Abstract
Study Objectives:
The mechanism of early neurological deterioration (END) in patients with stroke remains unclear. We assessed the relationship between nocturnal oxygen desaturation (NOD) in the stroke unit (SU) and END, especially occurring at nighttime, following acute stroke.
Methods:
A retrospective analysis was performed on a total of 276 patients with ischemic stroke who were admitted to the SU between July 2013 and June 2015. The oxygen desaturation index was calculated from pulse oximetry data sampled every 1 minute during 9 hours on the first night (10:00 PM to 7:00 AM) after admission, and NOD was defined as oxygen desaturation index ≥ 5 events/h. END was defined as an increase of ≥ 2 points from the baseline National Institutes of Health Stroke Scale during 7 days after onset. We compared clinical characteristics and NOD between patients with and without END.
Results:
Among the included patients (mean age 69.2; male 55.4%), 42 patients (15.2%) experienced END. The proportion of NOD was significantly greater in the END group (45.2% versus 12.8%, P < .001). After adjusting for confounders, NOD was independently associated with END (odds ratio 7.57; 95% confidence interval 3.14–18.27). Among END patients, 47.6% patients (n = 20) had END during nighttime. Moreover, NOD was more frequent in patients with END during nighttime compared to those with END during daytime (73.7% versus 26.1%, P = .002).
Conclusions:
NOD in the SU was associated with END, especially during nighttime, after ischemic stroke. This suggests that treatment of sleep-disordered breathing could be a modifiable factor to possibly reduce the risk of neurological worsening among acute stroke patients.
Citation:
Kim TJ, Ko SB, Jeong HG, Kim CK, Kim Y, Nam K, Mo H, An SJ, Choi HA, Yoon BW. Nocturnal desaturation is associated with neurological deterioration following ischemic stroke: a retrospective observational study. J Clin Sleep Med. 2017;13(11):1273–1279.
Keywords: during nighttime, early neurological deterioration, nocturnal desaturation, stroke unit, sleep-disordered breathing
INTRODUCTION
Early neurological deterioration (END) is defined as a substantial neurological worsening after initial symptom, and is associated with poor functional outcome.1–3 END is common, and occurs in approximately 25% of patients with acute ischemic stroke.1,2,4 Several clinical and radiological predictors for END have been reported, suggesting that multiple metabolic, hemo-dynamic, and systemic factors play a role in the development of END.1,2,5–7 The hypoxia during sleep-disordered breathing (SDB) may affect cardiac function and cerebral perfusion status, and this might lead to an ischemic event.3,6 Based on the selected patients who underwent polysomnography (PSG) in acute phase, sleep apnea or SDB was recognized as one of the risk factors for END.5,6,8 However, the results were based on the patients who could tolerate PSG in acute phase and no information exists on whether those patients deteriorated during nighttime.
In the stroke unit (SU), physiologic parameters such as blood pressure, heart rate, and systemic saturation using pulse oximetry are continuously monitored. With these well-organized physiologic data, we hypothesized that nocturnal oxygen desaturation (NOD) in the SU would be associated with END, especially END during nighttime. The aim of this study was to investigate whether NOD is associated with END during nighttime.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep-disordered breathing has been recognized as a risk factor for worse outcome after stroke. We assessed the relationship between nocturnal oxygen desaturation using continuously monitored systemic saturation data in the stroke unit and early neurological deterioration, especially occurring at nighttime, following acute stroke.
Study Impact: This study demonstrates that nocturnal desaturation in the stroke unit was associated with early neurological deterioration, especially during nighttime after stroke. This suggests that treatment of sleep-disordered breathing could be an important factor to prevent early neurological deterioration among acute stroke patients.
METHODS
Study Population
A total of 337 patients with acute ischemic stroke and transient ischemic attack (TIA) (within 7 days from stroke onset) who stayed in the SU on the first night of admission were screened from July 2013 to June 2015. A total of 276 patients older than 40 years (age range, 41–95 years) were enrolled for analysis. We excluded patients with the following conditions: lack of clinical information (n = 10); TIA (n = 27); age younger than 40 years (n = 9); and oxygen therapy due to desaturation (pulse oximetry saturation [SpO2] < 90%) on admission to SU care (n = 15). The number of patients in the young age group (age between 30 and 40 years) was very small (n = 9), and END within 7 days after stroke did not occur. Therefore, the data from the rest of the patients (age older than 40 years) were analyzed. This study was approved by the Institutional Review Board of the Seoul National University Hospital (IRB NO H-1212-087-450).
