividual change of the mediator. The intraindividual change of the mediator was the individual linear slope of change of the mediator extracted from the random effect in the mixed model, in which the mediator was regressed on the fixed and random effects intercept and time. The intraindividual change of the sleep variability variable was extracted the same way. The group variable (BBT-I versus SMAC) was a between-individual variable, coded as 1 (BBT-I) and 0 (SMAC).
Hypothesis 3: Baseline Sleep Variability Would Moderate Intraindividual Changes in Primary Sleep Outcomes
To examine whether baseline sleep variability moderated changes in sleep outcomes (SOL, WASO, TST, and SE), each outcome variable was regressed on the fixed effects of time, group, the respective baseline sleep variability, and the time × group, time × baseline sleep variability, group × baseline sleep variability, and the time × group × baseline sleep variability interactions.
RESULTS
Descriptives
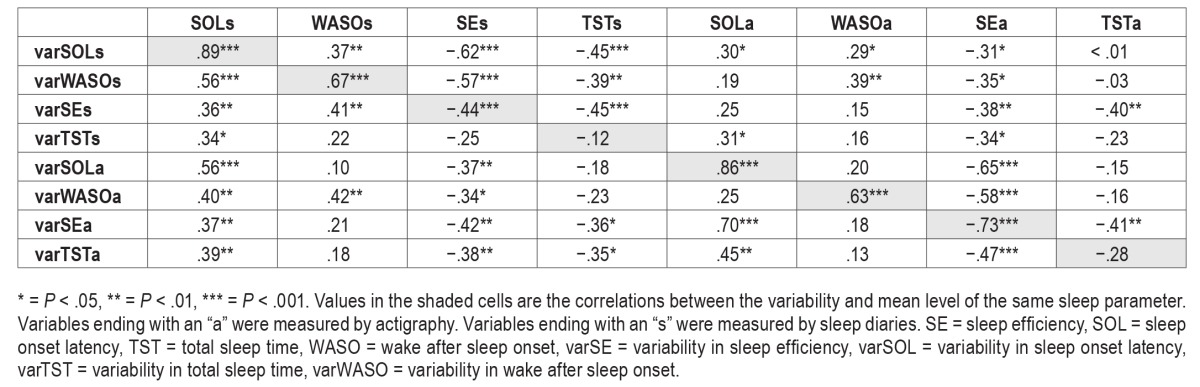
The means and standard deviations of the sleep variability variables at baseline, posttreatment, and follow-up are presented in Table 2. Table 3 presents the correlations between sleep variability and sleep outcomes at baseline. As expected, higher sleep variability was associated with poorer mean levels of sleep outcome except for TST.
Table 2.
Sleep variability variables by time and group.
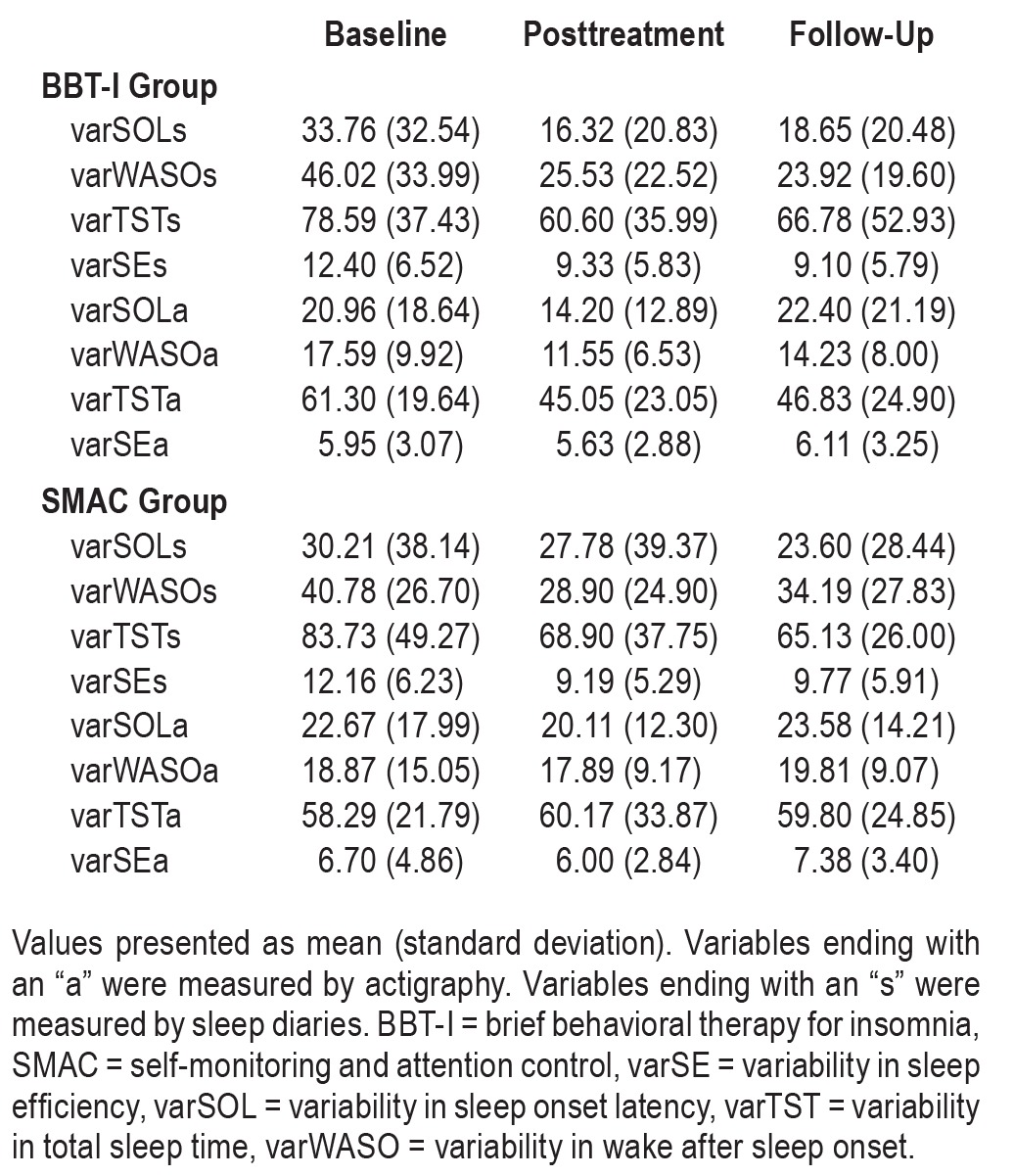
Table 3.
Correlations between sleep variability and sleep outcomes at baseline.
