Abstract
Study Objectives:
Neurocognitive deficits have been shown in school-aged children with sleep apnea. The effect of obstructive sleep apnea (OSA) on the neurodevelopmental outcome of preterm infants is unknown.
Methods:
A retrospective chart review was performed for all preterm infants (< 37 weeks) who had neonatal polysomnography (PSG) and completed neurodevelopmental assessment with the Bayley Scales of Infant and Toddler Development, 3rd Edition, between 2006 to 2015 at Riley Hospital. Exclusion criteria included grade IV intraventricular hemorrhage, tracheostomy, cyanotic heart disease, severe retinopathy of prematurity, craniofacial anomalies, or central and mixed apnea on PSG. Sleep apnea was defined as an apnea-hypopnea index (AHI) > 1 event/h. Regression analyses were performed to find a relationship between PSG parameters and cognitive, language, and motor scores.
Results:
Fifteen patients (males: n = 10) were eligible for the study. Median postmenstrual age at the time of the PSG was 41 weeks (37–46). Median AHI for the cohort was 17.4 events/h (2.2–41.3). Median cognitive, language, and motor scores were 90 (65–125), 89 (65–121), and 91 (61–112), respectively. Mean end-tidal CO2 (median 47 mm Hg [25–60]) negatively correlated with cognitive scores (P = .01) but did not significantly correlate with language or motor scores. AHI was not associated with cognitive, language, or motor scores.
Conclusions:
The median score for cognitive, language, and motor scores for preterm infants with neonatal OSA were within one standard deviation of the published norm. Mean end-tidal CO2, independent of AHI, may serve as a biomarker for predicting poor cognitive outcome in preterm infants with neonatal OSA.
Commentary:
A commentary on this article appears in this issue on page 1233.
Citation:
Bandyopadhyay A, Harmon H, Slaven JE, Daftary AS. Neurodevelopmental outcomes at two years of age for premature infants diagnosed with neonatal obstructive sleep apnea. J Clin Sleep Med. 2017;13(11):1311–1317.
Keywords: obstructive sleep apnea, polysomnography, infant, newborn
INTRODUCTION
Sleep plays an essential role in learning, memory consolidation, and cognition.1 Sleep-disordered breathing (SDB) results in periods of occlusion of the upper airway during sleep, often associated with oxygen desaturations, arousals, and impaired sleep quality. SDB in preschool children has been associated with impaired executive functioning, attention, receptive vocabulary, and behavioral problems.2 Even in the absence of obstructive sleep apnea (OSA) as diagnosed by polysomnography (PSG), SDB symptoms have been associated with reduced executive function and memory skills in 5-year-old children.3 Reduction in nocturnal arousals in preschool children with SDB was associated with improved attention and aggressive behavior.4 There is a strong association of SDB with deficits in cognitive abilities and academic achievement during school age, particularly in those who were born pre-term.5 Sleep apnea clearly has significant developmental and behavioral implications, and because children born preterm are more likely to receive a diagnosis of sleep apnea, compared to children born full term,6 it is important to assess the association of sleep apnea with neurocognitive outcomes in this population.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Preterm infants are an at-risk population for poor neurodevelopmental outcomes as well as sleep-disordered breathing (SDB). Sleep apnea has significant developmental and behavioral implications; however, the effect of neonatal SDB on the neurodevelopmental outcomes of preterm infants is unknown.
Study Impact: Although there is a high level of SDB in preterm infants, the median score for cognitive, language and motor scores were still within one standard deviation of the published norm. Mean end-tidal CO2, independent of apnea-hypopnea index, in polysomnography may be worth consideration as a biomarker for predicting poor cognitive outcome in this high-risk population.
Preterm infants are at high risk for suboptimal neurodevelopmental outcomes on account of a host of neonatal comorbidities and disrupted neuronal maturation.7 Risk factors for poor neurodevelopment include low gestational age, birth weight, presence of bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), severe neurological injury, pulmonary hypertension, sepsis, absence of antenatal steroids, patent ductus arteriosus (PDA) ligation, prolonged ventilation, prolonged total parenteral nutrition, and postnatal growth retardation.8–14 In addition to medical morbidities, preterm infants have reduced cerebral oxygenation during active sleep at post-term corrected age.15 Preterm infants with SDB and associated sleep disruption may be at increased risk of reduced cerebral oxygenation. However, the effect of neonatal OSA on the neurodevelopmental outcomes of preterm infants is unknown. Capnography during PSG is challenging,16 particularly in neonates. Although existing literature suggests no effect of permissive hypercapnia, during the first few days of life, on neurodevelopmental outcome,17,18 there are no published data on the effect of sustained hypercapnia during sleep on neuro-cognitive outcomes in preterm infants.