Baseline and Clinical Assessment
Baseline characteristics such as demographic data (age and sex) and vascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, a history of smoking [current smoker or ex-smoker], body mass index [BMI], initial systolic blood pressure [SBP] and diastolic blood pressure [DBP], and a history of stroke/TIA) were evaluated. We also collected information on history of pulmonary diseases related to hypoxemia, such as interstitial lung disease, miliary tuberculosis, chronic obstructive pulmonary disease, chronic asthma, cancer (lung cancer and advanced cancer with lung metastasis), pleural effusion, chronic pulmonary embolism, pulmonary hypertension, and aspiration pneumonia on admission. In addition, we obtained the information regarding the length of stay in the SU and general ward. Patients treated with oxygen therapy after SU monitoring were identified during hospitalization. The following laboratory evaluations were performed: white blood cell count, and initial fasting glucose, hemoglobin (Hb), triglyceride (TG), total cholesterol, high-density lipoprotein cholesterol, and fibrinogen levels. Blood samples were drawn after overnight fasting. Regarding vascular risk factors, hypertension was defined as a history of antihypertensive treatment or persistent elevated blood pressure (SBP ≥ 140 mm Hg or a DBP ≥ 90 mm Hg) after acute period.9 Hyperlipidemia was defined as a history of the use of lipid-lowering medication, a serum level of total cholesterol > 240 mg/dL, or a serum level of low-density lipoprotein cholesterol > 160 mg/dL.10 Diabetes mellitus was defined as a history of the use of insulin or oral hypoglycemic drugs, HbA1c ≥ 6.5%, fasting blood glucose ≥ 7.0 mmol/L, or non-fasting blood glucose ≥ 11.1 mmol/L.11
Ischemic stroke was categorized as large-artery atherosclerosis (LAA), small-vessel occlusion (SVO), cardioembolism, other determined, and undetermined, based on the Trial of Org 10172 in Acute Stroke Treatment criteria.12 The ischemic lesion locations were divided into the anterior circulation (anterior cerebral artery and/or middle cerebral artery territories), posterior circulation (posterior cerebral artery and/or vertebrobasilar artery territories), and multiple artery territory. All patients were initially evaluated for stroke severity based on the National Institutes of Health Stroke Scale (NIHSS) on admission to the SU. NIHSS is a scale to measure stroke severity (total score 0–42) and is composed of 15 items (level of consciousness, extraocular movements, visual fields, facial palsy, extremity strength, sensory function, ataxia, language, dysarthria, and extinction and inattention).13 NIHSS was performed every 4 hours in the SU and at least three times per day in the general ward by specialized nurses or stroke neurologists. Whenever END was suspected by the patients or nursing staff, NIHSS was performed in detail. END was defined as follows: an increase in NIHSS score ≥ 2 points from the baseline NIHSS score during the 7 days after symptom onset.14 The timing of END was captured by review of medical records.
Stroke Unit Monitoring Data and Nocturnal Oxygen Desaturation
Patients in the SU were continuously monitored with DASH 4000 monitors (General Electric, Boston, Massachusetts, United States) for cardiac rhythm; oxygen saturation (pulse oximetry), which was sampled every 2 second and averaged over 1 minute; and blood pressure (noninvasive automatic measurement every 15 minutes) during hospitalization. A high-resolution data acquisition system (BedmasterEX, Excel Medical Electronics, Jupiter, Florida, United States) was used to acquire physiologic digital data.
Nocturnal oxygen desaturation (NOD) was retrospectively evaluated using the oxygen desaturation index (ODI) during the first night following admission to the SU. ODI is a possible value to detect undiagnosed SDB.15–17 We used pulse oximetry saturation (SpO2) data from 10:00 PM to 7:00 AM (usual lights-out time in our SU). Artifacts regarding false desaturation due to sensing errors were deleted after manual review. ODI is an hourly average number of desaturation episodes, which is defined as at least 3% decrease in saturation from the mean saturation of the previous minute. The ODI was calculated over 9 hours.15,16,18 Patients were categorized into two groups based on their ODI as follows: normal (ODI < 5 events/h) and NOD group (ODI ≥ 5 events/h).15–18 We also checked the nocturnal mean blood pressure (MBP) and the time in which SpO2 was below 90% during overnight sleep.
