Abstract
Study Objectives:
Despite the overall improvement in posttraumatic stress disorder (PTSD) symptomatology with continuous positive airway pressure (CPAP) therapy, adherence to CPAP is far worse in veterans with PTSD compared to the general population with obstructive sleep apnea (OSA). The aim of this study was to compare the efficacy, adherence, and preference of CPAP versus mandibular advancement device (MAD) and the effect of these treatments on health outcomes in veterans with PTSD.
Methods:
Forty-two subjects with PTSD and newly diagnosed OSA by polysomnography were treated in a randomized, crossover trial of 12 weeks with CPAP alternating with MAD separated by a 2-week washout period. The primary outcome was the difference in titration residual apnea-hypopnea index (AHI) between CPAP and MAD. Secondary outcome measures included PTSD Checklist and health-related quality of life (Medical Outcomes Study 36-Item Short Form and Pittsburgh Sleep Quality Index).
Results:
Analyses were limited to the 35 subjects (mean age 52.7 ± 11.6 years) who completed the trial, regardless of compliance with their assigned treatment. CPAP was more efficacious in reducing AHI and improving nocturnal oxygenation than MAD (P < .001 and P = .04, respectively). Both treatments reduced PTSD severity and ameliorated scores of the Medical Outcomes Study Short Form 36 and Pittsburgh Sleep Quality Index, although no differences were detected between the CPAP and MAD arms. The reported adherence to MAD was significantly higher than CPAP (P < .001), with 58% preferring MAD to CPAP.
Conclusions:
Although CPAP is more efficacious than MAD at improving sleep apnea, both treatment modalities imparted comparable benefits for veterans with PTSD in relation to PTSD severity and health-related quality of life. MAD offers a viable alternative for veterans with OSA and PTSD who are nonadherent to CPAP.
Clinical Trial Registration:
Title: A Randomized Cross Over Trial of Two Treatments for Sleep Apnea in Veterans With Post-Traumatic Stress Disorder; URL: https://www.clinicaltrials.gov/ct/show/NCT01569022; Identifier: NCT01569022
Citation:
El-Solh AA, Homish GG, Ditursi G, Lazarus J, Rao N, Adamo D, Kufel T. A randomized crossover trial evaluating continuous positive airway pressure versus mandibular advancement device on health outcomes in veterans with posttraumatic stress disorder. J Clin Sleep Med. 2017;13(11):1327–1335
Keywords: continuous positive airway pressure, health outcomes, mandibular advancement device, posttraumatic stress disorder, randomized crossover trial
INTRODUCTION
Sleep-related breathing disorders are frequently encountered among United States veterans with posttraumatic stress disorder (PTSD).1 Veterans having PTSD suffer from nonrestorative sleep and nightmares leading to heightened state of arousal and anxiety, increased severity of depression, and poor quality of life.2 Accruing evidence suggests that patients with PTSD are at higher risk for sleep-disordered breathing than the general population.3,4 In a series of studies looking at postdeployment combat veterans with PTSD, rates of overall sleep disturbance symptoms approached 90%, with up to 70% considered to be at high risk for OSA.5,6 Concomitant sleep disorders have been shown to independently worsen outcomes. Compared with patients without sleep complaints, patients with PTSD and coexisting sleep disorders experience higher rates of suicidality,7 psychiatric distress,3 and substance abuse.
BRIEF SUMMARY
Current Knowledge/Study Rationale: In veterans with posttraumatic stress disorder (PTSD), the disturbed sleep can worsen the cognitive-behavioral manifestations of PTSD and contribute to poor mental and physical health outcomes. Because adherence to treatment with CPAP is less than optimal in this population, this study was undertaken to examine the clinical efficacy, compliance, and quality of sleep of mandibular advancement devices (MAD) compared to CPAP in veterans with OSA and PTSD.
Study Impact: Although CPAP is more efficacious in eliminating respiratory events, both MAD and CPAP result in similar beneficial changes in daytime sleepiness, PTSD symptomatology, and health-related quality of life measures in veterans with OSA and PTSD.
Continuous positive airway pressure (CPAP) remains the preferred treatment for OSA. The benefits of using CPAP therapy extend beyond the recognized improvement in excessive daytime sleepiness, cognitive function, and cardiovascular parameters in patients with PTSD. Recent clinical investigations suggest that treatment of OSA in veterans with PTSD may improve the underlying psychological disturbances and reduce nightmares.4,8,9 However, despite the overall improvement in PTSD symptomatology with CPAP therapy, adherence to treatment is far worse in these patients compared with the general population with OSA.10,11 The reasons behind this poor adherence have not been thoroughly investigated but anxiety disorder, nightmares, claustrophobia, and comorbid insomnia have been implicated in low CPAP usage. Additionally, the CPAP mask may act as a reminder of war imagery that leads to a significant number of patients to refuse using it. Mandibular advancement devices (MAD) have been used as an alternative treatment for CPAP-intolerant patients and proven beneficial in mild to moderate cases of OSA without PTSD. Moreover, MAD is a more preferred therapy than CPAP treatment.12,13 Yet, the efficacy of this treatment modality has not been examined in patients with PTSD and OSA.
We hypothesized that MAD is not inferior to CPAP in eliminating apneic events, improving quality of life (QOL) measures, and ameliorating PTSD symptoms in veterans with PTSD and concomitant OSA. Therefore, we conducted a pragmatic randomized crossover trial of 12 weeks of CPAP and 12 weeks of MAD in 42 consecutive outpatients with PTSD and newly diagnosed OSA with the aim of comparing efficacy, reported side effects, adherence, and preference of both MAD and CPAP. We also examined the effectiveness of these 2 treatments using the PTSD Checklist,14 the Pittsburgh Sleep Quality Index (PSQI),15 and a generic Medical Outcomes Study 36-Item Short Form (SF-36).16
METHODS
Participants
All study-related procedures were conducted on an outpatient basis in compliance with the Institutional Review Board of the VA Western New York Health Care System and the trial was registered at ClinicalTrials.gov (NCT01569022). Recruitment was conducted from August 2013 to April 2016. Potential participants were screened for preliminary eligibility and provided with a complete description of the study, after which written informed consent was obtained and participants were enrolled. Inclusion criteria were: (1) veteran aged 18 to 70 years with an established diagnosis of PTSD based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria as documented by the attending psychiatrist responsible for the patient psychiatric care; (2) documented OSA by polysomnography (apnea-hypopnea index [AHI] ≥ 5 events/h); and (3) ability to sign an informed consent. Veterans with central apnea defined as central apnea/hypopnea > 50% of the total respiratory events, coexisting narcolepsy, unstable medical or psychiatric condition, and presence of temporomandibular joint disease were excluded from participation.