The aim of our study was to assess cognitive/motor and language development at 2 years of age in preterm infants with documented neonatal OSA. Our hypothesis was that the severity of neonatal OSA would correlate with worsening cognitive, motor, and language development.
METHODS
This is a retrospective study evaluating preterm infants (< 37 weeks) cared for at Riley Hospital for Children at IU Health who received neonatal PSG between January 2006 and August 2015 prior to discharge from the neonatal intensive care unit and completed neurodevelopmental assessment with the Bayley Scales of Infant and Toddler Development, 3rd Edition (BSID-III), at 2 years of age at the Indiana University Newborn Follow-up Program. Exclusion criteria included grade IV intraventricular hemorrhage, tracheostomy, severe retinopathy of prematurity (stage 4 and stage 5), cyanotic heart disease, or syndromic craniofacial anomalies.
After institutional review board approval, demographics, medical morbidities, and results of neurodevelopmental testing were obtained from the hospital medical records. Post-menstrual age was defined as the sum of birth gestational age and chronological age. For the children who participated in the National Institute of Child Health and Human Development Neonatal Research Network (NRN) General Database and Follow-up study (inborn infants < 1000 g and/or < 27 weeks) data were obtained from the NRN local registry and for those outside of this cohort, data was manually extracted from medical records. Each baby had undergone a polysomnogram prior to discharge from the neonatal intensive care unit. The indications for the PSG were clinical concern for significant upper airway obstruction or for oxygen titration in patients with BPD at term corrected age. BPD is one of the most common morbidities in premature neonates. Although multiple definitions have been proposed, one commonly accepted version includes oxygen dependence at 28 postnatal days and severity and stratification as mild, moderate, or severe at 36 weeks postconceptual age (gestational age < 32 weeks) or at 56 days (gestational age > 32 weeks) based on supplemental oxygen delivery (ie, room air, fraction of inspired oxygen (FiO2) 0.22–0.29, and FiO2 > 0.30, respectively).19 For the purpose of the study, we extracted the obstructive apnea-hypopnea index (AHI) from the medical records. Because most of the patients were on oxygen supplementation during the study, we did not include central or mixed apneas; their scoring was confounded by the presence of oxygen supplementation.
Polysomnography
PSGs were attended studies performed in the sleep laboratory and included the following monitoring parameters: electroencephalogram (EEG), electrooculogram, chin electromyogram, electrocardiogram, thermistor and pressure transducer airflow signals, respiratory inductance plethysmography or thoracic impedance measurement (prior to 2007), continuous oximetry, and end-tidal CO2 (ETCO2) monitoring. The collection software was Sandman v 9.0 (Embla systems LLC, Tonawanda, New York, United States). Oxyhemoglobin saturation was measured via pulse oximetry (Masimo, Irvine, California, United States). ETCO2 was measured at the nose using BCI Capnocheck (Smiths Medical PM, Inc Waukesha, Wisconsin, United States) with side-stream sampling. Nasal airflow was acquired with a nasal cannula (Salter Labs, Arvin, California, United States).
PSGs were staged per scoring guidelines from Anders et al.20 Before 2007, the institutional criteria followed for scoring respiratory events were: apnea for airflow limitation of 80% or greater from baseline for at least 2 breaths and hypopneas for airflow limitation of 30% or greater for at least 2 breaths duration associated with either ≥ 4% desaturation or an arousal. After 2007, the American Academy of Sleep Medicine (AASM) respiratory rules for children were followed for scoring respiratory events.21 Before 2007, institutional criteria were followed for scoring arousals and awakenings. During non-rapid eye movement (Quiet & Indeterminate) sleep, arousals were scored as change in EEG frequency from theta to alpha or beta or from delta to theta, alpha, or beta for 3 seconds to 15 seconds. After 15 seconds, the EEG change was scored as an awakening. All gross body movements were scored as either arousals or awakenings consistent with the duration of the event. In rapid eye movement (Active) sleep, in addition to an EEG frequency change, an additional elevation of electromyogram amplitude during the event, was also required. After 2007, arousal index was scored as per AASM scoring criteria.21 PSGs were reviewed and reported by board-certified pediatric sleep medicine physicians.
OSA was defined as AHI > 1 event/h. Severe sleep apnea was defined as AHI > 15 events/h. Data on mean ETCO2, mean oxygen saturations, arousal index, and sleep efficiency were collected. If the study was performed on oxygen, the mean saturations on oxygen support was reported. Because of the physiological limitations of rapid respiratory rate, low tidal volume, the higher effect of apparatus dead-space in neonates, and the ventilation perfusion mismatch that affects patients with BPD, we defined hypoventilation as a mean ETCO2 ≥ 45 mm Hg.