Imaging Information
Enrolled patients underwent brain magnetic resonance imaging (MRI) on a 1.5 T or 3.0 T superconducting magnet system (n = 271, 98.2%) or computed tomography (CT) (n = 5, 1.8%) on admission. Imaging interpretations were performed by two neurologists (T.J.K. and S.J.A.) blinded to clinical information. Disagreement was resolved by discussion and a third investigator's opinion (S.B.K). A significant stenosis of the parent artery was defined when stenosis is ≥ 50% or occlusion.18 When END occurred, follow-up brain imaging studies (MRI or CT) were planned to identify relevant lesions. The patterns of follow-up imaging were categorized as follows: no change, expansion of index stroke lesion, new lesion outside the vascular territory of index stroke lesion, edematous change (swelling of initial lesion), and hemorrhagic transformation.19
Statistical Analysis
Continuous variables and proportions of categorical variables were compared using Student t tests, Pearson χ2 tests, or Fisher exact test as appropriate. The association between END and NOD was analyzed using logistic regression analyses. Covariates with statistically significant differences (P < .05) by univariate analysis and those with clinically important factors were adjusted for multivariate analysis. In addition, Kaplan-Meier method was performed to compare the END rate and timing between the patients with and without NOD. For all analyses, a two-tailed value of P < .05 was considered statistically significant. Statistical analyses were performed using the SPSS program (version 22.0, IBM, Armonk, New York, United States).
RESULTS
Baseline Characteristics of the Patients With END