Measurements
Clinical Evaluation
A general medical history was recorded and a clinical examination was performed on each patient. Additional information on comorbidities, coexisting psychiatric disorders, daytime sleepiness,17 severity of PTSD (assessed by the PTSD Checklist (PCL-M),18 sleep quality (assessed by the PSQI15), quality of life (assessed by SF-3616), and medications use were obtained.
Polysomnography
Initial standard overnight polysomnography and CPAP titration were performed according to recognized standards. Sleep stages were recorded in 30-second epochs using the Rechtschaffen and Kales sleep scoring criteria.19 Each epoch was analyzed for the number of apneas, hypopneas, arousals, and oxygen desaturation. Apnea was defined as the absence of airflow for more than 10 seconds. Hypopnea was defined as reduction in airflow of at least 30% lasting at least 10 seconds associated with either a 4% decrease in arterial oxyhemoglobin saturation or an electroencephalographic arousal. An arousal was defined according to the criteria proposed by the Atlas Task Force.20 Severity of OSA was graded based on the AHI as mild OSA (5 ≥ AHI ≤ 15 events/h), moderate OSA (15 > AHI ≤ 30 events/h), or severe OSA (AHI > 30 events/h).
CPAP titration was conducted on a separate night in the sleep laboratory. Patients were initiated at a pressure of 4 cm H2O. The pressure was gradually increased by 1 cm H2O every 20 minutes until such a level at which apnea, hypopnea, snoring, and recurrent oxyhemoglobin desaturations, but not arousals were eliminated. The residual AHI was determined based on the optimal pressure recorded during CPAP titration. Resolution of OSA was considered to be achieved when AHI < 5 events/h. Following CPAP titration, a respiratory therapist provided education about the basic operation and care of the mask and CPAP device. An educational brochure on OSA and CPAP treatment was given to each patient during the education session. The respiratory therapist then selected and fit the patient with a comfortable nasal CPAP mask.
Dental Evaluation
Following a dental examination, alginate impressions were taken of the upper and lower arches, and dental models were made with dental stone. Custom MAD appliances were then fabricated for each patient. The MAD provided full coverage of the upper and lower dental arches. The initial protrusion was set at 75% of maximal protrusion (corresponding to a protrusion of 10 ± 0.4 mm [mean ± standard error]). Patients were asked to use the MAD on a daily basis over a 4-week period during which the device was incrementally advanced to the maximum comfortable limit. If a patient reported that snoring, sleepiness, or morning headache persisted without side effects such as tooth pain or jaw muscle pain, the dentist advanced the MAD. Conversely, if the patient reported side effects, the jaw position of the MAD was set back. These adjustments continued until a maximum subjective effect was achieved. Following final adjustments of the device, the treatment effect of MAD was assessed with a polysomnographic evaluation.
Intervention
Participants were asked to acclimate to CPAP and MAD for 4 weeks (total) during which adjustments to both modes of therapy were made aiming to optimize comfort and abolish snoring. If the interface was found to be uncomfortable, the patient was given the opportunity to change the mask. None of the participants were exposed to dual therapy or had access to both devices at the same time. Weekly phone calls were made to inquire about side effects or problems with CPAP or MAD. At the end of the acclimatization period, patients underwent a 2-week washout. After washout, they were randomly assigned in a 1:1 ratio via a presealed and numbered opaque white envelope to one of the two treatment modalities (CPAP or MAD). This included the assignment to receive 12 weeks of treatment with MAD and CPAP in alternating order, with an intervening 1-week washout. For each intervention, a clinic visit was scheduled at the beginning, middle, and end of treatment. During each visit, a review of medications, adherence assessment, and adverse event surveillance were made when applicable. In addition, the following surveys were completed: the Epworth Sleepiness Scale (ESS),17 PCL-M,18 the PSQI,15 and the SF-36.16
Assessment Instruments
ESS is a short questionnaire validated to measure excessive daytime sleepiness in patients with OSA.17 It measures the likelihood of falling asleep in 8 different situations, with a score of 0–3 for each situation. The sum of individual scores for the eight items gives the final ESS score, ranging from 0–21. An ESS score > 10 suggests excessive daytime sleepiness.
The SF-36 is a generic 36-item Short Form Medical Outcomes Study.16 It has 8 main domains: physical functioning, role limitation due to physical problems, role limitation due to emotional problems, social functioning, mental health, energy/vitality, bodily pain, and general health perception. Each dimension item score is coded, summed, and transformed into a scale from 0 to 100 (worst to best possible health). The PSQI is a self-rating questionnaire that consists of 7 dimensions of sleep quality including: subjective sleep quality, sleep latency, sleep duration, sleep efficiency, daytime dysfunction, sleep fragmentation, and use of sleep aid medications.15 The possible scores range from 0–21, with a score greater than 5 indicative of impaired sleep quality. The PTSD Checklist is a 17-item self-report measure (1–5 points each) that assesses PTSD symptoms in relation to stressful military experiences. PTSD symptom severity scores are determined by summing the participants' answers to all 17 items from 1 (“not at all”) to 5 (“extremely”) (range 17–85)14 with 5- to 10-point change indicating statistically significant response to treatment.
Adherence
CPAP use was objectively measured using a downloadable monitoring smartcard, whereas MAD adherence was derived from a diary in which participants recorded nightly use (from the time it is applied until it is removed) as well as any problems or side effects they were experiencing.