Supplemental Oxygen Protocol
Oxygen titration goals were to maintain oxygen saturation (SaO2) at 90% or greater. If the baseline SaO2 was less than 90%, for ≥ 5 minutes then supplemental oxygen was initiated and the level adjusted to maintain saturations > 90%.
Bayley Scales of Infant and Toddler Development
Toddler neurodevelopment was assessed with the BSID-III.22 This validated tool uses developmental play tasks to compare motor, language, and cognitive development compared to standardized age norms.
Statistics
Univariate regression analysis was performed to find a relationship between cognitive, language, and motor composite scores and neonatal course as well as PSG parameters (AHI and mean ETCO2). For significant outcomes on univariate analyses, we performed multivariate regression analysis. Pearson correlation was performed to find a relationship between AHI and mean ETCO2. We divided the group based on mean ETCO2 less than or equal to 45 mm Hg and compared the neonatal course, sleep study parameters and language, cognitive and motor scores between the 2 groups by Wilcoxon nonparametric rank-sum test and Fisher exact test, as applicable. Where conceptual models called for it, multivariable analyses were performed; although with the small sample size, models were performed with one covariate at a time using Bonferroni corrections to control for type I error. Analyses were performed with SAS v9.4 (SAS Institute, Cary, North Carolina, United States).
RESULTS
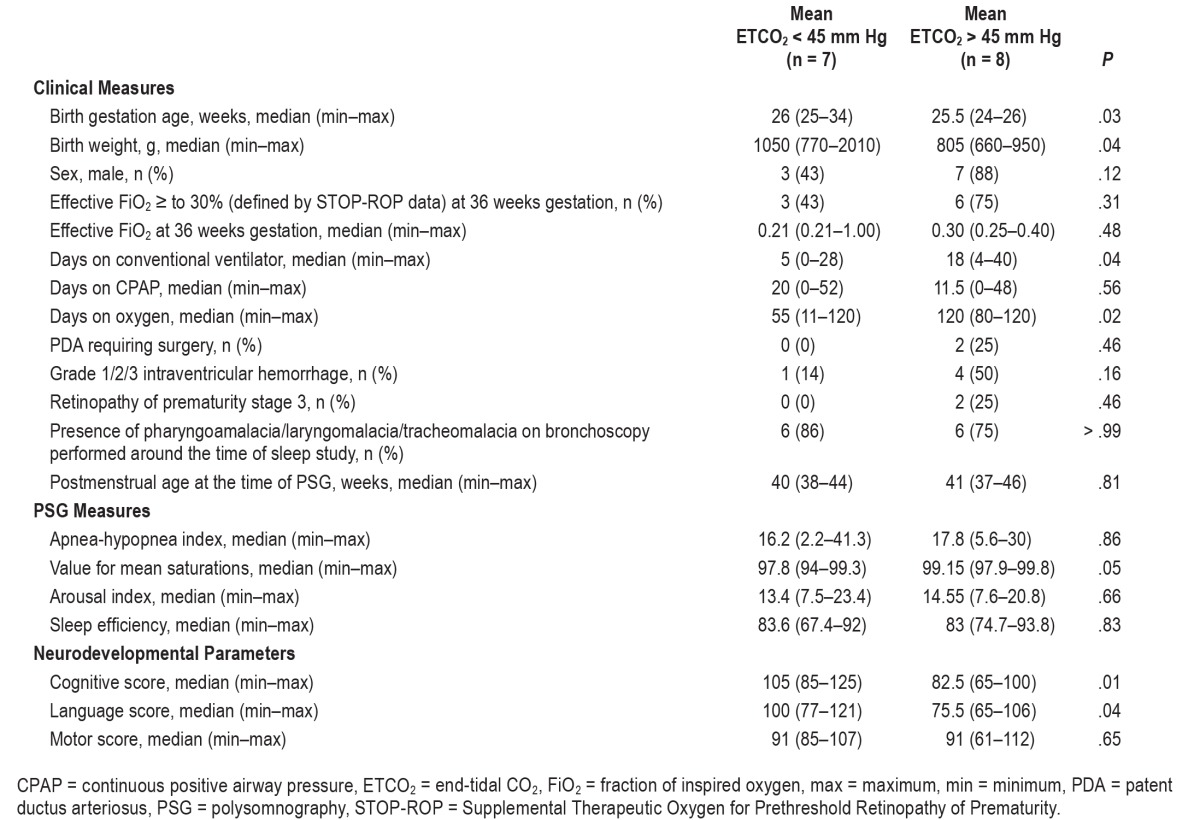
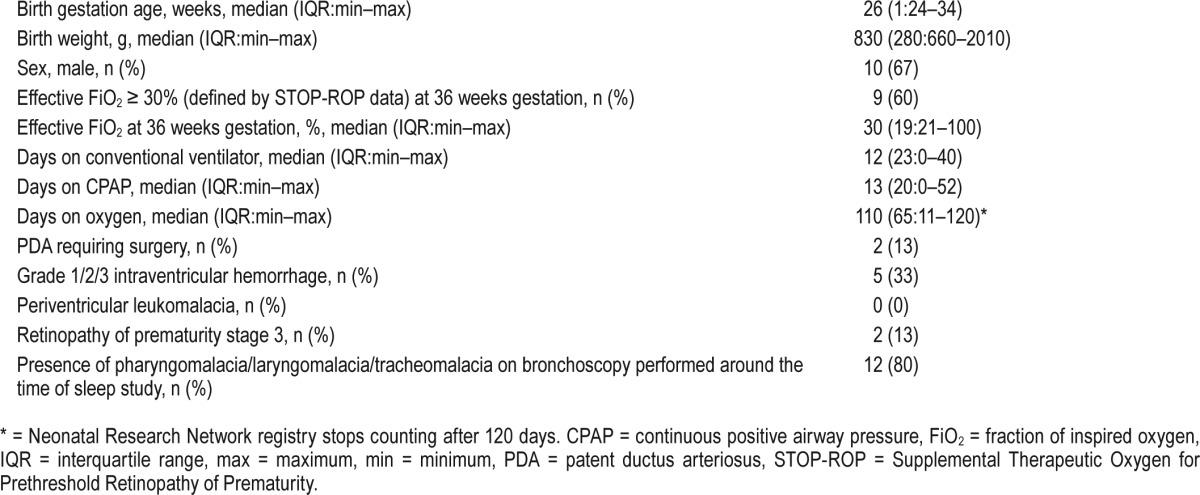
