Abstract
Study Objectives:
This study aims to report the effect of positive airway pressure (PAP) therapy on quality of life (QoL) measures in the clinical sleep-disordered breathing (SDB) population.
Methods:
We examined general QoL measures assessed by European Quality of Life-5D (EQ-5D) and sleep-specific QoL by examining Functional Outcomes of Sleep Questionnaire (FOSQ) scores before and after PAP therapy retrospectively in a clinical SDB population using paired and two-sample t tests. Age and socioeconomic status (SES) effect modification on pre-PAP QoL measures were investigated utilizing the interaction terms.
Results:
A total of 2,027 patients with SDB initiated PAP therapy between January 1, 2010 and December 31, 2014. The mean age of the cohort was 56.2 years (standard deviation = 13.2), with 45.8% female and 76.9% Caucasians. EQ-5D change after exclusion of those with normal QoL was 0.042 (0.152) in all patients, 0.051 (0.150) in patients who were PAP adherent by self-report, and 0.050 (0.132) in patients who were objectively PAP adherent (n = 704 of 1,011 with available objective adherence data, 69.6%). Change in FOSQ after excluding those with normal FOSQ was 1.9 (2.9) in all patients, 2.2 (2.9) in patients who were PAP adherent by self-report, and 2.3 (2.9) in patients who were objectively PAP adherent. Those with (1) worse QoL at baseline and younger age and (2) worse QoL at baseline and residing in lower SES strata had worse outcomes after PAP therapy (P < .05).
Conclusions:
We found consistent improvement in global and sleep-specific QoL measures after PAP therapy, hence providing evidence of PAP benefit in the clinical population and rationale for targeted efforts to optimize QoL in younger and lower SES subgroups.
Citation:
Walia HK, Thompson NR, Katzan I, Foldvary-Schaefer N, Moul DE, Mehra R. Impact of sleep-disordered breathing treatment on quality of life measures in a large clinic-based cohort. J Clin Sleep Med. 2017;13(11):1255–1263.
Keywords: age, quality of life, sleep-disordered breathing, socioeconomic status
INTRODUCTION
Sleep-disordered breathing (SDB) is common, with estimates of 15% to 30% in the general population and characterized by intermittent episodes of breathing cessation associated with hypoxia, autonomic nervous system fluctuations, microarousals, and sleep disruption.1 These adverse physiologic consequences and disruption of sleep in SDB can lead to experienced decrements in quality of life (QoL).2 Globally, there has been a shift to emphasize the importance of QoL and patient-reported outcomes measures, in addition to the standard traditional objective clinical and biologic measures of disease. Increasing focus has also been dedicated to consideration of longitudinal follow-up of these measures to track treatment responsiveness in clinical care.3 The recognition of the importance of collection of QoL measures hinges on increasing appreciation of the complex interrelationships of the personal and social context relative to disease. In fact, QoL has been the focus of several initiatives via the Patient Centered Outcomes Research Initiative reflecting the prioritization of these measures by patients and has been incorporated as a new area of focus in Healthy People 2020.
Accordingly, the American Academy of Sleep Medicine (AASM) has recognized that the longitudinal care model for SDB encompasses two main domains; one to reduce cardiovascular-related risk factors and the other to improve sleep-related symptoms and QoL.4 The rationale behind this is that the prevalence of impaired QoL associated with SDB has been reported to be as high as 80%5 and that this compromise in QoL is often the primary reason for a patient to seek attention from a medical provider. Furthermore, QoL impairment is one of the determining factors for instituting standard therapies.6
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep-disordered breathing is associated with impaired quality of life. There are limited clinical data demonstrating changes in quality of life measures with use of positive airway pressure therapy in those with sleep-disordered breathing.
Study Impact: This study showed improvement in generic quality of life and sleep-specific quality-of- life measures after the use of positive airway pressure therapy in a sleep-disordered breathing clinical population. Older patients and those in a higher socioeconomic subgroup had better quality of life measures after positive airway pressure therapy. This information will guide clinicians in terms of expected quality of life outcomes with sleep-disordered breathing treatment.
Although the European Quality of Life-5D (EQ-5D)7 and Functional Outcomes of Sleep Questionnaire (FOSQ)8 are both validated instruments for the measurement of QoL, the EQ-5D is considered a more global QoL instrument and the FOSQ is a more sleep-specific measure of QoL, especially in those with SDB. In a meta-analysis involving approximately 1,200 subjects, continuous positive airway pressure (CPAP) improved the physical component of QoL domains as well as vitality, utilizing specific QoL instruments despite lack of improvement in overall QoL scores; however, authors report that global QoL instruments may not accurately reflect QoL in patients with SDB.9 One randomized controlled trial (RCT) demonstrated significant improvement in FOSQ measures in patients with mild to moderate levels of SDB and excessive daytime sleepiness.10
Clearly there are some data demonstrating improvements in certain subscales of the global EQ-5D measures; nonetheless, the effect of SDB treatment on the overall EQ-5D score appears to be equivocal. QoL measures have not been systematically assessed in populations not selected for sleepiness symptoms in the clinical setting. The AASM identified improvements in QoL to be one of the best markers of effectiveness of SDB treatment in alignment with national priorities3; lack of clinical data for QoL measurement in clinical SDB was identified as an opportunity for improvement and an existing knowledge gap. Therefore, we leveraged a large clinical dataset irrespective of and unselected for baseline sleep apnea severity, degree of sleepiness, or comorbid conditions set to examine whether treatment of SDB translates into improvement in QoL measures. We hypothesized that there are significant and clinically meaningful improvements in validated QoL measures (utilizing generic EQ-5D and the sleep-specific FOSQ) before and after SDB treatment with positive airway pressure (PAP). Because QoL is likely modified by individual, social, and economic factors, we further elucidated the effect modification of age and socioeconomic status on the change of QoL measure in response to PAP treatment.11
METHODS
Study Sample
Electronic medical record (EMR) data (Epic Systems Corporation, Verona, Wisconsin, United States) were extracted for patients presenting for outpatient clinic visits in the Cleveland Clinic Sleep Disorders Center between January 1, 2010 and December 31, 2014. Inclusion criteria included those age 18 years or older, diagnosis of SDB determined if patient reported “yes” to PAP usage during the follow-up visit, and at least one post-PAP visit within a 1-year follow-up period. Patients were required to have a baseline visit (reporting no PAP usage) prior to the visit of self-reported PAP use. For the post-PAP visit, the visit closest to 6 months after the baseline visit was assessed. The post-PAP visit was required be within 30 days and 1 year of the baseline visit. Baseline demographic and clinical comorbidities data from the EMR regarding the patient's age, sex, race, median income by ZIP code, and history of comorbid conditions were obtained by the usage of International Classification of Diseases, Ninth Revision codes. Socioeconomic status was measured using median income for each patient's ZIP code using 2010 United States Census data. Baseline body mass index (BMI) values (kg/m2) were calculated based on available weight and height data at baseline. This study was approved by Institutional Review Board of the Cleveland Clinic.