Statistical Analysis
The analysis was designed to establish noninferiority of MAD compared with CPAP for the primary outcome. We limited our evaluation to the 35 subjects who completed the trial regardless of adherence to their assigned treatment. All analyses were conducted by observers blinded to the subjects' identity. The results are expressed as mean ± standard deviation (SD) or median with interquartile range when indicated. Categorical variables were displayed as numbers and percentages. The primary endpoint of the study was tested by comparing the upper limit of the 95% confidence interval for the CPAP-MAD difference in residual AHI with the a priori noninferiority margin using the paired t test. Health outcomes including ESS, PCL-M, SF-36, and PSQI were assessed using hierarchical- level modeling with fixed effects for treatment (two treatment conditions), and sequence (the treatment by period interaction).21,22 In all models, random effects included subjects nested within sequence as a sampling cluster. This approach allowed direct between-treatment comparisons as well as post hoc comparisons between each treatment and baseline. A potential order effect of the different regimens was investigated through analysis of variance expected versus observed frequencies were compared with the chi-square statistic, applying Yates correction. Carryover was evaluated separately for each outcome with unpaired t tests comparing sum of the scores between those who started on CPAP and those who started on MAD.23 A carryover effect is demonstrated if the summed values are different between the two treatment groups (ie, CPAP first and MAD first). Effect sizes were assessed using the Cohen d relating the magnitude of group difference to the SD, and may be interpreted as follows: small, 0.20 to 0.49; medium, 0.50 to 0.79; and large, 0.80 or more.24 A statistically significant difference among means was defined by a value of P < .05. The P value was corrected for multiple comparisons using the Bonferroni adjustment when indicated. Statistical analysis was conducted using STATA version 13.0 (StataCorp LP, College Station, Texas, United States).
Sample Size Determination
Power for the proposed crossover trial was calculated with the XSAMPSI routine in STATA version 12.1 (StataCorp LP, College Station, Texas, United States) following steps described by Senn.25 In the assessment of noninferiority of oral appliance to CPAP therapy, noninferiority was defined as a difference between the proportions of treatment effectiveness of less than 25%. With a one-sided significance level of 5%, a power of 80%, and an assumed proportion of treatment effectiveness of 90%, a minimum of 36 participants would be required. This difference was based on the detection of a large effect (δ = 0.80).24 Allowing for a 15% attrition rate, a total sample of 42 subjects was targeted for enrollment.
RESULTS
A total of 127 veterans were considered for study participation between August 2013 and April 2016. Fifty-four patients agreed to enroll in the study but 12 patients either failed to return for scheduled visits or withdrew from further participation because of lack of time or other pressing matters. The patients who declined to participate were not different from the participants in terms of age, ESS score, or severity of apnea during sleep (P > .2). Forty-two were randomized to CPAP or MAD. During the course of the trial, 7 patients were lost to follow-up, leaving 35 patients for statistical analysis (Figure 1). Overall, the study sample included predominantly men, who were middle aged and obese. The mean age of the studied population was 52.7 ± 11.6 years. The mean body mass index (BMI) was 32.5 ± 5.6 kg/m2 and mean ESS score was 11.8 ± 5.6. At enrollment, 57% of patients had excessive daytime sleepiness with ESS score greater than 10. Hyper-tension and depression were the predominant comorbidities in the study population with a prevalence rate of 65% and 60%, respectively. Polysomnographic data showed a mean AHI of 34.7 ± 29.7 events/h with a mean arousal index of 33.4 ± 26.4 events/h and mean nadir oxygen saturation of 82.9 ± 5.6%. Thirty percent had mild OSA with a mean AHI of 10.0 ± 2.3 events/h, 23% had moderate OSA with mean AHI of 21.5 ± 4.8 events/h, and 47% had severe OSA with a mean AHI of 58.9 ± 28.9 events/h.
Figure 1. Study flow chart: randomization, treatment, and follow-up.
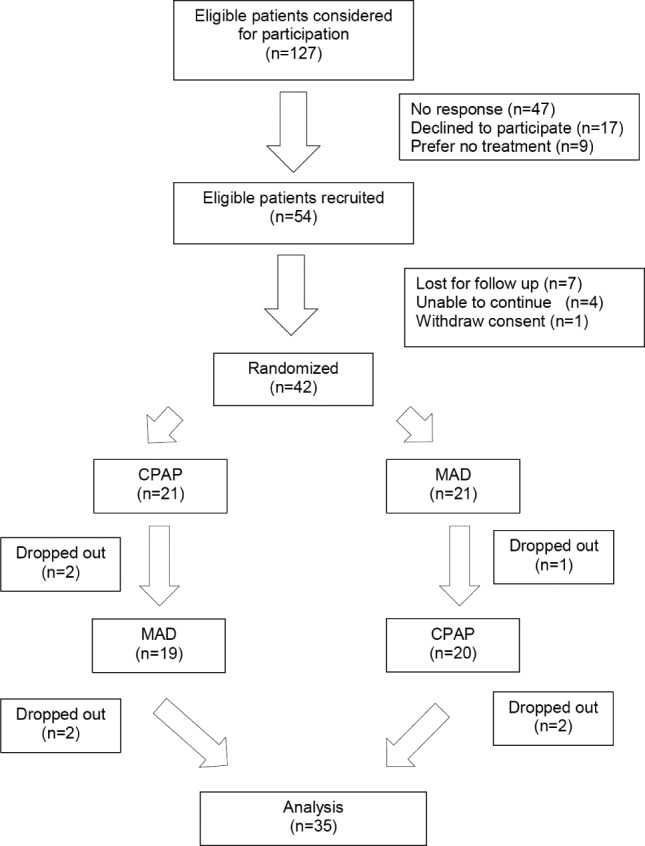
CPAP = continuous positive airway pressure, MAD = mandibular advancement device.