Fifteen patients (males: n = 10) were eligible for the study. Baseline characteristics and neonatal intensive care course has been described in Table 1. Eleven patients (73%) were on oxygen support during PSG. Median AHI for the cohort was 17.4 events/h (2.2–41.3). Median value of mean saturation was 99% (94% to 99.8%). Median arousal index was 14 events/h (7.5–23.4). Median sleep efficiency was 83% (67.4% to 93.8%). Median ETCO2 during PSG was 47 mm Hg (25–60). The sleep apnea was obstructive in nature. Median age at performance of BSID-III testing was 22 months (corrected for prematurity). Median cognitive composite score, language composite score, and motor composite score were 90 (interquartile range [IQR]:min-max 25: 65–125), 89 (IQR:min-max 32: 65–121) and 91 (IQR:min-max 16.75: 61–112, 1 missing data), respectively, for the entire study sample.
Table 1.
Baseline characteristics and neonatal course of the entire study population (n = 15).
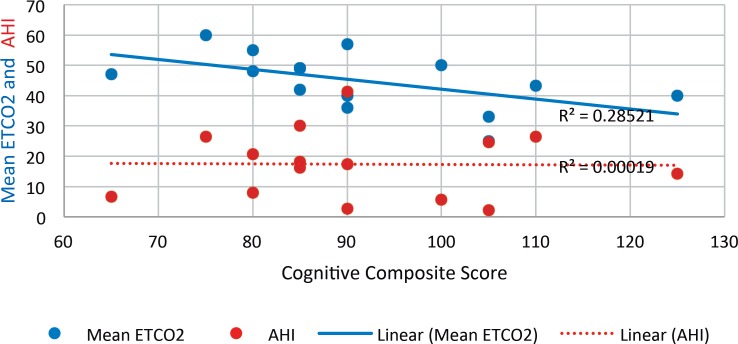
AHI and mean ETCO2 correlation did not reach statistical significance. Mean ETCO2 negatively correlated with cognitive scores (P = .04) but not language or motor scores (Figure 1). AHI did not correlate with cognitive, language, or motor scores. In a multivariable model (using one covariate at a time to maintain power) after adjusting for birth gestational age, days on ventilator, days on oxygen and birth weight, a higher mean ETCO2 was associated with lower cognitive scores (P = .01). Table 2 is a comparison of baseline characteristics, neonatal course, sleep study parameters, and language, motor, and cognitive scores in the study sample divided into 2 groups based on mean ETCO2 < 45 or > 45 mm Hg. Median language and cognitive scores were significantly lower in the group with mean ETCO2 > 45 mm Hg.
Figure 1. Relationship between cognitive composite score versus AHI and mean ETCO2.
AHI = apnea-hypopnea index, ETCO2 = end-tidal CO2.
Table 2.
Comparison of baseline characteristics, neonatal course and sleep study parameters in the two groups based on mean ETCO2 < 45 mm Hg.