Changes in Sleep Variability in BBT-I Compared to SMAC
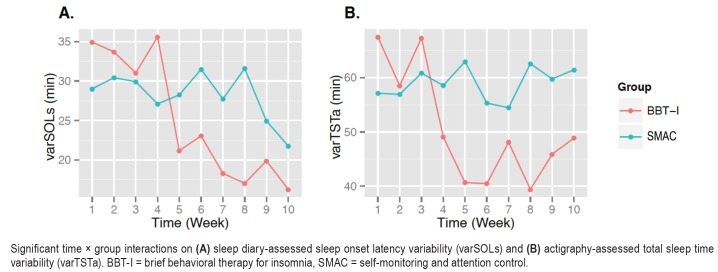
As shown in Table 4, the time × group interaction effect was significant on varSOLs and varTSTa, indicating that there were significant reductions of variability in SOLs and TSTa following BBT-I compared to SMAC (see Figure 2). The effect sizes of the interactions ranged from medium to large. Seventeen percent of between-individual variability in the changes of SOLs across time and 29% of between-individual variability in the changes of TSTa across time were explained by group. var-SOLs decreased by about 48% in BBT-I recipients versus about 22% in SMAC. varTSTa decreased by about 24% in BBT-I but increased by 3% in SMAC. Because sleep variability, especially variability in SOLs, was strongly correlated with the mean level of sleep outcomes, we examined whether the time × group interactions on varSOLs and varTSTa remained significant when the time-varying mean levels of SOLs and TSTa were included in the models. The time × group interaction on varSOLs became nonsignificant (b = −.86, P = .307). However, the time × group interaction on varTSTa remained significant even when the time-varying mean level of TSTa were included (b = −2.37, P = .009).
Table 4.
Fixed effects (P values) of time, group, and time × group interactions on sleep variability variables and behavioral mediators.
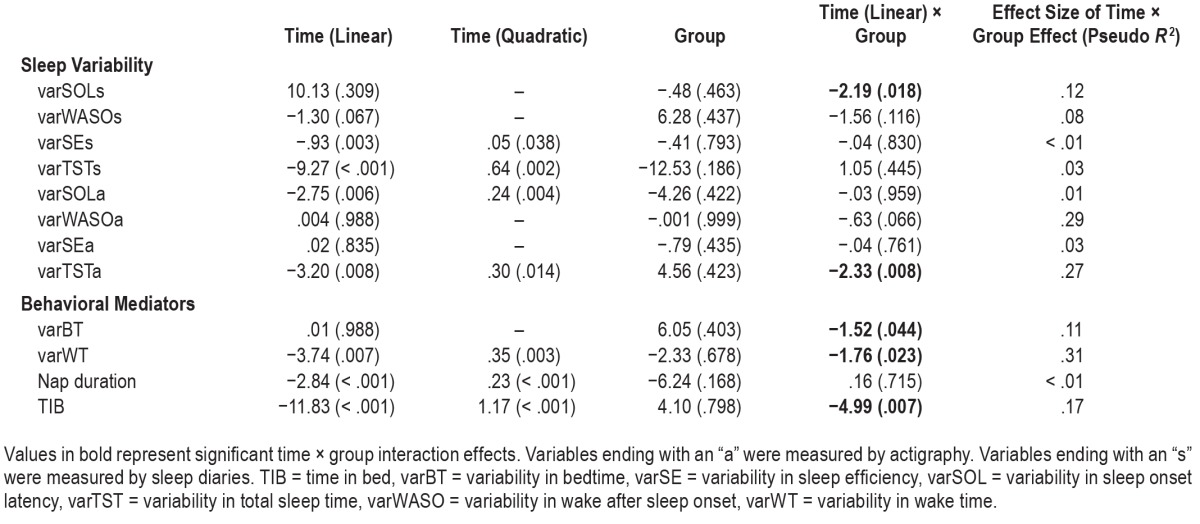
Figure 2. Significant time × group interactions.