Among a total of 276 patients (male 55.4%, and female 44.6%, with a mean age of 69.2 ± 11.1 years), 42 patients (15.2%) experienced END within 7 days after stroke onset (Table 1). The baseline characteristics including stroke risk factors and a history of pulmonary diseases were not different between patients with or without END (Table 1). Initial NIHSS was not different in patients with and without END, whereas discharge NIHSS was higher in patients with END due to neurologic deterioration. There was a tendency of longer length of stay in the SU in patients with END compared with those without END (2.5 ± 1.4 versus 2.1 ± 1.3, P = .055). These baseline characteristics showed similar trend according to sexes except discharge NIHSS (Table S1 in the supplemental material).
Table 1.
Baseline characteristics of the patients.
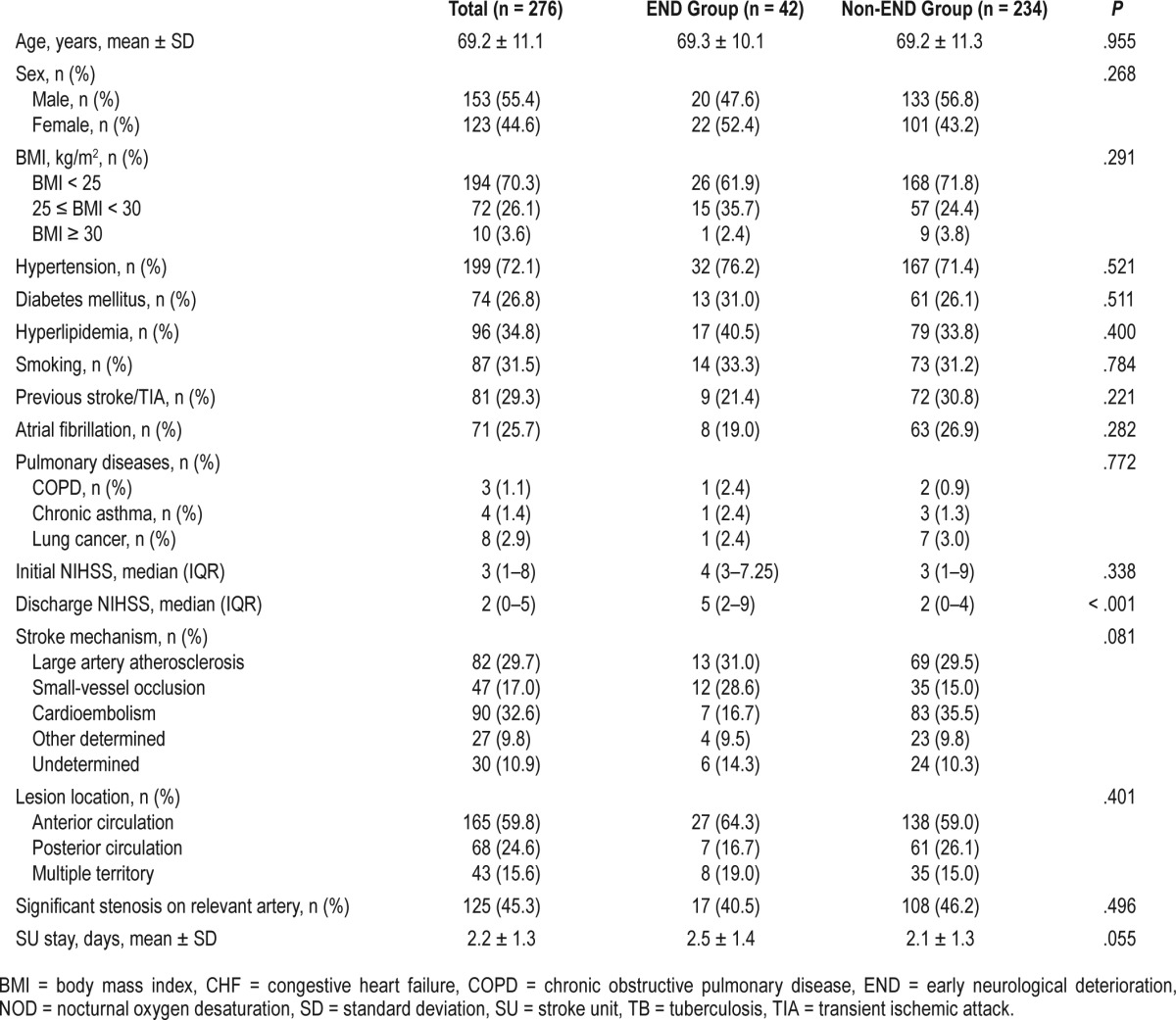
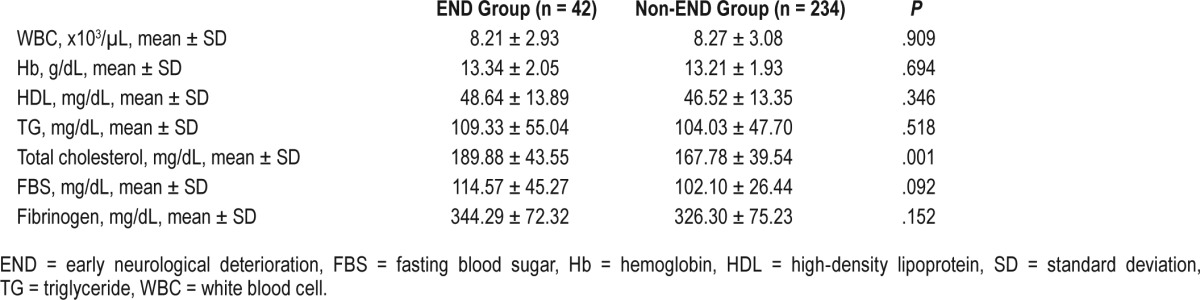
In addition, stroke subtypes were not different in patients with and without END (P = .081, Fisher exact test); however, SVO was more frequent in the END group whereas cardioembolism was dominant in the non-END group. Moreover, the location of stroke lesion (anterior versus posterior circulation) and the degree of stenosis in the parent artery were not different between the two groups. Regarding physiologic variables, nocturnal MBP was significantly higher in the patients with END compared to those without END (97.4 ± 15.1 versus 92.5 ± 13.3, P = .032). Moreover, the percentage of patients with oxygen therapy after SU monitoring was not different between the two groups (Table 2). Blood test results were similar in the two groups, except for higher total cholesterol level in patients with END (Table 3). These results were consistent with analyzed data categorized by sex (Table S2 and Table S3 in the supplemental material).
Table 2.
Monitoring data in the stroke unit from patients with and without early neurologic deterioration.
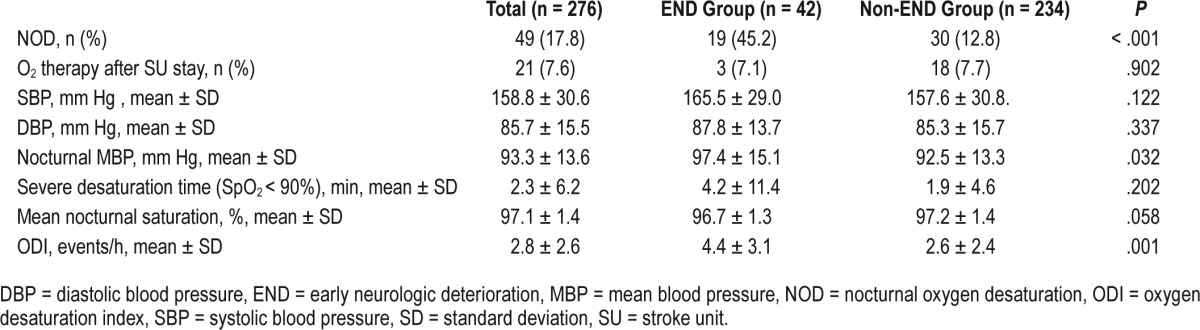
Table 3.
Laboratory data from patients with and without early neurological deterioration.