DISCUSSION
This study suggests a high level of sleep apnea in preterm infants with a high prevalence of BPD. Ours is the first study to explore the relationship between OSA and cognitive outcomes in preterm infants. Our study shows that the mean ETCO2 was associated with lower cognitive scores. A high mean ETCO2 during PSG could potentially serve as a biomarker for predicting lower cognitive outcomes in this high-risk population. However, the median score for all 3 composite scores was still within one standard deviation of the published norm. There have been concerns that BSID-III testing may underestimate developmental delay.23,24 In the light of this knowledge, our results may be underestimating poor cognitive, language, and motor outcome in these infants.
Despite excluding infants with severe neurological injury, tracheostomy, and severe retinopathy of prematurity, our patients remained highly morbid. Sixty percent of our patients were on greater than 30% FiO2 at 36 weeks of corrected age, consistent with severe BPD. Infants with BPD are known to suffer frequent episodes of hypoxemia and desaturations,25 which could impair the development of cognitive functions.26 Additionally, 80% of our patients had some form of airway obstruction confirmed by airway endoscopy (pharyngo/laryngo/ tracheomalacia) at the time of sleep study. Our patients had an elevated median AHI compared to published norms for children,27 which supports existing data that preterm infants are more likely to have SDB.5,6,28 This may be multifactorial, including upper airway obstruction29 and/or chronic lung parenchymal involvement with low pulmonary reserve. Our patients also had an elevated mean ETCO2, which could result from a combination of airway obstruction and ventilation perfusion mismatch secondary to BPD.
Seventy-three percent of our patients were on oxygen at the time of neonatal PSG. This may have led to underestimation of their uncorrected mean oxygen saturations. However, oxygen supplementation typically will not prevent obstructive episodes from occurring and the infant may still experience upper airway obstructive events resulting in arousals and sleep fragmentation. As these patients were continuously being treated with oxygen, it is unlikely that prolonged uncorrected hypoxia contributed to the neurocognitive effect in our study. Our patients had increased AHI and arousal index as well as poor sleep efficiency, suggesting that despite oxygen supplementation, preterm neonates are at risk for adverse outcomes of sleep apnea. A review of the literature does not reveal normative data on arousal indices of neonates. In a study by Traeger et al.,30 arousal index in 2- to 9-year-old children was reported as 8.8 ± 3.8 events/h (2.8–18.3). Our cohort had a higher arousal index with a sleep efficiency of 83%. Normative data on neonatal PSG is important to decide whether this elevated arousal index is a function of age or a manifestation of OSA. Regardless, this poor fragmentation of sleep may certainly affect cognitive outcomes.
It is known that sleep apnea can cause impaired executive functioning and behavior problems. Functional magnetic resonance imaging data in adults have suggested that SDB significantly alters functional connectivity in the cerebellar, frontal, parietal, temporal, occipital, limbic and basal ganglia regions. This impairment in network organization may result in altered responses in autonomic, cognitive, and sensorimotor functions.31 Our results did not show any association between AHI and composite scores. Despite the significantly elevated AHI of our study sample, the cognitive scores were within one standard deviation of published norms. Although Ng and Chan32 have reviewed normative data on infant PSG, the reviewed studies do not include neonates. Therefore, what is considered severe OSA in the older child and is currently being extrapolated to neonates in clinical practice may not apply to neonates.33 However, we were limited by small sample size.
AHI and mean ETCO2 did not correlate significantly in our study, which is consistent with existing literature.34 It has been suggested that the utility of nocturnal capnometry may go beyond apnea detection and quantification of hypoventilation syndromes. Instead it may reflect the balance of apnea and postapnea duration, which are not captured by AHI.35 Jaimchariyatam et al. suggested that nocturnal elevation of CO2 may be due to a ventilatory impairment in the balance between accrual of CO2 (longer apnea duration) and more importantly the unloading of CO2 (longer postapnea duration). This study proposed use of exhaled CO2 as a physiological marker of disease severity that is independent of AHI.35
Our study had a high median ETCO2 (47, 25–60 mm Hg). Sixty percent of our patient sample had BPD. Permissive hypercapnia-intentionally allowing alveolar hypoventilation and increased partial pressure of carbon dioxide (PaCO2) has been advocated as a means to decrease ventilator-induced lung injury and BPD.36 Existing literature suggests no effect of permissive hypercapnia on neurodevelopmental outcome.17,18 Additionally, the Childhood Adenotonsillectomy, or CHAT, trial did not report any correlation of percentage of total sleep time (TST) with ETCO2 > 50 mm Hg with changes on the cognitive and behavioral assessment at median age of 7.1 years. The median ETCO2, while asleep, of that study sample was 45 mm Hg,34 which is a little lower than in our study. However, our study did show a significant association between nocturnal hypercarbia and cognitive composite scores. There are two significant points to consider. Most of the cited literature has correlated TST above ETCO2 > 50 mm Hg with neurodevelopmental outcome. In our study, we have utilized a separate measure (mean ETCO2) for correlation. The second point of interest is that in the study on infants with permissive hyper-capnia,18 neurocognitive outcomes were correlated to arterial CO2 levels only within the first 14 days of life. However, in our study, ETCO2 was estimated at a median chronological age of 4 months. Studies have demonstrated in adults that the current ETCO2 sampling devices may underestimate arterial CO2 by 5–10 mm Hg, based on the pathophysiology of the patient.16,37,38 This underestimation may be greater in children, who physiologically have a higher respiratory rate, which may be further worsened with BPD. Additionally, the dilutional effect of any concurrent oxygen supplementation during ETCO2 measurement could also have played a role. Thus, the arterial PaCO2 may have been much higher in our sample of preterm infants, even when corrected to full term. An alternate definition from current polysomnographic pediatric hypoventilation criteria may therefore be necessary in neonates and young infants.