Treatment Efficacy
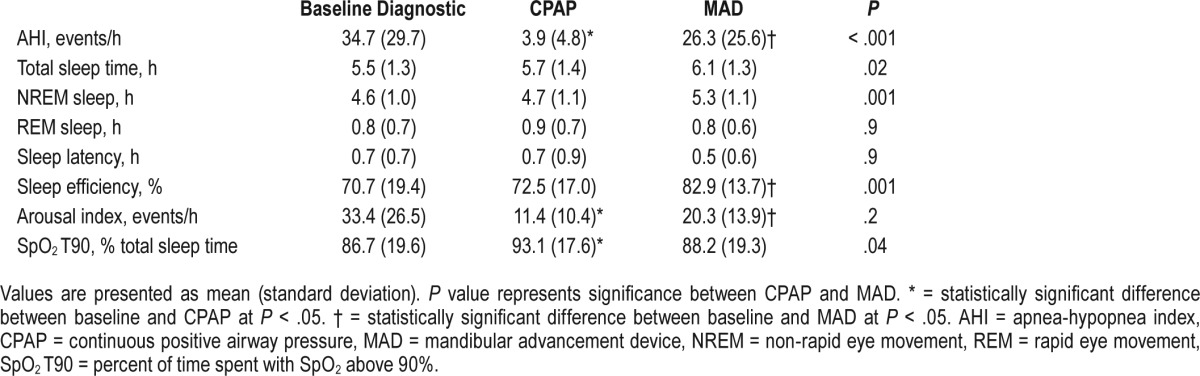
After titration and acclimatization with each device, the mean (SD) CPAP pressure was 8.9 ± 2.6 cm H2O (range, 4–14 cm H2O), whereas the mean mandibular advancement was 7.2 ± 2.8 mm (range, 1.1–14 mm). There was a significant difference in the efficacy of the two intervention modalities on sleep apnea treatment. Although AHI dropped with both CPAP and MAD treatment, sleep titration studies showed that the mean residual AHI was significantly higher for MAD compared to CPAP (26.3 ± 25.6 events/h versus 3.9 ± 4.8 events/h; P < .001, respectively) (Table 1). In total, 71% of CPAP titrated participants had complete resolution of their sleep apnea with CPAP, compared with only 14% with MAD (P < .001). The failure rate of normalizing AHI was 92% in patients with moderate and severe OSA for MAD and 26% for CPAP (P < .001). Other metrics of sleep-disordered breathing showed improvement with both treatments compared to baseline; however, participants on MAD had a longer total sleep time and higher sleep efficiency during the titration study than those on CPAP (Table 1). Conversely, subjects titrated with CPAP had a lower arousal index and an improved percent sleep time spent with SpO2 above 90% than those titrated with MAD.
Table 1.
Polysomnographic characteristics of CPAP and MAD titration.
Both CPAP and MAD resulted in significant improvement in ESS by 1.6 (95% CI 0.59 to 2.68) (P = .003) and 2.3 (95% CI 1.11 to 3.45) (P < .001) with estimated effect sizes (Cohen d) of 0.35 (95% CI 0.16 to 0.62) and 0.48 (95% CI 0.24 to 0.74), respectively. ESS scores after the washout period were similar to baseline, indicating a return to pretreatment sleepiness levels (P = .52).
PTSD Severity
PTSD symptoms, as assessed by PCL-M, improved following treatment with both MAD and CPAP (Figure 2). Compared to baseline, PCL-M scores decreased by 4.29 ± 12.0 and 6.22 ± 8.0 after 12 weeks of treatment with CPAP and MAD with estimated effect sizes (Cohen d) of 0.27 (95% CI 0.01 to 0.59; P = .04) and 0.47 (95% CI 0.28 to 0.73, P < .001), re -spectively. There was no significant difference in the extent of PTSD improvement using either CPAP or MAD (mean difference 1.97 [95% CI -2.89 to 6.83], P = .42).
Figure 2. PTSD response to treatment with CPAP and MAD.
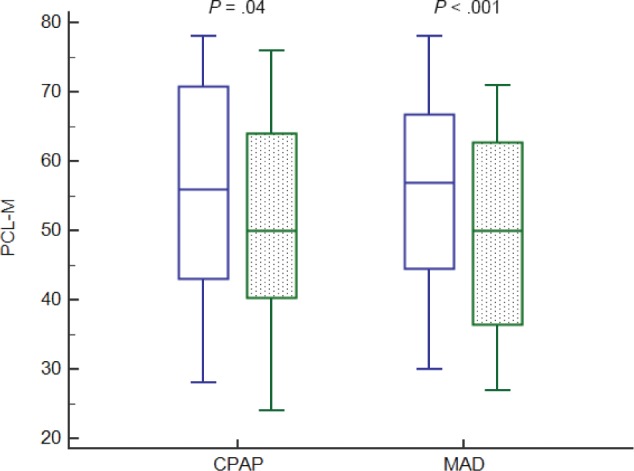
CPAP = continuous positive airway pressure, MAD = mandibular advancement device, PCL-M = PTSD Checklist-Military version; PTSD = posttraumatic stress disorder.
Quality of Life Measures
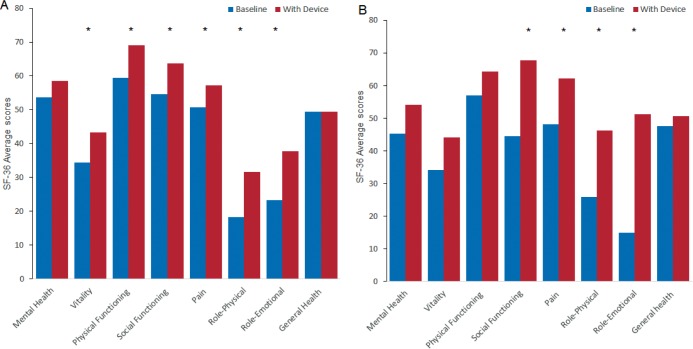
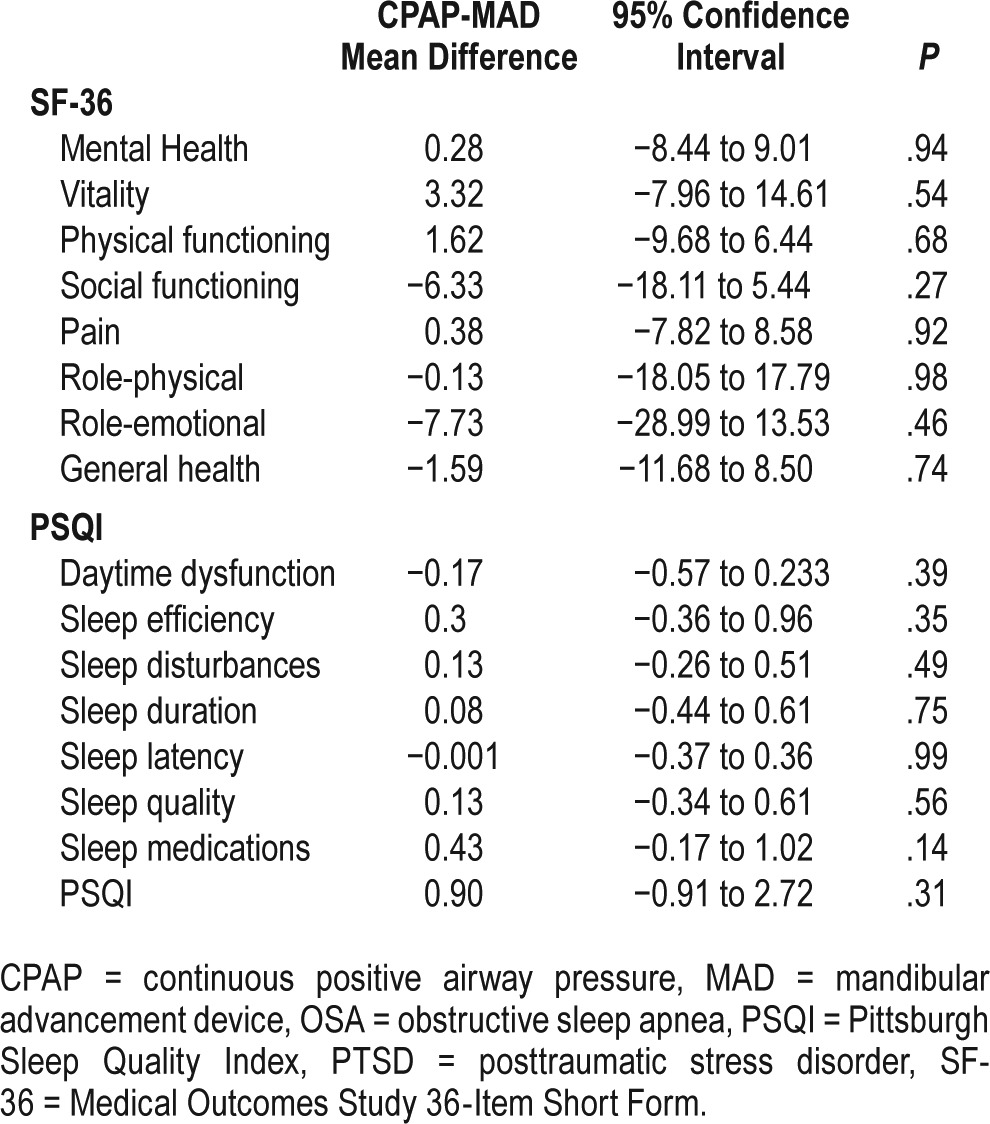