Correlation Between END and NOD
The proportion of patients with NOD was significantly higher in the END group compared to the non-END group (45.2% versus 12.8%, P < .001). Likewise, mean NOD was lower in patients with END compared to those without END (96.7 ± 1.3 versus 97.2 ± 1.4, P = .058), although not statistically significant. However, the duration of severe desaturation (SpO2 < 90%) was not different between the two groups.
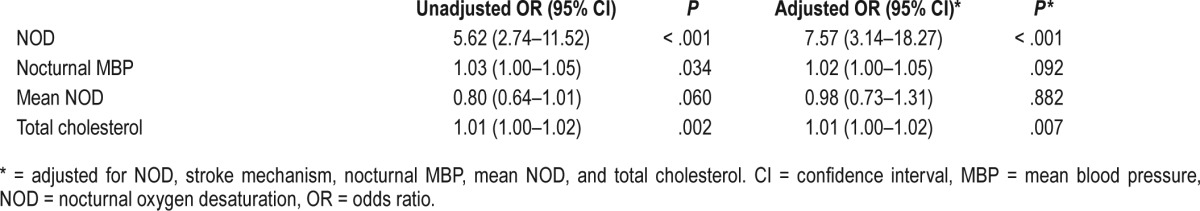
Patients with NOD had higher odds for development of END (odds ratio 7.57; 95% confidence interval, 3.14–18.27). The strong association between NOD and END was consistent in both sexes (male: odds ratio 6.86, 95% confidence interval 2.10–22.41; female odds ratio 5.57, 95% confidence interval 1.82–17.10) (Table S4 in the supplemental material). In addition, total cholesterol level was also associated with END (odds ratio 1.01, 95% confidence interval 1.00–1.02, P = .007) after adjusting for the relevant confounding variables (Table 4). However, mean NOD and nocturnal MBP were not associated with END after adjusting for confounders.
Table 4.
Factors associated with early neurological deterioration.
Association Between NOD and Timing of END
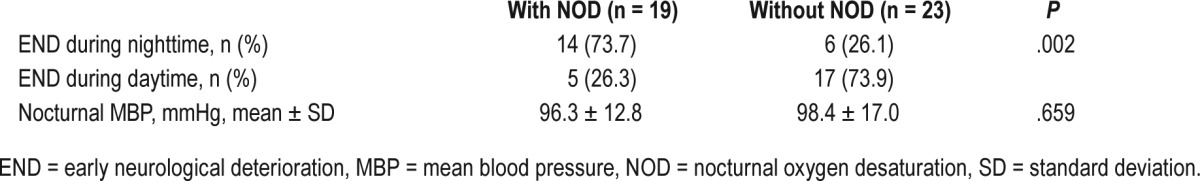
Among the 42 patients with END, 20 patients (47.6%) had END during nighttime. Among the 19 patients with NOD, most of them (14/19, 73.7%) had END during nighttime. However, in 23 patients without NOD, most patients (17/23, 73.9%) had END during daytime, and only 26.1% of patient had END during nighttime, which was statistically different (P = .002). In addition, 61.9% of patients (n = 26) experienced END while in the SU within 24 hours after admission (Figure 1 and Figure S1 in the supplemental material). Among them, 61.5% patients (n = 16) had END during nighttime, and 38.5% patients had END in the daytime. Moreover, patients who experienced END during nighttime had higher percentage of the presence of NOD (n = 10, 62.5%) compared to patients with END in the daytime. This suggested that the presence of NOD was strongly associated with END during nighttime (Table 5 and Figure 1). However, the nocturnal MBP was not different between the patients with and without NOD (96.3 ± 12.8 mm Hg versus 98.4 ± 17.0 mm Hg, P = .659) (Table 5).
Figure 1. Kaplan-Meier event curves for END from admission time to 7 days after admission.
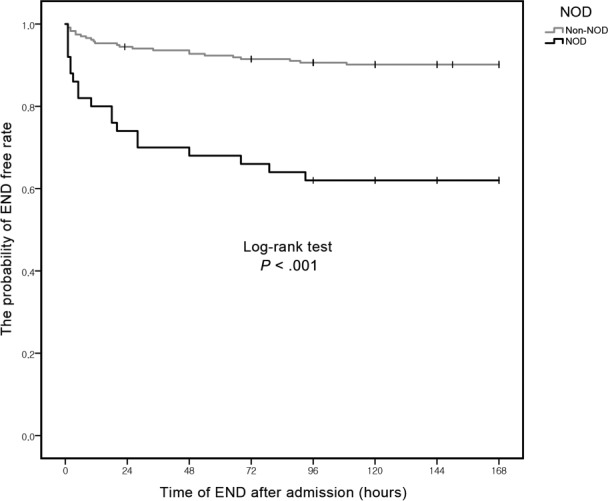
The probability of END-free was different between patients with and without nocturnal desaturation. END = early neurological deterioration, NOD = nocturnal oxygen desaturation
Table 5.