Significant time × group interactions on (A) sleep diary-assessed sleep onset latency variability (varSOLs) and (B) actigraphy-assessed total sleep time variability (varTSTa). BBT-I = brief behavioral therapy for insomnia, SMAC = self-monitoring and attention control.
Behavioral Mediators on Changes in Sleep Variability
As expected, varBT, varWT, and TIB decreased significantly in BBT-I compared to SMAC (see Table 4 and Figure 3). The effect sizes ranged from medium to large. Eleven percent, 31%, and 17% of the between-individual variability in the changes of varBT, varWT, and TIB across time were explained by group. The main effect of time was significant on nap duration; however, the time × group interaction was not significant. The result indicated that BBT-I, compared to SMAC, did not have a significant effect in reducing nap duration. One of the prerequisites for significant mediation of treatment effects on sleep variability is that there are significant treatment effects on the mediator. Nap duration did not meet this prerequisite and thus was excluded from the subsequent mediation analysis.
Figure 3. Significant time × group interactions.
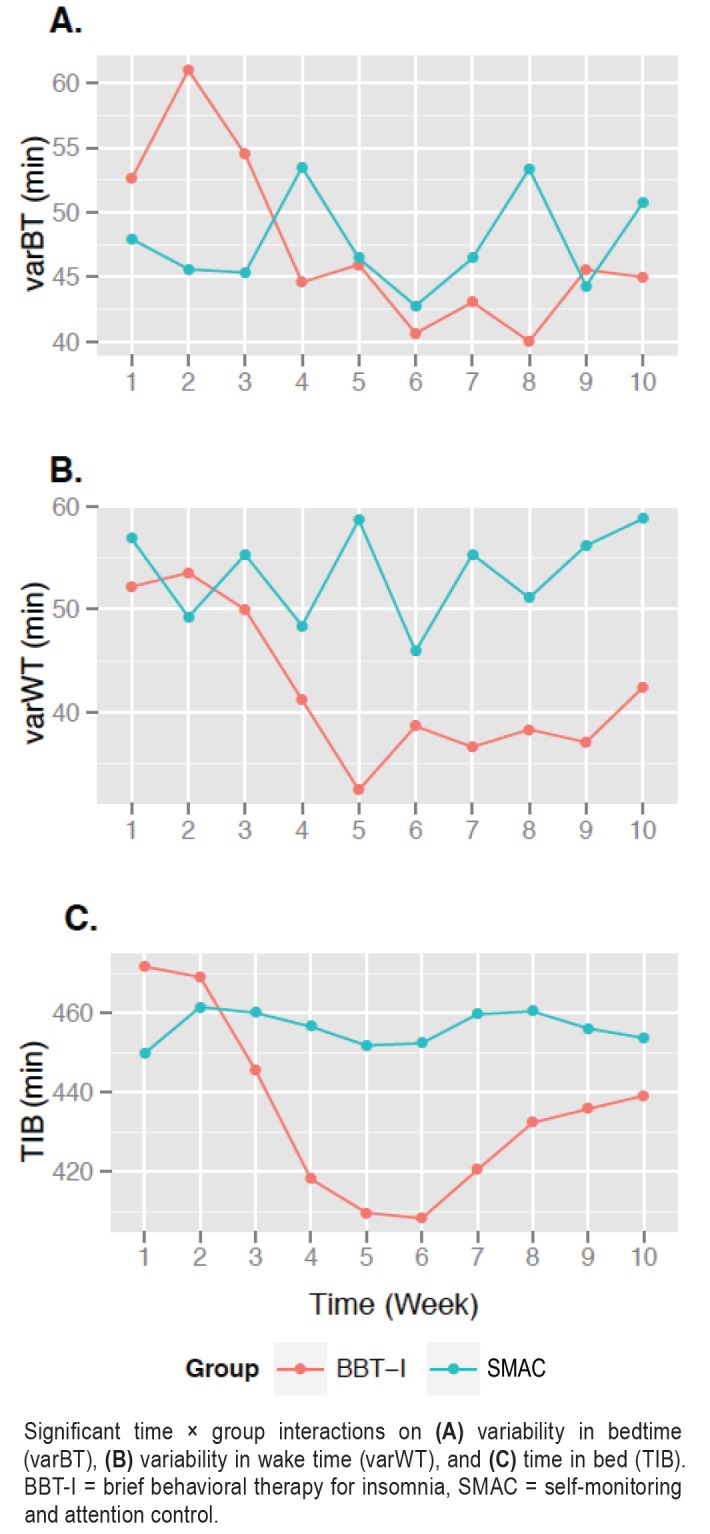
Significant time × group interactions on (A) variability in bedtime (varBT), (B) variability in wake time (varWT), and (C) time in bed (TIB). BBT-I = brief behavioral therapy for insomnia, SMAC = self-monitoring and attention control.