SDB encompasses a wide spectrum ranging from primary snoring to OSA. As per AASM guidelines, PSG criteria for pediatric hypoventilation have been defined as at least 25% of TST with hypercapnia (PaCO2 > 50 mm Hg). However, we did not have consistent reporting of percentage of TST with hypercapnia in all PSG. We therefore used mean ETCO2 as a surrogate for hypoventilation. Paruthi et al. demonstrated that percentage TST ETCO2 > 50 mm Hg did not correlate with changes in cognitive or behavioral measures at 5 to 9.9 years of age.34 Data on prematurity were not available for this study sample.34 Based on our study results, mean ETCO2 may be a more sensitive marker to detect cognitive outcome and its role should be explored further, particularly in the neonatal age group.
Limitations
Our study comprises a small cohort of preterm infants due to strict exclusion criteria and to exclude the confounding effects of known predictors of poor neurodevelopmental outcome. We did not have data on a repeat PSG prior to BSID-III testing to confirm persistence or resolution of SDB in the interim. We did not have data on any interventions, such as adenoidectomy or tonsillectomy, performed between the PSG and BSID-III testing. We were unable to correlate ETCO2 with blood gas CO2 level at the time of PSG, as these data were unavailable. The coadministration of oxygen with CO2 during neonatal PSG could have introduced a dilutional effect of ETCO2 estimation. These are limitations of a retrospective study with access to existing available data. We also did not have consistent data on maternal education or socioeconomic status in our patients, which could play a role in the neurodevelopmental outcomes. Finally, this is the experience of a single center with institutional practice biases that could affect the study results.
CONCLUSIONS
In conclusion, there is a high prevalence of SDB in preterm infants with dynamic airway collapse and bronchopulmonary dysplasia in the neonatal period. Mean ETCO2 negatively correlates with cognitive outcome. Preterm neonates with SDB should be closely monitored for cognitive delays. This study highlights the importance of normative data on neonatal sleep apnea to better interpret thresholds of clinical significance.
DISCLOSURE STATEMENT
All authors have seen and approved this manuscript. Some of the original data collection was supported by grants from the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) for the Neonatal Research Network, including for the Generic Database Study, and follow-up visit of high risk infants. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors report no conflicts of interest. This work was presented as a thematic poster presentation at Sleep Conference, June 13, 2016, Denver, Colorado.