Unpaired t tests for carryover were nonsignificant for all quality heath outcome measures (all values of P > .05). Eight components of the SF-36 were collected in the studied group. The CPAP treatment arm resulted in significant improvement in 6 specific individual domains: vitality, pain, physical functioning, social functioning, role physical, and role emotional (Figure 3). The MAD arm showed improvement in 4 domains: social functioning, pain, role physical, and role emotional (Figure 3). The mental health and the standardized general health component score of the SF-36 did not have statistically significant change from baseline with either treatment.
Figure 3. SF-36 subdomain scores at baseline and at 12-week follow-up with CPAP (A) and with MAD (B).
* = P < .05. CPAP = continuous positive airway pressure, MAD = mandibular advancement device, SF-36 = Medical Outcomes Study 36-Item Short Form.
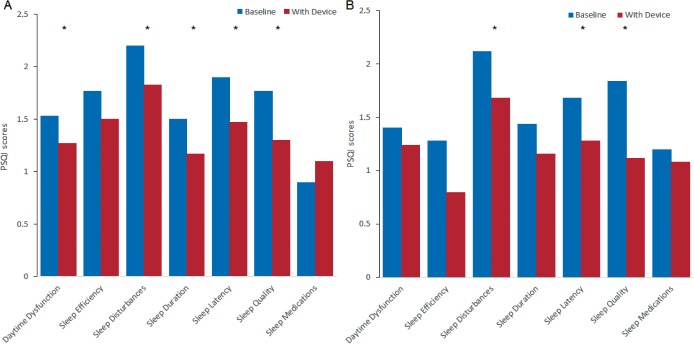
PSQI showed a significant decrease between baseline and end of treatment in the following specific items for the CPAP arm: daytime dysfunction, sleep disturbances, sleep duration, sleep latency, and sleep quality (Figure 4). The overall PSQI score showed improvement from 11.57 ± 4.02 to 9.63 ± 3.52 (P = .001). In the MAD arm, sleep disturbances, sleep latency, and sleep quality had significant decrease compared to baseline (Figure 4). The PSQI also exhibited a significant drop from 10.96 ± 3.04 to 8.36 ± 2.58 (P < .001). However, there were no significant differences between treatments in scores for SF-36 and PSQI (Table 2).
Figure 4. PSQI subdomain scores at baseline and at 12-week follow-up with CPAP (A) and with MAD (B).
* = P < .05. CPAP = continuous positive airway pressure, MAD = mandibular advancement device, PSQI = Pittsburgh Sleep Quality Index.
Table 2.
Quality of life response to OSA treatment in veterans with PTSD (n = 35).
Adherence and Reported Side Effects
Adherence to CPAP was significantly lower compared to MAD (P < .001). The mean nightly use of CPAP during nights used was only 3.4 ± 2.48 h/night. In contrast, MAD average device reported use per night was 5.66 ± 2.43 h/night. The 3 predominant reasons reported for CPAP nonadherence were mask discomfort (33%), claustrophobia (28%), and dry mouth (17%). Fifty-seven percent reported that the machine interfered with either falling asleep or maintaining sleep. Of interest, 10 of 19 patients with 50% or less use of therapy for an average of 4 h/night reported insomnia prior to CPAP treatment.
Alternatively, minor side effects were common with the oral appliance, particularly in the first month of treatment. These side effects included dryness of the mouth (26%), tooth discomfort or pain (19%), jaw pain (38%), and excessive salivation (15%). In most patients, the side effects were mild and improved with time. In no patient did any symptoms of temporomandibular joint dysfunction develop. Treatment preference results showed that 10 patients (29%) preferred CPAP; 20 (58%) preferred MAD; and 5 (13%) preferred neither. The medication regimen in both groups was unchanged, except 1 patient was taken off benzodiazepine at the beginning of CPAP treatment and 2 other patients had their dose of antidepressant drugs adjusted.