The Electronic Outcome Data Collection System
The Knowledge Program at the Cleveland Clinic is an electronic data collection system of disease-based patient-reported outcomes that are entered into the patient's EMR at the point of care, normally making these measures immediately available to providers.12 Patient-reported outcomes data include outcomes such as EQ-5D and FOSQ collected at every outpatient sleep visit. Patient-reported sleep duration is also collected through this platform. In addition, there is a query for PAP usage and number of days used per week and number of hours used per day to be filled by the patient. PAP adherence was defined based on the Centers for Medicare and Medicaid Services (CMS) adherence criteria, which is ≥ 5 days per week and ≥ 4 hours per day of PAP usage in the past 4 weeks.13
Sleep Study Data Base
Apnea-hypopnea index (AHI), BMI (kg/m2), and neck circumference (cm) were collected for patients who underwent sleep studies at Cleveland Clinic. Objective PAP adherence data was collected by contacting the primary durable medical equipment company (most commonly used from the Cleveland Clinic) for patients to extract data on the subset of patients for whom adherence data was available. Self-reported PAP adherence and objective PAP adherence were both calculated based on CMS criteria. Related sleep study data were obtained from attended overnight polysomnography studies performed on patients at the Cleveland Clinic using the Polysmith (Nihon Kohden Corporation, Tokyo, Japan) system following standard clinical guidelines. The recording montage included (F3- M2, C3-M2, O1-M2, F4-M1, C4-M1, O2-M1) bilateral electrooculography, submental and bilateral anterior tibial electromyography, thoracic and abdominal respiratory inductance plethysmography, and finger pulse oximetry. Nasal airflow and nasal pressure were measured using oronasal thermistor and nasal cannula, respectively. Hypopnea was defined as airflow ≥ 50% in the nasal pressure channel for ≥ 10 seconds resulting in an arousal or ≥ 3% oxygen desaturation.14 Apnea was defined as a decrease in amplitude of oronasal thermistor signal (or alternative) by 90% for ≥ 10 seconds based on AASM event definition criteria. AHI was defined as the number of hypopneas and apneas per hour of sleep.
Functional Outcomes of Sleep Questionnaire
The FOSQ is a sleep-specific questionnaire that assesses the effect of sleep disorders and excessive daytime sleepiness on activities of daily living. The FOSQ contains 30 items, and is divided into 5 scales: activity level, vigilance, intimacy and sexual relationship, general productivity, and social outcomes.8 During initial FOSQ development, a convenience sample of individuals was used to show test-retest reproducibility and internal reliability. The psychometric properties of FOSQ have been tested in a RCT and it has been shown to be a sensitive instrument in measuring difference in PAP adherence.15 Participants are asked if sleepiness interferes with performing a given task; responses range from 1 (extreme difficulty) to 4 (no difficulty). Responses are averaged (excluding missing responses) to create a subscale score of 1 to 4, and then subscale scores are summed (5 to 20 for the total score) with greater scores indicating less effect of sleepiness on daily life.16 A score of more than 17.9 is considered to be the threshold for persons with normal sleep-related QoL. A change of 2.0 or more points in the FOSQ score is considered to indicate a clinically meaningful improvement in daily functioning.17
European Quality of Life
The EQ-5D was developed by a multidisciplinary group of researchers from 5 European countries. There are 5 questions covering the areas of mobility, self-care, usual activity, pain/ discomfort, and anxiety and depression. Each question has 3 response categories, level 1 (no problems), level 2 (some problems), and level 3 (inability or extreme problems). Values range from −0.109 (worst possible health state) to 1 (best possible health state). The minimally important difference is considered to be 0.040 (US algorithm) for EQ-5D.18
Statistical Analyses
Primary Analyses
Unadjusted means for the pre-PAP, post-PAP, and changes in score were computed for FOSQ and EQ-5D on all patients and stratified by PAP adherence. Paired t tests were used to determine whether within-group scores significantly improved after PAP treatment. The effect size was calculated using Cohen d, where values of 0.2, 0.5, and 0.8 represent small, medium, and large effect sizes, respectively. Two-sample t tests were used to compare whether change in score differed according to PAP adherence both for self-report and objective PAP adherence. Analyses were performed after excluding patients with normal baseline QoL because they may not demonstrate improvement in QoL with SDB therapy.3 We also calculated changes in subgroups of EQ-5D and FOSQ (see supplemental material).
Secondary Analysis
To account for potential confounding variables related to better post-PAP outcomes, separate multivariable linear regression models for each of the scales were created. For each, the post-PAP score was the response variable and was adjusted for the pre-PAP score. In each model, the following covariates were included: age (years), sex, race (Caucasian, African-American, and other), smoking status (current, former, never), median income by ZIP code (based on 2010 United States Census data), AHI, BMI (kg/m2), and neck circumference (cm). Also, the following comorbidities were included: cancer, chronic renal failure, diabetes, depression, coronary artery disease, hyper-tension, stroke, and atrial fibrillation.
The effects of age and socioeconomic status (median income by ZIP code) were examined to determine whether each was dependent on pre-PAP scores. This was achieved by including interaction terms between the pre-PAP score and of age and socioeconomic status in separate models. We determined a priori that any interaction terms found to be nonsignificant would be removed from the model.
Multiple imputation was used19 to create and analyze 10 imputed datasets. Incomplete variables were imputed under fully conditional specification20 using the default settings of the Multiple Imputation by Chained Equations (mice) 2.13 package.21 Model parameters were estimated with multivariable linear regression applied to each imputed dataset separately. All computations were done in R, version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria, http://www.R-project.org). Values of P < .05 were considered statistically significant.