ACKNOWLEDGMENTS
The authors are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AHI
apnea-hypopnea index
- BSID
Bayley Scales of Infant and Toddler Development
- BPD
bronchopulmonary dysplasia
- CO2
carbon dioxide
- EEG
electroencephalogram
- ETCO2
end-tidal CO2
- FiO2
fraction of inspired oxygen
- NEC
necrotizing enterocolitis
- NRN
Neonatal Research Network
- OSA
obstructive sleep apnea
- PaCO2
partial pressure of carbon dioxide
- PDA
patent ductus arteriosus
- PSG
polysomnography
- SDB
sleep-disordered breathing
- TST
total sleep time
REFERENCES
- 1.Walker MP. The role of sleep in cognition and emotion. Ann N Y Acad Sci. 2009;1156:168–197. doi: 10.1111/j.1749-6632.2009.04416.x. [DOI] [PubMed] [Google Scholar]
- 2.Landau YE, Bar-Yishay O, Greenberg-Dotan S, Goldbart AD, Tarasiuk A, Tal A. Impaired behavioral and neurocognitive function in preschool children with obstructive sleep apnea. Pediatr Pulmonol. 2012;47(2):180–188. doi: 10.1002/ppul.21534. [DOI] [PubMed] [Google Scholar]
- 3.Gottlieb DJ, Chase C, Vezina RM, et al. Sleep-disordered breathing symptoms are associated with poorer cognitive function in 5-year-old children. J Pediatr. 2004;145(4):458–464. doi: 10.1016/j.jpeds.2004.05.039. [DOI] [PubMed] [Google Scholar]
- 4.Biggs SN, Walter LM, Jackman AR, et al. Long-term cognitive and behavioral outcomes following resolution of sleep disordered breathing in preschool children. PLoS One. 2015;10(9):e0139142. doi: 10.1371/journal.pone.0139142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Emancipator JL, Storfer-Isser A, Taylor HG, et al. Variation of cognition and achievement with sleep-disordered breathing in full-term and preterm children. Arch Pediatr Adolesc Med. 2006;160(2):203–210. doi: 10.1001/archpedi.160.2.203. [DOI] [PubMed] [Google Scholar]
- 6.Raynes-Greenow CH, Hadfield RM, Cistulli PA, Bowen J, Allen H, Roberts CL. Sleep apnea in early childhood associated with preterm birth but not small for gestational age: a population-based record linkage study. Sleep. 2012;35(11):1475–1480. doi: 10.5665/sleep.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8(1):110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambalavanan N, Baibergenova A, Carlo WA, Saigal S, Schmidt B, Thorpe KE. Early prediction of poor outcome in extremely low birth weight infants by classification tree analysis. J Pediatr. 2006;148(4):438–444.e431. doi: 10.1016/j.jpeds.2005.11.042. [DOI] [PubMed] [Google Scholar]
- 9.Asztalos EV, Church PT, Riley P, Fajardo C, Shah PS. Neonatal factors associated with a good neurodevelopmental outcome in very preterm infants. Am J Perinatol. 2017;34(4):388–396. doi: 10.1055/s-0036-1592129. [DOI] [PubMed] [Google Scholar]
- 10.Nakanishi H, Uchiyama A, Kusuda S. Impact of pulmonary hypertension on neurodevelopmental outcome in preterm infants with bronchopulmonary dysplasia: a cohort study. J Perinatol. 2016;36(10):890–896. doi: 10.1038/jp.2016.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alshaikh B, Yusuf K, Sauve R. Neurodevelopmental outcomes of very low birth weight infants with neonatal sepsis: systematic review and meta-analysis. J Perinatol. 2013;33(7):558–564. doi: 10.1038/jp.2012.167. [DOI] [PubMed] [Google Scholar]
- 12.Bourgoin L, Cipierre C, Hauet Q, et al. Neurodevelopmental outcome at 2 years of age according to patent ductus arteriosus management in very preterm infants. Neonatology. 2016;109(2):139–146. doi: 10.1159/000442278. [DOI] [PubMed] [Google Scholar]
- 13.Belfort MB, Rifas-Shiman SL, Sullivan T, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128(4):e899–e906. doi: 10.1542/peds.2011-0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meyers JM, Bann CM, Stoll BJ, et al. Neurodevelopmental outcomes in postnatal growth-restricted preterm infants with postnatal head-sparing. J Perinatol. 2016;36(12):1116–1121. doi: 10.1038/jp.2016.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decima PF, Fyfe KL, Odoi A, Wong FY, Horne RS. The longitudinal effects of persistent periodic breathing on cerebral oxygenation in preterm infants. Sleep Med. 2015;16(6):729–735. doi: 10.1016/j.sleep.2015.02.537. [DOI] [PubMed] [Google Scholar]
- 16.Sanders MH, Kern NB, Costantino JP, et al. Accuracy of end-tidal and transcutaneous PCO2 monitoring during sleep. Chest. 1994;106(2):472–483. doi: 10.1378/chest.106.2.472. [DOI] [PubMed] [Google Scholar]
- 17.Carlo WA, Stark AR, Wright LL, et al. Minimal ventilation to prevent bronchopulmonary dysplasia in extremely-low-birth-weight infants. J Pediatr. 2002;141(3):370–375. doi: 10.1067/mpd.2002.127507. [DOI] [PubMed] [Google Scholar]