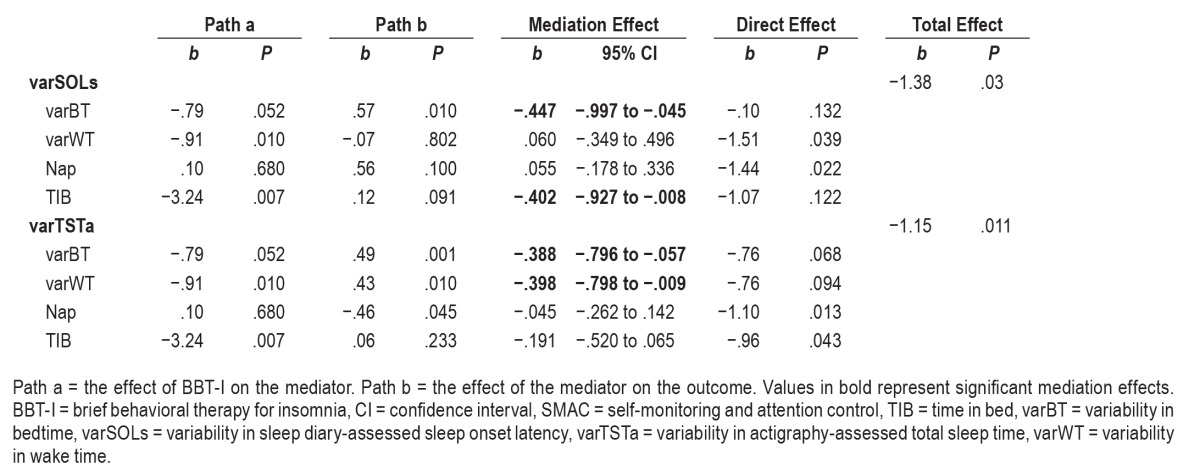
As shown in Table 5, the intraindividual changes of varBT and TIB significantly mediated the effects of BBT-I on the intraindividual changes of varSOLs. The indirect effect of BBT-I on varSOLs through the reduction of varBT was significant and explained almost all of the total effect of BBT-I on varSOLs (the regression coefficient of the total effect decreased from −1.38 to −.10 when the indirect effect was taken into account). The indirect effect of BBT-I on varSOLs through the reduction of TIB was significant also and explained some of the total effect of BBT-I on varSOLs (the regression coefficient of the total effect decreased from −1.38 to −1.07 when the indirect effect was taken into account).
Table 5.
Mediated effects of the changes in varSOLs and varTSTa in BBT-I versus SMAC.
Likewise, the intraindividual changes of varBT and varWT significantly mediated the effects of BBT-I on the intraindividual changes of varTSTa (see Table 5). The indirect effect of BBT-I on varTSTa through the reduction of varBT was significant and explained some of the total effect of BBT-I on varTSTa (the regression coefficient of the total effect decreased from −1.15 to −.76 when the indirect effect was taken into account). The indirect effect of BBT-I on varTSTa through the reduction of varWT was significant and explained some of the total effect of BBT-I on varTSTa (the regression coefficient of the total effect decreased from −1.15 to −.76 when the indirect effect was taken into account).
Moderations of Baseline Sleep Variability on Changes in Sleep Outcomes in BBT-I Compared to SMAC
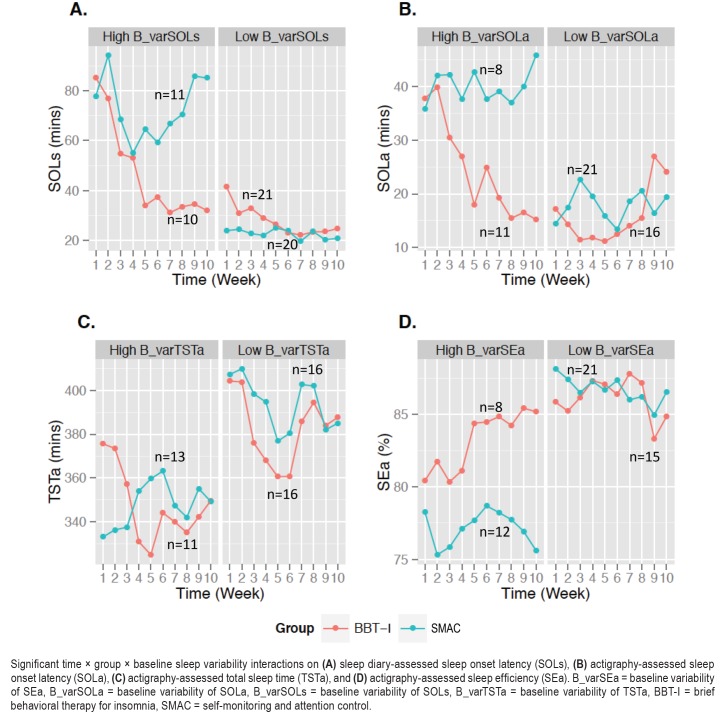
As shown in Table 6, the time × group × baseline variability 3-way interaction effects were significant on SOLs, SOLa, SEa, and TSTa. As shown in Figure 4, these interactions indicated that the improvements in SOLs, SOLa, SEa, and TSTa in BBT-I compared to SMAC were greater in individuals who had higher baseline sleep variability than those who had lower baseline sleep variability. The effect sizes of the 3-way interactions ranged from medium to very large. These interaction effects remained significant even when baseline levels of SOLs, SOLa, SEa, and TSTa were included in the models, indicating that the interaction effects were not explained by worse baseline sleep in individuals who had higher baseline sleep variability.