RESULTS
From the initial query, we obtained data for 2,963 patients with SDB who were older than 18 years. From this, we excluded 714 patients for the following reasons: had only one visit (n = 459), never reported PAP use (n = 168), or reported PAP use for every visit in the study period (n = 87), indicating that they had initiated PAP before our study period started. An additional 38 patients whose only post-PAP visit was less than 30 days or more than 365 days after the baseline visit were excluded. Finally, 184 patients failed to complete both the EQ-5D and FOSQ at both the pre- and post-PAP visits and were excluded. Thus, our final cohort consisted of 2,027 patients with SDB who initiated PAP between January 1, 2010 and December 31, 2014 (Figure 1). A total of 620 patients had pre-PAP EQ-5D index of 1. Thus, 1,407 patients were left after excluding those with normal QoL (by EQ-5D). A total of 907 patients had pre-PAP FOSQ ≥ 18. Thus, 1,120 patients were left after excluding those with normal QoL (by FOSQ).
Figure 1. Flow diagram for study sample.
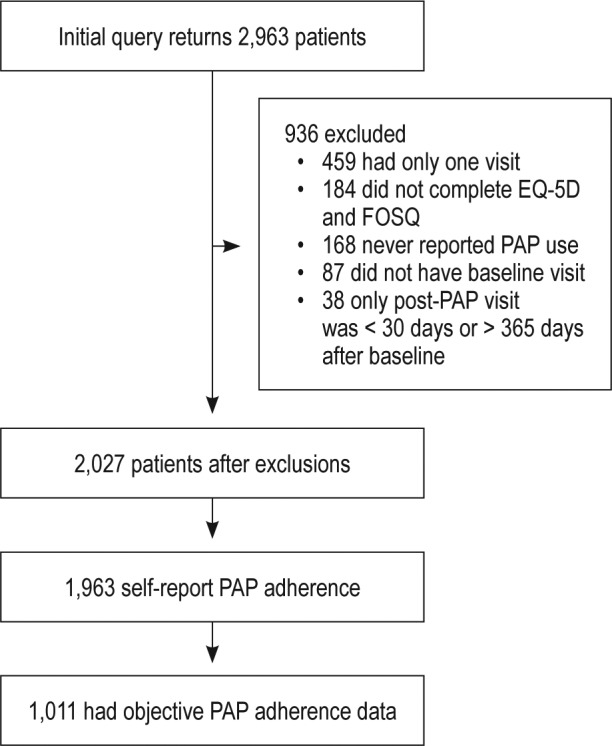
EQ-5D = European Quality of Life-5D, FOSQ = Functional Outcomes of Sleep Questionnaire, PAP = positive airway pressure.
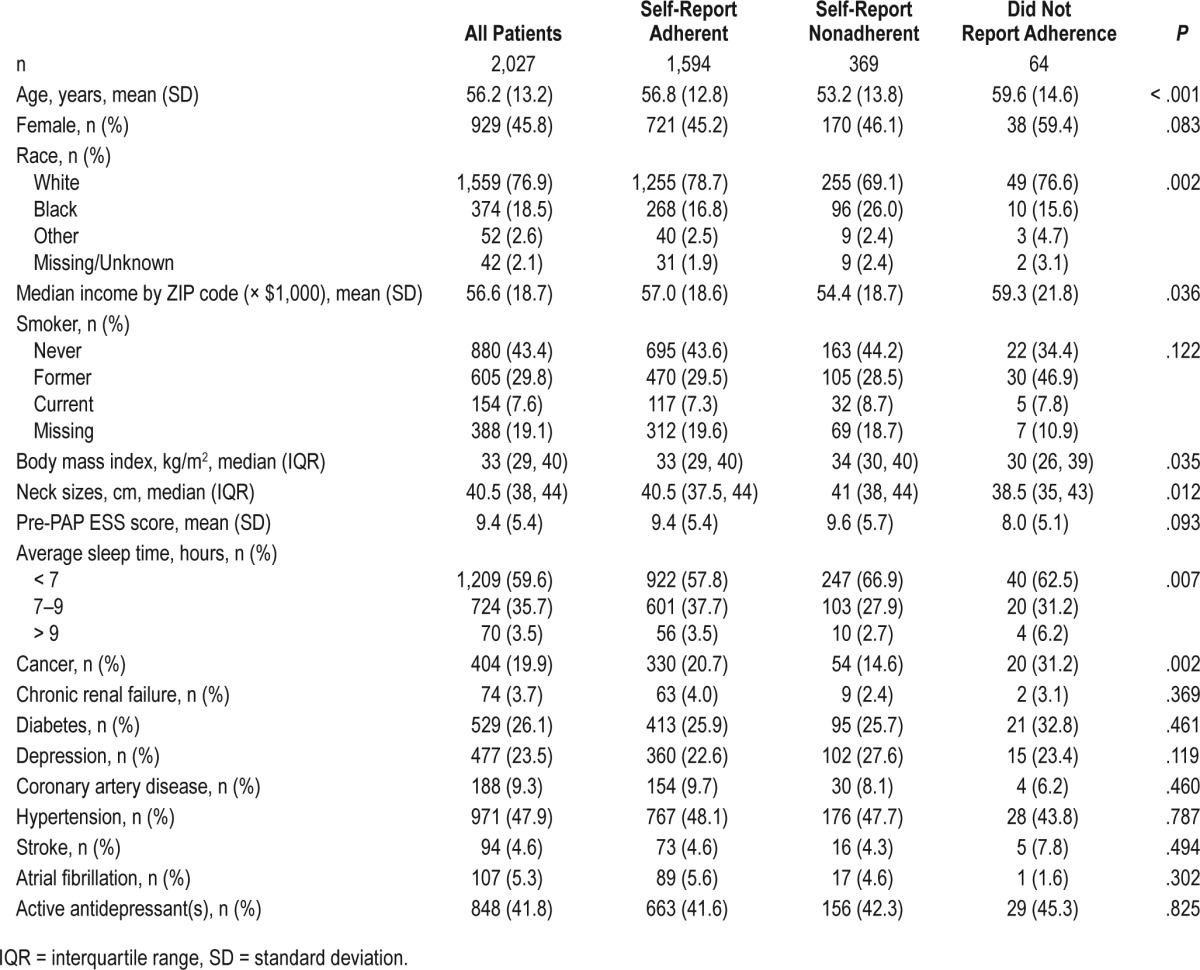
Demographic characteristics were stratified based on self-reported PAP adherence. The mean age of the cohort was 56.2 years (standard deviation = 13.2), with 45.8% female and 76.9% Caucasian (Table 1). Prevalence of hypertension was 47.9% in the overall cohort and 42% used antidepressants. Of the 1,963 patients who had self-reported PAP adherence data at follow-up, 1,594 (81.2%) were PAP adherent based on CMS criteria. Objective adherence data were available in 1,011 patients, of whom 704 (69.6%) were adherent based on CMS criteria. Agreement between objective and self-report adherence was 80.8%.