Association between nocturnal oxygen desaturation and the timing of early neurological deterioration.
Follow-Up Imaging Patterns of the Patients With END
Among the 42 patients with END, 39 patients (92.9%) underwent follow-up brain imaging (MRI or CT) to identify any changes after END detection. Most patients with END had “enlargement of lesion” (51.3%) pattern, and new lesion occur-rence on new vascular territory was 12.8%. Edematous change and hemorrhagic transformation were observed only in 5.2% of patients. However, degree of desaturation, measured by ODI, was not different among the patients with various causes of END (P = .559).
DISCUSSION
In this study, we found that patients with NOD on the first night in the SU had approximately eight times higher odds for the development of END in both sexes. Moreover, NOD was especially associated with END during nighttime.
END is clinically important because it is strongly related to poor functional outcome.1,2 As shown in our patients, those with END had higher discharge NIHSS scores even with similar admission NIHSS scores. Several metabolic, hemo-dynamic, and systemic factors for END have been reported, including initial stroke severity, degree of stenosis in the proximal vessel, systemic hypotension, elevation of serum total cholesterol level, seizure, or atrial fibrillation.1,2,5,7,20–22 In line with these studies, higher level of total cholesterol was associated with END in our patients. Endothelial dysfunction, arterial atherosclerosis, and microcirculatory disturbance was considered as possible mechanisms for END due to elevated total cholesterol.21,22 However, preceding systemic hypotension or seizure was not identified in our patients in whom END developed. In addition, the degree of stenosis in the parent vessels was also not different among patients with and without END. Moreover, nocturnal MBP was higher in patients with END compared with those without END. However, NOD occurred more frequently in patients with END compared with those without END (45.2% versus 12.8%, P < .001), which suggested that NOD was a possible mediator for the development of END. This is consistent with previous studies.3,6 Moreover, we found that NOD was more frequently observed in patients with END during nighttime compared with those with END during daytime. Among END patients, 61.9% of patients (n = 26) were detected END within 24 hours after admission during SU monitoring. Taken together, NOD is a possible risk factor for END, especially END during nighttime.
Nocturnal desaturation during SDB may decrease cerebral perfusion and cardiac function, and may trigger compensatory blood pressure surges.22–24 In addition, the hypoxia induced by SDB could impair cerebral autoregulation and activated platelet aggregability.6,22–24 These mechanisms might have led to the development of END during nighttime in our patient. In previous papers, END rate was different based on stroke mechanisms; LAA and SVO were more associated with END.1,25,26 However, we could not find any difference in terms of stroke mechanisms among patients with and without END, although patients with END had a slightly higher proportion of SVO. In patients with LAA, the degree of stenosis in the parent artery may be associated with the development of END.1,22,27 However, in our patients, the degree of stenosis was not significantly different in patients with END compared with those without END. It remains to be determined how NOD differently affects neurological deterioration in patients with various stroke mechanisms. The beneficial effect of SU care has been validated previously.28–30 In this study, continuous monitoring of pulse oximetry was useful in selecting high-risk patients for neurologic deterioration at nighttime. Pulse oximetry is a simple and validated monitoring tool in the SU. If analyzed appropriately, pulse oximetry might provide important information regarding NOD, and might be more easily applicable to acute stroke patients compared with PSG, which is usually performed on highly select patients.
There are several limitations to our study. First, this is a retrospective study based on prospectively collected data during hospitalization. Moreover, we did not measure true sleep duration during monitoring at the SU. In addition, we did not have information on a previous history of sleep apnea in our patients. Therefore, a certain degree of bias is inevitable. Second, although patients with high infarct volume possibly have a higher risk for neurologic deterioration,1,31 volumetric analysis of stroke lesion was not performed. However, the stroke severity, assessed by initial NIHSS score, was not different between the two study groups. Third, we calculated ODI using continuous pulse oximetry data on the first night at the SU. The data resolution of SpO2 was measured every 2 seconds and averaged over 1 minute, which might have affected the lower sensitivity of NOD than PSG in our patients. Moreover, desaturation level at the time when END occurred does not exist in our database. Although patients with SDB tended to show similar desaturation patterns in every sleep spell, the direct temporal relationship between desaturation and END was not available in our data. Fourth, the stroke severity of our patients was relatively mild (median NIHSS score of 3) compared with other studies. However, this result was consistent with our country's nationwide registry data of ischemic stroke (median initial NIHSS score of 3 [1–8]) rather than a selection bias.32 Moreover, severe stroke patients usually were admitted to the NeuroICU in our institution, and those with desaturation (median NIHSS 13) were also excluded because they were treated with oxygen therapy from the beginning (n = 15). However, baseline characteristics between included and excluded patients, including END rate, were not different between the two groups except for the higher prevalence of diabetes mellitus in excluded patients (Table S5 in the supplemental material). With these limitations, however, we think that our data are valid in presenting a correlation between NOD and “END during nighttime” in consecutive patients who were admitted to the SU.