Table 6.
Moderation effects of baseline sleep variability on time × group interaction effects on sleep outcomes.
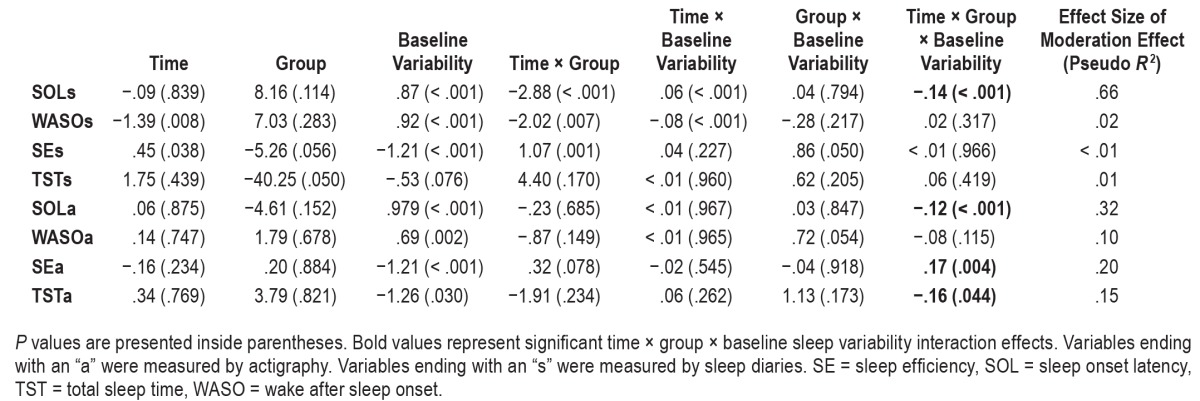
Figure 4. Significant time × group × baseline sleep variability interactions.
Significant time × group × baseline sleep variability interactions on (A) sleep diary-assessed sleep onset latency (SOLs), (B) actigraphy-assessed sleep onset latency (SOLa), (C) actigraphy-assessed total sleep time (TSTa), and (D) actigraphy-assessed sleep efficiency (SEa). B_varSEa = baseline variability of SEa, B_varSOLa = baseline variability of SOLa, B_varSOLs = baseline variability of SOLs, B_varTSTa = baseline variability of TSTa, BBT-I = brief behavioral therapy for insomnia, SMAC = self-monitoring and attention control.
As shown in Figure 4A, SOLs decreased by about 65% from 85 minutes to about 30 minutes following BBT-I in older adults who had higher baseline SOLs variability. SOLs decreased by about 40% following BBT-I in older adults who had lower baseline SOLs variability. A slope difference test indicated that the greatest decrease of SOLs was observed in BBT-I recipients who had higher baseline variability in SOLs, significantly greater than the decreases of SOLs in BBT-I recipients who had lower baseline variability in SOLs and SMAC participants (t values for slope differences: −4.63 to −6.35, P < .001). This pattern of results was corroborated by actigraphy data (see Figure 4B). The greatest decrease in SOLa was observed in BBT-I recipients who had high baseline variability in SOLa (SOLs decreased by 60% from about 37 minutes to 15 minutes), significantly greater than the decreases of SOLa in BBT-I recipients who had low baseline variability in SOLa and SMAC participants (t values for slope differences: −3.19 to 6.05, P = .001 to .003).
A similar pattern of interaction was observed with SEa. The greatest improvements in SEa were observed in BBT-I recipients who had high baseline variability in SEa, significantly greater than the improvements observed in BBT-I recipients who had low baseline SEa variability and SMAC participants (see Figure 4D; t values for slope differences; 3.83 to 5.94, P < .001). Unexpectedly, there was a decrease in TSTa in BBT-I recipients who had high baseline TSTa variability, significantly different from the slope of change of TSTa in SMAC participants who had high baseline TSTa variability (t value for slope difference: −2.26, P = .028).
DISCUSSION
The current study examined, in a randomized controlled trial of BBT-I for older adults, whether (1) there were significant reductions of variability in SOL, WASO, TST, and SE in BBTI compared to SMAC; (2) changes in bedtime and wake time variability, nap duration, and TIB mediated the reductions of sleep variability; and (3) baseline variability in SOL, WASO, SE and TST moderated intraindividual changes in SOL, WASO, TST, and SE in BBT-I compared to SMAC.