Table 1.
Baseline characteristics.
Primary Analyses
Displayed in Table 2 are mean EQ-5D and FOSQ scores before and after PAP as well as the mean change in score for all patients and stratified by PAP adherence (both self-report and objective). For the entire cohort, all scores significantly improved after PAP treatment (P < .0001 for both). For both the EQ-5D and FOSQ, improvement was greater when patients who reported highest QoL state were excluded [EQ-5D 0.043 (0.153) and FOSQ 1.9 (2.9) respectively]. For self-reported PAP-adherent patients, scores significantly improved for both scales (P < .001 for both). Among self-reported nonadherent patients, the EQ-5D index did not show significant change for all patients (P = .26) or among patients whose baseline EQ-5D was less than 1 (P = .32). However, significant improvements were observed for self-reported nonadherent patients in FOSQ (P < .001). When all patients were included, Cohen d was 0.08 for the EQ-5D and 0.32 for the FOSQ. When excluding patients with normal baseline QoL, Cohen d was 0.27 for the EQ-5D and 0.67 for the FOSQ, indicating small and medium effect sizes, respectively. For both scales, effect size was larger in adherent patients than nonadherent patients. Results were generally similar for the subgroup of patients with objective PAP adherence data.
Table 2.
Unadjusted means (standard deviations) for pre-PAP, post-PAP, and change in score for all patients, individual items and stratified by PAP adherence.
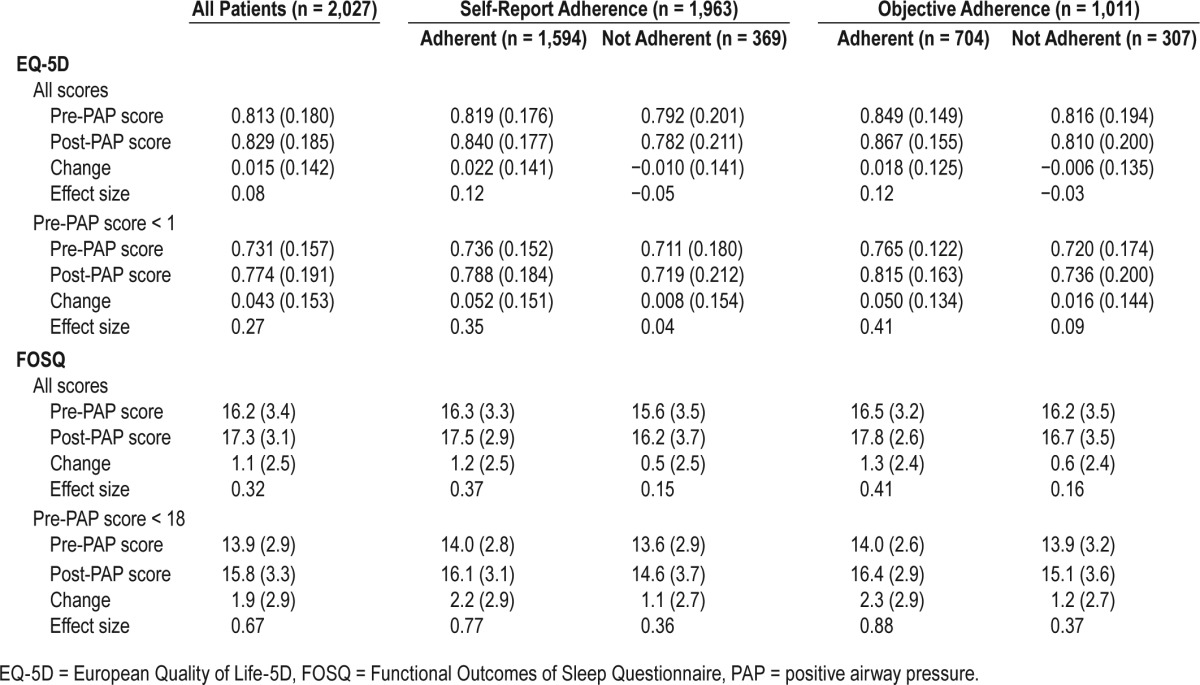
For both scales, self-reported adherent patients had significantly greater improvement in scores than nonadherent patients (P < .001 for both).
For both scales, objectively adherent patients had significantly more improvement in scores than nonadherent patients after excluding those with normal QoL indices (EQ-5D 0.050 [0.132] and FOSQ 2.3 [2.9] [P < .01 for both]) respectively.
Prior to PAP treatment, 44.7% of patients (907 of 2,027) had an FOSQ score ≥ 18. After PAP treatment, 60.9% of patients (1,235 of 2,027) had an FOSQ score ≥ 18 (P < .001). Among the 1,120 patients who had a pre-PAP FOSQ total score < 18, 37.1% (415 of 1,120) improved to a score of 18 or higher at the post-PAP visit (P < .001). Of the 363 patients who were objectively PAP adherent and with FOSQ < 18, 152 (41.9%) improved to a score of 18 or higher at post-PAP whereas 52 of 169 (30.8%) nonadherent patients improved to a score of 18 or higher at post-PAP (P = 0.018).
Secondary Analyses
Table 3 and Table 4 display results from multivariable linear regression models. After adjusting for the demographic and clinical variables, patients of “other race” had significantly worse post-PAP FOSQ scores than Caucasians. Patients who were taking one or more antidepressants before PAP initiation had statistically significant worse scores for EQ-5D and FOSQ. After adjusting for antidepressant use and other covariates, patients who had a history of depression had worse post-PAP FOSQ scores than patients without such a history. Patients with a history of cancer had better post-PAP EQ-5D index values than patients without a history of cancer. Patients who had a history of stroke had worse post-PAP FOSQ scores.
Table 3.
Multivariable linear regression model results for post-PAP EQ-5D index.