DISCUSSION
To the best of our knowledge, this is the first randomized comparative trial comparing CPAP and MAD based on PSG titration of both treatments in veterans with PTSD. Both therapies had salutatory effects on polysomnographic variables during follow-up, but CPAP therapy was significantly more efficacious in improving AHI and oxyhemoglobin saturation levels. Consistent with previous investigations,26–31 both treatment modalities were comparable in improving subjective sleepiness, functional outcomes, and health perceptions.
Four separate meta-analyses were conducted comparing MAD against CPAP in OSA.32–35 With a total of 13 studies selected for review (746 patients), the estimated overall difference in AHI was 7.03 events/h (95% CI 5.41, 8.66), with CPAP having lower posttreatment AHI than MAD. CPAP produced an improvement of approximately 3 times that of the combined estimate for MAD. The difference in AHI between the two treatment modalities was more accentuated in our study, as severe sleep apnea accounted for 47% of the total apneic population. A lower baseline AHI, lower BMI, and younger age were all associated with better treatment responses to oral appliance.36,37 In this follow-up, we observed a higher rate of MAD failure to normalize AHI as the severity of sleep apnea worsened. There was no other discernible trend pointing to a higher MAD efficacy but the relatively small sample size could have been a limiting factor in our analysis. Lateral cephalometry can identify craniofacial characteristics that could have an effect on treatment response, although no definitive clinical recommendations are available because of inherent methodological weaknesses of the currently available studies.38
Reported improvements in subjective daytime sleepiness and health perceptions were found in both treatment groups, underscoring the therapeutic benefit of CPAP and MAD therapy at all timepoints during the follow-up period, even in patients with severe OSA. Similar findings in different studies using the same questionnaires (pooled) were reported in a review article by Chan and asociates.39 Gagnadoux and colleagues30 found comparative subjective improvements among patients treated with CPAP or MAD using the Nottingham Health Profile questionnaire. For CPAP, a significant improvement was observed for 2 out of 6 domains of health-related quality of life, namely emotional reaction and energy. For MAD, health-related quality of life was significantly improved for 4 out of 6 domains, namely physical mobility, pain, emotional reaction, and sleep. We have observed parallel improvement in social functioning, pain, role physical, and role emotional with both CPAP and MAD with no significant difference between the two treatments. Energy (vitality) was the only measure in both the study by Gagnadoux et al. and the current investigation to show improvement with CPAP but not MAD, which may reflect an acute alteration of energy balance secondary to sleep consolidation given the higher efficacy of CPAP compared to MAD.
Results from recent investigations have revealed that adequate treatment of OSA with CPAP has been linked to amelioration of symptoms of PTSD including nightmares.8,40,41 In one of these studies involving 40 veterans with combat-related PTSD, a positive association was established between the reduction in PCL-M and the average of hours of CPAP use per night.40 Similarly, Orr and colleagues41 showed significant reduction in PTSD symptoms following 6 months of treatment with CPAP. In this study, we were able to show that the amelioration in PTSD severity extended also to OSA treatment with MAD. Surprisingly, the effect size improvements in PTSD Checklist were comparable for both CPAP and MAD despite the lower efficacy of MAD. Evidence of equivalent health outcomes between oral appliances and CPAP suggests that treatment effectiveness may not be captured solely by reduction in AHI. Theoretically many patients with incomplete efficacy on oral appliance are no worse off than when on fully efficacious CPAP in terms of treatment effectiveness. As the overall effectiveness of treatment intervention in sleep-disordered breathing depends on adherence to treatment, it follows that treatment effectiveness can be expressed as a composite of efficacy and hours of treatment usage.42 In support of this argument, studies that have evaluated noninvasive treatment of sleep-disordered breathing have uniformly reported a superior rate of adherence to MAD over CPAP across the entire AHI spectrum.42 Alternatively,43 although greater nightly adherence to MAD compared with CPAP therapy has been the driving hypothesis for the lack of difference in health outcomes, other physiologic indices may be at play. AHI, the defining measure of sleep apnea, appraises only the respiratory component of the disease and may not account for the myriad of inflammatory markers that are upregulated in patients with this ailment.44 Studies of a sleep apnea cohort have documented higher concentrations of proinflammatory cytokines such as tumor necrosis factor, interleukin-1, and interleukin-6 in patients with sleep apnea compared with controls; however, the association of these markers with AHI has not been universal.45,46 Considering that these biomarkers have been linked to impaired endovascular and cognitive functioning,47,48 abatement of these inflammatory biomarkers following treatment whether by using CPAP or MAD49,50 may infer additional benefit beyond the rectification of respiratory abnormalities by either modality.
Systematic reviews have extensively examined physiological, psychological and motivational factors associated with treatment adherence in patients with sleep apnea.51 Patient characteristics such as age, sex, BMI, race, education, and socioeconomic status have been examined as possible predictors of CPAP adherence without consistent findings.52 Factors such as education, telephone calls, and reinforcement alone had also no effect on CPAP utilization.53 Data are scarce on the relevance of these variables in patients with PTSD and no uniform predictors of adherence have been formalized in this population. However, presence of nightmares, claustrophobia, and concomitant insomnia have been implicated in low CPAP adherence in veterans with PTSD.10,11 In our cohort, concomitant insomnia was a factor in CPAP nonadherence as more than half of those who participated reported difficulty falling asleep. Collen and colleagues11 have reported a greater use of CPAP in veterans with PTSD using sedating medications than those not prescribed these agents. We did not find a link between use of hypnotics and CPAP adherence in our participants; however, we did not account for sedatives sold over the counter or antipsychotic medications.