Reduction of Sleep Variability in BBT-I
Consistent with our hypothesis, BBT-I was efficacious in reducing variability in some sleep parameters, SOLs, and TSTa, with medium to large effect sizes. These findings are consistent with prior studies in young and middle-aged adults.13,14 Extending from previous research, this study showed that a 4-session BBT-I was efficacious in reducing sleep variability in older adults with chronic insomnia. The decreases in percentage in SOLs variability and TSTa variability were comparable to those in a prior study implementing a 4-session CBT-I in middle-aged adults.13 The current study included a SMAC group that provided a more stringent test of the effects of the treatment of insomnia in reducing sleep variability than prior studies, in which a control group was not included.4,13,14 A prior randomized controlled trial of a 12-month exercise intervention showed that SOLs variability significantly decreased in the intervention group, compared to the health education control group, by about 20% (∼15 minutes) from baseline to posttreatment.24 It is fairly impressive that 4 sessions of BBT-I over 4 weeks was adequate in reducing SOLs by 65% (∼55 minutes) in the current study, and that the treatment gains were maintained at 3-month follow-up. However, it should be noted that the time × group effect on varSOLs became nonsignificant when the changes of the mean level of SOLs were taken into account. This finding might indicate that the reduction of SOLs variability might be concomitant of the reduction of mean SOLs.
The significant reduction of varTSTa in BBT-I compared to SMAC was novel. Significant reduction of objective TST variability was not found in prior trials of CBT-I in middle-aged adults13 nor in exercise interventions for older adults with chronic insomnia4,24 Additionally, the time × group interaction on varTSTa remained significant even when the mean levels of TSTa were taken into account. Taken together, the present study provided compelling evidence that BBT-I is an efficacious and efficient treatment for reducing variability in some sleep parameters in older adults with chronic insomnia.
Bedtime Variability, Wake Time Variability, and TIB Mediated Reduction in Sleep Variability
Reductions of bedtime variability, wake time variability, and TIB mediated the time × group interaction effect on sleep variability. This was the first study to empirically examine the putative mechanisms by which behavioral techniques bring about reduction of sleep variability. The findings are consistent with the theoretical underpinning of CBT-I11,12 Specifically, the mediation of bedtime variability on the effect of BBT-I on varSOLs can be considered a full mediation.25 Establishing a more consistent bedtime helps stabilize circadian rhythm. A consistent bedtime might also lead to increased homeostatic sleep drive by preventing one from going to bed too early, and subsequently restore the “automaticity” of sleep12 (ie, falling asleep more easily without having to try). Reducing TIB helps decrease sleep-incompatible activities in bed and strengthen sleep cues, which could also explain the increased consistency of SOLs.
Likewise, the significant mediations of the effect of BBT-I on the reduction of varTSTa by increased bedtime consistency and wake time consistency are consistent with the theoretical underpinning of behavioral therapy for insomnia. Although nap duration decreased across time in the sample, BBT-I did not have a significant effect on the decrease of nap duration compared to SMAC. A lack of treatment effects on nap duration precluded the possibility of mediation effects of nap duration on treatment outcomes.
Sleep Variability Moderates Effectiveness of BBT-I on Sleep Improvements
The moderation effects of baseline sleep variability on the effects of BBT-I on the improvements of SOLs, SOLa, SEa, and TSTa were novel. This was the first study to examine the moderation effects of sleep variability on the intraindividual changes of sleep outcomes in treatment. Older adults who had high baseline sleep variability appeared to benefit from BBT-I to a significantly greater extent than those who had low baseline sleep variability. This pattern of relationships was observed in both subjective and objective SOL and objective SE and TST. These findings are congruent with prior studies in young and middle-aged adults in which higher baseline sleep variability was found to be associated with better subjective sleep quality at posttreatment.14 The current study extended beyond examining the snapshots of changes at pretreatment and posttreatment and provided evidence for the moderating role of sleep variability on the trajectories of changes in sleep outcomes in BBT-I for older adults.
The moderation effects of sleep variability on the changes of sleep outcomes were not explained by worse baseline sleep parameters. It does not appear that the higher responsiveness to BBT-I in older adults who had high baseline sleep variability was due to poorer sleep at baseline. However, it should be noted that older adults who had low baseline variability did have much better sleep, especially objective sleep (see Figure 4). Their average SOLa was below 30 minutes and SEa was above 85%. There might be a ceiling/flooring effect in detecting significant improvements in objective sleep in this subgroup of participants. They were included in the study because they did show poorer sleep measured by sleep diaries, the recommended diagnostic tool for chronic insomnia.26
The literature on motivation suggests that if a person is more aware of the discrepancy of their current state and the ideal state, he/she is more motivated to make changes to achieve the ideal state, especially when the strategies for change are perceived as within one's control.27 Older adults who have more variable sleep might be more receptive to making changes with respect to setting regular sleep schedules and stimulus control. Older adults who have insomnia but have much less variable sleep patterns might not see the link between bedtime and wake time consistency and their insomnia. It is possible that perceived treatment ineffectiveness might negatively influence treatment outcomes by hampering patients' motivation not only in adhering to the behavioral components but also in overall engagement in treatment, quality of the therapeutic alliance, and/or treatment-related self-efficacy. Additional research is needed before any definitive conclusions can be made. Additionally, the group with lower SOLs variability was more educated. It is possible that this group might benefit more from the cognitive component of CBT-I, which was not included in the behavioral-only treatment of insomnia in this study.