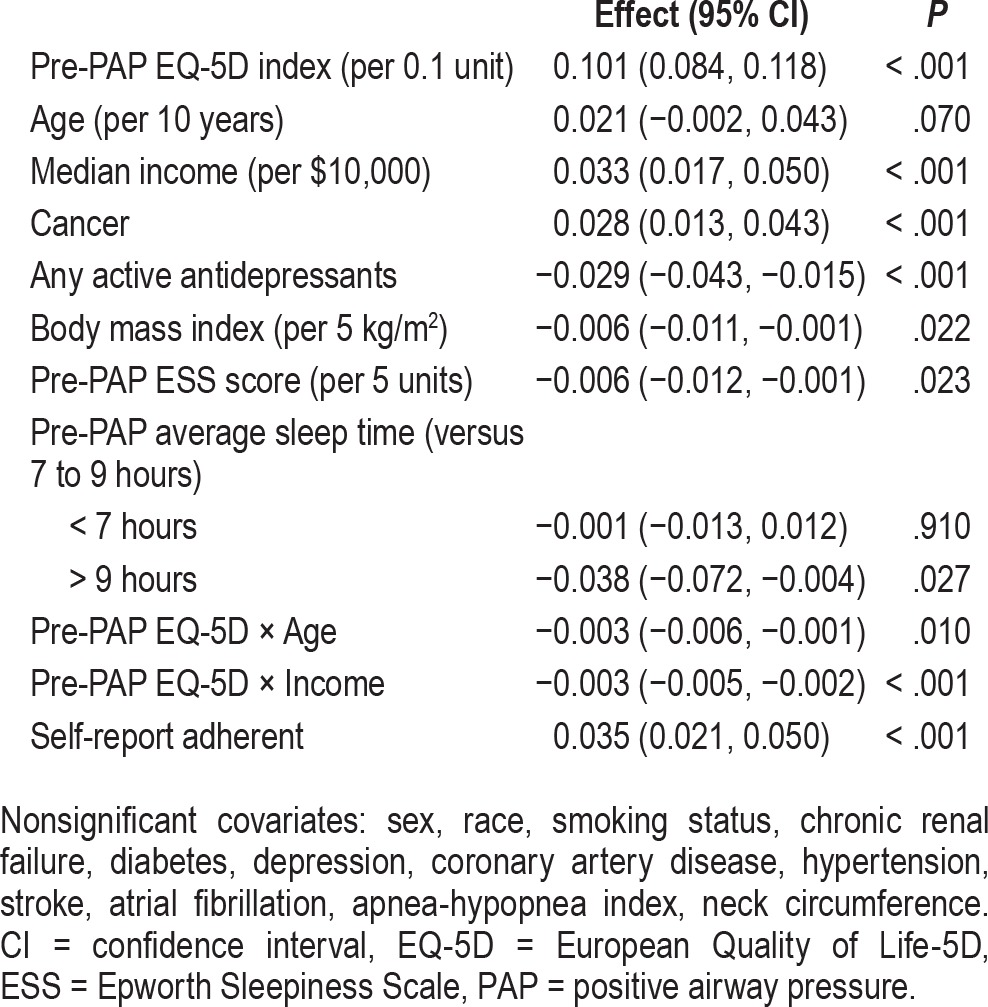
Table 4.
Multivariable linear regression model results for post-PAP FOSQ total score.
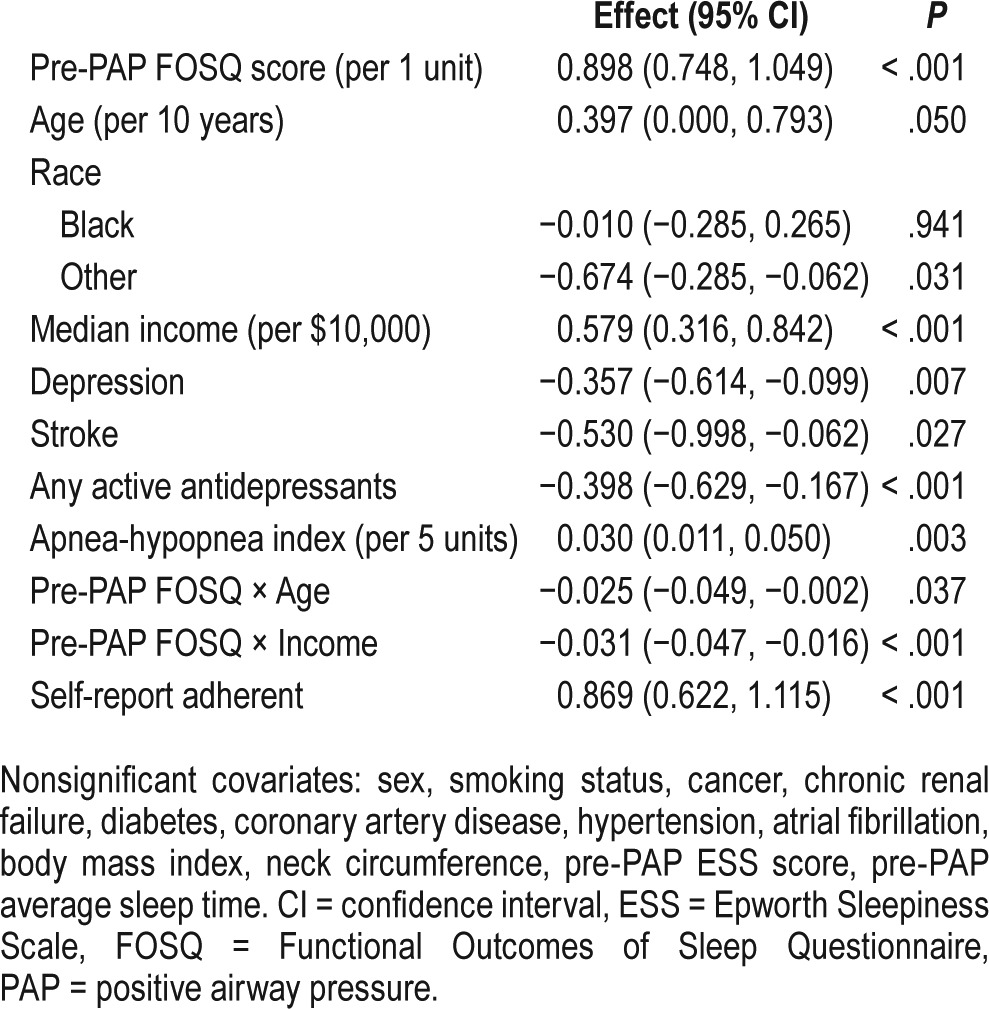
Higher AHI was associated with better post-PAP scores for the FOSQ. Higher BMI was associated with worse EQ-5D. Neck circumference was not significantly associated with post-PAP outcomes. Pre-PAP sleepiness as measured by the Epworth Sleepiness Scale was associated with worse post-PAP EQ-5D. Compared to patients who slept 7 to 9 hours, patients who slept more than 9 hours had significantly worse post-PAP EQ-5D index values. After adjustment for covariates, self-report adherence was associated with better scores for both scales.