Several limitations should be considered in relation to our study. First, participants were selected from a specialized sleep disorders clinic with a research interest in alternatives to CPAP therapy and therefore, referral bias cannot be excluded. Second, daytime sleepiness and insomnia were subjectively assessed and no objective measures of these parameters were obtained. Consequently, we are unable to ascertain the underlying causes and the reproducibility of insomnia and sleep disturbances in this population. Third, the protocol is designed to provide a 2-week washout period to minimize any carryover effects from previous assigned therapy. This period is more than adequate because 2 previous studies that used crossover design to compare the efficacy of mandibular appliances with nasal CPAP failed to detect any significant carryover effects.54,55 Fourth, the residual AHI for MAD-treated subjects was higher than previously reported.42 The subjective titration by self-reporting may have resulted in suboptimal response to MAD.56 Advancing the oral appliance during a titration polysomnogram could have reduced the difference in residual AHI between CPAP and MAD. However, it is unlikely that the health outcomes would have been significantly different had the device been advanced during a titration sleep study for those with incomplete response because the magnitude of the MAD-attributed benefits on health outcomes would have been potentially larger, leading to a much smaller effect size. Fifth, we have relied on participants' diary to denote MAD adherence, which may have overestimated compliance. However, recordings from oral appliance devices with embedded microsensors have found no difference between objective and subjective MAD adherence.57
In conclusion, the results of our study support titrated MAD as an effective treatment for veterans with PTSD and OSA. Although less efficacious than CPAP, MAD was associated with comparable improvement in PTSD severity and functional outcomes.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. All authors have no conflicts of interest to disclose. The study was supported by a Merit Review Grant (CX000478) from the Department of Veterans Affairs (AES). The views expressed in this study do not communicate an official position of the Department of Veterans Affairs.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- MAD
mandibular advancement device
- OSA
obstructive sleep apnea
- PCL
PTSD Checklist
- PSQI
Pittsburgh Sleep Quality Index
- PTSD
posttraumatic stress disorder
- REM
rapid eye movement
- SD
standard deviation
- TST
total sleep time
REFERENCES
- 1.Yesavage JA, Kinoshita LM, Kimball T, et al. Sleep-disordered breathing in Vietnam veterans with posttraumatic stress disorder. Am J Geriatr Psychiatry. 2012;20(3):199–204. doi: 10.1097/JGP.0b013e3181e446ea. [DOI] [PubMed] [Google Scholar]
- 2.Lettieri CJ, Williams SG, Collen JF. OSA syndrome and posttraumatic stress disorder: clinical outcomes and impact of positive airway pressure therapy. Chest. 2016;149(2):483–490. doi: 10.1378/chest.15-0693. [DOI] [PubMed] [Google Scholar]
- 3.Krakow B, Melendrez D, Johnston L, et al. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis. 2002;190(7):442–452. doi: 10.1097/00005053-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291–298. doi: 10.1016/s0022-3999(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 5.Colvonen PJ, Masino T, Drummond SP, Myers US, Angkaw AC, Norman SB. Obstructive sleep apnea and posttraumatic stress disorder among OEF/OIF/ OND veterans. J Clin Sleep Med. 2015;11(5):513–518. doi: 10.5664/jcsm.4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ocasio-Tascon ME, Alicea-Colon E, Torres-Palacios A, Rodriguez-Cintron W. The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath. 2006;10(2):70–75. doi: 10.1007/s11325-005-0043-9. [DOI] [PubMed] [Google Scholar]
- 7.Krakow B, Artar A, Warner TD, et al. Sleep disorder, depression, and suicidality in female sexual assault survivors. Crisis. 2000;21(4):163–170. doi: 10.1027//0227-5910.21.4.163. [DOI] [PubMed] [Google Scholar]
- 8.Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA) J Clin Sleep Med. 2014;10(6):631–636. doi: 10.5664/jcsm.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youakim JM, Doghramji K, Schutte SL. Posttraumatic stress disorder and obstructive sleep apnea syndrome. Psychosomatics. 1998;39(2):168–171. doi: 10.1016/S0033-3182(98)71365-9. [DOI] [PubMed] [Google Scholar]
- 10.El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495–1500. doi: 10.1093/sleep/33.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667–672. doi: 10.5664/jcsm.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37(5):1000–1028. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 13.Chan AW. Bias, spin, and misreporting: time for full access to trial protocols and results. PLoS Med. 2008;5(11):e230. doi: 10.1371/journal.pmed.0050230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weathers FW, Litz BT, Herman DS, Huska JA, Keane TM. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at: 9th Annual Conference of the ISTSS; 1993; San Antonio, TX. [Google Scholar]
- 15.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 16.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31(3):247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Weathers FW, Litz BT, Huska JA, Keane TM. PCL-M for DSM-IV. Boston, MA: National Center for PTSD - Behavioral Science Division; 1994. [Google Scholar]
- 19.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: Brain Information Service/Brain Research Institute, University of California; 1968. [Google Scholar]
- 20.EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15(2):173–184. [PubMed] [Google Scholar]
- 21.Jones B, Kenward MG. Design and Analysis of Cross-over Trials. 2nd ed. Boca Raton, FL: Chapman & Hall/CRC; 2003. [Google Scholar]
- 22.Raudenbush S, Bryk A. Hierarchical Linear Models: Applications and Data Analysis Methods. 2nd ed. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 23.Wellek S, Blettner M. On the proper use of the crossover design in clinical trials: part 18 of a series on evaluation of scientific publications. Dtsch Arztebl Int. 2012;109(15):276–281. doi: 10.3238/arztebl.2012.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Erlbaum; 1988. [Google Scholar]
- 25.Senn S. Cross-over Trials in Clinical Research. Chichester, NY: John Wiley & Sons; 1993. [Google Scholar]
- 26.Gotsopoulos H, Chen C, Qian J, Cistulli PA. Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med. 2002;166(5):743–748. doi: 10.1164/rccm.200203-208OC. [DOI] [PubMed] [Google Scholar]