Alternatively, the co-occurrence of insomnia and high sleep variability might indicate elevated dysregulation of autonomic functioning.28 There is increasing evidence suggesting that circadian disorganization is associated with dysregulation of the autonomic system.29 It is possible that older adults with insomnia who also have high sleep variability might have elevated baseline dysregulation of autonomic arousal and more room for improvements to occur in the treatment of insomnia. Increased consistency of circadian timing and relaxation strategies in BBT-I might improve autonomic functioning and subsequently sleep outcomes, especially in older adults who have high sleep variability.
Limitations and Strengths
This study has several limitations. First, sleep diaries were collected on paper. Although they were instructed to complete their diaries daily, participants could have completed the diaries not as instructed, such as filling out several days of diaries at once, and resulted in biases and inaccuracies. Second, the majority of the sample are White. Findings might have limited generalizability to non-White populations. Third, this trial is an efficacy trial conducted in an academic setting. Future studies are needed to examine whether findings generalize to non-academic community settings. Lastly, polysomnography data of sleep were not collected. Although diary is the gold standard for diagnosing and evaluating insomnia, polysomnography measures of sleep might aid the interpretation of sleep improvements, especially improvement in SE. Given that TIB was not standardized in the calculation of sleep diary- and actigraphy-assessed SE, changes of SE across treatment might be attributable to simply a reduction of TIB. Nonetheless, there were significant increases in TST in the treatment group, indicating that improvement in SE was not due to a reduction of TST. Despite the aforementioned issues, the current study is a well-controlled clinical trial that allowed for more stringent tests of the effectiveness of BBT-I in reducing sleep variability than currently available in the literature. In particular, it has several methodological strengths: (1) the use of both sleep diaries and actigraphy in assessing sleep; (2) assessments of sleep for a total of 70 days from pretreatment to follow-up; (3) the use of a self-monitoring and attention control group that allowed for the evaluation of the efficacy of BBT-I on sleep variability beyond the effects of self-monitoring and therapist's attention, and (4) the use of mixed modeling and mediation models to examine intra-individual changes and putative mechanisms of change in treatment.
CONCLUSIONS
The current study found that a 4-session BBT-I is efficacious in reducing some parameters of sleep variability in older adults. The reduction of sleep variability was mediated by the increase of bedtime and wake time consistency and the decrease of TIB. Older adults who had higher baseline sleep variability benefited more from this BBT-I than those who had lower baseline sleep variability. Given the ease of assessing sleep variability, sleep variability could be a useful and cost-effective measure clinicians use to identify older adults who will benefit more from behavioral sleep interventions, or those who might not benefit much from behavioral sleep interventions. Future research is needed to explore the mechanisms by which sleep variability affects treatment efficacy.
DISCLOSURE STATEMENT
All authors have read and approved this manuscript. The authors report no conflicts of interest. The project described was supported by Award Number AG024459 (Christina S. McCrae, PhD, PI) from the National Institute on Aging (NIA). Additional support was provided by an Institutional Training Grant Award Number AG020499 (Michael Marsiske, PhD, Director) from the NIA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA.
ACKNOWLEDGMENTS
The authors thank Dr. Michael Marsiske for his statistical consultation.