A sensitivity analysis where PAP adherence was treated as a continuous variable indicated that, for each additional hour of PAP usage per night, the post-PAP EQ-5D index and FOSQ total score improved by 0.006 and 0.21 units, respectively (P < .001 for both). Results for other predictors were very similar to the models for which adherence was treated as binary.
Effect of Age and Socioeconomic Status and PAP Usage on EQ-5D and FOSQ
Patients with worse baseline QoL indices and of lower socioeconomic status had worse post-PAP QoL scores compared to those of higher socioeconomic status and had similarly worse baseline QoL indices (P < .05) (Figure 2). Younger patients with worse baseline QoL scores had worse post PAP QoL indices compared to older patients with similar baseline scores (P < .05) (Figure 3).
Figure 2. Effect modification of PAP usage and EQ-5D and FOSQ by socioeconomic status in SDB (interactions adjusted for all the variables).
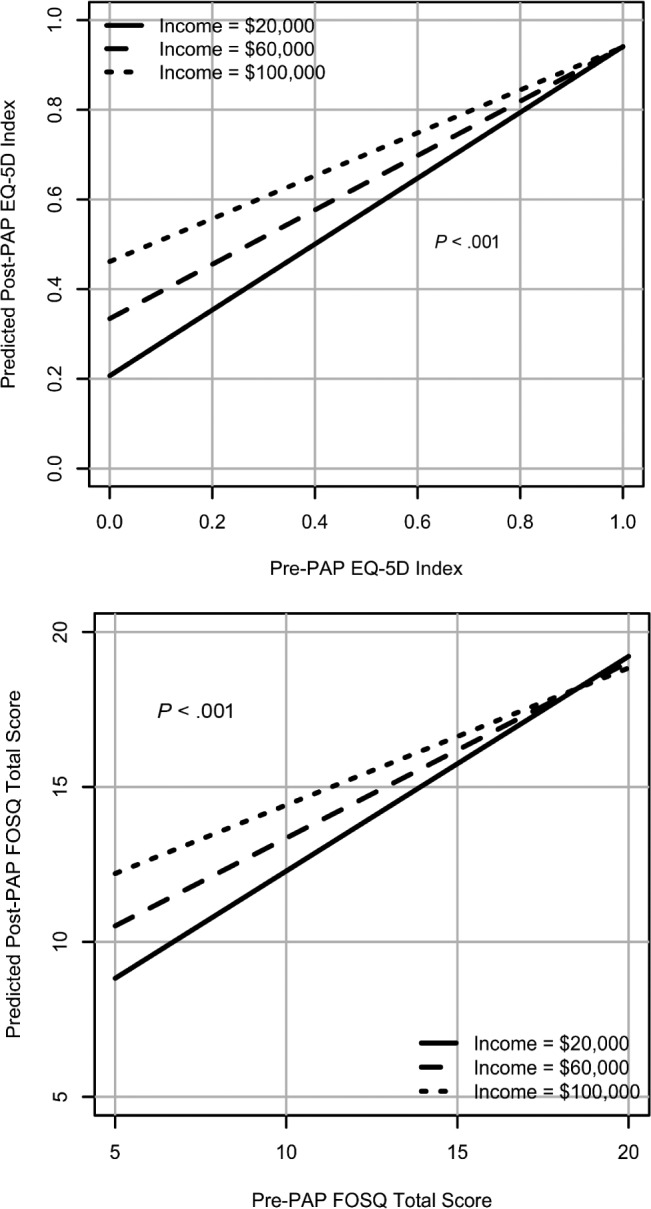
EQ-5D = European Quality of Life-5D, FOSQ = Functional Outcomes of Sleep Questionnaire, PAP = positive airway pressure, SDB = sleep-disordered breathing.
Figure 3. Effect modification of PAP usage and EQ-5D and FOSQ by age in SDB (interactions adjusted for all the variables).
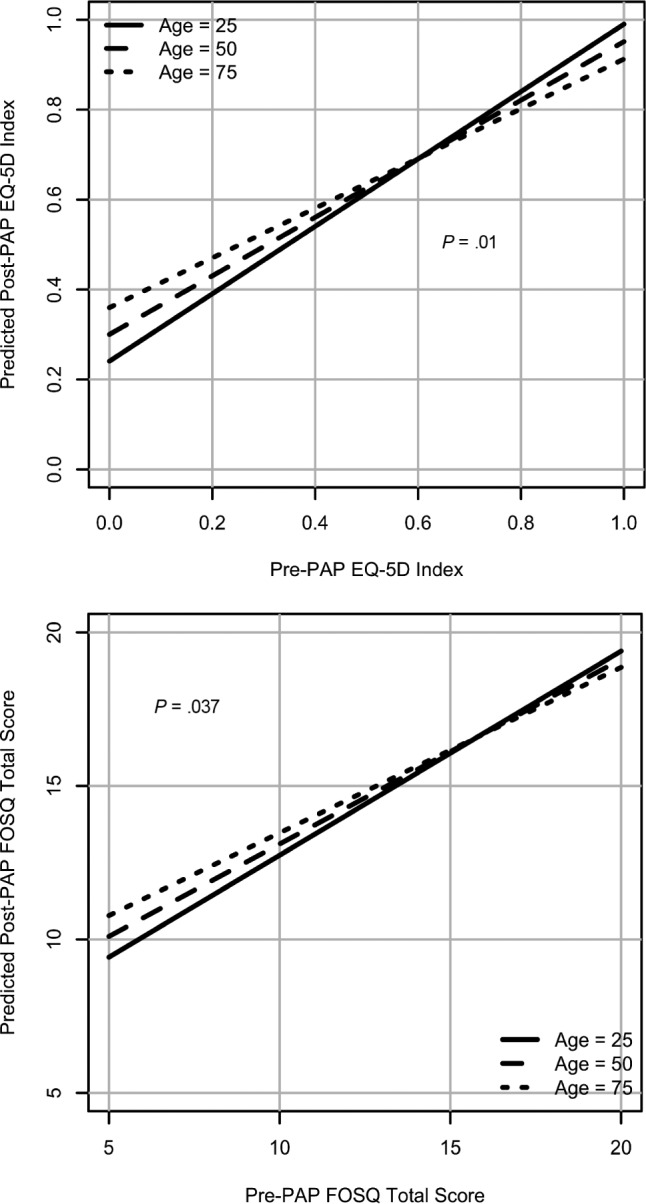
EQ-5D = European Quality of Life-5D, FOSQ = Functional Outcomes of Sleep Questionnaire, PAP = positive airway pressure, SDB = sleep-disordered breathing.