- 18.Thome UH, Genzel-Boroviczeny O, Bohnhorst B, et al. Neurodevelopmental outcomes of extremely low birthweight infants randomised to different PCO2 targets: the PHELBI follow-up study. Arch Dis Child Fetal Neonatal Ed. 2017;102(5):F376–F382. doi: 10.1136/archdischild-2016-311581. [DOI] [PubMed] [Google Scholar]
- 19.Baraldi E, Carraro S, Filippone M. Bronchopulmonary dysplasia: definitions and long-term respiratory outcome. Early Hum Dev. 2009;85(10):S1–S3. doi: 10.1016/j.earlhumdev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Anders TF, Emde RN, Parmelee AH. A Manual of Standardized Terminology, Techniques and Criteria for Scoring of States of Sleep and Wakefulness in Newborn Infants. Los Angeles, CA: UCLA Brain Information Service/BRI Publications Office, NINDS Neurological Information Network; 1971. [Google Scholar]
- 21.American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules. Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [PMC free article] [PubMed] [Google Scholar]
- 22.Bayley N. Bayley Scales of Infant and Toddler Development. San Antonio, TX: Harcourt Assessment, Psychological Corp; 2006. [Google Scholar]
- 23.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW. Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164(4):352–356. doi: 10.1001/archpediatrics.2010.20. [DOI] [PubMed] [Google Scholar]
- 24.Spittle AJ, Spencer-Smith MM, Eeles AL, et al. Does the Bayley-III Motor Scale at 2 years predict motor outcome at 4 years in very preterm children? Dev Med Child Neurol. 2013;55(5):448–452. doi: 10.1111/dmcn.12049. [DOI] [PubMed] [Google Scholar]
- 25.Sekar K, Duke JC. Sleep apnea and hypoxemia in recently weaned premature infants with and without bronchopulmonary dysplasia. Pediatr Pulmonol. 1991;10(2):112–116. doi: 10.1002/ppul.1950100213. [DOI] [PubMed] [Google Scholar]
- 26.Goldstein RF, Thompson RJ, Oehler JM, Brazy JE. Influence of acidosis, hypoxemia, and hypotension on neurodevelopmental outcome in very low birth weight infants. Pediatrics. 1995;95(2):238–243. [PubMed] [Google Scholar]
- 27.Mindell JA, Owens JA. A Clinical Guide to Pediatric Sleep: Diagnosis and Management of Sleep Problems. 3rd ed. Philadelphia, PA: Wolters Kluwer; 2015. [Google Scholar]
- 28.Abu-Shaweesh JM, Martin RJ. Neonatal apnea: what's new? Pediatr Pulmonol. 2008;43(10):937–944. doi: 10.1002/ppul.20832. [DOI] [PubMed] [Google Scholar]
- 29.Gobbi R, Baiardi S, Mondini S, et al. Technique and preliminary analysis of drug-induced sleep endoscopy with online polygraphic cardiorespiratory monitoring in patients with obstructive sleep apnea syndrome. JAMA Otolaryngol Head Neck Surg. 2017;143(5):459–465. doi: 10.1001/jamaoto.2016.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traeger N, Schultz B, Pollock AN, Mason T, Marcus CL, Arens R. Polysomnographic values in children 2-9 years old: additional data and review of the literature. Pediatr Pulmonol. 2005;40(1):22–30. doi: 10.1002/ppul.20236. [DOI] [PubMed] [Google Scholar]
- 31.Park B, Palomares JA, Woo MA, et al. Disrupted functional brain network organization in patients with obstructive sleep apnea. Brain Behav. 2016;6(3):e00441. doi: 10.1002/brb3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng DK, Chan CH. A review of normal values of infant sleep polysomnography. Pediatr Neonatol. 2013;54(2):82–87. doi: 10.1016/j.pedneo.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Sheldon SH, Ferber R, Kryger MH. Principles and Practice of Pediatric Sleep Medicine. 1st ed. Philadelphia, PA: Elsevier, Saunders; 2005. [Google Scholar]
- 34.Paruthi S, Rosen CL, Wang R, et al. End-tidal carbon dioxide measurement during pediatric polysomnography: signal quality, association with apnea severity, and prediction of neurobehavioral outcomes. Sleep. 2015;38(11):1719–1726. doi: 10.5665/sleep.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jaimchariyatam N, Dweik RA, Kaw R, Aboussouan LS. Polysomnographic determinants of nocturnal hypercapnia in patients with sleep apnea. J Clin Sleep Med. 2013;9(3):209–215. doi: 10.5664/jcsm.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thome UH, Ambalavanan N. Permissive hypercapnia to decrease lung injury in ventilated preterm neonates. Semin Fetal Neonatal Med. 2009;14(1):21–27. doi: 10.1016/j.siny.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 37.Stock MC. Capnography for adults. Crit Care Clin. 1995;11(1):219–232. [PubMed] [Google Scholar]
- 38.Kasuya Y, Akça O, Sessler DI, Ozaki M, Komatsu R. Accuracy of postoperative end-tidal Pco2 measurements with mainstream and sidestream capnography in non-obese patients and in obese patients with and without obstructive sleep apnea. Anesthesiology. 2009;111(3):609–615. doi: 10.1097/ALN.0b013e3181b060b6. [DOI] [PubMed] [Google Scholar]