In conclusion, NOD in the SU is associated with END, especially END during nighttime, in patients with acute ischemic stroke. Further large-scale studies on intervening NOD are needed to confirm our findings.
DISCLOSURE STATEMENT
This study was supported in part by the Seoul National University Research Fund (0420120950) and in part by National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013M3A9B2076531). The funding organization had no role in the study design, conduct, and analyses conducted during this study or the preparation. The authors report no conflicts of interest. All authors have read and approved the submitted manuscript.
ABBREVIATIONS
- BMI
body mass index
- CHF
congestive heart failure
- COPD
chronic obstructive pulmonary disease
- CT
computed tomography
- DBP
diastolic blood pressure
- END
early neurological deterioration
- Hb
hemoglobin
- HDL
high-density lipoprotein
- LAA
large-artery atherosclerosis
- MBP
mean blood pressure
- MRI
magnetic resonance imaging
- NIHSS
National Institutes of Health Stroke Scale
- NOD
nocturnal oxygen desaturation
- ODI
oxygen desaturation index
- PSG
polysomnography
- SBP
systolic blood pressure
- SDB
sleep-disordered breathing
- SU
stroke unit
- SVO
small-vessel occlusion
- TG
triglyceride
- TIA
transient ischemic attack
- WBC
white blood cell
REFERENCES
- 1.Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J. 2008;84(994):412–417. doi: 10.1136/pgmj.2007.066118. [DOI] [PubMed] [Google Scholar]
- 2.Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. QJM. 2006;99(9):625–633. doi: 10.1093/qjmed/hcl082. [DOI] [PubMed] [Google Scholar]
- 3.Palamarchuk I, Kimpinski K, Lippert C, Hachinski V. Nocturnal deterioration after ischemic stroke and autonomic dysfunction: hypothesis and implications. Cerebrovasc Dis. 2013;36(5-6):454–461. doi: 10.1159/000356093. [DOI] [PubMed] [Google Scholar]
- 4.Miyamoto N, Tanaka Y, Ueno Y, et al. Demographic, clinical, and radiologic predictors of neurologic deterioration in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2013;22(3):205–210. doi: 10.1016/j.jstrokecerebrovasdis.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 5.Siegler JE, Martin-Schild S. Early neurological deterioration (END) after stroke: the END depends on the definition. Int J Stroke. 2011;6(3):211–212. doi: 10.1111/j.1747-4949.2011.00596.x. [DOI] [PubMed] [Google Scholar]
- 6.Iranzo A, Santamaría J, Berenguer J, Sánchez M, Chamorro A. Prevalence and clinical importance of sleep apnea in the first night after cerebral infarction. Neurology. 2002;26;58(6):911–916. doi: 10.1212/wnl.58.6.911. [DOI] [PubMed] [Google Scholar]
- 7.Weimar C, Mieck T, Buchthal J, Ehrenfeld CE, Schmid E, Diener HC. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol. 2005;62(3):393–397. doi: 10.1001/archneur.62.3.393. [DOI] [PubMed] [Google Scholar]
- 8.Roffe C. Hypoxaemia and stroke. Rev Clin Gerontol. 2001;11:323–335. [Google Scholar]
- 9.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;16;285(19):2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Vijan S. In the clinic. Type 2 diabetes. Ann Intern Med. 2010;152(5):ITC31–15. doi: 10.7326/0003-4819-152-5-201003020-01003. [DOI] [PubMed] [Google Scholar]
- 12.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 13.Brott T, Adams HP, Jr, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870. doi: 10.1161/01.str.20.7.864. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto N, Kimura K, Yokota C, et al. Early neurological deterioration represents recurrent attack in acute small non-lacunar stroke. J Neurol Sci. 2004;217(2):151–155. doi: 10.1016/j.jns.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi M, Fujimoto K, Urushibata K, Uchikawa S, Imamura H, Kubo K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest. 2003;124(3):936–941. doi: 10.1378/chest.124.3.936. [DOI] [PubMed] [Google Scholar]