DISCUSSION
In this large, single tertiary care center clinical SDB cohort, there were significant and clinically meaningful improvements noted in generic QoL and sleep-related measures utilizing EQ-5D and FOSQ after the institution of PAP therapy even after consideration of comorbid conditions and sleepiness status. Although the improvement was noted in all patients irrespective of baseline quality of life status, findings were more pronounced after excluding those with normal baseline quality of life status with small to medium effect sizes observed in EQ-5D and FOSQ, respectively. The improvements were more robust in those who were PAP adherent. It was also noted that younger patients and those with worse baseline QoL indices had less improvement compared to older patients with worse baseline QoL. Moreover, patients in lower socioeconomic strata with poorer QoL at baseline had less improvement in QoL scores compared to those in higher socioeconomic strata and worse baseline QoL.
Although there are trials that have shown improvement in QoL measures in SDB following PAP therapy using different QoL instruments, clinical data showing this effectiveness are limited,3 mainly because of small sample sizes. There are also limitations to the nonclinical data including generalizability as trials included mainly sleepy individuals. Furthermore, there are no known data to demonstrate effect modification of age and SES. In a clinical study of patients who were PAP adherent, improvement of the World Health Organization QoL in 41 patients was noted.22 Another study of 50 patients, predominantly men on CPAP for OSA, showed improvement in the sleep apnea QoL index23 after 6 months of treatment. Furthermore, there are other small clinical studies showing improvement in QoL with PAP by Medical Outcomes Study Short Form-36 (SF-36) questionnaires24 and sleep apnea QoL index.25 A multicenter clinical effectiveness study showed improvement in FOSQ in all domains and similar to our study showed greater improvement in patients who were PAP adherent.26 Our study differs from the existing clinical studies because it includes a robustly large number of patients and utilizes both sleep-related and global QoL instruments.
In an RCT involving participants with mild to moderate levels of SDB and excessive daytime sleepiness, the mean improvement in FOSQ was noted to be 1.73 ± 2.50.10 A study comparing FOSQ score in participants receiving auto-PAP therapy or fixed-pressure CPAP therapy after laboratory titration showed similar improvements.27 A RCT involving only women showed improvement in QoL measures after 3 months of PAP therapy as determined by the Quebec Sleep Questionnaire.28 A recent meta-analyses showed improvement in the medical and physical components of SF-36 with the use of CPAP therapy29 in patients with OSA.
A recent study showed that individuals with severe OSA who lived in lower income neighborhoods did not face obstacles in obtaining PAP treatment; however, they did observe that those living in higher income neighborhoods had a 27% higher rate of PAP acceptance.30 In our study, despite adjusting for PAP adherence, we noted more improvement in QoL with use of PAP in those residing in higher SES strata. There is a paucity of data showing effectiveness of PAP therapy on QoL measures stratified by age group. There is one study to date demonstrating improvements in all domains of QoL following PAP therapy in elderly patients with severe SBD.31 We observed similar results in that older people with worse baseline QoL showed more improvement compared to younger patients.
The strengths of our study include large sample size, adjustment for potential confounding factors, and utilization of standardized QoL questionnaires collected systematically in the clinic-based setting. Furthermore, this is in alignment with the AASM quality measures highlighting the need to assess and measure QoL parameters in the clinical setting3 as well in concordance with national priorities. However, there are some limitations to consider. This is a single-center, retrospective study. Part of the observed improvement in QoL scores may be due to regression to the mean. There is a lack of control arm to compare changes in these measures without PAP therapy; however, this lays the foundation to confirm these changes in a prospective trial setting. Both subjective and objective PAP adherence was considered. As multiple comparisons between outcomes and covariates were performed, there is increased likelihood of type I error. SES was an assessment based on median income by ZIP code and therefore may not have effectively incorporated important aspects of SES. A prospective investigation of subgroups including different SES and age confirming the current findings of similar QoL and enhanced responsiveness in the older age group and those living in higher SES would be valuable and inform SDB treatment guidelines. Future research should assess changes in QoL measures with SDB treatment and focus on prioritizing patient-centric measures, tailoring by subgroups the most predisposed to lack of responsiveness to treatment from a QoL perspective.