ABBREVIATIONS
- BBT-I
brief behavioral therapy for insomnia
- CBT-I
cognitive behavioral therapy for insomnia
- LOCF
last observation carried forward
- MMSE
Mini Mental State Examination
- SE
sleep efficiency
- SEa
sleep efficiency assessed by actigraphy
- SEs
sleep efficiency assessed by sleep diaries
- SMAC
self-monitoring and attention control
- SOL
sleep onset latency
- SOLa
sleep onset latency assessed by actigraphy
- SOLs
sleep onset latency assessed by sleep diaries
- TIB
time in bed
- TST
total sleep time
- TSTa
total sleep time assessed by actigraphy
- TSTs
total sleep time assessed by sleep diaries
- varBT
variability of bedtime
- varSE
variability of sleep efficiency
- varSEa
variability of sleep efficiency assessed by actigraphy
- varSEs
variability of sleep efficiency assessed by sleep diaries
- varSOL
variability of sleep onset latency
- varSOLa
variability of sleep onset latency assessed by actigraphy
- varSOLs
variability of sleep onset latency assessed by sleep diaries
- varTST
variability of total sleep time
- varTSTa
variability of total sleep time assessed by actigraphy
- varTSTs
variability of total sleep time assessed by sleep diaries
- varWASO
variability of wake after sleep onset
- varWASOa
variability of wake after sleep onset assessed by actigraphy
- varWASOs
variability of wake after sleep onset assessed by sleep diaries
- varWT
variability of wake time
- WASO
wake after sleep onset
- WASOa
wake after sleep onset assessed by actigraphy
- WASOs
wake after sleep onset assessed by sleep diaries
REFERENCES
- 1.Mallon L, Broman JE, Hetta J. Relationship between insomnia, depression, and mortality: a 12-year follow-up of older adults in the community. Int Psychogeriatr. 2000;12(3):295–306. doi: 10.1017/s1041610200006414. [DOI] [PubMed] [Google Scholar]
- 2.Stewart R, Besset A, Bebbington P, et al. Insomnia comorbidity and impact and hypnotic use by age group in a national survey population aged 16 to 74 years. Sleep. 2006;29(11):1391–1397. doi: 10.1093/sleep/29.11.1391. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 4.Baron KG, Reid KJ, Malkani RG, Kang J, Zee PC. Sleep variability among older adults with insomnia: associations with sleep quality and cardiometabolic disease risk. Behav Sleep Med. 2017;15(2):144–157. doi: 10.1080/15402002.2015.1120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buysse DJ, Cheng Y, Germain A, et al. Night-to-night sleep variability in older adults with and without chronic insomnia. Sleep Med. 2010;11(1):56–64. doi: 10.1016/j.sleep.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okun ML, Reynolds CF, 3rd, Buysse DJ, et al. Sleep variability, health-related practices, and inflammatory markers in a community dwelling sample of older adults. Psychosom Med. 2011;73(2):142–150. doi: 10.1097/PSY.0b013e3182020d08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemola S, Ledermann T, Friedman EM. Variability of sleep duration is related to subjective sleep quality and subjective well-being: an actigraphy study. Plos One. 2013;8(8):e71292. doi: 10.1371/journal.pone.0071292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):126–133. doi: 10.7326/M15-2175. [DOI] [PubMed] [Google Scholar]
- 9.Irwin MR, Cole JC, Nicassio PM. Comparative meta-analysis of behavioral interventions for insomnia and their efficacy in middle-aged adults and in older adults 55+years of age. Health Psychol. 2006;25(1):3–14. doi: 10.1037/0278-6133.25.1.3. [DOI] [PubMed] [Google Scholar]
- 10.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwartz DR, Carney CE. Mediators of cognitive-behavioral therapy for insomnia: a review of randomized controlled trials and secondary analysis studies. Clin Psychol Rev. 2012;32(7):664–675. doi: 10.1016/j.cpr.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 12.Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–243. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Ortuno MM, Edinger JD. Internight sleep variability: its clinical significance and responsiveness to treatment in primary and comorbid insomnia. J Sleep Res. 2012;21(5):527–534. doi: 10.1111/j.1365-2869.2012.01010.x. [DOI] [PubMed] [Google Scholar]
- 14.Suh S, Nowakowski S, Bernert RA, et al. Clinical significance of night-to-night sleep variability in insomnia. Sleep Med. 2012;13(5):469–475. doi: 10.1016/j.sleep.2011.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Academy of Sleep Medicine. International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 16.Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories-IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 17.Yesavage JA. Geriatric Depression Scale: consistency of depressive symptoms over time. Percept Motor Skill. 1991;73(3 Pt 1):1032. doi: 10.2466/pms.1991.73.3.1032. [DOI] [PubMed] [Google Scholar]
- 18.McCrae CS, Williams J, Dautovich ND, et al. Impact of brief behavioral treatment for insomnia (BBT-I) on sleep and cognition in older adults with insomnia: the rest randomized controlled trial. Sleep. 2017;40(suppl 1):A124–A125. [Google Scholar]
- 19.Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am J Psychiatry. 2009;166(6):639–641. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- 20.Bates D, Mächler M, Bolker BM, Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 21.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12(2):121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- 22.Tofighi D, MacKinnon DP. RMediation: an R package for mediation analysis confidence intervals. Behav Res Methods. 2011;43(3):692–700. doi: 10.3758/s13428-011-0076-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacKinnon DP, Fritz MS. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behav Res Methods. 2007;39(3):384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buman MP, Hekler EB, Bliwise DL, King AC. Exercise effects on night-to-night fluctuations in self-rated sleep among older adults with sleep complaints. J Sleep Res. 2011;20(1):28–37. doi: 10.1111/j.1365-2869.2010.00866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 27.Bandura A. Self-regulation of motivation and action through goal systems. In: Hamilton V, Bower GH, Frijda NH, editors. Cognitive Perspectives on Emotions and Motivation. Netherlands: Springer; 1988. pp. 37–61. [Google Scholar]
- 28.Vgontzas AN, Fernandez-Mendoza J, Liao DP, Bixler E. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durgan DJ, Young ME. The cardiomyocyte circadian clock emerging roles in health and disease. Circ Res. 2010;106(4):647–658. doi: 10.1161/CIRCRESAHA.109.209957. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.