- 16.Maziere S, Pepin JL, Siyanko N, et al. Usefulness of oximetry for sleep apnea screening in frail hospitalized elderly. J Am Med Dir Assoc. 2014;15(6):447.e9–e14. doi: 10.1016/j.jamda.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y. Oxygen desaturation index from nocturnal oximetry: a sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg. 2012;114(5):993–1000. doi: 10.1213/ANE.0b013e318248f4f5. [DOI] [PubMed] [Google Scholar]
- 18.Kim TJ, Ko SB, Jeong HG, et al. Nocturnal desaturation in the stroke unit is associated with wake-up ischemic stroke. Stroke. 2016;47(7):1748–1753. doi: 10.1161/STROKEAHA.116.013266. [DOI] [PubMed] [Google Scholar]
- 19.Kang DW, Latour LL, Chalela JA, Dambrosia J, Warach S. Early ischemic lesion recurrence within a week after acute ischemic stroke. Ann Neurol. 2003;54(1):66–74. doi: 10.1002/ana.10592. [DOI] [PubMed] [Google Scholar]
- 20.Alawneh JA, Moustafa RR, Baron JC. Hemodynamic factors and perfusion abnormalities in early neurological deterioration. Stroke. 2009;40(6):e443–e450. doi: 10.1161/STROKEAHA.108.532465. [DOI] [PubMed] [Google Scholar]
- 21.Terasawa Y, Iguchi Y, Kimura K, et al. Neurological deterioration in small vessel disease may be associated with increase of infarct volume. J Neurol Sci. 2008;269(1-2):35–40. doi: 10.1016/j.jns.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Kwon HM, Lim JS, Park HK, Lee YS. Hypertriglyceridemia as a possible predictor of early neurological deterioration in acute lacunar stroke. J Neurol Sci. 2011;309(1-2):128–130. doi: 10.1016/j.jns.2011.06.057. [DOI] [PubMed] [Google Scholar]
- 23.Gibson GJ. Sleep disordered breathing and the outcome of stroke. Thorax. 2004;59(5):361–363. doi: 10.1136/thx.2003.020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diomedi M, Placidi F, Cupini LM, Bernardi G, Silvestrini M. Cerebral hemodynamic changes in sleep apnea syndrome and effect of continuous positive airway pressure treatment. Neurology. 1998;51(4):1051–1056. doi: 10.1212/wnl.51.4.1051. [DOI] [PubMed] [Google Scholar]
- 25.Terasawa Y, Iguchi Y, Kimura K, et al. Neurological deterioration in small vessel disease may be associated with increase of infarct volume. J Neurol Sci. 2008;269(1-2):35–40. doi: 10.1016/j.jns.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 26.Steinke W, Ley SC. Lacunar stroke is the major cause of progressive motor deficits. Stroke. 2002;33(6):1510–1516. doi: 10.1161/01.str.0000016326.78014.fe. [DOI] [PubMed] [Google Scholar]
- 27.Tei H, Uchiyama S, Ohara K, Kobayashi M, Uchiyama Y, Fukuzawa M. Deteriorating ischemic stroke in 4 clinical categories classified by the Oxfordshire Community Stroke Project. Stroke. 2000;31(9):2049–2054. doi: 10.1161/01.str.31.9.2049. [DOI] [PubMed] [Google Scholar]
- 28.Stroke Unit Trialists' Collaboration. Organised inpatient (stroke unit) care for stroke. Cochrane Database Syst Rev. 2013;(9):CD000197. doi: 10.1002/14651858.CD000197.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Candelise L, Gattinoni M, Bersano A, et al. Stroke-unit care for acute stroke patients: an observational follow-up study. Lancet. 2007;369(9558):299–305. doi: 10.1016/S0140-6736(07)60152-4. [DOI] [PubMed] [Google Scholar]
- 30.Ko SB. Multimodality monitoring in the neurointensive care unit: a special perspective for patients with stroke. J Stroke. 2013;15(2):99–108. doi: 10.5853/jos.2013.15.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong DC, Yenari MA, Albers GW, O Brien M, Marks MP, Moseley ME. Correlation of perfusion-and diffusion-weighted MRI with NIHSS score in acute (< 6.5 hour) ischemic stroke. Neurology. 1998;50(4):864–869. doi: 10.1212/wnl.50.4.864. [DOI] [PubMed] [Google Scholar]
- 32.Kim BJ, Park JM, Kang K, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J Stroke. 2015;17(1):38–53. doi: 10.5853/jos.2015.17.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