DISCLOSURE STATEMENT
Work for this study was performed at the Cleveland Clinic. All authors have seen and approved the manuscript. This study was funded by the Cleveland Clinic Neurological Institute-Center of Research Outcomes and Evaluation Scholar Award and National Heart, Lung, and Blood Institute (NHLBI) R21HL108226 and R01HL109493. Dr. Mehra reports that she has received NIH funding for which she has served as Principal Investigator (NHLBI R01HL109493, R21HL108226). Her institution has received positive airway pressure machines and equipment from Philips Respironics for use in NIH-funded research. She has received honorarium from the American Academy of Sleep Medicine for speaking. She serves as an Associate Editor for the journal Chest. She has received royalties from Up to Date. Nancy Foldvary-Shaefer receives grant support from UCB, Inc, Jazz Pharmaceuticals plc, and ResMed and serves on the Speaker's Bureau for Jazz Pharmaceuticals plc. Nicolas Thompson has received grant support from Novartis Pharmaceuticals. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the Cleveland Clinic Home Care durable medical equipment company for providing the objective adherence data for positive airway pressure therapy. We also thank Michael Herbert for providing variables from the sleep study data set. We thank Srividya Ramachandran for assistance with editorial services.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- CMS
Centers for Medicare and Medicaid Services
- EMR
electronic medical record
- EQ-5D
European Quality of Life-5D
- FOSQ
Functional Outcomes of Sleep Questionnaire
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PRO
patient reported outcomes
- QoL
quality of life
- RCT
randomized controlled trial
- SDB
sleep-disordered breathing
- SES
socioeconomic status
REFERENCES
- 1.Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47(1):194–202. doi: 10.1183/13993003.01148-2015. [DOI] [PubMed] [Google Scholar]
- 2.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24(1):96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 3.Aurora RN, Collop NA, Jacobowitz O, Thomas SM, Quan SF, Aronsky AJ. Quality measures for the care of adult patients with obstructive sleep apnea. J Clin Sleep Med. 2015;11(3):357–383. doi: 10.5664/jcsm.4556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flemons WW. Clinical practice. Obstructive sleep apnea. N Engl J Med. 2002;347(7):498–504. doi: 10.1056/NEJMcp012849. [DOI] [PubMed] [Google Scholar]
- 5.Jenkinson C, Stradling J, Petersen S. Comparison of three measures of quality of life outcome in the evaluation of continuous positive airways pressure therapy for sleep apnoea. J Sleep Res. 1997;6(3):199–204. doi: 10.1046/j.1365-2869.1997.00043.x. [DOI] [PubMed] [Google Scholar]
- 6.Marquis P, Caron M, Emery MP, Acquadro C. The role of health-related quality of life data in the drug approval processes in the US and Europe: a review of guidance documents and authorizations of medicinal products from 2006 to 2010. Pharm Med. 2011;25(3):147–160. [Google Scholar]
- 7.Rabin R, de Charro F. EQ-5D: a measure of health status from the EuroQol Group. Ann Med. 2001;33(5):337–343. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 8.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 9.Jing J, Huang T, Cui W, Shen H. Effect on quality of life of continuous positive airway pressure in patients with obstructive sleep apnea syndrome: a meta-analysis. Lung. 2008;186(3):131–44. doi: 10.1007/s00408-008-9079-5. [DOI] [PubMed] [Google Scholar]
- 10.Weaver TE, Mancini C, Maislin G, et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am J Respir Crit Care Med. 2012;186(7):677–683. doi: 10.1164/rccm.201202-0200OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Development of the World Health Organization WHOQOL-BREF quality of life assessment. The WHOQOL Group. Psychol Med. 1998;28(3):551–558. doi: 10.1017/s0033291798006667. [DOI] [PubMed] [Google Scholar]
- 12.Katzan I, Speck M, Dopler C, et al. The Knowledge Program: an innovative, comprehensive electronic data capture system and warehouse. AMIA Annu Symp Proc. 2011;2011:683–692. [PMC free article] [PubMed] [Google Scholar]
- 13.Billings ME, Kapur VK. Medicare long-term CPAP coverage policy: a cost-utility analysis. J Clin Sleep Med. 2013;9(10):1023–1029. doi: 10.5664/jcsm.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iber C, Ancoli-Israel S, Chesson A, Quan SF for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 15.Billings ME, Rosen CL, Auckley D, et al. Psychometric performance and responsiveness of the functional outcomes of sleep questionnaire and sleep apnea quality of life instrument in a randomized trial: the HomePAP study. Sleep. 2014;37(12):2017–2024. doi: 10.5665/sleep.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gooneratne NS, Weaver TE, Cater JR, et al. Functional outcomes of excessive daytime sleepiness in older adults. J Am Geriatr Soc. 2003;51(5):642–649. doi: 10.1034/j.1600-0579.2003.00208.x. [DOI] [PubMed] [Google Scholar]
- 17.Chasens ER, Ratcliffe SJ, Weaver TE. Development of the FOSQ-10: a short version of the Functional Outcomes of Sleep Questionnaire. Sleep. 2009;32(7):915–919. doi: 10.1093/sleep/32.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo N, Johnson J, Coons SJ. Using instrument-defined health state transitions to estimate minimally important differences for four preference-based health-related quality of life instruments. Med Care. 2010;48(4):365–371. doi: 10.1097/mlr.0b013e3181c162a2. [DOI] [PubMed] [Google Scholar]
- 19.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- 20.Van Buuren S, Brand JP, Groothuis-Oudshoorn CG, Rubin DB. Fully conditional specification in multivariate imputation. J Stat Comput Simul. 2006;76(12):1049–1064. [Google Scholar]
- 21.van Buuren S. Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45(3):1–67. [Google Scholar]
- 22.Diamanti C, Manali E, Ginieri-Coccossis M, et al. Depression, physical activity, energy consumption, and quality of life in OSA patients before and after CPAP treatment. Sleep Breath. 2013;17(4):1159–1168. doi: 10.1007/s11325-013-0815-6. [DOI] [PubMed] [Google Scholar]
- 23.Avlonitou E, Kapsimalis F, Varouchakis G, Vardavas CI, Behrakis P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath. 2012;16(2):563–569. doi: 10.1007/s11325-011-0543-8. [DOI] [PubMed] [Google Scholar]
- 24.D'Ambrosio C, Bowman T, Mohsenin V. Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure--a prospective study. Chest. 1999;115(1):123–129. doi: 10.1378/chest.115.1.123. [DOI] [PubMed] [Google Scholar]
- 25.Coman AC, Borzan C, Vesa CS, Todea DA. Obstructive sleep apnea syndrome and the quality of life. Clujul Med. 2016;89(3):390–395. doi: 10.15386/cjmed-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183(9):1238–1244. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 28.Campos-Rodriguez F, Queipo-Corona C, Carmona-Bernal C, et al. Continuous positive airway pressure improves quality of life in women with OSA. A randomized-controlled trial. Am J Respir Crit Care Med. 2016;194(10):1286–1294. doi: 10.1164/rccm.201602-0265OC. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn E, Schwarz EI, Bratton DJ, Rossi VA, Kohler M. Effects of CPAP and mandibular advancement devices on health-related quality of life in OSA: a systematic review and meta-analysis. Chest. 2017;151(4):786–794. doi: 10.1016/j.chest.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Kendzerska T, Gershon AS, Tomlinson G, Leung RS. The effect of patient neighborhood income level on the purchase of continuous positive airway pressure treatment among patients with sleep apnea. Ann Am Thorac Soc. 2016;13(1):93–100. doi: 10.1513/AnnalsATS.201505-294OC. [DOI] [PubMed] [Google Scholar]
- 31.Martinez-Garcia MA, Chiner E, Hernandez L, et al. Obstructive sleep apnoea in the elderly: role of continuous positive airway pressure treatment. Eur Respir J. 2015;46(1):142–151. doi: 10.1183/09031936.00064214. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