- 27.Engleman HM, McDonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166(6):855–859. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 28.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62(4):354–359. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 30.Gagnadoux F, Fleury B, Vielle B, et al. Titrated mandibular advancement versus positive airway pressure for sleep apnoea. Eur Respir J. 2009;34(4):914–920. doi: 10.1183/09031936.00148208. [DOI] [PubMed] [Google Scholar]
- 31.Doff MH, Hoekema A, Wijkstra PJ, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: a 2-year follow-up. Sleep. 2013;36(9):1289–1296. doi: 10.5665/sleep.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoeahypopnoea syndrome: a systematic review and economic analysis. Health Technol Assess. 2009;13(4):iii–iv. xi–xiv, 1–119, 143–274. doi: 10.3310/hta13040. [DOI] [PubMed] [Google Scholar]
- 33.Giles TL, Lasserson TJ, Smith BJ, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;(1):CD001106. doi: 10.1002/14651858.CD001106.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Lim J, Lasserson TJ, Fleetham J, Wright J. Oral appliances for obstructive sleep apnoea. Cochrane Database Syst Rev. 2006;(1):CD004435. doi: 10.1002/14651858.CD004435.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharples LD, Clutterbuck-James AL, Glover MJ, et al. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev. 2016;27:108–124. doi: 10.1016/j.smrv.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Lowe AA, Fleetham JA, Park YC. Cephalometric and physiologic predictors of the efficacy of an adjustable oral appliance for treating obstructive sleep apnea. Am J Orthod Dentofacial Orthop. 2001;120(6):639–647. doi: 10.1067/mod.2001.118782. [DOI] [PubMed] [Google Scholar]
- 37.Mehta A, Qian J, Petocz P, Darendeliler MA, Cistulli PA. A randomized, controlled study of a mandibular advancement splint for obstructive sleep apnea. Am J Respir Crit Care Med. 2001;163(6):1457–1461. doi: 10.1164/ajrccm.163.6.2004213. [DOI] [PubMed] [Google Scholar]
- 38.Alessandri-Bonetti G, Ippolito DR, Bartolucci ML, D'Anto V, Incerti-Parenti S. Cephalometric predictors of treatment outcome with mandibular advancement devices in adult patients with obstructive sleep apnea: a systematic review. Korean J Orthod. 2015;45(6):308–321. doi: 10.4041/kjod.2015.45.6.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan AS, Lee RW, Cistulli PA. Dental appliance treatment for obstructive sleep apnea. Chest. 2007;132(2):693–699. doi: 10.1378/chest.06-2038. [DOI] [PubMed] [Google Scholar]
- 40.El-Solh AA, Vermont L, Homish GG, Kufel T. The effect of continuous positive airway pressure on post-traumatic stress disorder symptoms in veterans with post-traumatic stress disorder and obstructive sleep apnea: a prospective study. Sleep Med. 2017;33:145–150. doi: 10.1016/j.sleep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Orr JE, Smales C, Alexander TH, et al. Treatment of OSA with CPAP is associated with improvement in PTSD symptoms among veterans. J Clin Sleep Med. 2017;13(1):57–63. doi: 10.5664/jcsm.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sutherland K, Phillips CL, Cistulli PA. Efficacy versus effectiveness in the treatment of obstructive sleep apnea: CPAP and oral appliances. Journal of Dental Sleep Medicine. 2015;2(4):175–181. [Google Scholar]
- 43.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. J Clin Sleep Med. 2014;10(2):215–227. doi: 10.5664/jcsm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med. 2013;9(10):1003–1012. doi: 10.5664/jcsm.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svensson M, Venge P, Janson C, Lindberg E. Relationship between sleep-disordered breathing and markers of systemic inflammation in women from the general population. J Sleep Res. 2012;21(2):147–154. doi: 10.1111/j.1365-2869.2011.00946.x. [DOI] [PubMed] [Google Scholar]
- 46.Thunstrom E, Glantz H, Fu M, et al. Increased inflammatory activity in nonobese patients with coronary artery disease and obstructive sleep apnea. Sleep. 2015;38(3):463–471. doi: 10.5665/sleep.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marsland AL, Petersen KL, Sathanoori R, et al. Interleukin-6 covaries inversely with cognitive performance among middle-aged community volunteers. Psychosom Med. 2006;68(6):895–903. doi: 10.1097/01.psy.0000238451.22174.92. [DOI] [PubMed] [Google Scholar]
- 48.Haensel A, Bardwell WA, Mills PJ, et al. Relationship between inflammation and cognitive function in obstructive sleep apnea. Sleep Breath. 2009;13(1):35–41. doi: 10.1007/s11325-008-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nizankowska-Jedrzejczyk A, Almeida FR, Lowe AA, et al. Modulation of inflammatory and hemostatic markers in obstructive sleep apnea patients treated with mandibular advancement splints: a parallel, controlled trial. J Clin Sleep Med. 2014;10(3):255–262. doi: 10.5664/jcsm.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yokoe T, Minoguchi K, Matsuo H, et al. Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation. 2003;107(8):1129–1134. doi: 10.1161/01.cir.0000052627.99976.18. [DOI] [PubMed] [Google Scholar]
- 51.Olsen S, Smith S, Oei TP. Adherence to continuous positive airway pressure therapy in obstructive sleep apnoea sufferers: a theoretical approach to treatment adherence and intervention. Clin Psychol Rev. 2008;28(8):1355–1371. doi: 10.1016/j.cpr.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Alves C, Caminha JM, da Silva AM, Mendonca D. Compliance to continuous positive airway pressure therapy in a group of Portuguese patients with obstructive sleep apnea syndrome. Sleep Breath. 2012;16(2):555–562. doi: 10.1007/s11325-011-0542-9. [DOI] [PubMed] [Google Scholar]
- 53.Weaver TE. Adherence to positive airway pressure therapy. Curr Opin Pulm Med. 2006;12(6):409–413. doi: 10.1097/01.mcp.0000245715.97256.32. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. 2006;29(2):244–262. doi: 10.1093/sleep/29.2.244. [DOI] [PubMed] [Google Scholar]
- 55.Ferguson KA, Ono T, Lowe AA, al-Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. 1997;52(4):362–368. doi: 10.1136/thx.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Almeida FR, Parker JA, Hodges JS, Lowe AA, Ferguson KA. Effect of a titration polysomnogram on treatment success with a mandibular repositioning appliance. J Clin Sleep Med. 2009;5(3):198–204. [PMC free article] [PubMed] [Google Scholar]
- 57.Vanderveken OM, Dieltjens M, Wouters K, De Backer WA, Van de Heyning PH, Braem MJ. Objective measurement of compliance during oral appliance therapy for sleep-disordered breathing. Thorax. 2013;68(1):91–96. doi: 10.1136/thoraxjnl-2012-201900. [DOI] [PMC free article] [PubMed] [Google Scholar]